Summary
In the leaf epidermis, stomatal pores allow gas exchange between plants and the environment. The production of stomatal guard cells requires the lineage cells to divide asymmetrically. In this Insight review, we describe an emerging picture of how intrinsic molecules drive stomatal asymmetric cell division in multidimensions, from transcriptional activities in the nucleus to the dynamic assembly of the polarity complex at the cell cortex. Given the significant roles of stomatal activity in plant responses to environmental changes, we incorporate recent advances in external cues feeding into the regulation of core molecular machinery required for stomatal development. The work we discuss here is mainly based on the dicot plant Arabidopsis thaliana with summaries of recent progress in the monocots.
Keywords: Asymmetric cell division, Stomatal development, Cell-division potential, Cell-fate decision, Transcription factors, Cell polarity, Polarity scaffold proteins
I. Introduction
Stomata are turgor-driven microscopic pores in higher plants. The controlled stomatal movement allows efficient gas exchange, restricts excessive water evaporation, and defends pathogen egress. The formation of stomatal complexes in both dicot and monocot plants requires tightly regulated lineage specification, asymmetric cell division, cell-fate transition, and cell-fate differentiation, all of which are programmed by intrinsic developmental pathways and influenced by extrinsic environmental changes.
In dicot plant Arabidopsis, the specification of the stomatal lineage cells in young seedlings occurs largely randomly, involving the conversion of a subset of protodermal cells into meristemoid mother cells (MMCs). One MMC divides asymmetrically to produce two daughter cells. The small daughter cell becomes a meristemoid and the large one becomes a stomatal lineage ground cell (SLGC), each of which might undergo additional asymmetric cell divisions (ACDs) before terminally differentiating into stomatal guard cells and pavement cells, respectively. The meristemoids often undergo successive self-renewing ACDs in an inward spiral manner, whilst the SLGCs divide asymmetrically to place the newly generated guard cells away from the existing ones (Fig. 1a).
Fig. 1. Stomatal division and differentiation in dicots and monocots.
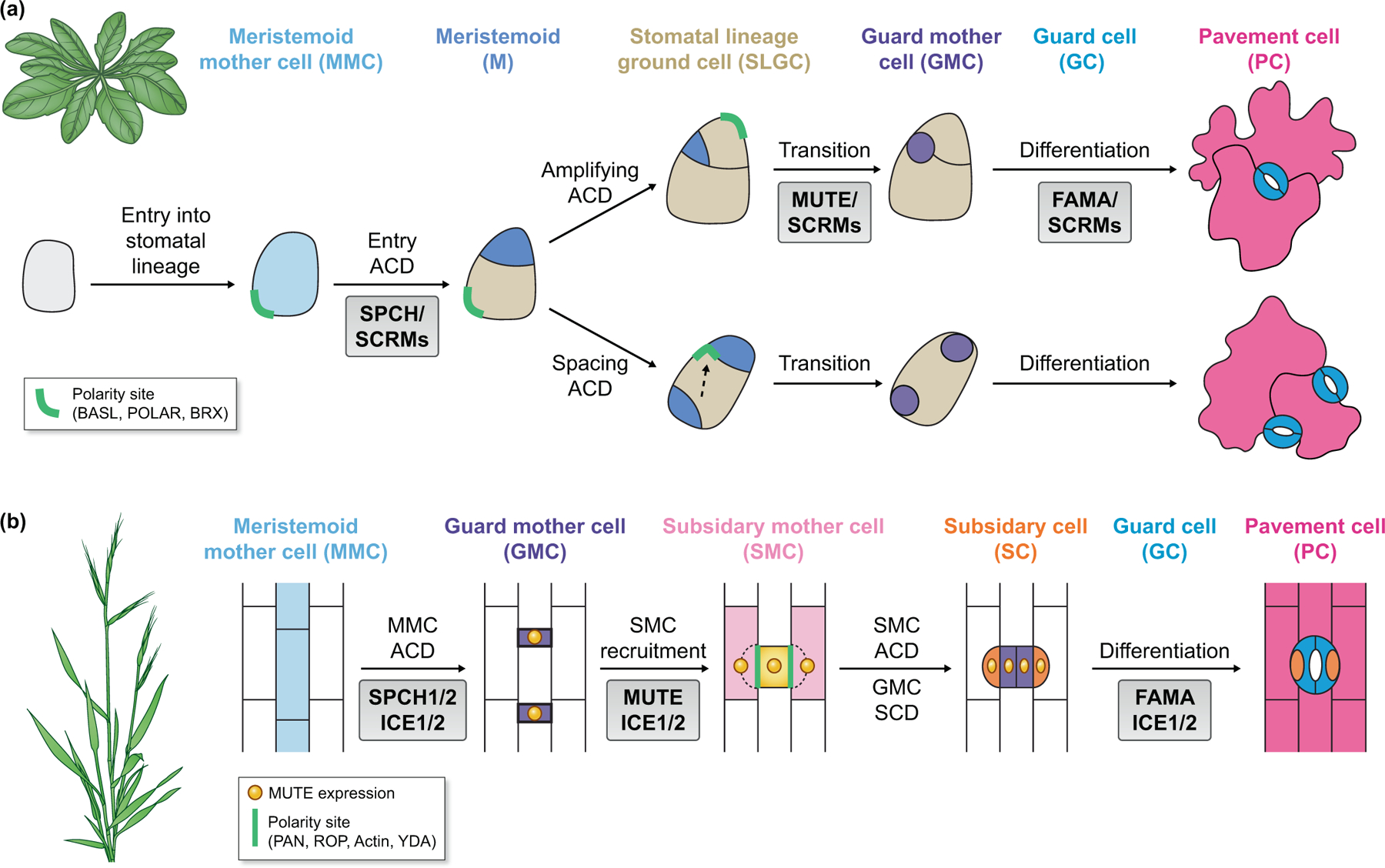
Diagrams show cell types, asymmetric cell division (ACD) and stomatal differentiation progresses in Arabidopsis (a, example for dicots) and Brachypoium (b, example for monocots). Key regulators for successive cell-fate transitions are specified in grey boxes. Particularly interesting cellular events (polarity site and mobile transcription factor MUTE) are highlighted accordingly.
