Abstract
Background/Objective:
Sleep is a critically important behavior which influences diverse aspects of health, functioning, and longevity. An increasing literature suggests the importance of sleep regularity, also referred to as sleep inconsistency, sleep variability, or intraindividual variability in sleep. Given there is no brief, subjective measure of sleep regularity, the purpose of this study was to develop the Sleep Regularity Questionnaire (SRQ) and to begin the process of examining its psychometric properties using a construct-validation approach.
Participants/Methods:
In an online study of sleep and health, participants (n = 3249; Mage (SD) = 42.77(16.73); 48.5% female; 77.3% white) completed the in-development SRQ, as well as the Insomnia Severity Index and the Pittsburgh Sleep Quality Index.
Results:
An exploratory factor analysis followed by a confirmatory factor analysis revealed a two factor structure, represented by circadian regularity and sleep continuity regularity, with good model fit indices (X2 = 50.9, df = 7, p < .001; RMSEA = .06; CFI= .99; NFI = .99; IFI = .99; TLI = .98). Test-retest reliability, as well as concurrent, convergent and incremental validity were examined, with promising results.
Conclusions:
Preliminary psychometrics suggest that the SRQ is a valid and stable instrument for the assessment of sleep regularity in adults that is related to, but distinct from, other established sleep constructs. Future research will benefit from assessing the validity of the SRQ in various clinical samples and how it compares to measures of sleep regularity calculated from prospective daily assessments.
Keywords: Sleep Regularity, Sleep Inconsistency, Intraindividual Variability, Measurement, Self-report, Psychometrics
1. Introduction
Sleep is a vital, multidimensional process critical to human functioning. While having consistent nights of sleep with adequate quality and quantity is considered a marker of healthy aging,1,2 poor sleep has been linked to adverse physical, psychological, and cognitive consequences. These adverse outcomes can, in turn, reduce overall quality of life and increase both morbidity and mortality.3 Prior research aimed at evaluating the associations between sleep and adverse outcomes has largely been conducted on the aggregate mean/average level of sleep characteristics for an individual over a period of time. Using average sleep characteristics is a generally straightforward way to classify individuals into “poor” sleepers and “good” sleepers based on their self-reported sleep quality on measures such as the Pittsburgh Sleep Quality Index (PSQI)4 and the Insomnia Severity Index (ISI)5; however, the practice of asking for aggregate retrospective recall of sleep habits, obscures the large amount of nightly inconsistency in sleep within an individual. As such, researchers could be drawing inaccurate or incomplete conclusions about the degree to which an individual’s sleep contributes to a variety of health outcomes. Even when sleep is measured prospectively via sleep diary or actigraphy, the common practice of calculating means/averages could obscure the great level of night-to-night inconsistency often present.
For example, two individuals who spend, on average, 45 minutes awake per night, may have drastically different nightly sleep patterns, with one being awake very close to 45 minutes every night (Person A) and the other oscillating between around 30 minutes and 60 minutes per night (Person B; Figure 1).
Figure 1.
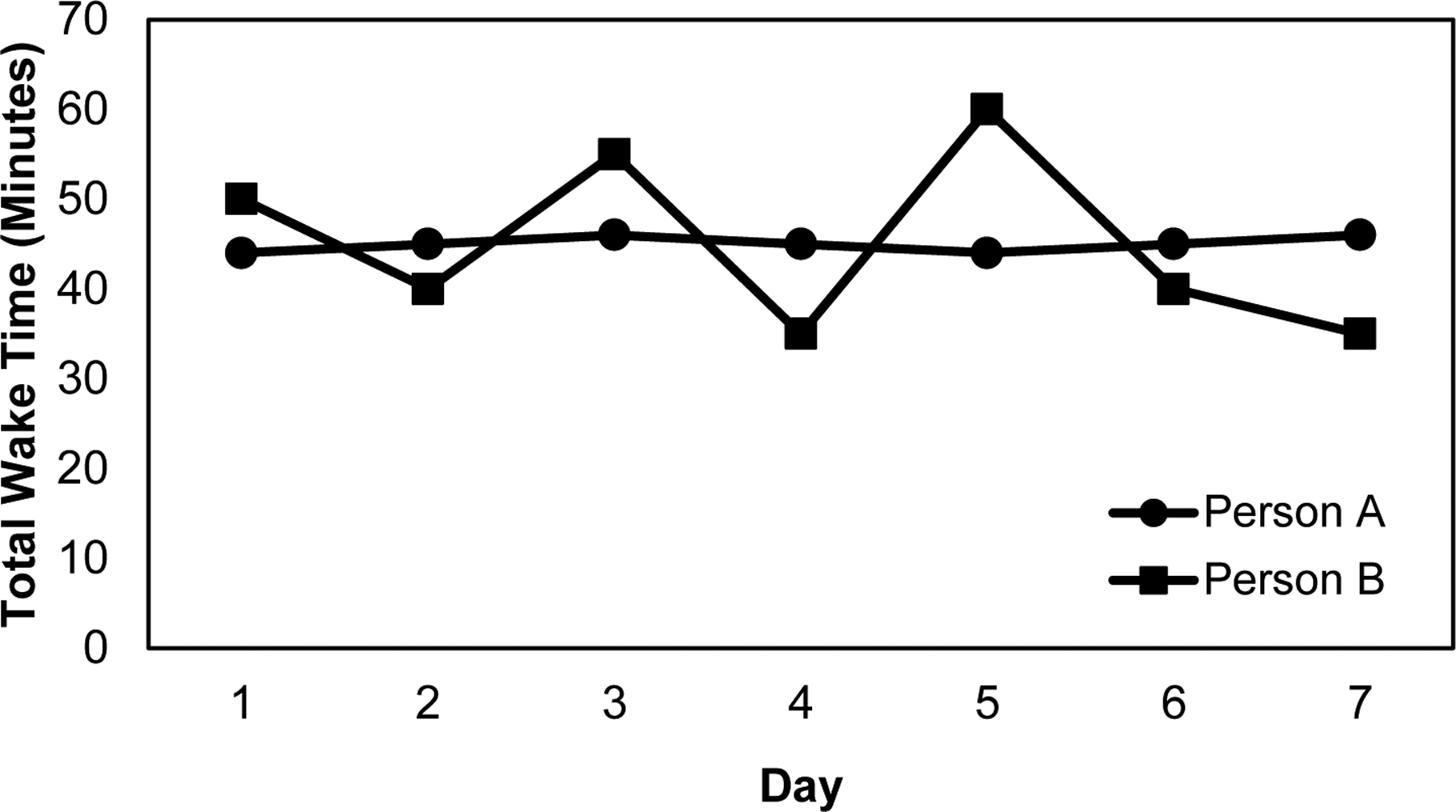
Example of total wake time over a week of two hypothetical people with the same amount of time awake on average but different levels of inconsistency.
