Abstract
Background:
Connectivity between the anterior insula (AI) and the bed nucleus of the stria terminalis (BNST) may play a role in negative emotions that drive compulsive drinking in patients with alcohol use disorder (AUD). We hypothesized that reductions in drinking during cognitive behavioral therapy (CBT), an effective treatment that teaches regulation (coping) skills for managing negative emotions during abstinence, would be associated with reductions in resting state functional connectivity (RSFC) between the AI and the BNST.
Methods:
We included 18 patients with a DSM-V diagnosis of AUD who were 1) seeking treatment and 2) drinking heavily at baseline. We measured RSFC as a Pearson’s correlation between the BNST and multiple insula regions of interest (ROIs) at baseline and after completion of 12 weeks of a single-arm clinical trial of outpatient CBT. We also assessed the number of heavy drinking days over the previous 28 days (NHDD) at both timepoints. We used one-sample t-tests to evaluate AI-BNST RSFC at baseline, paired t-tests to evaluate changes in AI-BNST RSFC from pre-CBT to post-CBT, and linear regression to evaluate the relationship between changes in AI-BNST RSFC and NHDD.
Results:
We found that there was significant positive RSFC between the AI and the BNST at baseline (p=0.0015). While there were no significant changes in AI-BNST RSFC from pre- to post-CBT at the group level (p=0.42), we found that individual differences in reductions in AI-BNST RSFC from pre- to post-CBT were directly related to reductions in NHDD from pre- to post-CBT (r = 0.73, p=0.0008).
Conclusions:
These findings provide evidence that reduced AI-BNST RSFC may be a mechanism of drinking reduction in AUD and that AI-BNST RSFC may be a target for CBT and possibly other treatments.
Keywords: BNST, CBT, functional connectivity, insula, negative affect
INTRODUCTION
Alcohol use disorder (AUD) is a chronic condition that causes significant morbidity and mortality (Sacks et al., 2015; White et al., 2020). Though effective pharmacologic and behavioral treatments are available, considerable heterogeneity among treatment responses exist (Naqvi and Morgenstern, 2015; Witkiewitz et al., 2019). Identifying the neural systems that underlie behavior change in currently effective treatments may allow for the discovery of targets for novel, more effective treatments (Naqvi and Morgenstern, 2015; Ray et al., 2020; Witkiewitz et al., 2019). In this study we focused on resting state functional connectivity between the anterior insula (AI) and the bed nucleus of the stria terminalis (BNST) as a neural correlate of behavior change in AUD during treatment with cognitive behavioral therapy (CBT).
The role of the insula in addiction was established by Naqvi and colleagues, who showed that smokers with insular strokes quit smoking with ease (Naqvi et al., 2007). They proposed a model in which the anterior insula conveys homeostatically relevant information about the interoceptive impact of substance use and withdrawal to subcortical motivation systems that drive craving and relapse, especially under more deliberative, self-regulated conditions, such as during treatment (Naqvi et al., 2014). Pre-clinical work has shown that the BNST, part of the extended amygdala, plays an important role in negative reinforcement behaviors associated with both acute (Olive et al., 2002) and prolonged alcohol deprivation and withdrawal (Koob and Volkow, 2016). The BNST has been implicated in experimental models of diffuse (generalized) anxiety states that lack a discreet external object, such as responses to prolonged or uncertain threat (Davis et al., 2010; Goode et al., 2019; Walker et al., 2009) and contextual fear conditioning (Sullivan et al., 2004). However, no study as of yet has examined the role of the BNST in withdrawal in AUD or any other substance use disorder in a human clinical population. Rodent tract tracing studies have demonstrated inputs to the BNST from both the agranular (Reynolds and Zahm, 2005) and dysgranular (Shi and Cassell, 1998) insula, regions corresponding to AI in humans. A human probabilistic tractography study (Flook et al., 2020) has shown structural connectivity between ventral AI and BNST. A study using optogenetics in rodents (Centanni et al., 2019) showed that excitatory inputs from agranular insula to the BNST drive a negative emotion phenotype induced by alcohol abstinence in rodents. One model that emerges from this is that inputs from the AI may serve to convey information about the interoceptive impact of alcohol withdrawal to the BNST, which promotes negative emotions that drive compulsive drinking. In this model, abstinence causes an increase in functional coupling between AI and BNST, which leads to increased negative emotion that then motivates drinking (negative reinforcement).
CBT is a behavioral treatment commonly delivered to outpatients who have a goal of initiating abstinence, often in combination with medications (Kranzler and Soyka, 2018; McCrady et al., 2014; Witkiewitz et al., 2019; Witkiewitz and Marlatt, 2011). A major impediment to initiating abstinence is anxiety and other negative emotions that are triggered by abstinence (withdrawal). CBT is designed in part to teach “coping skills” aimed at improving patients’ ability to modulate anxiety and other negative emotions that hamper drinking reduction (Longabaugh and Morgenstern, 1999). CBT has been proposed to work at the neural level by targeting prefrontal cortex (PFC) systems that govern executive function and goal-directed behavior, in particular their regulation of cortical and subcortical systems that generate both incentive-salience (e.g., cue-induced craving) and negative emotions such as anxiety (Naqvi and Morgenstern, 2015). According to this model, CBT may facilitate drinking reduction by down-regulating AI-BNST functional connectivity that would otherwise be increased by abstinence.
Resting state functional connectivity (RSFC) is a functional MRI technique that captures the spontaneous synchronous neural activity of anatomically and functionally related neural circuitry (Biswal et al., 1995). RSFC is known to be related to stable trait variables, but it can also index changes related to experience-dependent plasticity (Laumann and Snyder, 2021; Newbold et al., 2020), making it a useful probe for changes due to a behavioral treatment. RSFC has been used in studies investigating differences between patients with substance use disorders (SUDs) and healthy controls, relationships between RSFC and SUD severity, and using baseline RSFC to predict treatment outcomes (Wilcox et al., 2018). To our knowledge, only two studies (Holla et al., 2020; Perini et al., 2020) have examined how RSFC changes during treatment in AUD, and neither of these examined how changes in RSFC relate to changes in alcohol use. Furthermore, while several studies in AUD have examined RSFC between the insula and other cortical (Dai et al., 2021; Dean et al., 2020; Halcomb et al., 2019; Karch et al., 2015; Perini et al., 2020; Vergara et al., 2017) and subcortical areas (Orban et al., 2013), none have examined RSFC of the insula with the BNST.
Here, we examined RSFC between the BNST and the insula in treatment seeking patients with AUD who were drinking heavily at baseline who then received a course of outpatient CBT for abstinence initiation as part of a single-arm mechanistic clinical trial. We first examined RSFC between BNST and multiple anterior and posterior insula regions during a minimum 24-hour abstinent period prior to beginning CBT, in order to identify insula regions in which elevated positive RSFC with the BNST may reflect withdrawal due to drinking reduction. We then examined the relationships between changes in insula-BNST RSFC and changes in drinking behavior during CBT. We predicted that there would be significant positive RSFC between the anterior insula and the BNST (AI-BNST RSFC) at baseline, that AI-RSFC would be reduced after completing CBT at the group level, and that reductions in AI-BNST would be correlated with individual differences in reductions in the number of heavy drinking days.