(a) In Arabidopsis, SPCH and SCRMs initiate stomatal asymmetric division of the meristemoid mother cell (MMC) (light blue) to produce a small daughter cell meristemoid (M) (blue) and a large daughter cell, stomatal lineage ground cell (SLGC) (pink). The polarity complex (green) (assembled by scaffold proteins BASL, POLAR, and BRX) is expressed in MMC and SLGC. After a few rounds of meristemoid amplifying ACD, MUTE enables cell-fate transition to guard mother cell (GMC) (purple). FAMA then promotes cell-fate differentiation to guard cell (GC) (dark blue). The SLGC spacing division involves directional switch of the polarity site (dashed arrow) and generates a satellite meristemoid distal to the polarity site.
(b) In Brachypodium, the formation of both guard cells (dark blue) and subsidiary cells (SC) (red) require ACD (MMC ACD and SMC ACD, respectively). Key regulators and possible partners of cell-fate determination (grey boxes) are summarized from research in Brachypodium, rice and maize. In grasses, the expression of SPCHs in MMC (light blue) may partner with ICE1 and ICE2 to initiate entry ACD that produces a small daughter cell (GMC, purple) and a large daughter pavement cell (PC) (light pink). The formation of GMC induces the recruitment of adjacent subsidiary mother cells (SMCs) (pink) and direct the orientation of SMC ACD. The expression of MUTE (yellow) in GMC specifies the GC fate (dark blue). MUTE also moves out of GMC to recruit SMC and specify the SC fate. The polarity proteins (green) include the PAN receptor-like proteins that recruit Type I ROP GTPases to the SMC surface contacting nascent GMCs. ROPs induce the polarization of F-actin regulators, which promote both the polarization of PAN polarization and directional nuclear migration in SMC. YDA has a predominant role in the suppression of stomatal formation and shows polar accumulation at the interface between GMC and SMC.
In many monocot plants, stomatal guard cell complexes (two guard cells companioned by two subsidiary cells) are linearly aligned along the longitudinal axis. At the leaf base, stomatal precursor cells divide asymmetrically to produce a small guard mother cell (GMC) that differentiates into guard cells and a large daughter cell that differentiates into a pavement cell. The two neighboring epidermal cells lateral to GMC are recruited to become subsidiary mother cells (SMCs), each of which divides asymmetrically to produce a subsidiary cell adjacent to GMC. The subsidiary cells intimately connected to the GCs enable the four-celled stomatal complexes to move more efficiently for gas exchange (Hepworth et al., 2018; Nunes et al., 2020) (Fig. 1b).
II. Significance of ACD in stomatal development and patterning
In multicellular organisms, stem cells undergo asymmetric cell division (ACD) for self-renewal and generation of daughter cells with specialized identity and function. Stem cell ACD is fundamentally important for plant development, given that organ morphogenesis and patterning mainly occur post-embryonically in plants and require continuous generation of new cell types throughout the whole lifespan. Stomatal ACD is controlled by both cell-autonomous and non-cell-autonomous mechanisms to ensure controlled cell-fate specification, proper distribution, and patterning. The initiation of stomatal lineage cells involves a positive feedback loop of the basic helix-loop-helix (bHLH) transcription factor SPEECHLESS (SPCH) with its partners, the bHLH SCREAMs (SCRM/ICE1 and SCRM2) (Horst et al., 2015). The cell-autonomous function of SPCH as a transcription factor is manifested by its direct binding to the promoters of key genes required for cell-fate transition and differentiation in stomatal development (Fig. 2a and 2b) (Lau et al., 2014). SPCH also drives the expression of the intrinsic polarity factors, BREAKING OF ASYMMETRY IN THE STOMATAL LINEAGE (BASL) and POLAR, which regulate division orientation and cell-fate specification in stomatal ACD (Dong et al., 2009; Pillitteri et al., 2011; Lau et al., 2014). Targets of SPCH and SPCH itself are regulated by peptide- and phytohormone-mediated non-cell-autonomous pathways that contribute to the modulation of stomatal production in response to changing environments (Lau et al., 2014).
Fig. 2. Regulatory networks of SPCH and MUTE in Arabidopsis stomatal development.
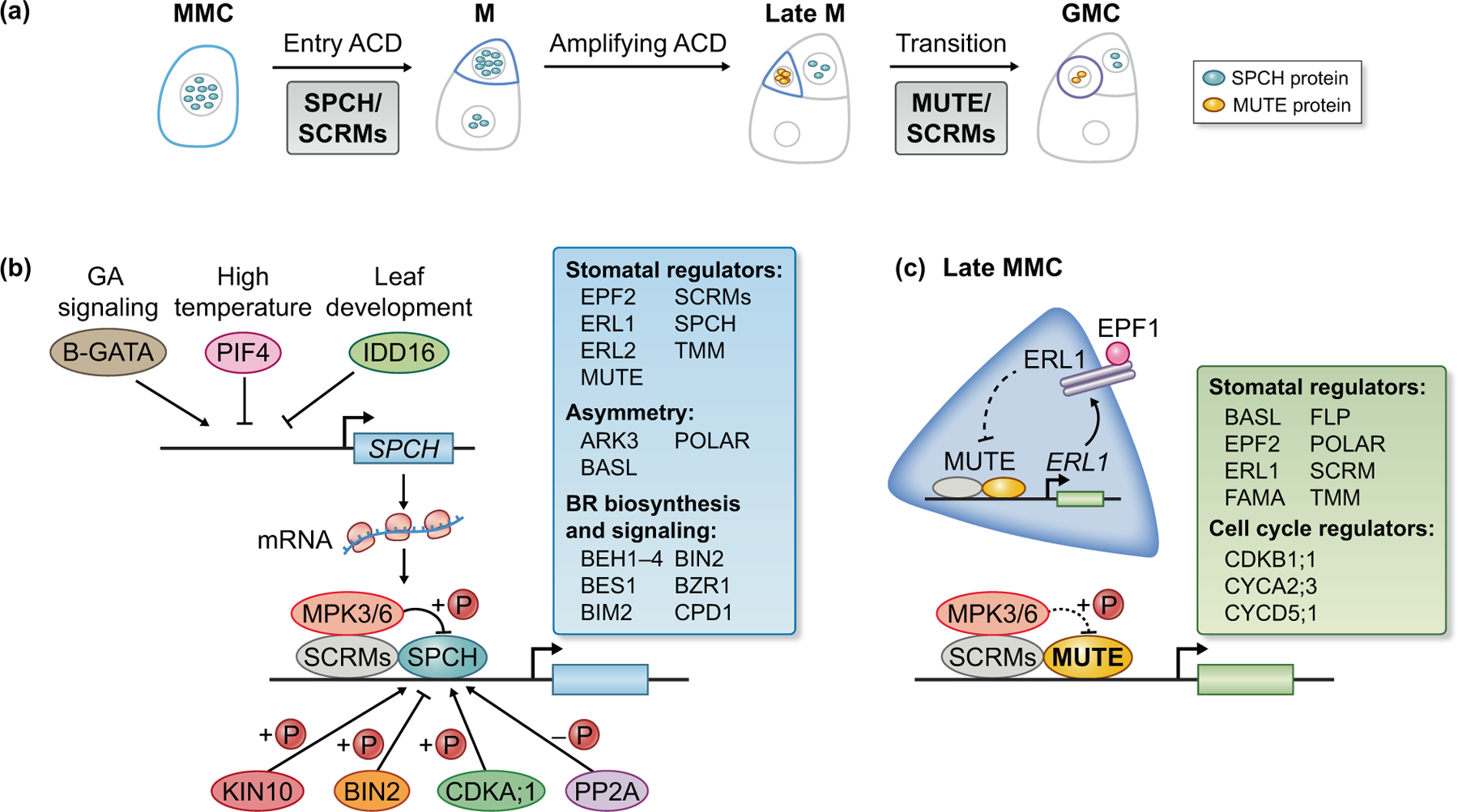
(a) Diagrams depict the protein expression levels of SPCH (blue ovals) and MUTE (yellow ovals) in stem-cell-like stomatal ACD. The density of ovals represents protein amount.