Sleep regularity is a comparatively new area of research in the sleep literature. Previous work has shown that many sleep characteristics were highly inconsistent, with older adults having poorer and more inconsistent sleep than younger adults.6 Specifically, older adults had more inconsistent wake after sleep onset (WASO) and sleep quality ratings.6 Lack of regularity in sleep, especially inconsistent WASO7,8 and sleep efficiency,8 has been found to be associated with poorer objective sleep quality.7,8 Similarly, lower regularity in sleep duration has been associated with subjective sleep quality and subjective well-being.9 Importantly, sleep regularity has been shown to decrease following treatment for insomnia and may serve as a marker for high treatment responsiveness.10
Lower regularity in sleep duration has been found to be associated with higher HbA1c,7 lower regularity in sleep onset was associated with higher body mass index (BMI),7 and lower regularity in a variety of sleep characteristics including bedtime, wake time, time in bed, terminal wakefulness, wake after sleep onset, sleep onset latency, and number of nighttime awakenings were associated with higher levels of pro-inflammatory markers,11,12 suggesting that sleep regularity (i.e., the opposite of sleep inconsistency) is related to other objective markers of health.7,11,12 Some associations between sleep and health, such as the association of regularity in nightly total sleep time and daytime napping with obesity,13 have been found to be independent of the association with average sleep duration, further demonstrating the importance of examining sleep regularity in addition to average sleep when looking at sleep’s role in health outcomes.14 Furthermore, preliminary evidence indicates that greater bedtime and rise time regularity in older adults is associated with sleeping more than seven hours per night.15 This finding is particularly concerning because sleeping fewer than seven hours per night is associated with greater mortality risk.16
To date, researchers have examined associations among sleep regularity and a variety of mental and physical health outcomes by utilizing subjective (i.e., diary) and objective (e.g., actigraphy) prospective daily assessments of sleep. While each of these methods are relatively easy to implement, they require intense, repeated data collection across numerous days and are not able to be rapidly scored and interpreted within a clinical setting or in large epidemiological survey designs. Therefore, there is a need for a brief subjective measure of sleep regularity.
The purpose of this study is to determine whether a newly developed retrospective questionnaire, the Sleep Regularity Questionnaire (SRQ), could fill this void as a valid and reliable measure of sleep regularity and whether self-reported sleep regularity is distinct from, and adds unique information above, other commonly employed aggregate measures of self-reported sleep. Based on a theory-driven method of scale construction, we adopted a construct-validation approach17 for developing a measure that assesses the regularity of sleep. This approach involved a substantive validity phase during which the initial item pool was developed, a structural validity phase where the factor-structure was evaluated and confirmed, and an external validity phase where concurrent, convergent and incremental validity of the scale were assessed alongside theoretically supported and relevant measures.
2. Materials and Method
2.1. Participants and Procedure
The present study was a secondary analysis of data from the Investigating Sleep Longitudinally Across Normal Development (ISLAND) study. Data from participants (N = 4,678) who completed sleep and health questionnaires as part of this observational online study were included in the present study. Inclusion and exclusion criteria were limited to ensuring a relatively equal number of men and women living in the United States were recruited across age groups covering the adult lifespan. Enrollment was limited such that once the target had been met for a given sex/age group (~500/group), enrollment for that age/sex group was closed.
The study protocol and procedures were approved by the Virginia Commonwealth University Institutional Review Board (IRB# HM20008543). Participants were recruited via an online platform (Amazon’s Mechanical Turk; MTurk) where individuals are compensated for the completion of various surveys and tasks. The study did not require written informed consent; however, participants were able to view a research participant information and consent form and to agree to participate by answering “yes” to a question presented after the document had been viewed. After providing informed consent, participants completed a series of measures associated with sleep, physical health and well-being, and mental health and well-being.
MTurk was identified as an appropriate data collection tool for the parent study, as MTurk tends to provide more demographically diverse samples than other standard internet data collection methods while providing the same level of reliability among data as traditional paper-and-pencil methods.18 In addition, other validated sleep measures have been demonstrated to have similar psychometric properties, whether completed online or in-person.19
To address threats to validity associated with online data collection, two validity checks were implemented as part of the survey: (a) an instructional manipulation check which asked participants to answer an item with a specific response and (b) a consistency check which compared participants’ responses to an age question at the beginning of the survey to a birthdate question at the end. Participants failing the instructional validity check (N = 587), the consistency check (N = 176), or for whom the age validity check could not be completed (i.e., both the age and birth year questions were not answered; N = 251) were excluded from analyses. Additionally, duplicate responses determined to be from the same participant were excluded from analyses and the respondent’s first response was always used (N = 380). After removal of outliers during data screening procedures (N = 35), the final sample for the study was N = 3,249.
2.2. Measures
2.2.1. Demographic Information
Participants self-reported their age, gender, and race/ethnicity.
2.2.2. Sleep Regularity
The Sleep Regularity Questionnaire (SRQ) was used to assess the degree to which individuals engage in consistent sleep behaviors. During the substantive validity phase measure items were developed by a licensed clinical psychologist with over a decade of experience and expertise in behavioral sleep medicine. Specific questions were built upon items from existing, validated sleep measures (i.e., daily sleep diaries, the PSQI, and the ISI) by asking about regularity of characteristics usually measured on the aggregate level. The SRQ started with questions focused on the beginning of the night (e.g., “I go to bed about the same time every night.”) and continued through to the morning (e.g., “I get out of bed at the same time each morning.”). SRQ items were selected to assess the full breadth of sleep-related behaviors. Participants rated each of the 10 items, on a scale from 0 (not at all) to 4 (very much) with regard to how much they agreed with each of the statements. The full SRQ is presented in Appendix A.
2.2.3. Sleep Quality
Subjective sleep quality was assessed using the Pittsburgh Sleep Quality Index (PSQI),4 a 19-item self-report questionnaire with both Likert and open-ended response formats. Items assess average sleep patterns using the open-ended response format, e.g., “During the past month, when have you usually gone to bed?”. Items assess frequency of sleep disturbances using items such as, “During the past month, how often have you had trouble sleeping because you … Cannot get to sleep within 30 minutes?”, which are rated on a Likert scale ranging from 0 Not during the past month to 3 Three or more times a week. The PSQI is the most widely used measure of sleep quality and disturbances20 and will serve as a measure of convergent validity for the SRQ.
2.2.4. Insomnia Symptoms
Insomnia symptoms were measured via the Insomnia Severity Index (ISI; e.g., “How worried/distressed are you about your current sleep pattern?”). The ISI contains seven items rated on four-point Likert scales.5 The ISI is a widely used scale which has been deemed a valid and reliable measure of insomnia symptoms among both community and clinical-based samples.5,21 The ISI was used as a measure of concurrent validity for the SRQ.
2.2.5. Depression Symptoms
Depression symptoms were measured via the Patient Health Questionnaire-2 (PHQ-2; e.g., “Over the last 2 weeks, how often have you been bothered by the following problems…Little interest or pleasure in doing things”). The PHQ-2 contains two items rated on four-point Likert scales.22 Scores on the PHQ-2 range from 0–6, with higher scores indicating more depressive symptoms. The PHQ-2 is a widely used measure which has been deemed a valid and reliable screening measure of depression symptoms among both community and clinical-based samples.23 The PHQ-2 was used as an outcome measure to assess incremental validity of the SRQ.
