Abstract
Introduction:
Transplant eligibility for hepatocellular carcinoma (HCC) is determined by the imaging identification of tumor burden within Milan criteria. Transjugular intrahepatic portosystemic shunts (TIPS) reduce portal hypertension but may impact HCC visualization. It was hypothesized that the presence of pre-transplant TIPS would correlate with occult HCC and reduced survival.
Methods:
A single-center retrospective case control study was performed among liver transplant recipients with HCC (2000–2017). The primary endpoint was occult disease on explant pathology. Backward stepwise elimination of candidate variables was modeled with logistic regression (LR). Secondary endpoints included disease-free survival (DFS) and overall survival (OS), evaluated with Kaplan-Meier curves and Cox regression analysis.
Results:
40 of 640 patients had TIPS and more frequently exhibited occult disease (80.0% vs. 43.1%, p<0.001, odds ratio [OR] 4.16, p<0.001). Explant tumor burden was equivalent between TIPS subgroups; accordingly, TIPS status was not independently associated with reduced DFS or OS. However, exceeding Milan criteria was associated with reduced DFS (hazard ratio 3.21, p=0.001), and TIPS status in patients with a single suspected lesion (N=316) independently correlated with explant tumor burdens beyond Milan criteria (OR 13.47; p=0.001). Equivalent explant tumor burdens between TIPS groups suggests that occult disease in TIPS patients was due to reduced diagnostic capacity by imaging. Portal venous thrombosis similarly correlated with occult disease, suggesting a mechanism through altered hepatic perfusion.
Conclusion:
TIPS on pre-transplant imaging are associated with occult HCC on explant pathology. TIPS are not independently associated with reduced DFS or OS, but are associated with exceeding Milan criteria for patients with a single suspected lesion. The presence of TIPS may alter the sensitivity of imaging and necessitate a higher index of suspicion.
Keywords: occult, Milan, imaging, pathology, survival
Hepatocellular carcinoma (HCC) is the most common primary malignant neoplasm in patients with cirrhosis and the second leading cause of cancer-related death worldwide. Only 28% of HCC patients with unresectable disease survive three years without intervention (1). Transplantation provides the best outcome, but organ availability is insufficient (2). Organs are therefore allocated to patients who fall within Milan criteria, which consists of one lesion smaller than five centimeters in diameter or up to three lesions each smaller than three centimeters in diameter without macroscopic vascular invasion. Under these circumstances, overall and recurrence-free survival rates at four years reach 85% and 92%, respectively (3).
Evaluation for HCC is unique among solid tumors in that the diagnosis is largely determined by pre-transplant imaging (4). However, despite evaluation at regular intervals, up to 42% of liver explants reveal unexpected (occult) intrahepatic HCC on explant pathology (5). Patients with tumor burdens within Milan criteria on explant pathology demonstrate longer recurrence-free survival compared to those who exceed Milan criteria (3). The clinical utility of these parameters is therefore rooted in how well the tumor burden can be determined by pre-transplant imaging (6), with strong reliance on characteristic perfusion patterns such as arterial hyperenhancement and venous washout that are dependent on the blood supply to the lesions (7).
Transjugular intrahepatic portosystemic shunts (TIPS) connect the portal and hepatic veins in order to reduce manifestations of portal hypertension. There are many conceivable mechanisms by which TIPS could impact the development of occult disease, but there exists little evidence that they cause tumor seeding or progression. Rather, data demonstrate that TIPS alter perfusion to hepatic parenchyma, which may have consequences on imaging characteristics. In an experimental non-cirrhotic swine model, the presence of TIPS led to alterations in hepatic arterial blood flow on scintigraphy (8). Increased arterial blood flow was subsequently demonstrated in cirrhotic patients with TIPS, with 88% of the 25 patients demonstrating a rise in arterial peak velocity by intravascular doppler sonography after TIPS placement (9). Non-invasive volume perfusion computed tomography (CT) in 23 patients with portal hypertension similarly demonstrated that TIPS increased hepatic arterial perfusion and decreased total portal venous perfusion to the liver (10). These alterations reflect the compensatory changes that take place through autonomic regulation of hepatic arterial tone in order to maintain constant hepatic perfusion, known as the arterial buffer response (11).
Portal venous thrombus (PVT) similarly decreases portal venous flow and increases hepatic arterial flow (12). PVT is associated with atypical characteristics of HCC on axial imaging such as decreased arterial phase hypervascularity (13) and venous washout (13,14), leading to delays in diagnosis (14). It was therefore hypothesized that the alterations in vascular flow induced by the presence of TIPS would negatively impact the ability to accurately detect the presence of HCC among patients undergoing surveillance and that these occult lesions could portend inferior survival among liver transplant recipients.