(b) Regulatory networks around SPCH. Upstream transcription factors responding to developmental or environmental cues can regulate SPCH transcription. PIF4 in the high temperature signaling pathway and IDD16 in the organ morphogenesis pathway may directly bind to the SPCH promoter and suppress SPCH expression, whilst B-GATA in GA signaling promotes SPCH expression. At the protein level, SPCH is tightly regulated by phosphorylation and dephosphorylation. The bHLH transcription factors, ICE1 and SCRM2, bring MPK3/6 close to SPCH for phosphorylation. MPK3/6- and BIN2-mediated phosphorylation promotes SPCH degradation, whereas the CDKA;1 and KIN10 kinase-mediated phosphorylation and the phosphatase PP2A-mediated dephosphorylation promote SPCH stabilization. CDKA;1 also phosphorylates SPCH for activation. Downstream of SPCH, based on the work from (Lau et al., 2014), SPCH directly regulates the expression of key genes in stomatal development, divisional asymmetry, and hormonal signaling, particularly BR biosynthesis and signaling (blue box).
(c) Regulatory networks around MUTE. MUTE (yellow) is expressed in late meristemoids and promotes the cell-fate transition to GMC. Based on the work from (Han et al., 2018), MUTE directly controls the key genes functioning in stomatal development and cell-cycle control (green box). An autocrine signaling regulation (diagram on the left) was proposed to contribute to the restricted expression of MUTE in late meristemoids. MUTE directly induces the expression of ERL1 that perceives signaling of the secreted peptide EPF1. Downstream of ERL1, the YDA MAPK cascade was suspected to inhibit MUTE function (dashed arrow).
III. The SPEECHLESS transcription factor initiates stomatal ACD
The absence of SPCH leads to an epidermis devoid of stomata, while elevated protein levels of SPCH result in the over proliferation of stomatal lineage cells in Arabidopsis (MacAlister et al., 2007; Lampard et al., 2008). Given the pivotal roles of SPCH in stomatal production, the expression and activity levels of SPCH are tightly modulated at the transcriptional and post-transcriptional levels (Fig. 2b). Red light induces the expression of the LLM-domain B-class GATA genes that act upstream of SPCH to promote stomatal production (Klermund et al., 2016). Also, high-temperature signaling induces the expression of the bHLH transcription factor PHYTOCHROMEINTERACTING FACTOR 4 (PIF4) that binds to the E-boxes within the SPCH promoter to suppress SPCH expression. In turn, SPCH directly binds to the PIF4 promoter to suppress PIF4 expression (Lau et al., 2018). The negative feedback loop of PIF4-SPCH can quickly lower SPCH expression to reduce stomatal production, thereby restricting water loss from plants under warmer conditions. Furthermore, the expression of SPCH was found directly inhibited by a C2H2 zinc finger transcription factor INDETERMINATE DOMAIN (IDD) 16 that functions in plant organ morphogenesis (Qi et al., 2019).
At the post-transcriptional level, the stability and function of SPCH proteins are heavily influenced by phosphorylation and de-phosphorylation (Fig. 2b). The Mitogen-Activated Protein Kinase (MAPK) cascade, composed of the MAPKK Kinase YODA (YDA), MAPK Kinase 4 and 5 (MKK4/5), and MAPK 3 and 6 (MPK3/6) in Arabidopsis, transduces signals from the cell-surface receptors, including TOO MANY MOUTHS (TMM) and the ERECTA family (Nadeau & Sack, 2002; Shpak et al., 2005). Activated MAPKs trigger an increased level of SPCH phosphorylation for degradation, thus conferring a strong inhibition on stomatal production (Bergmann et al., 2004; Wang et al., 2007; Lampard et al., 2008). Recently, the bHLH partners, ICE1/SCRM and SCRM2, were found to scaffold MPK3/6 into proximity to SPCH (Putarjunan et al., 2019). In addition, the GSK3-like BRASSINOSTEROID INSENSITIVE 2 (BIN2) kinase, a key regulator in Brassinosteroid signaling, phosphorylates SPCH for degradation (Gudesblat et al., 2012). On the other hand, the stabilization of SPCH involves Sucrose non-fermenting-1 (SNF1)-related kinase 1 (SnRK1)- and CYCLIN-DEPENDENT KINASEs A(CDKA;1)-mediated protein phosphorylation (Yang et al., 2015; Han et al., 2020) and Protein Phosphatase 2A (PP2A)-mediated dephosphorylation (Bian et al., 2020). Interestingly, the activity of SPCH in promoting stomatal development requires phosphorylation of the Serine 186 residue that can be modified by BIN2, MPK3/6, or CDKA;1 (Yang et al., 2015). Thus, there is an elegant balance of phosphorylation and dephosphorylation underlying SPCH protein stability and functionality for plants to optimize stomatal production in changing developmental and growth conditions.