2.2.6. Anxiety Symptoms
Anxiety symptoms were measured via the Generalized Anxiety Disorder 2-item scale (GAD-2; e.g., “Over the last 2 weeks, how often have you been bothered by the following problems…Feeling nervous, anxious, or on edge”). The GAD-2 contains two items rated on four-point Likert scales.24 Scores on the GAD-2 range from 0–6, with higher scores indicating more anxiety symptoms. The GAD-2 is a widely used measure which has been deemed a valid and reliable screening measure of anxiety symptoms among both community and clinical-based samples.25 The GAD-2 was used as an outcome measure to assess incremental validity of the SRQ.
2.2.7. Physical Health
Physical health was self-rated using a single item which asked, “In general, would you say your physical health is…”. Respondents answered using a 0–100 slider bar visual analog scale with five anchors (i.e., poor, fair, good, very good, excellent). Similar single-item measures of physical health have been widely used to assess individual’s perceptions of global physical health.26 Self-rated physical health was used as outcome measures to assess incremental validity of the SRQ.
2.3. Statistical Analyses
2.3.1. Data Screening
Analyses were conducted using SPSS v.26 and AMOS v.26.27 The characteristics of the sample were described with counts/percentages for nominal variables and means/standard deviations for continuous variables. Patterns of missing data were assessed via Little’s MCAR test. Univariate outliers were assessed via z-scores, and multivariate outliers were assessed via Mahalanobis distance. Univariate and multivariate skewness and kurtosis coefficients as well as visual inspection of Q-Q plots were used to assess normality. The data were then split in half randomly using SPSS’s select cases function (random 50%) assigning 1640 participants to sample 1 and 1644 to sample 2.
2.3.2. Sample Size
Determining sample size for factor analyses is not an exact science, but a sample size of 1000 is considered “excellent”.28 Even after dividing the sample in half to allow for split-half cross validation, each subsample contained >1000 participants and is adequately powered to determine and confirm factor structure.
2.3.3. Exploratory Factor Analysis
In order to determine the factor structure of the SRQ, an exploratory factor analysis (EFA) assuming no a priori factor structure was performed using principal axis factoring and a promax rotation using all 10 items of the SRQ from sample 1, a random subsample of half of the participants of the overall sample. Kaiser-Meyer-Olkin (KMO) and Bartlett’s tests were used to evaluate whether the data were suitable for factor analysis.29 Factor analysis serves several related functions in scale development, including: (1) determining the number of latent variables/factors underlying a set of items thus condensing a larger set of variables to ease understanding and interpretation, (2) determining the meaning of the factors, and (3) identifying and eliminating items that are not closely related to one of the factors. Factor analysis was employed in the present study to serve these functions.
2.3.3.1. Rotation Selection
An oblique promax rotation was selected for use, as factors were expected to be related to one another.30 If factors were not found to be related at least .15–.30 or higher, an orthogonal varimax rotation would be used.
2.3.3.2. Simple Structure
Several indices were used to determine the factor structure of the SRQ. First, a scree plot with the cutoff eigenvalue of 1 was utilized to visually identify the number of factors.31 An item was chosen to load onto a specific factor if the highest loading eigenvalue exceeded an absolute value of .50, with all cross loadings being at least .15 less than the item’s highest factor loading.32,33 Items not reaching these factor loading guidelines were deleted, and the EFA was rerun among retained items until a final structure was confirmed.
2.3.4. Confirmatory Factor Analysis
A confirmatory factor analysis (CFA) was performed on the data from sample 2 specifying the factor structures that had emerged in the EFA and omitting items not loading with simple structure in the EFA. Additional removal of multivariate outliers was performed during this step. Model fit was evaluated using previously established guidelines.30 Specifically, a goodness of fit index (GFI) of ≥ .90 was considered good, a root mean square error of approximation (RMSEA) of ≤ .08 was considered good and ≤ .10 adequate, and a comparative fit index (CFI) normed fit index (NFI), Tucker-Lewis index (TLI), and incremental fit index (IFI) were all considered good at ≥ .95 and adequate at ≥ .90.
2.3.4.1. Measurement Invariance
In order to determine whether the CFA differed between males and females, an invariance design was run as a function of gender.
2.3.4.2. Reliability and Validity Assessment
Reliability via internal consistency and test-retest coefficients was investigated. Internal consistency was evaluated using item-total correlations and Cronbach’s αs for the subscale scores for the revised SRQ, and test-retest reliability of the scale was assessed using an intraclass correlation coefficient (ICC). To examine test-retest reliability, 233 individuals completed a second administration of the SRQ at an average of 78.07 days after the first assessment (range = 5–153 days, SD = 25.13 days). This group was 52.4% male, predominantly White (85%), and had a mean age of 53.85 (SD = 17.17, range = 19–89).
In order to examine the scale’s concurrent and convergent validity, SRQ total scores and subscale scores were correlated with participants PSQI and ISI total scores, and were compared using t-tests between individuals categorized as ‘good’ and ‘poor’ sleepers based on PSQI and ISI. Lastly, incremental validity was ascertained via hierarchical multiple regressions, with PSQI or ISI total scores entered in the first block and one SRQ subscale score in the second block. Twelve hierarchical regressions were conducted to assess the SRQ’s ability to predict depression symptoms, anxiety symptoms, and self-rated physical health above and beyond the PSQI and ISI.
3. Results
3.1. Participants
Demographic characteristic from the final sample are presented in Table 1.
Table 1.
Sample Demographic Information
| Sample Demographic Information | Whole Sample (N = 3249) | Sample 1, EFA (N = 1640) | Sample 2, CFA (N = 1609) | |||
|---|---|---|---|---|---|---|
| Variable | N | % | N | % | N | % |
| Age [Range / Mean(SD)] | 19–99 | 42.77 (16.73) | 19–87 | 42.41 (16.67) | 19–99 | 43.14 (16.80) |
| Racial/Ethnic Group | ||||||
| White | 2512 | 77.3 | 1283 | 78.2 | 1229 | 76.4 |
| Black | 209 | 6.4 | 103 | 6.3 | 106 | 6.6 |
| Asian | 174 | 5.4 | 84 | 5.1 | 90 | 5.6 |
| Hispanic/Latino Only | 145 | 4.5 | 66 | 4.0 | 79 | 4.9 |
| More than one race/ethnicity, not Hispanic/Latino | 77 | 2.4 | 35 | 2.1 | 42 | 2.6 |
| More than one race/ethnicity, Hispanic/Latino | 67 | 2.1 | 30 | 1.8 | 37 | 2.3 |
| Other Race | 43 | 1.3 | 26 | 1.6 | 17 | 1.1 |
| Native American | 16 | 0.5 | 10 | 0.6 | 6 | .4 |
| Pacific Islander | 6 | 0.2 | 3 | 0.2 | 3 | .2 |
| Gender | ||||||
| Female | 1575 | 48.5 | 810 | 43.2 | 765 | 47.5 |
| Male | 1466 | 45.1 | 708 | 49.4 | 758 | 47.1 |
| Other | 208 | 6.4 | 122 | 7.4 | 86 | 5.3 |
EFA = exploratory factor analysis, CFA = confirmatory factor analysis.