MATERIALS AND METHODS
Participants
Treatment-seeking participants with AUD were recruited through the Substance Treatment and Research Service (STARS) at the New York State Psychiatric Institute (NYSPI)/Columbia University Irving Medical Center and provided informed consent in accordance with the NYSPI Institutional Review Board (IRB) from 07/2015–12/2019, after the conclusion of enrollment. We aimed to recruit 20–25 participants based on previous, similarly designed studies (Abler et al., 2012; DeVito EE et al., 2012; Kober et al., 2010; Ritche et al., 2011). Participants were recruited in the local New York City community through advertisements on radio, the internet and print media. Participants were eligible if they 1) were between the ages of 18 and 65; 2) were English-speaking and able to provide informed consent and comply with study procedures, 3) were right-handed; 4) had an average of at least 4 heavy drinking days (defined as ≥ 4 standard drinks for women and ≥ 5 drinks for men) per week over the last 28 days, 5) met Diagnostic and Statistical Manual for Mental Disorders 5th edition (DSM-5) criteria for Alcohol Use Disorder (AUD); 6) had a current goal of abstaining from alcohol use; 7) were seeking treatment and were willing to not seek additional treatment, apart from attending Alcoholics Anonymous (AA); and 8) were willing to abstain from alcohol completely for 24 hours on 3 separate occasions. Participants were excluded if they had 1) any current moderate or severe substance use disorder other than alcohol, nicotine, or caffeine use disorders; 2) a lifetime history of bipolar disorder, schizophrenia, or schizoaffective disorder; 3) a diagnosis of any current psychiatric disorder that in the investigator’s judgment might require intervention over the course of the study; 8) history of severe alcohol withdrawal (e.g., seizure, delirium tremens, complicated or resistant alcohol withdrawal); 9) significant risk for suicide or violence; 10) legally mandated to receive treatment; 11) sufficient social instability to preclude study participation; 12) currently taking any psychotropic medications; 13) significant cognitive impairment; or 14) neurological or medical conditions that would interfere with MRI scanning.
Overall Study Structure
All participants received a diagnostic assessment using the MINI (Sheehan et al., 1998), along with psychiatric and medical evaluation. After meeting eligibility criteria, participants underwent a baseline assessment of drinking levels along with a battery of self-report scales related to drinking behavior (Supplement). For the baseline assessment, participants were instructed to abstain from alcohol for at least 24 hours prior to coming to the clinic. Participants who exhibited moderate or severe alcohol withdrawal (score >9 on the Clinical Institute for Withdrawal from Alcohol, CIWA-AR (Sullivan et al., 1989), or who were unable to abstain for >24 hours at the baseline assessment were excluded from MRI scanning. Participants then underwent the pre-CBT MRI scan after abstaining for at least 24 hours. Following the MRI session, all participants received up to 12 once-weekly sessions of manualized CBT over 12 weeks (single arm; no control intervention). After 12 weeks, participants repeated the alcohol assessment battery. They then underwent a post-CBT MRI scan after abstaining for at least 24 hours. The overall study procedures, relationship to brief abstinence periods, and the timing of the various assessments are illustrated in Table 1.
Table 1:
Summary of Study Procedures and Assessments
| Screening | *Baseline Assessment | *Pre-CBT MRI | CBT Wk 6 | Post-CBT Assessment | *Post-CBT MRI | |
|---|---|---|---|---|---|---|
| TLFB-NHDD | x | x | x | x | x | x |
| HAM-A | x | x | ||||
| Psychiatric and Medical Evaluation | x | x | ||||
| CIWA | x | x | x | x | ||
| Vital signs | x | x | x | x | x | x |
| BAL | x | x | x | x | x | x |
| Urine drug screen | x | x | x | |||
| Time since last drink | x | x | x | x | ||
| Alcohol Battery | x | x |
After >24-h abstinence
Clinical Outcome
The primary clinical outcome was the number of heavy drinking days (NHDD), calculated as the number of days over the previous 28 days on which ≥ 5 drinks for men or ≥ 4 drinks for women were consumed, as assessed using the Timeline Followback (TLFB) method (Sobell and Sobell, 1995). This was calculated at both the baseline assessment (pre-CBT NHDD), and the post-CBT assessment at 12 weeks (post-CBT NHDD). NHDD given is a commonly used outcome in clinical trials for AUD (Kranzler et al., 2014; Simpson et al., 2018). Further, recent evidence suggests that reductions in heavy drinking (vs. achieving abstinence) may correspond to clinically meaningful outcomes (Falk et al., 2019).
CBT Sessions
Participants received up to 12 sessions of CBT, administered by a masters or doctoral level clinician with experience delivering CBT, adhering to the Project Match CBT Manual (Kadden et al., 2003). Each session lasted one hour and included the following modules: 1) Introduction to Coping Skills; 2) Coping with Craving and Urges to Drink; 3) Managing Thoughts About Alcohol and Drinking; 4) Problem Solving; 5) Drink Refusal Skills; 6) Planning for Emergencies and Coping with Lapses; 7) Seemingly Irrelevant Decisions; 8) up to 5 elective modules on various topics as relevant. Additionally, sessions included assessments of drinking levels and psychiatric symptoms. Only participants who completed a minimum of 6 sessions were included in the analyses.
MRI Acquisition and Pre-Processing
The scanning procedures for both pre- and post-treatment MRI scanning sessions were the same. Participants first completed a 7-day TLFB to quantify recent drinking, including the number of drinks over the last 24 hours. They were administered a breath alcohol test, a urine drug test, vital signs, the CIWA-Ar, the Alcohol Urge Questionnaire (AUQ) (Bohn et al., 1995) and a urine drug screen immediately prior to both scans, along with a 7-day Timeline Followback (Sobell and Sobell, 1995) to ascertain drinking levels over the week prior the MRI scan, as well as a question about the number of hours since the last drink. Participants completed a single 10-minute resting state scan during which they were instructed to rest with eyes open while looking at a fixation cross. They also completed a Regulation of Craving task (Kober et al., 2010; Naqvi et al., 2015; Suzuki et al., 2019) in the MRI scanner, the results of which are not reported here.
MRI data were collected on a 3T MR750 GE Scanner. Functional images were acquired with a single band EPI sequence with a TR of 2000ms, TE of 25ms, flip angle of 77°, 64×64 in plane matrix, field of view 19.2 cm, and 45 3mm slices in an ascending, interleaved order. High resolution (1mm isotropic) structural T1 images were also acquired with a flip angle of 12°, 256×256 in-plane matrix, and field of view 25.6 cm. Data were processed using fMRIPrep version 1.5.10, which performs standard pre-processing steps including alignment to individual’s anatomical data, movement correction, distortion correction, and atlas alignment into MNI volume space.(Esteban et al., 2019) We then used Ciftify version 2.3.3, which allows for the adaptation of Human Connectome Project Pipelines to legacy data in which T2w and fieldmaps were not acquired.(Dickie et al., 2019). Specifically, Ciftify performs surface-based extraction and surface atlas alignment of grey matter voxels to improve co-registration of functional maps between individuals and with standard surface atlases (Dickie et al., 2019; Glasser et al., 2013). Standard post-processing procedures were implemented using the ABCD-DCAN pipelines (https://github.com/DCANLabs/dcan_bold_processing/blob/master/dcan_bold_proc.py), which were used to minimize movement-related artifacts as adapted from recommendations from Power et al. (Power et al., 2014), with a framewise displacement (FD) threshold of 0.2 mm for frame censoring after a notch bandpass filter to remove motion related to respiration (Fair et al., 2019)
ROI Selections
We selected a probabilistic BNST region of interest (ROI) developed by Theiss and colleagues that is publicly available at a threshold of 10% (https://identifiers.org/neurovault.image:39103) (Theiss et al., 2017). We used insula ROIs provided by Farb and colleagues (Farb et al., 2013a, 2013b) in which the insula is subdivided into subunits based on sulcal and gyral patterns corresponding cytoarchitectonic areas: dorsal accessory gyrus (DAC), ventral accessory gyrus (VAC), anterior short gyrus (AS), ventral short gyrus (VS), middle short gyrus (MS), posterior short gyrus (PS), anterior long gyrus (AL), and posterior long gyrus (PL). We transformed these ROI volumes into MNI space and projected them onto a cortical surface map in order facilitate parcel-wise analysis, as follows: the insula ROIs were projected onto mid-thickness left and right-hemisphere surfaces (generated as the average of the white and pial surfaces) using the ribbon-constrained volume-to-surface projection procedure available in Connectome Workbench 1.0. This procedure samples data from voxels within the gray matter ribbon (i.e., between the white and pial surfaces) that lie in a cylinder orthogonal to the local mid-thickness surface weighted by the extent to which the voxel falls within the ribbon (Glasser et al., 2013). All ROIs are illustrated in Fig. 1.