The orthologs of SPCH have been identified and characterized in monocots. There are two SPCH genes in rice and Brachypodium and mutant analyses suggested that SPCH1 and SPCH2 function largely redundantly for the initiation of the stomatal lineage in both plant species (Raissig et al., 2016; Wu et al., 2019). As in Arabidopsis, these SPCHs may also heterodimerize with the partner ICE1/SCRM in monocots (Raissig et al., 2016; Wu et al., 2019). Interestingly, monocot SPCHs together with ICE1/SCRM were recently suggested to partner with the SHORT ROOT(SHR) /SCARECROW(SCR) module to regulate stomatal development (Wu et al., 2019), hinting that mobile SHR may commonly serve as a positional cue derived from the vasculature to assist asymmetric division of outer cell layers in plants.
IV. The fate of small daughter cell – the meristemoid
After a stomatal ACD in Arabidopsis, the small daughter cell meristemoid is projected to differentiate into stomatal guard cells (Fig. 2a). Prior to terminal differentiation, the meristemoid undergoes a few cycles of self-renewing ACD, a process fine-tuned by an antagonistic regulation of ethylene and glucose signaling (Gong et al., 2021). The expression of MUTE in late meristemoids switches on the one-time symmetric cell division of GMC by promoting CYCD5 and possibly CYCD7, too (Pillitteri et al., 2007; Han et al., 2018; Weimer et al., 2018). Towards stomatal differentiation, MUTE directly binds to the promoters and activates the expression of FAMA and FOUR LIPS (FLP) (Han et al., 2018), the key transcription factors for terminal differentiation of stomatal guard cells (Lai et al., 2005; Ohashi-Ito & Bergmann, 2006) (Fig. 2c). It remains unknown how MUTE, potentially a direct target of SPCH, is only expressed in late meristemoids but not as broadly as SPCH in early meristemoids and SLGCs. However, how MUTE is restricted from excessive expression in GMCs can be explained by the proposed autocrine regulation between MUTE and the peptide-ligand signaling (Qi et al., 2017) (Fig. 2c). In this model, MUTE directly induces the expression of the ERECTA-like 1 (ERL1) receptor-like kinase that perceives signaling of the secreted peptide EPIDERMAL PATTERNING FACTOR 1 (EPF1). This peptide-ligand signaling, in turn, triggers the canonical YDA MAPK cascade to suppress nuclear factors, likely including MUTE, in stomatal differentiation (Qi et al., 2017) (Fig. 2c). The divisional behavior of the meristemoid is also regulated by auxin signaling because mutations in the auxin efflux transporters resulted in abnormal stomatal differentiation (Le et al., 2014). Also, the acquisition of GMC fate is presaged by the PIN3-mediated depletion of auxin levels from the meristemoid but the underlying mechanism and possible connection with MUTE remain obscure (Le et al., 2014).
In monocots, ZmMUTE (maize) and OsMUTE (rice) showed conserved regulation of GMC identity, like AtMUTE in Arabidopsis. These mute mutants fail to produce GMC symmetric division and normal stomatal complexes (Wang et al., 2019; Wu et al., 2019). Interestingly, the grass MUTE proteins may not be restricted to the GMCs, as the YFP-tagged BdMUTE was found to diffuse into the neighboring subsidiary mother cells (SMCs) (Raissig et al., 2017; Wang et al., 2019) (Fig. 1b). Accordingly, in Brachypodium, the absence of MUTE results in the loss of SMC identity, the integrity of the four-celled stomatal complex, and regular stomatal movement (Raissig et al., 2017; Wang et al., 2019). Thus, MUTE in grasses appears to function autonomously to specify GMC fate and non-autonomously to specify SMC fate.
V. Differentiation of large daughter cell – the SLGC
After a stomatal ACD in Arabidopsis, the large daughter cell SLGC is distinguishingly marked by the presence of a polarity module comprised of a few scaffold proteins, including BASL, POLAR, and the BREVIS RADIX (BRX) family (Dong et al., 2009; Pillitteri et al., 2011; Rowe et al., 2019). The SLGCs, relative to the meristemoids, have more restricted cell-division potential and are projected to exit from stomatal fate to ultimately become pavement cells. Recently, Ho et al. employed transcriptional profiling of the polarized BRXL2-enriched SLGCs and identified the chromatin architectural protein DEK and the transcription factor MYB16, both of which contribute to the latent status of SLGC between cell division and endoreduplication (Ho et al., 2021). In addition, asymmetric divisions of SLGCs are fine-tuned by feedback crosstalk between SPCH and the cytokinin (CK) signaling components (Vatén et al., 2018).
The restricted division potential of SLGCs is also enforced by polarized BASL that by directly interacting with YDA enriches a “SPCH-targeting MAPK signaling module” (Zhang et al., 2015; Zhang et al., 2016). Thus, the SLGCs differ from the meristemoids by increased levels of MAPK signaling, resulting in elevated SPCH phosphorylation thus lower SPCH protein abundance in the SLGCs. The BRX proteins become polarized in a BASL-interdependent manner and the palmitoylation of BRX helps to tether the BRX-BASL complex to the plasma membrane (Rowe et al., 2019) (Preprint). In the root, BRX interacts with PROTEIN KINASE ASSOCIATED WITH BRX (PAX) to polarly accumulate to the rootward end of developing protophloem sieve elements, where BRX and PAX function as a molecular rheostat to modulate auxin flux and the timing of protophloem differentiation (Marhava et al., 2018). Whether the levels of auxin signaling in SLGCs are similarly modulated by BRX in stomatal development remains unknown.