Note: Table represents whole sample after removal of 35 outliers from Sample 2 during CFA analyses. Sample did not significantly differ on any demographic variables.
3.2. Data Screening
To assess whether data were missing completely at random (MCAR), Little’s MCAR test was used. Little’s MCAR test was found to be significant, indicating that data were missing not at random, X2(17) = 59.92, p < .001. Upon further inspection, data were only missing for PSQI total score. Therefore, no action was taken to impute the missing data, as the PSQI was selected to serve as a measure by which to validate the SRQ, and using SRQ items to impute the PSQI would falsely inflate the correlation between the scales. Instead, the participants with missing PSQI total score were excluded from that analysis only. Univariate and multivariate outliers comprised a very small amount of the total sample (< 1–2%), so these values were left in the sample.34
Structural Validity
3.3. EFA
KMO (.884) and Bartlett’s (X2(45) = 2519.60, p < .001) tests were performed with results suggesting that the data were suitable for factor analysis.29 The promax rotation was maintained, as all factors were correlated at .23–.85 (all ps <.001). A scree plot31 revealed a pronounced inflection point at the second-highest eigenvalue. Specifically, the first two factors accounted for 60.32% of the items’ cumulative variance, with 50.02% explained by factor one and 10.30% explained by factor two.
The item loadings for the two factors in this EFA appear in the table below. The four items that did not achieve simple structure were considered not to be a meaningful part of the factor solution and are listed at the bottom of the table (Table 2).
Table 2.
1st EFA factor loadings
| Original SRQ Item | Factor 1 | Factor 2 |
|---|---|---|
| 6 | .966 | −.131 |
| 5 | .911 | −.068 |
| 1 | .707 | .093 |
| 8 | .549 | .304 |
| 4 | −.031 | .807 |
| 3 | −.068 | .778 |
| Removed Items | ||
| 2 | .435 | .376 |
| 7 | .404 | .280 |
| 10 | .259 | .252 |
| 9 | .131 | .317 |
Items pertaining to factor one (bed time, wake time, rise time, and sleep time) may best be described as “circadian regularity”, whereas items pertaining to factor two (number of nighttime awakenings, duration of nighttime awakenings) may best be described as “sleep continuity regularity.”
As factors with fewer than three items are often unstable, a second EFA was run with only the six retained items and the same loading criteria to see if the factor structure would change. All six items loaded onto one factor as determined by the scree plot and eigenvalues. This factor accounted for 60.95% of the items’ cumulative variance. All six scale items pertaining to a single factor may best be characterized as “sleep regularity.”
3.4. CFA
After the removal of 35 multivariate outliers from the subsample, the two-factor model indicated by the exploratory factor analysis was run. All of the scale items loaded significantly onto their respective first-order latent factors, all ps < .001. The correlation between circadian regularity and sleep continuity regularity was .58, p < .001. Model fit was evaluated with previously stated criteria, and was determined to be in the good range, with the exception of the X2 value (X2 = 239.53, df = 8, p < .001; GFI = .948; RMSEA = .134; CFI = .962, NFI = .961; IFI = .962; TLI = .929); however X2 tests are extremely sensitive to large sample sizes, suggesting that this is probably not a good estimate of fit for our sample.35
Based on modification indices, it was determined that correlating the uniqueness terms of items 5 and 6 would greatly improve model fit. Items 5 and 6 ask about the regularity with which someone wakes up (item 5) and gets out of bed (item 6). As the time someone wakes up and the time someone gets out of bed are naturally highly correlated, it was determined to be appropriate to include this modification to the model. Therefore, the two-factor model was re-run after the addition of a correlation path between items 5 and 6 (Figure 2). All of the scale items loaded significantly onto their respective first-order latent factors, all ps < .001. The correlation between circadian regularity and sleep continuity regularity was .66, p < .001. Model fit was evaluated with previously stated criteria, and was determined to be in the good range, with the exception of the X2 value (X2 = 50.9, df = 7, p < .001; GFI = .989; RMSEA = .062; CFI = .993, NFI = .992; IFI = .993; TLI = .985).
Figure 2.
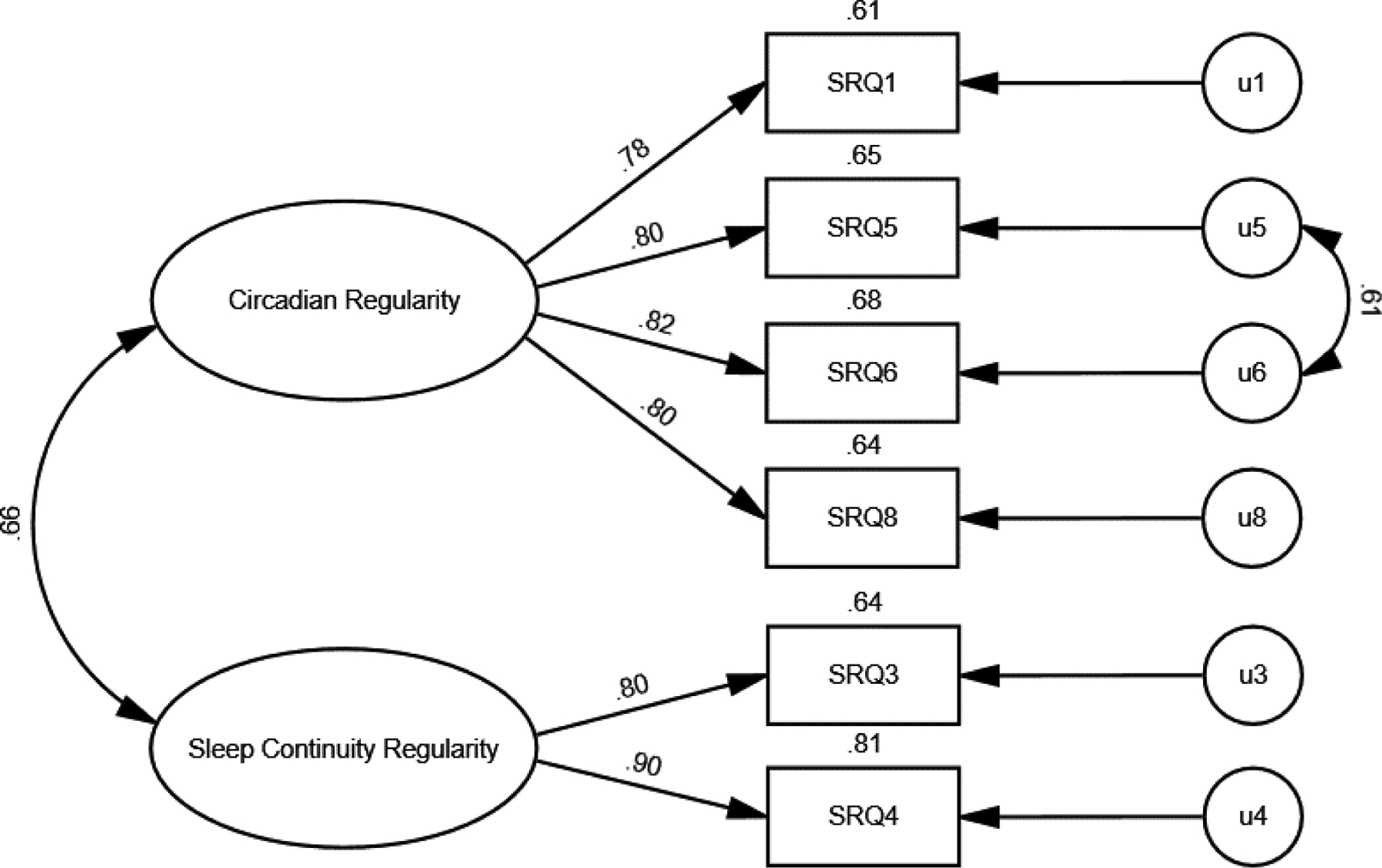
Two-factor CFA with multivariate outliers removed from sample and a correlation path drawn between the uniqueness terms for items 5 and 6. Standardized regression weights are shown. Numbers on top of the boxes are squared multiple correlations (the amount of variability in the particular scale item accounted for by the latent factor).