Figure 1:
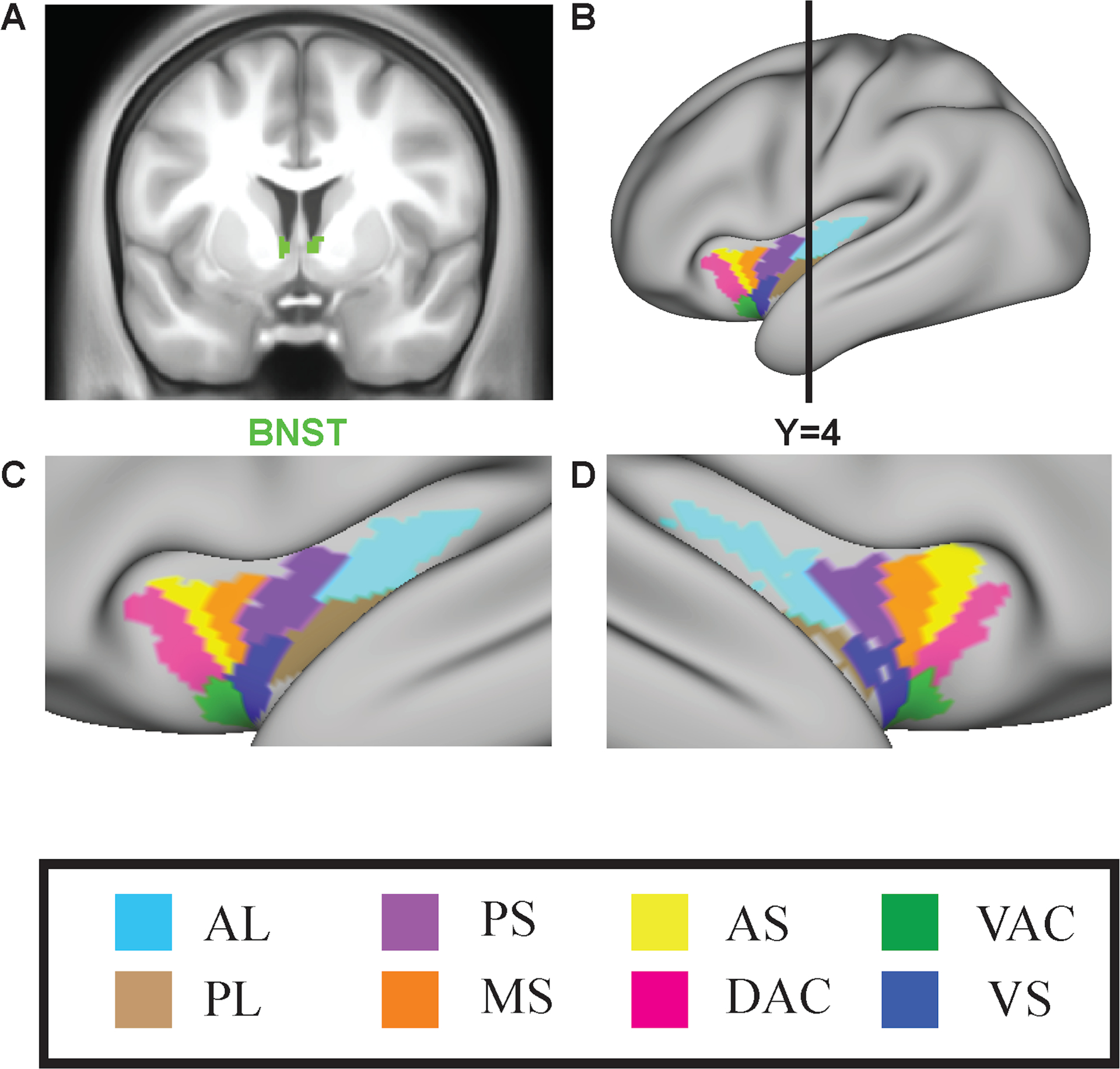
Insula regions of interest (ROIs). A: BNST ROI shown in coronal section. B. Surface map showing left cortical surface with insula ROIs shown, and also the A-P position of the coronal slice in A. C. Close-up of the left insula. D. Close-up of the right insula. AL= anterior long gyrus, PL = posterior long gyrus, PS = posterior short gyrus, MS = middle short gyrus, AS = dorsal anterior short gyrus, DAC = dorsal accessory gyrus, VAC = ventral accessory gyrus, VS = ventral anterior short gyrus, BNST=bed nucleus of the stria terminalis
Calculation of Insula-BNST RSFC
For each insula ROI, connectivity with the ipsilateral BNST ROI was calculated. First, the BOLD time-courses of all of the voxels in each ROI were averaged, creating a parcellated-time series. The parcellated time-series of the BNST was then correlated with the parcellated time-series of each of the insula regions, respectively, using Pearson’s correlations. A parameter (Pearson’s R) indexing RSFC with the BNST (insula-BNST RSFC) was thus generated for each insula ROI, for each participant, at each timepoint (pre- and post-CBT).
Statistical Analysis of Insula-BNST RSFC
All AI-BNST RSFC correlations were Fisher z-transformed prior to analyses. We performed one-sample t-tests for each BNST-insula ROI Pearson’s R value (i.e., to detect a difference from zero), with significance thresholds Bonferroni-corrected for 16 multiple comparisons, corresponding to 8 insula regions in both right and left hemispheres (α = 0.05/16= 0.003125). All subsequent analyses were restricted to insula ROI’s that showed significantly positive RSFC with the BNST at baseline (pre-CBT), under the assumption that only areas with positive RSFC at baseline would undergo changes during treatment.
To test differences between pre-CBT and post-CBT insula-BNST RSFC, we performed paired t-tests between pre-CBT and post-CBT RSFC values for each insula ROI, Bonferroni correcting significance thresholds for multiple comparisons. Because sex differences in AI-BNST structural connectivity have been found (Flook et al., 2020), we also investigated differences in pre-CBT insula-BNST RSFC between men and women using a 2-sample t-test.
We performed a linear regression to examine the relationship between the changes in insula-BNST RSFC (pre-CBT minus post-CBT), which was treated as the independent variable, and changes in NHDD (pre-CBT minus post-CBT), which was treated as the dependent variable, adjusting for the difference (pre-CBT minus post-CBT) in percentage of frames censored. Thus, for both metrics, positive values indicate a reduction from pre-CBT to post-CBT. Because of the small sample size and potential influence of outliers, robust regressions were used (Rousseeuw and Leroy, 1987). Because of the reductions in smoking have been shown to inversely correlate with relapse in subjects with AUD (Friend and Pagano, 2005), we repeated this linear regression, adjusting for the differences in cigarettes smoked per day, averaged over 28 days. We also repeated the linear regression using sex as a covariate, given sex differences in AI-BNST structural connectivity cited above.
RESULTS
Demographic Data
We screened 445 participants, of which 108 (24.3%) were not seeking treatment or were entered into another treatment trial, 66 (14.8%) did not complete screening process, 75 (16.9%) did not meet minimum AUD criteria, and 158 (35.5%) were excluded for psychiatric or medical conditions, cognitive impairment, or other exclusionary criteria. Of the 38 participants initially enrolled in the treatment study, 3 did not undergo scanning (1 medically unstable, 1 had a tattoo, 1 head too large for the scanner), and 1 underwent only underwent task, but not resting state, fMRI scanning due to late arrival. Of the 34 participants who underwent resting state scanning, 21 underwent both pre- and post-CBT MRI sessions. None of these were excluded because of an inability to abstain for more than 24 hours or because they demonstrated significant withdrawal after 24 hours of abstinence. Of the remaining 21 participants, 3 were excluded from analyses due to >50% frames censored in either scan (Power et al., 2014). For CONSORT diagram for this trial, see Fig. 2. Table 2 describes demographic characteristics of the remaining 18 participants who were included in the analyses.