In Brachypodium, despite two copies of YDA were identified, mutating BdYDA1 only was sufficient to give rise to severe stomatal clusters and abnormal recruitment of subsidiary cells (Abrash et al., 2018). Interestingly, BdYDA1-YFP showed polar accumulation in some of the stomatal lineage cells, most frequently at the interface between GMC and the prospective SMC (Abrash et al., 2018). Given the fact that Brachypodium does not have BASL, whether the four BdBRX proteins contribute to the polarization of YDA should be investigated in the future. Previously in maize, the asymmetric division of SMC was found to be driven by the polarized receptor-like protein PANGLOSS 1 (PAN1) that acts with the Rho of Plants (ROP) small GTPases to form an actin patch in SMC adjacent to GMC (Cartwright et al., 2009; Humphries et al., 2011; Facette et al., 2015) (Fig. 1b). It is worth noting that the polarity site in both systems (maize and Arabidopsis) plays roles in directional nuclear migration and division orientation in developing stomata. However, not much has been known, particularly in Arabidopsis, about how polarity proteins impinge on the spatial organization of the cytoskeleton elements and/or drive directional sometimes opposite nuclear migration before and after an ACD (Muroyama et al., 2020).
VI. Timed assembly of polarity components – before, during, and after stomatal ACD
The polarity platform represented by BASL was found to maintain before, during, and after a stomatal ACD (Dong et al., 2009). However, the ACD mother cell (MMC) fundamentally differs from the daughter cell (SLGC) with regards to cell-division potential (high in MMC and low in SLGC, Fig. 3). Although both contain the seemingly “same” polarity complex, the MMC expresses high levels of SPCH and undergoes more active stem-cell-like division, whereas the SLGC contains low levels of SPCH and is restricted from active cell division (Fig. 3). The distinct activities of MMC and SLGC might result from the distinct signaling components and functions that are dynamically organized by the polarity platform before, during, and after an ACD, respectively.
Fig. 3. Timed assembly of polarity components during stomatal ACD.
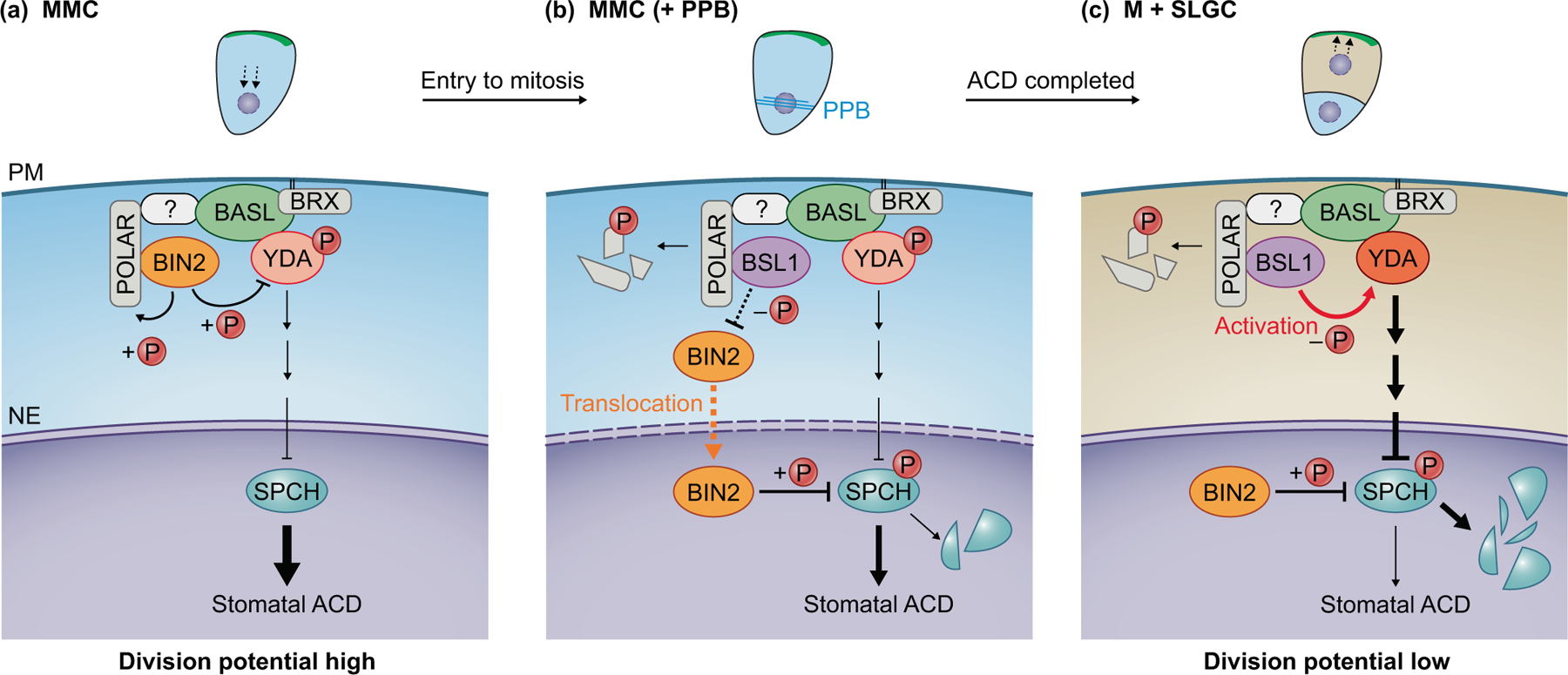
Diagrams on top show subsequent events during a stomatal asymmetric cell division (ACD) (a-c). The formation of the preprophase band (PPB, blue lines) indicates the cell entering mitosis. The direction of nuclear migration (dashed arrows) was found against and towards the polarity site, before and after the division, respective. Diagrams below detail the specific changes in the components of the polarity complex leading to differential division potential of the cell at different stages.
(a) Before an ACD (in MMC, meristemoid mother cell), BASL (green) is polarized at the cell cortex through the interaction with BRX that is palmitoylated to attach to the plasma membrane (grey). Polarization of POLAR (grey) is largely dependent on BASL, but its association with the polarity complex may need an unidentified component (box with a question mark). Polarized POLAR recruits the BIN2 GSK3-like kinases (orange) to inhibit the kinase activity of YDA (pink), leading to alleviated MAPK-mediated suppression on SPCH (blue). A high level of SPCH sustains a high level of cell-division potential in the MMCs. PM, plasma membrane. NE, nuclear envelope.