Finally, a CFA was conducted on the one-factor solution that emerged via the second EFA. All scale items loaded significantly onto their respective first-order latent factor, all ps < .001. Model fit was poor according to previously stated criteria (X2 = 1044.71, df = 9, p < .001; GFI = .827; RMSEA = .268; CFI = .832, NFI = .831; IFI =.832; TLI = .719). Thus, the two factor model with the additional correlation path was retained for all remaining analyses.
3.4.1. Measurement Invariance
In order to determine whether the CFA on the final solution differed for men and women, an invariance design was employed as a function of self-reported gender. The analysis evaluated the difference between an unconstrained model, which assumes that the groups are yielding different parameter values when the model is applied to the data, and a constrained model, which assumes that the groups are yielding equivalent parameter values. Three sets of comparisons were of interest: measurement weights, structural covariances, and measurement residuals.
The first two sets of comparisons were non-significant (all ps > .520). However, the measurement residual comparison did reach statistical significance, X2(14) = 31.16, p = .005, suggesting that men and women differed in the magnitude of the uniqueness terms of the manifest variables in the model. Bonferroni-corrected post-hoc comparisons (α = .0083) suggested that none of the error terms individually were noninvariant across the two groups (all zs < 2.64). It may therefore be concluded that the responses of men and women can both be described by the CFA. The CFA generally showed overall strong invariance across men and women.
External Validity
3.4.2. Reliability and Validity Assessment
Sample 2, the subsample used for CFA analyses, was used for internal consistency and validity assessments. The Cronbach’s α for the total six-item questionnaire was .87. For the four-item circadian regularity subscale, the Cronbach’s α was .88, and .78 for the two-item sleep continuity regularity subscale. Average inter-item correlation ranged from .38 to .85. Test-retest analyses revealed an ICC of .78 [95% CI=. 71 to .83], indicating acceptable reliability over an average of 78 days. Participants’ total score on the SRQ, circadian regularity subscale, and sleep continuity regularity subscale were significantly negatively correlated with participants’ PSQI total score (r = −.37, p < .001; r = −.40, p < .001; r = −.21, p < .001, respectively), indicating that individuals with higher scores on the SRQ (indicating greater sleep regularity) had lower scores on the PSQI (indicating better sleep quality). Participants’ total score on the SRQ, circadian regularity subscale, and sleep continuity regularity subscale were significantly negatively correlated with participants scores on the ISI at total scale score (r = −.39, p < .001; r = −.42, p < .001; r = −.21, p < .001, respectively), indicating that individuals with higher scores on the SRQ (indicating greater sleep regularity) had lower scores on the ISI (indicating fewer insomnia symptoms). Independent samples t-tests revealed that there was significant difference between levels of sleep regularity for good and poor sleepers. Participants with good sleep quality defined by the PSQI had significantly higher sleep regularity (M = 24.91) than those with poor sleep quality defined by the PSQI (M = 21.10), t(1457.52)= −15.244, p < .001. Similarly, participants with ISI-defined good sleep had significantly higher sleep regularity (M = 23.98) than those with ISI-defined poor sleep (M = 20.42), t(1310.32)= 13.61, p < .001. Hierarchical regressions revealed varied levels of incremental validity. For the most part, while the SRQ predicted significant variance above and beyond both the PSQI and ISI, the amount of additional variance predicted was small, with the exception of a medium sized effect of circadian regularity predicting depressive symptoms above and beyond the PSQI (ΔR2 = 0.16, p < .001, f2 = 0.19), see Table 3.
Table 3.
Incremental Validity of the Sleep Regularity Questionnaire.
| PHQ-2 | GAD-2 | Physical Health VAS | ||||
|---|---|---|---|---|---|---|
| ΔR2 | f 2 | ΔR2 | f 2 | ΔR2 | f 2 | |
| Sleep Continuity Regularity | 0.004 | 0.004 | 0.003* | 0.003 | 0.001 | 0.001 |
| Circadian Regularity | 0.160* | 0.190 | 0.003* | 0.003 | 0.021* | 0.021 |
| Insomnia Severity Index | ||||||
| Sleep Continuity Regularity | 0.002 | 0.002 | 0.000 | 0.000 | 0.000 | 0.000 |
| Circadian Regularity | 0.006* | 0.006 | 0.003* | 0.003 | 0.018* | 0.018 |
Note: PHQ-2 = Patient Health Questionnaire – 2 item; GAD-2 = Generalized Anxiety Disorder – 2 item; Physical Health VAS = 0 – 100 visual analog scale.
p < .05.
4. Discussion
In the present study, a series of analyses was conducted in order to derive and initially test the psychometric properties of a brief questionnaire aimed at assessing the regularity of sleep habits—the Sleep Regularity Questionnaire (SRQ). This questionnaire was designed to serve as a quick, retrospective self-report measure of sleep regularity. EFA revealed a two-factor “circadian regularity” and “sleep continuity regularity” structure of the SRQ. Model fit indices from the CFA showed good fit of the two-factor structure. The summed-total of the final six items of the measure and the four-item circadian regularity subscale demonstrated good internal consistency and the two-item sleep continuity regularity subscale demonstrated acceptable internal consistency. Moreover, test-retest analyses revealed stability over an average of an 11-week period. Invariance analyses suggested that the SRQ is appropriate for use among both men and women, as there were no differences in factor structure between sexes. The SRQ evidenced both concurrent and convergent validity, as it was negatively associated with sleep quality measured via the PQSI and insomnia symptoms measured via the ISI. Finally, the circadian regularity subscale showed some promise for incremental validity in predicting mental health and physical health above commonly used sleep scales.