Methods:
Study population
A single-center retrospective case control study was performed among patients who underwent liver transplantation between November 2000 and July 2017 and were found to have HCC or hepatocholangiocarcinoma on explant pathology. No patients were excluded. The study was evaluated by our Institutional Review Board and met eligibility criteria for review exemption (protocol # 832467).
Imaging
Magnetic resonance imaging (MRI) was performed at 1.5 tesla (T) or 3.0 T with gadolinium. Our routine scanning includes T-1 weighted and opposed phase gradient echo, T-2 weighted, diffuse weighted, and fat suppressed T-1 weighted imaging obtained before and after gadolinium administration. All CT imaging was performed with intravenous contrast.
Determination of TIPS status and occult disease
TIPS status was determined at the time of the last pre-transplant imaging. CT and MRI images at our institution are reviewed in a multidisciplinary tumor conference by expert body radiologists. The identification of malignancy and indeterminate nodules on pre-transplant imaging was based on report extraction. Occult malignancy was defined as the presence of any HCC lesions on explant pathology that were not identified on the last pre-transplant imaging. A completely necrotic, treated lesion on imaging or explant was considered an HCC lesion. Dysplastic nodules were excluded.
Variables and endpoints
The primary endpoint was occult disease on explant pathology. Secondary endpoints included disease-free survival (DFS) and overall survival (OS). Demographic and clinical variables were abstracted for inclusion in the multivariable model for occult disease, including the allocation and biochemical Model for End Stage Liver Disease (MELD) scores, alpha-fetoprotein (AFP) level, etiology of liver disease, time between diagnosis of liver disease and transplantation, time between diagnosis of cancer and transplantation, type and number of pre-transplant treatments (systemic or liver directed), time between last imaging and transplantation, type of last pre-transplant imaging, presence and extent of PVT on imaging based on a classification system in the literature (15), and presence of indeterminate lesions on imaging. To account for the fact that some patients received multiple imaging modalities, we reported the most sensitive and specific modality performed within three months of the final imaging, prioritizing MRI first, CT second, and ultrasound third (16). Histopathological characteristics of the tumor, including differentiation and invasion, were determined by a liver pathologist. The most de-differentiated grade identified in each specimen was coded. Warm and cold ischemia times were also abstracted to incorporate as potential confounders in the survival analysis.
Statistical analysis
Descriptive statistics are reported as frequencies for binary or categorical variables, mean and standard deviation for normally distributed continuous variables, and median and interquartile range (IQR) for non-parametric continuous variables. Normality of continuous variables was evaluated by skewness and kurtosis testing. Univariate analysis between the two TIPS subgroups was performed with proportion tests for categorical values, t tests for normal continuous values, and Wilcoxon rank sum for non-parametric continuous values. To determine the relationship between clinical variables and occult disease, backward stepwise elimination of candidate variables was modeled with logistic regression (LR) for categorical dependent variables and linear regression for continuous dependent variables, combining forward selection using a p-value for variable entry set to 0.10 and variable removal with a p-value set to 0.20 for elimination (17mina. Goodness of fit was determined by R-squared or pseudo R-squared values and is listed with the corresponding table for each model. Given that the patients with TIPS retained some differences from those without TIPS such as higher MELD score, we performed a secondary propensity score analysis that included independent variables hypothesized to be associated with occult disease including patient age, biochemical MELD score, preoperative AFP, pre-transplant treatments, wait time, and most recent imaging prior to transplant. Propensity score was confirmed to be balanced graphically and objectively (20). One match was used per observation. Alpha was set to 0.05 for all statistical analysis.
For evaluation of DFS and OS, Kaplan-Meier survival analysis was performed. Recurrence incorporated local or distant disease. Censorship occurred for death by another cause, lack of follow-up, or the end of this study. Univariable Cox regression analysis was utilized to identify relationships between clinical characteristics and survival. Covariates demonstrating modest significance (p<0.20) on univariate analysis and clinically relevant variables were tested in the multivariable model and removed utilizing manual backward elimination until only variables with p<0.10 remained. The prediction models were evaluated with Harrell’s C concordance statistic and the number of covariates was limited to maintain model stability. Statistical analysis was performed using STATA version 15.1 (StataCorp LLC).
Results:
Cohort characteristics and demographics
The database was comprised of 640 patients who underwent transplantation and had HCC (N=633, 98.9%) and/or hepatocholangiocarcinoma (N=23, 3.6%). The median age at the time of transplant was 58.6 years (IQR: 53.9–64.0) in this largely male (82.5%) population. The leading etiology of liver disease was hepatitis C (73.0%) and the majority (85.8%) had a diagnosis of HCC prior to transplant. The median time between transplant and last follow up was 79 months (IQR: 34–125), 103 months in patients who survived throughout the study period (IQR: 67–140). Of the 63 patients exhibiting PVT, 36 (57.1%) were present only in the trunk and 18 (28.6%) were present only in one or more branches. 23 (36.5%) were occlusive and 15 (23.8%) extended into the mesenteric and/or splenic veins.