(b) During an ACD, BSL1 (purple) associates with the BASL polarity complex. The polarization of BSL1 coincides with the formation of the PPB upon MMC entering mitosis. Polarized BSL1 inhibits BIN2’s function at the PM involving the BSL1 phosphatase activity and mainly through dissociating BIN2 from the PM. Consequently, the BIN2 inhibition on YDA is released, leading to elevated SPCH degradation (broken blue oval). At the PM, BIN2 may trigger the turnover of POLAR through phosphorylation (broken grey box) that lowers the PM-association of BIN2. When BIN2 is enriched in the nucleus, it promotes SPCH degradation via phosphorylation.
(c) After an ACD, the BSL1-BASL-YDA polarity complex is inherited by SLGC (stomatal lineage ground cell). BSL1 directly activates YDA through dephosphorylation, so that elevated MAPK signaling confers strong suppression on SPCH and lowered cell-division potential. Therefore, the participation of BSL1 in the polarity complex during the transition from MMC to SLGC jointly regulates BIN2 localization and YDA activity, enabling the transition from high division potential to low division potential in MMC and SLGC, respectively. This process is essential for the progression of stomatal ACD and the specification of the two daughter cells with distinct developmental trajectories.
More specifically, the YDA MAPK signaling cassette associates with BASL polarity in both MMC and SLGC. The positive feedback between BASL and the YDA MAPK signaling confers a strong inhibition on the division-promoting activity of SPCH in SLGC (Zhang et al., 2015) (Fig. 3c). However, the suppression on SPCH must be alleviated to allow high division potential in MMC. It was recently reported that the GSK3-like BIN2 kinases are recruited to the polarity site through direct interaction with POLAR (Houbaert et al., 2018). Therefore, in MMC, the cortically enriched BIN2 can inhibit YDA kinase activity, thereby releasing the YDA MAPK-mediated inhibition to allow SPCH to accumulate in MMC (Kim et al., 2012; Houbaert et al., 2018) (Fig. 3a). Thus, although the YDA MAPK cascade is polarized in both MMC and SLGC, the participation of BIN2 in the polarity site suppresses YDA to maintain the high division potential of MMC(Guo & Dong, 2019).
Then, the next question was how the inhibition of BIN2 on YDA can be alleviated after division in SLGC. Recently, members of the BRI1 SUPPRESSOR (BSU1) family were identified to interact with BASL and the founding member BSL1 joins the polarity site and functions as a molecular switch to enable the transition from MMC to SLGC (Guo et al., 2021). Time-lapse examination of fluorescent protein-tagged BSL1 showed that BSL1 becomes polarized slightly later than BASL in MMC and coincides with the formation of the preprophase band (an indication of the entry to mitosis) (Fig. 3b). The association of BSL1 with the polarity complex on one hand leads to the translocation of BIN2 from the plasma membrane to the nucleus, likely due to its dephosphorylation activity. Consequently, the BIN2-mediated inhibition of YDA is released, whilst nuclear BIN2 may directly bind and phosphorylate SPCH for degradation (Gudesblat et al., 2012). On the other hand, polarized BSL1 directly binds to YDA for dephosphorylation and activation, leading to elevated MAPK signaling and further suppressed SPCH (Guo et al., 2021). Therefore, through the joint regulation of BIN2 (translocation) and YDA (activation), the assembly of BSL1 in the polarity complex, upon the MMC entry to mitosis, can quickly lower the protein level of SPCH and tune down the division potential of MMC, thereby enabling the progression towards SLGC in stomatal ACD (Guo et al., 2021).
VII. Outstanding questions
Stomatal development has proven to provide an excellent platform for dissecting molecular mechanisms underlying coordinated activities of cell polarity, self-renewing division, and cell-fate differentiation in plant development. A few outstanding questions remain in the field. First, surrounding the master regulator SPCH, is there a phosphocode-dependent regulatory dichotomy of the SPCH protein in degradation and activity? How is MUTE, the terminator of stomatal ACD, tightly restricted to express in a specific population of meristemoids? Does this process involve chromatin remodeling as suggested by (Lee et al., 2019)? How grass MUTE proteins can travel and induce the formation of the specialized subsidiary cell is also a big open area. The fast-expanding list of polarized proteins participating in the regulation of Arabidopsis stomatal ACD raised a long-standing question - whether a complementary polarity domain exists and contributes to the maintenance of cell polarity. It was also clear that, although the BASL polarity site is largely defined by intrinsic mechanisms (Chan et al., 2020), the mechanism for polarity switch during SLGC spacing division, presumably driven by signals released from the neighboring GMC, remains unknown. Also, YDA was found to commonly polarize in both monocot and dicot stomatal ACD cells. How YDA becomes polarized in the grasses and whether YDA polarization overlaps with the PAN signaling module are all uncharacterized.
The application of newer technologies, such as CRISPR-Cas-aided reverse genetics, cell-type-specific knocking out essential genes, and TurboID-based proximity labeling combined with quantitative proteomics, etc., will be greatly helpful for identifying new regulators and better characterizing known regulators in vivo. Successful basic research creates new directions and will ultimately lead to the pathways to produce plants with improved traits for agricultural applications.
Acknowledgements
X.G. is supported by grants from the National Institute of Health (GM109080 and GM131827 to J.D.). L.W. is supported by fellowships from the China Scholarship Council and the Waksman Busch Program at Rutgers. Current research program in J.D.’s lab is supported by NIH GM131827, NSF 825885, and NSF 1952823.