The two-factor solution which emerged from the EFA and CFA represents two distinct aspects of sleep regularity, which largely aligns with the two-process model of sleep.36 In the two process model, Process S represents a homeostatic process, whereas Process C represents a circadian process. The “sleep continuity regularity” factor is most closely aligned with Process S, as items in this factor pertain to nighttime awakenings, which are related to an individual’s homeostatic sleep drive. On the other hand, the “circadian regularity” factor aligns mostly clearly with Process C, as items in this factor pertain to an individual’s natural sleep rhythm. Though the scale item pertaining to sleep duration regularity may at face value be more aligned with Process S, results of the factor analysis revealed that it was more related to Process C. The duration of sleep obtained on any given night is likely the result of a complex interplay between both Process S and C. Our results suggest that the typical regularity of nightly sleep duration is more closely associated with variables commonly associated with circadian processes. Our proposed measure of sleep regularity appears to measure the regularity of the two-processes of sleep regulation, lending credence to our interpretation of the factor analyses, with the EFA and CFA resulting in a two factor model that mirrors the two-process model of sleep.36 The items that did not maintain factor structure are qualitatively unique, as they do not assess sleep or wake time but instead query about feeling refreshed, napping, and weekday-weekend shift. Taken as a whole, the psychometric properties of the SRQ in the present study suggest that it may be a strong measure of sleep regularity for use among English-speaking adults. By developing a brief, self-report measure of sleep regularity, researchers and clinicians alike will be able to quickly and easily assess the consistency or inconsistency in which an individual sleeps and investigate whether this regularity might be an important factor in an individual’s sleep, functional, and/or health outcomes.
The SRQ is a unique measure among the numerous self-report sleep questionnaires frequently used in both clinical and research settings. Commonly used self-report measures of sleep query about sleep disturbance,4 insomnia symptoms,5,37 daytime sleepiness,38 and beliefs and attitudes about sleep.39 However, none of the aforementioned questionnaires contain a single item pertaining to the regularity or inconsistency in which these sleep-related thoughts and behaviors occur. This is troubling given the recent evidence6,7,10,13–15 suggestive of the importance of sleep regularity above and beyond habitual sleep habits. The SRQ aims to fill this void with a simple, short, easy to administer and score, retrospective questionnaire.
Recent evidence suggests that sleep regularity measured through actigraphy differs between men and women.11 Specifically, inconsistency in terminal wakefulness, time awake after sleep onset, sleep onset latency, number of times awake during the night, and time in bed measured via actigraphy all loaded onto a latent “sleep inconsistency” variable for women but not for men. Furthermore, sleep inconsistency was associated with markers of inflammation for women but not for men. It is possible that, while regularity in actigraphically measured sleep differs for men and women, the self-report measure presented in the present study may be a more universally appropriate measure of sleep regularity, given the findings of sex invariance across the factor structure.
While Cognitive-Behavioral Therapy for Insomnia (CBT-I) has been proven efficacious,40 the search for potential markers of treatment response is an important next step in order to increase the efficiency in which treatment is delivered. Sleep regularity has been shown to have important treatment implications. For example, it has been found that individuals with varying starting levels of sleep regularity respond differently to treatment for insomnia.10,41,42 While the exact nature of this association remains unclear, with some reporting that those with lower baseline regularity in sleep respond better to treatment10,42 and others reporting the opposite,41 what is clear is the potential utility of sleep regularity in aiding in patient and treatment selection.
4.1. Limitations and Future Directions
Additional prospective research on the SRQ is warranted. For example, a test-retest study in which the interval of days between assessments is shorter (i.e., an average of 2–4 weeks), or multiple time points are used, may provide more powerful evidence of the measure’s stability. Such examinations would better permit the evaluation of whether sleep regularity is a stable trait or acts in more a state-like fashion. In addition, longitudinal data on various clinical samples would allow for evaluation of the SRQ in response to a focused intervention, such as CBT-I.40,43 A longitudinal study would allow for evaluation of whether improvement in sleep regularity over time, as measured by the SRQ and as a result of CBT-I, is associated with other positive sleep and health outcomes which have largely been elusive.44
This study established the concurrent and convergent validity of the SRQ by comparing scores to measures of commonly used aggregate-based sleep measures, such as the PSQI and the ISI; however, future studies should conduct comparisons of individuals’ SRQ scores to other well-established self-report measures of sleep to establish sleep regularity as a unique construct. Additionally, it will be important to validate the SRQ with conventional measures of sleep regularity. While the literature has shown sleep regularity to be associated with a number of poor health outcomes, sleep regularity in these studies has been measured using daily self-reported sleep diaries,e.g., 45, actigraphy,e.g., 46 polysomnography,e.g., 47 or some combination therein.e.g., 48 Therefore, future studies should examine whether the SRQ is a valid, brief self-report measure of the same sleep regularity computed via those methodologies.
The relative lack of racial and ethnic minorities in the present sample did not allow for the examination of whether the SRQ differed by racial and ethnic groups. In light of findings that racial and ethnic minorities are more prone to poor sleep,49,50 future research may benefit from examining the psychometric properties of the scale among different racial minority groups. Future studies should also examine and confirm the reported factor structure of the SRQ when administered in its recommended shortened form (i.e., 6 total items). Such an examination would assure that the obtained factor structure was not dependent on any item-order effects that may have been present in the original 10-item version of the SRQ. Lastly, examination of specific recall periods (e.g., usual sleep vs. previous 2-weeks vs previous 4-weeks) would allow for optimal instruction wording for the SRQ.
4.2. Conclusions
The SRQ appears to be a valid and stable instrument for the assessment of sleep regularity in adults that is related to, but distinct from, other established sleep constructs, and it adds to the prediction of sleep-related phenomena beyond existing sleep measures. This short, retrospective questionnaire could provide important information not already captured by existing sleep questionnaires. Its brevity lends itself to both clinical and large survey uses. Future work should include this questionnaire as part of routine batteries to investigate the utility of regular assessment of sleep regularity.
Supplementary Material
Highlights.
Sleep regularity is an important conceptualization of sleep behavior.
The Sleep Regularity Questionnaire (SRQ) is a brief, self-report scale.
Preliminary psychometrics suggest that the SRQ is a valid and stable instrument.
The SRQ is appropriate for use in both male and female samples.
Acknowledgements:
Funding:
This work was supported by the National Institute on Aging (NIA) of the National Institutes of Health (NIH) (Award Number K23AG049955; PI: Dzierzewski). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Declaration of interest:
Dr. Dzierzewski reports grants from National Institute on Aging, during the conduct of the study. Miss Donovan and Sabet have nothing to disclose.