Figure 2:
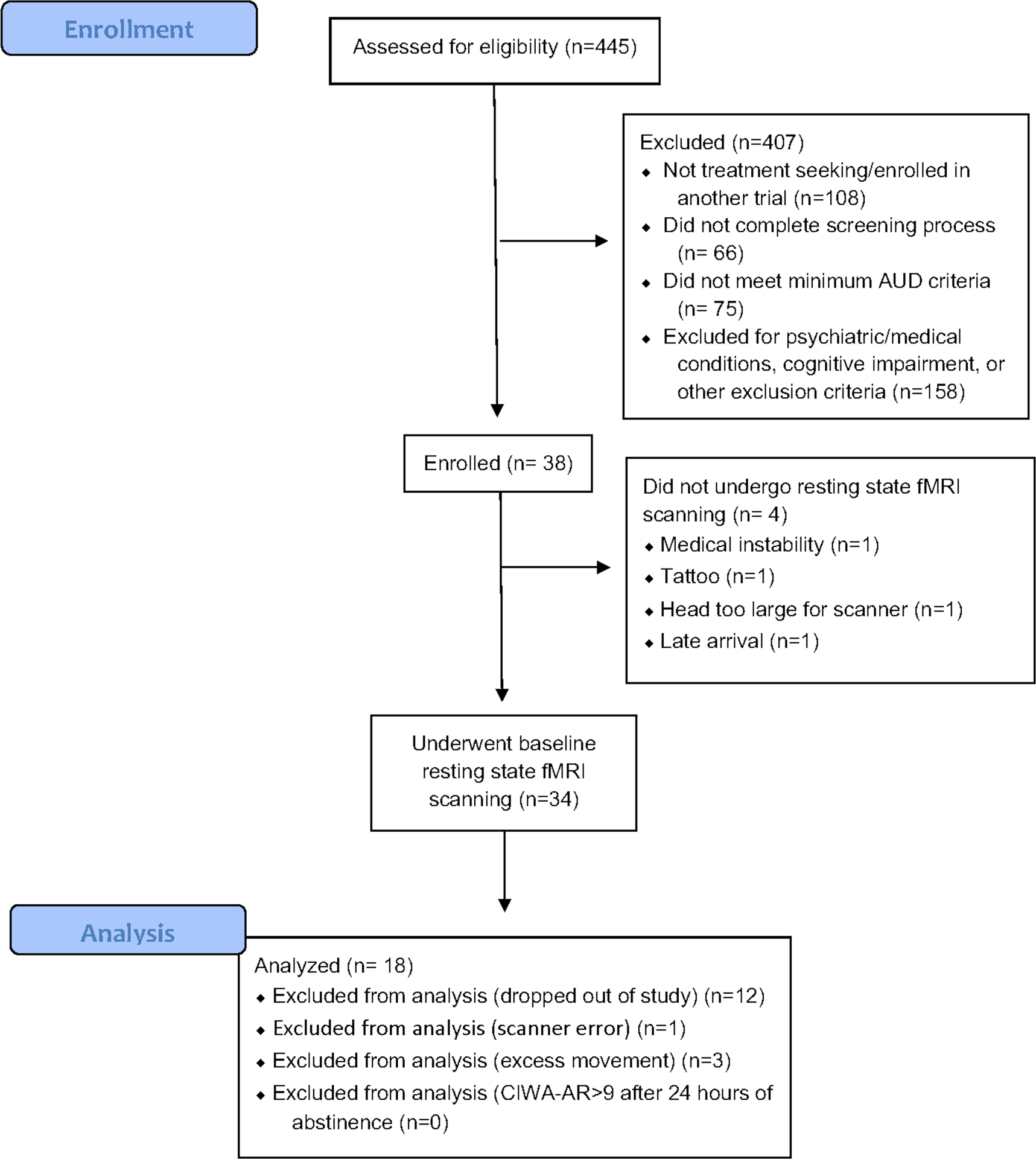
CONSORT diagram for the trial. CIWA-AR = Clinical Institute Withdrawal Assessment of Alcohol Scale, Revised
Table 2:
Subject Characteristics
| Initially consented (n=34) | Included in analyses (n=18) | ||||
|---|---|---|---|---|---|
| Mean ± SD | n (%) | Mean ± SD | n (%) | ||
| Age | 45.41 ± 11.35 | 47.11 ± 11.09 | |||
| Female | 14 (41.2) | 8 (44.4) | |||
| Hispanic | 9 (26.5) | 3 (16.7) | |||
| Race | |||||
| White | 23 (67.7) | 15 (83.3) | |||
| Black | 5 (14.7) | 2 (11.1) | |||
| Asian | 1 (2.9) | 1 (5.6) | |||
| Multi-racial | 1 (2.9) | 0 (0.0) | |||
| Other | 4 (11.8) | 0 (0.0) | |||
| DSM-5 Diagnoses | |||||
| MDD | 11 (32.4) | 4 (22.2) | |||
| GAD | 2 (5.9) | 0 (0.0) | |||
| Panic Disorder | 1 (2.9) | 0 (0.0) | |||
| Anorexia Nervosa | 1 (2.9) | 0 (0.0) | |||
| Cannabis Use Disorder (mild) | 1 (2.9) | 0 (0.0) | |||
| Cocaine Use Disorder (past) | 1 (2.9) | 0 (0.0) | |||
| Baseline NHDD | 22.74 ± 4.62 | 22.33 ± 4.10 | |||
| Baseline CIWA-Ar | 1.18 ± 1.42 | 0.72 ± 0.89 | |||
| Number of CBT sessions completed | 7.41 ± 4.03 | 9.72 ± 2.16 | |||
MDD = Major Depressive Disorder
GAD = Generalized Anxiety Disorder
CIWA = Clinical Institute Withdrawal Assessment of Alcohol Scale, Revised
NHDD= Number of Heavy Drinking Days
CBT = Cognitive Behavioral Therapy
Pre-CBCT and Post-CBT Insula-BNST RSFC
The left dorsal accessory gyrus (DAC), a dorsal anterior region, was the only insula ROI in either hemisphere to show significant positive RSFC with the BNST at baseline (t=3.76; p=0.0015); no other insula ROI showed significant positive connectivity with the BNST at baseline after correcting for multiple comparisons. Focusing on the left DAC, there were no significant differences in RSFC with the BNST between pre-CBT and post-CBT conditions (p=0.42; Fig. 3). In fact, a majority (10/18) had an increase in AI-BNST RSFC over time. There were also no significant differences in pre-CBT AI-BNST RSFC between males and females (t =0.9(16); p=0.37).