References
- Abrash E, Anleu Gil MX, Matos JL, Bergmann DC. 2018. Conservation and divergence of YODA MAPKKK function in regulation of grass epidermal patterning. Development 145(14): dev165860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergmann DC, Lukowitz W, Somerville CR. 2004. Stomatal development and pattern controlled by a MAPKK kinase. Science 304(5676): 1494–1497. [DOI] [PubMed] [Google Scholar]
- Bian C, Guo X, Zhang Y, Wang L, Xu T, DeLong A, Dong J. 2020. Protein phosphatase 2A promotes stomatal development by stabilizing SPEECHLESS in Arabidopsis. Proc Natl Acad Sci U S A 117(23): 13127–13137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartwright HN, Humphries JA, Smith LG. 2009. PAN1: a receptor-like protein that promotes polarization of an asymmetric cell division in maize. Science 323(5914): 649–651. [DOI] [PubMed] [Google Scholar]
- Chan J, Mansfield C, Clouet F, Dorussen D, Coen E. 2020. Intrinsic Cell Polarity Coupled to Growth Axis Formation in Tobacco BY-2 Cells. Curr Biol 30(24): 4999–5006.e4993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong J, MacAlister CA, Bergmann DC. 2009. BASL controls asymmetric cell division in Arabidopsis. Cell 137(7): 1320–1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Facette MR, Park Y, Sutimantanapi D, Luo A, Cartwright HN, Yang B, Bennett EJ, Sylvester AW, Smith LG. 2015. The SCAR/WAVE complex polarizes PAN receptors and promotes division asymmetry in maize. Nat Plants 1: 14024. [DOI] [PubMed] [Google Scholar]
- Gong Y, Alassimone J, Varnau R, Sharma N, Cheung LS, Bergmann DC. 2021. Tuning self-renewal in the Arabidopsis stomatal lineage by hormone and nutrient regulation of asymmetric cell division. Elife 10:e63335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gudesblat GE, Schneider-Pizoń J, Betti C, Mayerhofer J, Vanhoutte I, van Dongen W, Boeren S, Zhiponova M, de Vries S, Jonak C, et al. 2012. SPEECHLESS integrates brassinosteroid and stomata signalling pathways. Nat Cell Biol 14(5): 548–554. [DOI] [PubMed] [Google Scholar]
- Guo X, Dong J. 2019. To Divide or Differentiate: It Is about Scaffolding. Trends Plant Sci 24(6): 481–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo X, Park CH, Wang ZY, Nickels BE, Dong J. 2021. A spatiotemporal molecular switch governs plant asymmetric cell division. Nat Plants 7(5): 667–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han C, Liu Y, Shi W, Qiao Y, Wang L, Tian Y, Fan M, Deng Z, Lau OS, De Jaeger G, et al. 2020. KIN10 promotes stomatal development through stabilization of the SPEECHLESS transcription factor. Nat Commun 11(1): 4214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han SK, Qi X, Sugihara K, Dang JH, Endo TA, Miller KL, Kim ED, Miura T, Torii KU. 2018. MUTE Directly Orchestrates Cell-State Switch and the Single Symmetric Division to Create Stomata. Dev Cell 45(3): 303–315.e305. [DOI] [PubMed] [Google Scholar]
- Hepworth C, Caine RS, Harrison EL, Sloan J, Gray JE. 2018. Stomatal development: focusing on the grasses. Curr Opin Plant Biol 41: 1–7. [DOI] [PubMed] [Google Scholar]
- Ho CK, Bringmann M, Oshima Y, Mitsuda N, Bergmann DC. 2021. Transcriptional profiling reveals signatures of latent developmental potential in Arabidopsis stomatal lineage ground cells. Proc Natl Acad Sci U S A 118(17):e2021682118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horst RJ, Fujita H, Lee JS, Rychel AL, Garrick JM, Kawaguchi M, Peterson KM, Torii KU. 2015. Molecular Framework of a Regulatory Circuit Initiating Two-Dimensional Spatial Patterning of Stomatal Lineage. PLoS Genet 11(7): e1005374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houbaert A, Zhang C, Tiwari M, Wang K, de Marcos Serrano A, Savatin DV, Urs MJ, Zhiponova MK, Gudesblat GE, Vanhoutte I, et al. 2018. POLAR-guided signalling complex assembly and localization drive asymmetric cell division. Nature 563(7732): 574–578. [DOI] [PubMed] [Google Scholar]
- Humphries JA, Vejlupkova Z, Luo A, Meeley RB, Sylvester AW, Fowler JE, Smith LG. 2011. ROP GTPases act with the receptor-like protein PAN1 to polarize asymmetric cell division in maize. Plant Cell 23(6): 2273–2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim TW, Michniewicz M, Bergmann DC, Wang ZY. 2012. Brassinosteroid regulates stomatal development by GSK3-mediated inhibition of a MAPK pathway. Nature 482(7385): 419–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klermund C, Ranftl QL, Diener J, Bastakis E, Richter R, Schwechheimer C. 2016. LLM-Domain B-GATA Transcription Factors Promote Stomatal Development Downstream of Light Signaling Pathways in Arabidopsis thaliana Hypocotyls. Plant Cell 28(3): 646–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai LB, Nadeau JA, Lucas J, Lee EK, Nakagawa T, Zhao L, Geisler M, Sack FD. 2005. The Arabidopsis R2R3 MYB proteins FOUR LIPS and MYB88 restrict divisions late in the stomatal cell lineage. Plant Cell 17(10): 2754–2767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lampard GR, Macalister CA, Bergmann DC. 2008. Arabidopsis stomatal initiation is controlled by MAPK-mediated regulation of the bHLH SPEECHLESS. Science 322(5904): 1113–1116. [DOI] [PubMed] [Google Scholar]
- Lau OS, Davies KA, Chang J, Adrian J, Rowe MH, Ballenger CE, Bergmann DC. 2014. Direct roles of SPEECHLESS in the specification of stomatal self-renewing cells. Science 345(6204): 1605–1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau OS, Song Z, Zhou Z, Davies KA, Chang J, Yang X, Wang S, Lucyshyn D, Tay IHZ, Wigge PA, et al. 2018. Direct Control of SPEECHLESS by PIF4 in the High-Temperature Response of Stomatal Development. Curr Biol 28(8): 1273–1280.e1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le J, Liu XG, Yang KZ, Chen XL, Zou JJ, Wang HZ, Wang M, Vanneste S, Morita M, Tasaka M, et al. 2014. Auxin transport and activity regulate stomatal patterning and development. Nat Commun 5: 3090. [DOI] [PubMed] [Google Scholar]