Appendix A: Sleep Regularity Questionnaire (SRQ)
INSTRUCTIONS: Thinking of how you usually sleep, how much do you agree with the statements below. There are no right or wrong answers. Don’t spend too much time on any one statement.
| NOT AT ALL | A LITTLE BIT | SOMEWHAT | MODERATELY | VERY MUCH | |
|---|---|---|---|---|---|
| □ | □ | □ | □ | □ | |
| 2. It takes me about the same amount of time to fall asleep each night............ | □ | □ | □ | □ | □ |
| 3. I wake up for about the same number of times each night................... | □ | □ | □ | □ | □ |
| 4. I spend about the same amount of time awake each night.......................... | □ | □ | □ | □ | □ |
| 5. I wake up at about the same time each morning........................................ | □ | □ | □ | □ | □ |
| 6. I get out of bed at about the same time each morning................................ | □ | □ | □ | □ | □ |
| 7. I feel about equally refreshed upon waking up each morning....................... | □ | □ | □ | □ | □ |
| 8. I am asleep for about the same amount of time every night.................... | □ | □ | □ | □ | □ |
| 9. I take (or do not take) a similar number and duration of naps each day | □ | □ | □ | □ | □ |
| 10. My sleep on weekdays and weekends is about the same................ | □ | □ | □ | □ | □ |
FACTOR LOADINGS:
Circadian Regularity Factor: Questions 1, 5, 6, 8
Sleep Continuity Factor: 3, 4
REMOVED ITEMS (DID NOT LOAD ON A FACTOR):
2, 7, 9, 10
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Buysse DJ. Sleep Health: Can We Define It? Does It Matter? Sleep. 2014;37(1):9–17. doi: 10.5665/sleep.3298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Driscoll HC, Serody L, Patrick S, et al. Sleeping well, aging well: A descriptive and cross-sectional study of sleep in “successful agers” 75 and older. The American Journal of Geriatric Psychiatry. 2008;16(1):74–82. doi: 10.1097/JGP.0b013e3181557b69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grandner MA. Addressing sleep disturbances: An opportunity to prevent cardiometabolic disease? Int Rev Psychiatry. 2014;26(2):155–176. doi: 10.3109/09540261.2014.911148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry research. 1989;28(2):193–213. doi: 10.1016/0165-1781(89)90047-4 [DOI] [PubMed] [Google Scholar]
- 5.Bastien CH, Vallières A, Morin CM. Validation of the Insomnia Severity Index as an outcome measure for insomnia research. Sleep medicine. 2001;2(4):297–307. [DOI] [PubMed] [Google Scholar]
- 6.Shoji KD, Tighe CA, Dautovich ND, McCrae CS. Beyond mean values: Quantifying intraindividual variability in pre-sleep arousal and sleep in younger and older community-dwelling adults. Sleep Sci. 2015;8(1):24–30. doi: 10.1016/j.slsci.2015.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baron KG, Reid KJ, Malkani RG, Kang J, Zee PC. Sleep variability among older adults with insomnia: Associations with sleep quality and cardiometabolic disease risk. Behav Sleep Med. 2017;15(2):144–157. doi: 10.1080/15402002.2015.1120200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Buysse DJ, Cheng Y, Germain A, et al. Night-to-night sleep variability in older adults with and without chronic insomnia. Sleep medicine. 2010;11(1):56–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lemola S, Ledermann T, Friedman EM. Variability of Sleep Duration Is Related to Subjective Sleep Quality and Subjective Well-Being: An Actigraphy Study. PLOS ONE. 2013;8(8):e71292. doi: 10.1371/journal.pone.0071292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chan WS, Williams J, Dautovich ND, et al. Night-to-night sleep variability in older adults with chronic insomnia: Mediators and moderators in a randomized controlled trial of brief behavioral therapy (BBT-I). J Clin Sleep Med. 2017;13(11):1243–1254. doi: 10.5664/jcsm.6790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dzierzewski JM, Donovan EK, Kay DB, Sannes TS, Bradbrook KE. Sleep Inconsistency and Markers of Inflammation. Frontiers in Neurology. Published online2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Okun ML, Reynolds CF, Buysse DJ, et al. Sleep variability, health-related practices, and inflammatory markers in a community dwelling sample of older adults. Psychosom Med. 2011;73(2):142–150. doi: 10.1097/PSY.0b013e3182020d08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Patel SR, Hayes AL, Blackwell T, et al. The association between sleep patterns and obesity in older adults. Int J Obes (Lond). 2014;38(9):1159–1164. doi: 10.1038/ijo.2014.13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bei B, Seeman TE, Carroll JE, Wiley JF. Sleep and physiological dysregulation: a closer look at sleep intraindividual variability. Sleep. 2017;40(9). doi: 10.1093/sleep/zsx109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Paterson JL, Reynolds AC, Dawson D. Sleep schedule regularity is associated with sleep duration in older Australian adults: Implications for improving the sleep health and wellbeing of our ageing population. Clinical Gerontologist. 2018;(2). doi: 10.1080/07317115.2017.1358790 [DOI] [PubMed] [Google Scholar]
- 16.Grandner MA, Hale L, Moore M, Patel NP. Mortality associated with short sleep duration: The evidence, the possible mechanisms, and the future. Sleep Medicine Reviews. 2010;14(3):191–203. doi: 10.1016/j.smrv.2009.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Simms LJ, Watson D. The Construct Validation Approach to Personality Scale Construction. In: Robins RW, Fraley RC, Kruger RF, Eds. Handbook of Research Methods in Personality Psychology. New York: NY: Guilford Press; 2007:240–284. [Google Scholar]
- 18.Buhrmester M, Kwang T, Gosling SD. Amazon’s Mechanical Turk: A New Source of Inexpensive, Yet High-Quality, Data? Perspect Psychol Sci. 2011;6(1):3–5. doi: 10.1177/1745691610393980 [DOI] [PubMed] [Google Scholar]
- 19.Thorndike FP, Ritterband LM, Saylor DK, Magee JC, Gonder-Frederick LA, Morin CM. Validation of the Insomnia Severity Index as a Web-Based Measure. Behavioral Sleep Medicine. 2011;9(4):216–223. doi: 10.1080/15402002.2011.606766 [DOI] [PubMed] [Google Scholar]
- 20.Mollayeva T, Thurairajah P, Burton K, Mollayeva S, Shapiro CM, Colantonio A. The Pittsburgh sleep quality index as a screening tool for sleep dysfunction in clinical and non-clinical samples: A systematic review and meta-analysis. Sleep Medicine Reviews. 2016;25:52–73. doi: 10.1016/j.smrv.2015.01.009 [DOI] [PubMed] [Google Scholar]
- 21.Morin CM, Belleville G, Bélanger L, Ivers H. The Insomnia Severity Index: Psychometric Indicators to Detect Insomnia Cases and Evaluate Treatment Response. Sleep. 2011;34(5):601–608. doi: 10.1093/sleep/34.5.601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kroenke K, Spitzer RL, Williams JB. The Patient Health Questionnaire-2: validity of a two-item depression screener. Medical care. 2003;41(11):1284–1292. [DOI] [PubMed] [Google Scholar]
- 23.Manea L, Gilbody S, Hewitt C, et al. Identifying depression with the PHQ-2: A diagnostic meta-analysis. Journal of Affective Disorders. 2016;203:382–395. doi: 10.1016/j.jad.2016.06.003 [DOI] [PubMed] [Google Scholar]
- 24.Kroenke K, Spitzer RL, Williams JBW, Monahan PO, Löwe B. Anxiety Disorders in Primary Care: Prevalence, Impairment, Comorbidity, and Detection. Annals of Internal Medicine. 2007;146(5):317–325. doi: 10.7326/0003-4819-146-5-200703060-00004 [DOI] [PubMed] [Google Scholar]
- 25.Plummer F, Manea L, Trepel D, McMillan D. Screening for anxiety disorders with the GAD-7 and GAD-2: a systematic review and diagnostic metaanalysis. General Hospital Psychiatry. 2016;39:24–31. doi: 10.1016/j.genhosppsych.2015.11.005 [DOI] [PubMed] [Google Scholar]
- 26.Macias C, Gold PB, Öngür D, Cohen BM, Panch T. Are Single-Item Global Ratings Useful for Assessing Health Status? J Clin Psychol Med Settings. 2015;22(4):251–264. doi: 10.1007/s10880-015-9436-5 [DOI] [PubMed] [Google Scholar]
- 27.Arbuckle JL. IBM SPSS Amos 26 User’s Guide. Published online2019.