Figure 3:
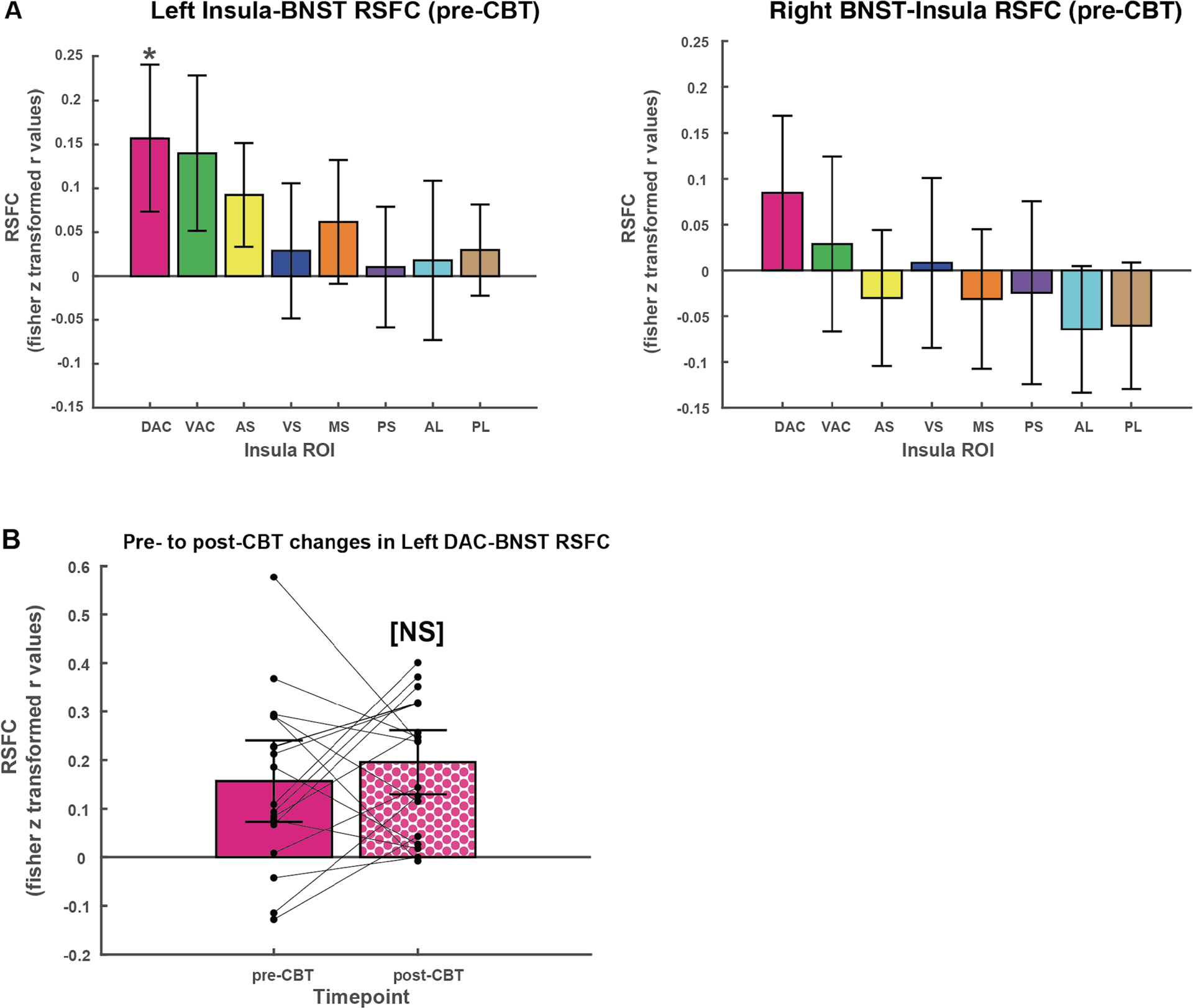
Left insula-BNST RSFC patterns. A. RSFC between insula ROIs and ipsilateral BNST at baseline (pre-CBT). Only the left DAC showed significant positive RSFC with the ipsilateral BNST at baseline, after correcting for multiple comparisons. B. Changes in RSFC between left dorsal anterior insula (DAC) and ipsilateral BNST, pre- and post-CBT. Points represent individual patients, with lines connecting pre- and post-CBT RSFC values. AL= anterior long gyrus, PL = posterior long gyrus, PS = posterior short gyrus, MS = middle short gyrus, AS = dorsal anterior short gyrus, DAC = dorsal accessory gyrus, VAC = ventral accessory gyrus, VS = ventral anterior short gyrus. BNST=bed nucleus of the stria terminalis. *significant at p<0.003, NS=not significant at p<0.05.
Relationships Between Changes in AI-BNST RSFC and Clinical Outcomes
NHDD was significantly reduced from pre-CBT (mean=22.33, SD=4.10) to post-CBT (mean=4.22, 5.89), with a large effect size (t=13.86 (17), p<0.0001, Cohen’s d=3.57). The reduction in NHDD strongly correlated with a reduction in the resting state functional connectivity between DAC and BNST (r = 0.73; p = 0.0008). This correlation was still significant after robust regression (p= 0.001). When sex was included as a covariate, differences in sex were not associated with changes in NHDD from pre- to post-CBT (p=0.46). Further, cigarettes smoked per day did not change significantly from pre-CBT (mean=1.47, SD=4.85) to post-CBT (mean=1.59, SD=4.89; t=−1, p=0.33), and the relationship between changes in NHDD and change in RSFC was still significant when adjusting for changes in smoking from pre- to post-CBT (p=0.003). Variability in percentage of frames censored was small (pre-CBT MRI: mean 0.095; SD=0.10; post-CBT MRI: mean 0.083, SD= 0.095), and the overall model did not significantly change in absence of adjusting for frames censored (r= 0.72, p=0.0007). When examining the scatter plot, patients in the right upper quadrant were those who had larger decreases in DAC-BNST RSFC, corresponding to larger decreases in NHDD; patients in the lower left quadrant had smaller decreases (as well as increases) in DAC-BNST RSFC corresponding to smaller decreases in drinking (Fig. 4).
Figure 4:
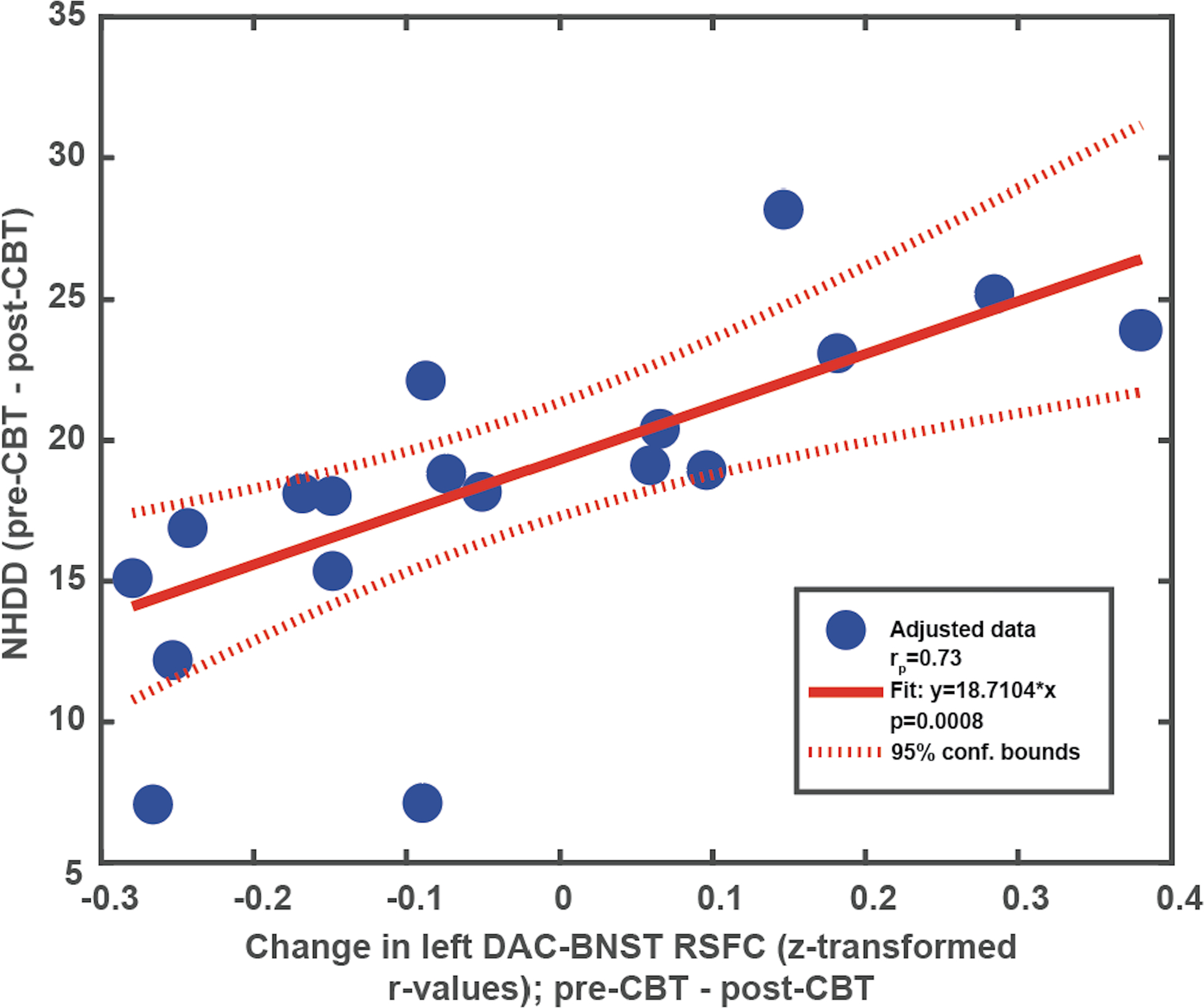
Relationship between NHDD (pre-CBT – post-CBT) and Left DAC-BNST RSFC (pre-CBT – post-CBT). Scatterplot and best fit line of linear regression model. BNST=bed nucleus of the stria terminalis. DAC=dorsal accessory gyrus. NHDD= Number of heavy drinking days
DISCUSSION
Here, we showed 1) significant positive RSFC between the anterior insula (AI) and the bed nucleus of the stria terminalis (BNST) in heavy drinking AUD participants at baseline and 2) a direct relationship between reductions in AI-BNST RSFC and reductions in heavy drinking after undergoing CBT treatment. This provides the first evidence in humans that AI-BNST RSFC is associated with changes in drinking behavior during a treatment for AUD.