- Lee LR, Wengier DL, Bergmann DC. 2019. Cell-type-specific transcriptome and histone modification dynamics during cellular reprogramming in the Arabidopsis stomatal lineage. Proc Natl Acad Sci U S A 116(43): 21914–21924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacAlister CA, Ohashi-Ito K, Bergmann DC. 2007. Transcription factor control of asymmetric cell divisions that establish the stomatal lineage. Nature 445(7127): 537–540. [DOI] [PubMed] [Google Scholar]
- Marhava P, Bassukas AEL, Zourelidou M, Kolb M, Moret B, Fastner A, Schulze WX, Cattaneo P, Hammes UZ, Schwechheimer C, et al. 2018. A molecular rheostat adjusts auxin flux to promote root protophloem differentiation. Nature 558(7709): 297–300. [DOI] [PubMed] [Google Scholar]
- Muroyama A, Gong Y, Bergmann DC. 2020. Opposing, Polarity-Driven Nuclear Migrations Underpin Asymmetric Divisions to Pattern Arabidopsis Stomata. Curr Biol 30(22): 4549–4552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadeau JA, Sack FD. 2002. Control of stomatal distribution on the Arabidopsis leaf surface. Science 296(5573): 1697–1700. [DOI] [PubMed] [Google Scholar]
- Nunes TDG, Zhang D, Raissig MT. 2020. Form, development and function of grass stomata. Plant J 101(4): 780–799. [DOI] [PubMed] [Google Scholar]
- Ohashi-Ito K, Bergmann DC. 2006. Arabidopsis FAMA controls the final proliferation/differentiation switch during stomatal development. Plant Cell 18(10): 2493–2505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillitteri LJ, Peterson KM, Horst RJ, Torii KU. 2011. Molecular profiling of stomatal meristemoids reveals new component of asymmetric cell division and commonalities among stem cell populations in Arabidopsis. Plant Cell 23(9): 3260–3275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillitteri LJ, Sloan DB, Bogenschutz NL, Torii KU. 2007. Termination of asymmetric cell division and differentiation of stomata. Nature 445(7127): 501–505. [DOI] [PubMed] [Google Scholar]
- Putarjunan A, Ruble J, Srivastava A, Zhao C, Rychel AL, Hofstetter AK, Tang X, Zhu JK, Tama F, Zheng N, et al. 2019. Bipartite anchoring of SCREAM enforces stomatal initiation by coupling MAP kinases to SPEECHLESS. Nat Plants 5(7): 742–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi SL, Lin QF, Feng XJ, Han HL, Liu J, Zhang L, Wu S, Le J, Blumwald E, Hua XJ. 2019. IDD16 negatively regulates stomatal initiation via trans-repression of SPCH in Arabidopsis. Plant Biotechnol J 17(7): 1446–1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi X, Han SK, Dang JH, Garrick JM, Ito M, Hofstetter AK, Torii KU. 2017. Autocrine regulation of stomatal differentiation potential by EPF1 and ERECTA-LIKE1 ligand-receptor signaling. Elife 6:e24102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raissig MT, Abrash E, Bettadapur A, Vogel JP, Bergmann DC. 2016. Grasses use an alternatively wired bHLH transcription factor network to establish stomatal identity. Proc Natl Acad Sci U S A 113(29): 8326–8331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raissig MT, Matos JL, Anleu Gil MX, Kornfeld A, Bettadapur A, Abrash E, Allison HR, Badgley G, Vogel JP, Berry JA, et al. 2017. Mobile MUTE specifies subsidiary cells to build physiologically improved grass stomata. Science 355(6330): 1215–1218. [DOI] [PubMed] [Google Scholar]
- Rowe MH, Dong J, Weimer AK, Bergmann DC. 2019. A Plant-Specific Polarity Module Establishes Cell Fate Asymmetry in the Arabidopsis Stomatal Lineage. bioRxiv: 614636. [Google Scholar]
- Shpak ED, McAbee JM, Pillitteri LJ, Torii KU. 2005. Stomatal patterning and differentiation by synergistic interactions of receptor kinases. Science 309(5732): 290–293. [DOI] [PubMed] [Google Scholar]
- Vatén A, Soyars CL, Tarr PT, Nimchuk ZL, Bergmann DC. 2018. Modulation of Asymmetric Division Diversity through Cytokinin and SPEECHLESS Regulatory Interactions in the Arabidopsis Stomatal Lineage. Dev Cell 47(1): 53–66.e55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Guo S, Qiao X, Guo J, Li Z, Zhou Y, Bai S, Gao Z, Wang D, Wang P, et al. 2019. BZU2/ZmMUTE controls symmetrical division of guard mother cell and specifies neighbor cell fate in maize. PLoS Genet 15(8): e1008377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Ngwenyama N, Liu Y, Walker JC, Zhang S. 2007. Stomatal development and patterning are regulated by environmentally responsive mitogen-activated protein kinases in Arabidopsis. Plant Cell 19(1): 63–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weimer AK, Matos JL, Sharma N, Patell F, Murray JAH, Dewitte W, Bergmann DC. 2018. Lineage- and stage-specific expressed CYCD7;1 coordinates the single symmetric division that creates stomatal guard cells. Development 145(6): dev160671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Z, Chen L, Yu Q, Zhou W, Gou X, Li J, Hou S. 2019. Multiple transcriptional factors control stomata development in rice. New Phytol 223(1): 220–232. [DOI] [PubMed] [Google Scholar]
- Yang KZ, Jiang M, Wang M, Xue S, Zhu LL, Wang HZ, Zou JJ, Lee EK, Sack F, Le J. 2015. Phosphorylation of Serine 186 of bHLH Transcription Factor SPEECHLESS Promotes Stomatal Development in Arabidopsis. Mol Plant 8(5): 783–795. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Guo X, Dong J. 2016. Phosphorylation of the Polarity Protein BASL Differentiates Asymmetric Cell Fate through MAPKs and SPCH. Curr Biol 26(21): 2957–2965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Wang P, Shao W, Zhu JK, Dong J. 2015. The BASL polarity protein controls a MAPK signaling feedback loop in asymmetric cell division. Dev Cell 33(2): 136–149. [DOI] [PMC free article] [PubMed] [Google Scholar]