- 28.Comrey AL, Lee HB. A First Course in Factor Analysis. 2nd ed.Lawrence Earlbaum Associates; 1992. [Google Scholar]
- 29.Tabachnick BG, Fidell LS. Using Multivariate Statistics, 6th Edition. 6th ed.Pearson; 2013. [Google Scholar]
- 30.Meyers LS, Gamst G, Guarino AJ. Applied Multivariate Research: Design and Interpretation. 3rd ed.SAGE Publications, Inc; 2016. [Google Scholar]
- 31.Cattell RB. The scree test for the number of factors. Multivariate Behavioral Research. 1966;1(2):245–276. doi: 10.1207/s15327906mbr0102_10 [DOI] [PubMed] [Google Scholar]
- 32.Devillis RF. Scale Development: Theory and Applications. Vol 26. SAGE Publications; 1991. [Google Scholar]
- 33.Worthington RL, Whittaker TA. Scale Development Research: A Content Analysis and Recommendations for Best Practices. The Counseling Psychologist. 2006;34(6):806–838. doi: 10.1177/0011000006288127 [DOI] [Google Scholar]
- 34.Cohen J, Cohen P, West SG, Aiken LS. Applied Multiple Regression/Correlation Analysis for the Behavioral Sciences. 3rd ed.Lawrence Earlbaum Associates; 2003. [Google Scholar]
- 35.Schermelleh-Engel K, Moosbrugger H, Müller H. Evaluating the Fit of Structural Equation Models: Tests of Significance and Descriptive Goodness-of-Fit Measures. Methods of Psychological Research. 2003;8(2):23–74. [Google Scholar]
- 36.Borbely AA. A two process model of sleep regulation. Human Neurobiology. 1982;1:195–204. [PubMed] [Google Scholar]
- 37.Okun ML, Kravitz Howard M, Sowers Mary Fran, Moul Douglas E., Buysse Daniel J., Hall Martica. Psychometric Evaluation of the Insomnia Symptom Questionnaire: a Self-report Measure to Identify Chronic Insomnia. Journal of Clinical Sleep Medicine. 2009;05(01):41–51. doi: 10.5664/jcsm.27391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. sleep. 1991;14(6):540–545. [DOI] [PubMed] [Google Scholar]
- 39.Morin CM, Vallières A, Ivers H. Dysfunctional Beliefs and Attitudes about Sleep (DBAS): Validation of a brief version (DBAS-16). Sleep. 2007;30(11):1547–1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dzierzewski JM, Griffin SC, Ravyts S, Rybarczyk B. Psychological interventions for late-life insomnia: Current and emerging science. Curr Sleep Medicine Rep. 2018;4(4):268–277. doi: 10.1007/s40675-018-0129-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sanchez-Ortuno MM, Edinger JD. Internight sleep variability: Its clinical significance and responsiveness to treatment in primary and comorbid insomnia. Journal of Sleep Research. 2012;21(5):527–534. [DOI] [PubMed] [Google Scholar]
- 42.Suh S, Nowakowski S, Bernert RA, et al. Clinical Significance of night-to-night sleep variability in insomnia. Sleep Med. 2012;13(5):469–475. doi: 10.1016/j.sleep.2011.10.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Alessi C, Martin JL, Fiorentino L, et al. Cognitive Behavioral Therapy for Insomnia in Older Veterans Using Nonclinician Sleep Coaches: Randomized Controlled Trial. J Am Geriatr Soc. 2016;64(9):1830–1838. doi: 10.1111/jgs.14304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McCrae CS, Curtis AF, Williams JM, et al. Efficacy of Brief Behavioral Treatment for Insomnia in Older Adults: Examination of Sleep, Mood, and Cognitive Outcomes. Sleep Medicine. Published online June 2, 2018. doi: 10.1016/j.sleep.2018.05.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dillon HR, Lichstein KL, Dautovich ND, Taylor DJ, Riedel BW, Bush AJ. Variability in Self-Reported Normal Sleep Across the Adult Age Span. J Gerontol B Psychol Sci Soc Sci. 2015;70(1):46–56. doi: 10.1093/geronb/gbu035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Khawaja IS, Hashmi AM, Westermeyer J, Thuras P, Hurwitz T. Nocturnal Awakening & Sleep Duration in Veterans with PTSD: An Actigraphic Study. Pak J Med Sci. 2013;29(4):991–996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cheek RE, Shaver JL, Lentz MJ. Lifestyle Practices and Nocturnal Sleep in Midlife Women with and without Insomnia. Biological Research For Nursing. 2004;6(1):46–58. doi: 10.1177/1099800404263763 [DOI] [PubMed] [Google Scholar]
- 48.McCrae CS, McNamara JPH, Rowe MA, et al. Sleep and affect in older adults: Using multilevel modeling to examine daily associations. Journal of Sleep Research. 2008;17(1):42–53. doi: 10.1111/j.1365-2869.2008.00621.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kaufmann CN, Mojtabai R, Hock RS, et al. Racial/Ethnic Differences in Insomnia Trajectories Among U.S. Older Adults. Am J Geriatr Psychiatry. 2016;24(7):575–584. doi: 10.1016/j.jagp.2016.02.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Whinnery J, Jackson N, Rattanaumpawan P, Grandner MA. Short and Long Sleep Duration Associated with Race/Ethnicity, Sociodemographics, and Socioeconomic Position. Sleep. 2014;37(3):601–611. doi: 10.5665/sleep.3508 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