We identified one ROI within the AI (the dorsal accessory gyrus, or DAC) that showed significant positive RSFC with the BNST at baseline that survived corrections for multiple comparisons. Contrary to our prediction, RSFC between this AI region and the BNST (AI-BNST RSFC) was not significantly reduced over time at the group level, despite a marked reduction in heavy drinking at the group. Indeed, AI-BNST RSFC change from pre- to post-CBT was variable among subjects: some participants (N=8) showed decreased RSFC as predicted, while others (N=10) showed increased AI-BNST RSFC. When we correlated these individual differences with changes in heavy drinking, we found a strong and significant positive correlation with changes in AI-BNST RSFC, even after accounting for changes in RSFC due to changes in smoking, as well as sex. In other words, while all participants experienced reductions in heavy drinking from pre- to post-CBT, individuals who showed reductions in AI-BNST RSFC showed greater reductions in heavy drinking, compared to individuals who showed increases in AI-BNST RSFC.
The model we have proposed would predict that reductions in AI-BNST RSFC, in addition to being correlated with reductions in heavy drinking, should also be positively correlated with reductions in self-reported anxiety and craving, along with increases in self-reported coping ability over time. We did not observe significant correlations between changes in DAC-BNST RSFC and changes in any of these measures (Fig. S1), possibly because of the small sample size or because other brain systems have a greater impact on these variables. Furthermore, while anxiety was measured at baseline, the second measurement was done at the midpoint of treatment (where it was actually increased, relative to baseline), not at the post-CBT MRI, which may explain the lack of relationship with changes in AI-BNST RSFC. Measuring self-reported anxiety closer to the time of the MRI scans may potentially reveal a stronger relationship between changes in anxiety and changes in AI-BNST RSFC. This may further explain the variability in change in RSFC from pre- to post-CBT; individuals who are better able to regulate anxiety through CBT over time may show a greater reduction in AI-BNST.
We found that the only insula ROI with significantly positive RSFC with the BNST at baseline was the left DAC, corresponding to anterior dysgranular insula (Farb et al., 2013a, 2013b). Previous anatomical findings would have predicted that the more ventral anterior insula ROI (ventral accessory gyrus, or VAC), corresponding to agranular insula, should have shown greater RSFC with the BNST at baseline. In rats, the BNST receives inputs from both agranular (Reynolds and Zahm, 2005) and dysgranular (Shi and Cassell, 1998) insula, but in human probabilistic tractography studies, only the ventral (agranular) insula and not the dorsal (dysgranular) insula has been shown to have structural connectivity with the BNST (Flook et al., 2020). Furthermore, evidence from rodents suggests that the protracted alcohol phenotype is shown to be dependent upon inputs from the agranular insula (Centanni et al., 2019) (this study did not address the dysgranular insula). While the more ventral ROI (VAC) did have positive RSFC with BNST at baseline that approached statistical significance, this did not survive correction for multiple comparisons. Moreover, we did not observe even a correlation of trend-level significance between changes in VAC-BNST RSFC and changes in heavy drinking from pre- to post-CBT (Table S1). While a small sample size makes drawing conclusions difficult, this difference between the DAC and the VAC may reflect a dissociation of function. Whereas the VAC has more has direct anatomical connections with the BNST, the DAC may play a greater role in cognitive regulation functions through its connections with PFC regions involved in self-regulation functions that are engaged by CBT. Anatomical studies in non-human primates have demonstrated direct connectivity between mid/dorsal anterior insula regions and dorsolateral prefrontal cortical (DLPFC) areas that mediate executive attention (Evrard, 2019). Cognitive regulation of cue-induced alcohol craving is associated with increased activity in both the mid/dorsal AI region, as well as in the DLPFC (Suzuki et al., 2019). In healthy individuals, increased perceived control during threat anticipation, a cognitive regulation process, has been correlated with reduced activation of the dorsal AI, but not ventral AI (Alvarez et al., 2015), while individuals who underwent a mindfulness-based stress reduction, another form of emotion regulation, demonstrated greater activity in dorsal AI during an interoceptive awareness task, compared to controls, with no effect on ventral AI (Farb et al., 2013b). Together, this suggests that the dorsal AI may be a target of executive attention processes that are critical for self-regulation, which may serve to modulate representations of the interoceptive impact of withdrawal according to self-regulation goals. This is consistent with the results of an exploratory voxel-wise analysis of RSFC between the DAC and the entire cortex, which demonstrated that reductions in NHDD from pre- to post-CBT were correlated with increased RSFC between the DAC and the dorsolateral PFC region corresponding to Brodmann’s Area 46 (Supplement).
The study is limited by several factors, which is why the results should be considered preliminary. First, the study included neither a healthy control group nor a control for the behavioral intervention. Because of a lack of healthy controls, we do not know if AI-BNST RSFC was significantly positive at baseline because it reflects a pathophysiological mechanism in AUD, as we hypothesize, or because it is a normal system that is engaged to the same degree in non-AUD populations. Because of the lack of a control for the behavioral intervention, we do not know if changes in AI-BNST RSFC over time were due to specific effects of CBT; they may instead have been due to a more general mechanism of behavior change or, more trivially, the mere passage of time. Another possibility is that changes in AI-BNST RSFC over time may reflect a consequence rather than a cause of reduced drinking. Specifically, it is possible that participants who showed greater drinking reductions over time would also have tended to have a longer time elapsed since their last heavy drinking day at the post-CBT MRI scan, leading to less withdrawal and therefore lower AI-BNST, compared to participants with smaller drinking reductions. Additionally, the reliability of the RSFC data may have been limited by the relatively short (10-minute) acquisition time (Gordon et al., 2017; Laumann et al., 2016), which may have decreased the sensitivity of our analyses for detecting changes in RSFC over time. In addition to all of this, the conclusions are limited by a small sample size. To address these limitations, future studies may include a longer MRI acquisition time; a larger sample size; a group of matched healthy control participants; a group of heavy drinking AUD participants who receive CBT after being fully detoxified, in order to isolate changes in RSFC due to CBT as opposed withdrawal; as a group in whom abstinence is imposed without behavioral treatment, for example with disulfiram, in order to isolate changes in RSFC that are due to withdrawal as opposed to CBT.
The direct relationship between reduced AI-BNST RSFC and reduced heavy drinking points to this region as a potential target for treatments for AUD. Promising medication approaches suggested by recent experiments in rodents include enhancement of endocannabinoid signaling, which reduces excitatory input from the AI to the BNST during protracted abstinence and also reduces the abstinence-related behavioral phenotype (Centanni et al., 2019), as well as kappa opioid antagonists, which reduce compulsive binge-like drinking when infused directly into the BNST (Haun et al., 2020). Such medications may exert effects on heavy drinking by down-modulating AI-BNST RSFC. These medications may work well in combination with behavioral therapies that are also targeted at AI-BNST RSFC, which may include CBT. Thus, AI-BNST RSFC may serve as a biomarker that can facilitate the development of novel, more effective combined medication and behavioral treatments for AUD.
Supplementary Material
ACKNOWLEDGEMENTS
This work was supported by National Institutes of Health Grant Nos. 1K23AA022771 (Principal Investigator NHN) and T32DA007294 (Principal Investigator FRL). We thank Norman Farb, PhD for providing us with the insula ROIs used in this study.
DISCLOSURES
FRL receives grant support from the NIDA, SAMHSA and US World Meds as well as a consultant for Major League Baseball. She also receives medication from Indivior for research. In addition, FRL was an unpaid member of a Scientific Advisory Board for Alkermes, Indivior, Novartis and US WorldMeds but did not personally receive any compensation in the form of cash payments (honoraria/consulting fees) or food/beverage (she declined food/beverages in each circumstance) nor receive compensation in the form of travel reimbursement. JJM has served as a consultant to Indivior and Novartis. GHP receives income and equity through family from Pfizer, inc. for unrelated projects. ABS, JS, and NHN report no disclosures.
Footnotes
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
ClinicalTrials.gov Identifier: NCT02316574
REFERENCES
- Alvarez RP, Kirlic N, Misaki M, Bodurka J, Rhudy JL, Paulus MP, Drevets WC (2015) Increased anterior insula activity in anxious individuals is linked to diminished perceived control. Transl Psychiatry 5:e591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abler B, Grön G, Hartmann A, Metzger C, Walter M (2012) Modulation of frontostriatal interaction aligns with reduced primary reward processing under serotonergic drugs. J Neurosci. 32:1329–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswal B, Yetkin ZF, Haughton VM, Hyde JS (1995) Functional connectivity in the motor cortex of resting human brain using echo‐planar MRI. Magn Reson Med 34:537–541. [DOI] [PubMed] [Google Scholar]
- Bohn MJ, Krahn DD, Staehler BA (1995) Development and initial validation of a measure of drinking urges in abstinent alcoholics. Alcohol Clin Exp Res 19:600–606. [DOI] [PubMed] [Google Scholar]
- Centanni SW, Morris BD, Luchsinger JR, Bedse G, Fetterly TL, Patel S, Winder DG (2019) Endocannabinoid control of the insular-bed nucleus of the stria terminalis circuit regulates negative affective behavior associated with alcohol abstinence. Neuropsychopharmacology 44:526–537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai X, Zhang J, Gao L, Yu J, Li Y, Du B, Huang X, Zhang H (2021) Intrinsic dialogues between the two hemispheres in middle-aged male alcoholics: a resting-state functional MRI study. Neuroreport 32:206–213. [DOI] [PubMed] [Google Scholar]
- DeVito EE, Worhunsky PD, Carroll KM, Rounsaville BJ, Kober H, Potenza MN (2012) A preliminary study of the neural effects of behavioral therapy for substance use disorders. Drug Alcohol Depend. 122:228–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M, Walker DL, Miles L, Grillon C (2010) Phasic vs Sustained Fear in Rats and Humans: Role of the Extended Amygdala in Fear vs Anxiety. Neuropsychopharmacology 35:105–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean SF, Fede SJ, Diazgranados N, Momenan R (2020) Addiction neurocircuitry and negative affect: A role for neuroticism in understanding amygdala connectivity and alcohol use disorder. Neurosci Lett 722:134773. [DOI] [PubMed] [Google Scholar]
- Dickie EW, Anticevic A, Smith DE, Coalson TS, Manogaran M, Calarco N, Viviano JD, Glasser MF, Essen DCV, Voineskos AN (2019) Ciftify: A framework for surface-based analysis of legacy MR acquisitions. Neuroimage 197:818–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esteban O, Markiewicz CJ, Blair RW, Moodie CA, Isik AI, Erramuzpe A, Kent JD, Goncalves M, DuPre E, Snyder M, Oya H, Ghosh SS, Wright J, Durnez J, Poldrack RA, Gorgolewski KJ (2019) fMRIPrep: a robust preprocessing pipeline for functional MRI. Nat Methods 16:111–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evrard HC (2019) The Organization of the Primate Insular Cortex. Front Neuroanat 13:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fair DA, Miranda-Dominguez O, Snyder AZ, Perrone A, Earl EA, Van AN, Koller JM, Feczko E, Tisdall MD, Kouwe A van der, Klein RL, Mirro AE, Hampton JM, Adeyemo B, Laumann TO, Gratton C, Greene DJ, Schlaggar BL, Hagler D, Watts R, Garavan H, Barch DM, Nigg JT, Petersen SE, Dale AM, Feldstein-Ewing SW, Nagel BJ, Dosenbach NUF (2019) Correction of respiratory artifacts in MRI head motion estimates. Neuroimage 208:116400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falk DE, O’Malley SS, Witkiewitz K, Anton RF, Litten RZ, Slater M, Kranzler HR, Mann KF, Hasin DS, Johnson B, Meulien D, Ryan M, Fertig J, Workgroup A (2019) Evaluation of Drinking Risk Levels as Outcomes in Alcohol Pharmacotherapy Trials. JAMA Psychiatry 76:374–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farb NAS, Segal ZV, Anderson AK (2013) Attentional Modulation of Primary Interoceptive and Exteroceptive Cortices. Cereb Cortex 23:114–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farb NAS, Segal ZV, Anderson AK (2013) Mindfulness meditation training alters cortical representations of interoceptive attention. Soc Cogn Affect Neur 8:15–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flook EA, Feola B, Avery SN, Winder DG, Woodward ND, Heckers S, Blackford JU (2020) BNST-insula structural connectivity in humans. Neuroimage. 210:116555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friend KB, Pagano ME (2005) Changes in cigarette consumption and drinking outcomes: Findings from Project MATCH. J Subst Abuse Treat 29:221–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasser MF, Sotiropoulos SN, Wilson AJ, Coalson TS, Fischl B, Andersson JL, Xu J, Jbabdi S, Webster M, Polimeni JR, Essen DC, Jenkinson M. (2013) The minimal preprocessing pipelines for the Human Connectome Project. Neuroimage 80:105–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goode TD, Ressler RL, Acca GM, Miles OW, Maren S (2019) Bed nucleus of the stria terminalis regulates fear to unpredictable threat signals. Elife 8:e46525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon EM, Laumann TO, Gilmore AW, Newbold DJ, Greene DJ, Berg JJ, Ortega M, Hoyt-Drazen C, Gratton C, Sun H, Hampton JM, Coalson RS, Nguyen AL, McDermott KB, Shimony JS, Snyder AZ, Schlaggar BL, Petersen SE, Nelson SM, Dosenbach NUF. (2017) Precision Functional Mapping of Individual Human Brains. Neuron 95:791–807.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halcomb ME, Chumin EJ, Goñi J, Dzemidzic M, Yoder KK (2019) Aberrations of anterior insular cortex functional connectivity in nontreatment-seeking alcoholics. Psychiatry Res Neuroimaging. 284:21–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haun HL, Griffin WC, Lopez MF, Becker HC (2020) Kappa opioid receptors in the bed nucleus of the stria terminalis regulate binge-like alcohol consumption in male and female mice. Neuropharmacology 167:107984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holla B, Biswal J, Ramesh V, Shivakumar V, Bharath RD, Benegal V, Venkatasubramanian G, Chand PK, Murthy P (2020) Effect of prefrontal tDCS on resting brain fMRI graph measures in Alcohol Use Disorders: A randomized, double-blind, sham-controlled study. Prog in Neuropsychopharmacol and Biol Psychiatry 102:109950. [DOI] [PubMed] [Google Scholar]
- Kadden R, Carroll K, Donovan D, Cooney N, Monti P, Abrams D, Litt M, Hester R (2003) Cognitive-Behavioral Coping Skills Therapy Manual: A Clinical Research Guide for Therapists Treating Individuals with Alcohol Abuse and Dependence. Rockville, MD: U.S. Department of Health and Human Services Public Health Service National Institutes of Health National Institute on Alcohol Abuse and Alcoholism. [Google Scholar]
- Karch S, Keeser D, Hümmer S, Paolini M, Kirsch V, Karali T, Kupka M, Rauchmann B-S, Chrobok A, Blautzik J, Koller G, Ertl-Wagner B, Pogarell O (2015) Modulation of Craving Related Brain Responses Using Real-Time fMRI in Patients with Alcohol Use Disorder. Plos One. 10:e0133034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kober H, Mende-Siedlecki P, Kross EF, Weber J, Mischel W, Hart CL, Ochsner KN (2010) Prefrontal–striatal pathway underlies cognitive regulation of craving. Proc Natl Acad Sci U S A 107:14811–14816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Volkow ND (2016) Neurobiology of addiction: a neurocircuitry analysis. Lancet Psychiatry. 3:760–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kranzler HR, Covault J, Feinn R, Armeli S, Tennen H, Arias AJ, Gelernter J, Pond T, Oncken C, Kampman KM (2014) Topiramate Treatment for Heavy Drinkers: Moderation by a GRIK1 Polymorphism. Am J Psychiatry 171:445–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kranzler HR, Soyka M (2018) Diagnosis and Pharmacotherapy of Alcohol Use Disorder: A Review. JAMA 320:815–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laumann TO, Snyder AZ (2021) Brain activity is not only for thinking. Curr Opin Behav Sci 40:130–136. [Google Scholar]
- Laumann TO, Snyder AZ, Mitra A, Gordon EM, Gratton C, Adeyemo B, Gilmore AW, Nelson SM, Berg JJ, Greene DJ, McCarthy JE, Tagliazucchi E, Laufs H, Schlaggar BL, Dosenbach NUF, Petersen SE (2016) On the Stability of BOLD fMRI Correlations. Cereb Cortex 103:4719–4732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longabaugh R, Morgenstern J (1999) Cognitive-behavioral coping-skills therapy for alcohol dependence. Current status and future directions. Alcohol Res Health 23:78–85. [PMC free article] [PubMed] [Google Scholar]
- McCrady BS, Owens MD, Borders AZ, Brovko JM (2014) Psychosocial Approaches to Alcohol Use Disorders Since 1940: A Review. J Stud Alcohol Drugs Suppl 75:68–78. [DOI] [PubMed] [Google Scholar]
- Naqvi NH, Gaznick N, Tranel D, Bechara A (2014) The insula: a critical neural substrate for craving and drug seeking under conflict and risk. Ann N Y Acad Sci 1316:53–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naqvi NH, Morgenstern J (2015) Cognitive Neuroscience Approaches to Understanding Behavior Change in Alcohol Use Disorder Treatments. Alcohol Res Curr Rev 37:29–38. [PMC free article] [PubMed] [Google Scholar]
- Naqvi NH, Ochsner KN, Kober H, Kuerbis A, Feng T, Wall M, Morgenstern J (2015) Cognitive Regulation of Craving in Alcohol‐Dependent and Social Drinkers. Alcohol Clin Exp Res 39:343–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naqvi NH, Rudrauf D, Damasio H, Bechara (2007) Damage to the Insula Disrupts Addiction to Cigarette Smoking Science 315:531–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newbold DJ, Laumann TO, Hoyt CR, Hampton JM, Montez DF, Raut RV, Ortega M, Mitra A, Nielsen AN, Miller DB, Adeyemo B, Nguyen AL, Scheidter KM, Tanenbaum AB, Van AN, Marek S, Schlaggar BL, Carter AR, Greene DJ, Gordon EM, Raichle ME, Petersen SE, Snyder AZ, Dosenbach NUF (2020) Plasticity and Spontaneous Activity Pulses in Disused Human Brain Circuits. Neuron 107:580–589.e6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olive MF, Koenig HN, Nannini MA, Hodge CW (2002) Elevated extracellular CRF levels in the bed nucleus of the stria terminalis during ethanol withdrawal and reduction by subsequent ethanol intake. Pharmacol Biochem Behav 72:213–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orban C, McGonigle J, Kalk NJ, Erritzoe D, Waldman AD, Nutt DJ, Rabiner EA, Lingford-Hughes AR (2013) Resting state synchrony in anxiety-related circuits of abstinent alcohol-dependent patients. Am J Drug Alcohol Abuse 39:433–440. [DOI] [PubMed] [Google Scholar]
- Perini I, Kämpe R, Arlestig T, Karlsson H, Löfberg A, Pietrzak M, Zangen A, Heilig M (2020) Repetitive transcranial magnetic stimulation targeting the insular cortex for reduction of heavy drinking in treatment-seeking alcohol-dependent subjects: a randomized controlled trial. Neuropsychopharmacology 45:842–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power JD, Mitra A, Laumann TO, Snyder AZ, Schlaggar BL, Petersen SE (2014) Methods to detect, characterize, and remove motion artifact in resting state fMRI. Neuroimage 84:320–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray LA, Grodin EN, Leggio L, Bechtholt AJ, Becker H, Ewing SWF, Jentsch JD, King AC, Mason BJ, O’Malley S, MacKillop J, Heilig M, Koob GF (2020) The future of translational research on alcohol use disorder. Addict Biol 26::e12903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds SM, Zahm DS. Specificity in the Projections of Prefrontal and Insular Cortex to Ventral Striatopallidum and the Extended Amygdala. J Neurosci 2005;25:11757–11767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchey M, Dolcos F, Eddington KM, Strauman TJ, Cabeza R (2011) Neural correlates of emotional processing in depression: changes with cognitive behavioral therapy and predictors of treatment response. J Psychiatr Res. 45:577–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rousseeuw PJ, Leroy AM (1987) Robust Regression and Outlier Detection. Wiley Series in Probability and Statistics. John Wiley & Sons, Inc, Hoboken, NJ. [Google Scholar]
- Sacks JJ, Gonzales KR, Bouchery EE, Tomedi LE, Brewer RD 20152010 National and State Costs of Excessive Alcohol Consumption. Am J Prev Med 49:e73–e79. [DOI] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Hergueta T, Baker R, Dunbar GC (1998) The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry 59 Suppl 20: 22–33. [PubMed] [Google Scholar]
- Shi C, Cassell MD (1998) Cortical, thalamic, and amygdaloid connections of the anterior and posterior insular cortices. J Comp Neurol 399:440–468. [DOI] [PubMed] [Google Scholar]
- Simpson TL, Saxon AJ, Stappenbeck C, Malte CA, Lyons R, Tell D, Millard SP, Raskind M (2018) Double-Blind Randomized Clinical Trial of Prazosin for Alcohol Use Disorder. Am J Psychiatry 175:1216–1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB (1995) Alcohol Timeline Followback users’ manual. Addiction Research Foundation. Toronto, Canada. [Google Scholar]
- Sullivan GM, Apergis J, Bush DEA, Johnson LR, Hou M, Ledoux JE (2004) Lesions in the bed nucleus of the stria terminalis disrupt corticosterone and freezing responses elicited by a contextual but not by a specific cue-conditioned fear stimulus. Neuroscience 128:7–14. [DOI] [PubMed] [Google Scholar]
- Sullivan JT, Sykora K, Schneiderman J, Naranjo CA, Sellers EM (1989) Assessment of Alcohol Withdrawal: the revised clinical institute withdrawal assessment for alcohol scale (CIWA‐Ar). Brit J Addict 84:1353–1357. [DOI] [PubMed] [Google Scholar]
- Suzuki S, Mell MM, O’Malley SS, Krystal JH, Anticevic A, Kober H (2019) Regulation of Craving and Negative Emotion in Alcohol Use Disorder. Biol Psychiatry Cog Neurosci Neuroimaging 5:239–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theiss JD, Ridgewell C, McHugo M, Heckers S, Blackford J (2017) Manual segmentation of the human bed nucleus of the stria terminalis using 3T MRI. Neuroimage 146: 288–292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vergara VM, Liu J, Claus ED, Hutchison K, Calhoun V (2017) Alterations of resting state functional network connectivity in the brain of nicotine and alcohol users. Neuroimage 151:45–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker DL, Miles LA, Davis M (2009) Selective participation of the bed nucleus of the stria terminalis and CRF in sustained anxiety-like versus phasic fear-like responses. Prog Neuro-psychopharmacology Biol Psychiatry 33:1291–1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White AM, Castle IP, Hingson RW, Powell P (2020) Using Death Certificates to Explore Changes in Alcohol‐Related Mortality in the United States, 1999 to 2017 Alcohol Clin Exp Res 44:178–187. [DOI] [PubMed] [Google Scholar]
- Wilcox CE, Abbott CC, Calhoun VD (2018) Alterations in resting-state functional connectivity in substance use disorders and treatment implications. Prog in Neuropsychopharmacol and Biol Psychiatry 91:79–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkiewitz K, Litten RZ, Leggio L (2019) Advances in the science and treatment of alcohol use disorder. Sci Adv 5:eaax4043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkiewitz K, Marlatt A (2011) Behavioral therapy across the spectrum. Alcohol Res Health 33:313–9. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