40 of the patients had TIPS. The indication for TIPS placement was exclusively variceal bleeding and/or ascites management. No patients rapidly decompensated after TIPS placement. 85.0% of patients with known pre-TIPS imaging evaluation underwent CT or MRI a median of 54 days (IQR: 10–126) prior to shunt placement and only one patient had malignancy at that time. Transplant was performed a median of 674 days (IQR: 309–1414) after TIPS placement. Interval ultrasounds were available in 80.0% of TIPS patients, identifying that 96.9% (31/32) of the evaluated shunts were patent.
The groups of patients with and without TIPS were not statistically different in terms of age, gender, etiology of liver disease, time on the waitlist, type of donation received (donation after brain death, donation after cardiac death, living donation, extended criteria donation), time between the most recent pre-transplant imaging and transplant, the incidence of one or more indeterminate lesions on pre-transplant imaging, and the presence of hepatocholangiocarcinoma. They also did not differ in terms of the incidence of PVT, extent of PVT into the splenic and/or mesenteric veins, presence of occlusive PVT, or thrombotic involvement of both the portal trunk and its branches (Table 1). A significant difference between subgroups included a higher biochemical MELD score in TIPS patients (17 [IQR: 15–22] vs. 12 [IQR: 9–18], p<0.001). Allocation MELDs were not dissimilar between groups (24[IQR: 21–28] vs. 25[IQR: 22–29]), as fewer exception points were granted to the TIPS group given their less frequent identification of pre-transplant HCC. Patients were equally dispersed over time, such that the allocation systems utilized were consistent between groups. TIPS patients were also more likely to not have undergone pre-transplant HCC therapy (70.0% vs. 27.4%, p<0.001), a difference that persisted when excluding patients with no known pre-transplant malignancy (80.5% versus 57.1% p=0.009).
Table 1.
Demographic and clinical characteristics of all patients by TIPS status.
| No TIPS (n=600) | TIPS (n=40) | P value | |
|---|---|---|---|
|
| |||
| Age at transplant, Median (IQR) | 58.4 (54.0–64.0) | 57.3 (52.7–64.7) | 0.57 |
|
| |||
| Male gender, N (%) | 45 (82.5%) | 33 (82.5%) | 0.99 |
|
| |||
| Etiology, N (%) | |||
| Hepatitis C | 438 (73.0%) | 29 (72.5%) | 0.95 |
| Hepatitis B | 42 (7.0%) | 0 (0.0%) | 0.08 |
| Alcohol | 42 (7.0%) | 4 (10.0%) | 0.48 |
| Autoimmune | 14 (2.3%) | 2 (5.0%) | 0.29 |
| Non-alcoholic steatohepatitis | 14 (2.3%) | 2 (5.0%) | 0.29 |
| Cryptogenic/other | 38 (6.3%) | 3 (7.5%) | 0.78 |
|
| |||
| MELD | |||
| Allocation, Median (IQR) | 25 (22–29) | 24 (21–28) | 0.08 |
| Biochemical, Median (IQR) | 12 (9–18) | 17 (15–22) | <0.001 |
| Exception granted, N (%) | 479 (80.0%) | 24 (60.0%) | 0.003 |
|
| |||
| AFP (ng/mL), Median (IQR) | |||
| At time of listing for transplant | 14 (6–41) | 6 (3–15) | 0.004 |
| Highest prior to-transplant | 18 (7–83) | 10 (5–34) | 0.02 |
| Most recent prior to transplant | 10 (4–28) | 5 (2–17) | 0.02 |
|
| |||
| Days on waitlist, Median (IQR) | 195 (64–397) | 267 (85–990) | 0.09 |
|
| |||
| Pre-transplant treatment*, N (%) | |||
| None | 163 (27.4%) | 28 (70.0%) | <0.001 |
| TACE | 378 (63.1%) | 10 (25.0%) | <0.001 |
| RFA | 76 (12.7%) | 3 (7.5%) | 0.33 |
| Resection | 14 (2.3%) | 0 (0.0%) | 0.33 |
| Radioembolization | 5 (0.8%) | 1 (2.5%) | 0.28 |
| Percutaneous ethanol injection | 3 (0.5%) | 0 (0.0%) | 0.65 |
| Number of treatments | 1 (0–2) | 2 (1–3) | 0.001 |
|
| |||
| Portal venous thrombosis, N (%) | |||
| Incidence | 57 (9.5%) | 6 (15.0%) | 0.26 |
| Site | |||
| Only trunk | 35 (61.4%) | 1 (16.7%) | 0.04 |
| Only branch | 14 (24.6%) | 4 (66.7%) | 0.03 |
| Trunk and branches | 6 (10.5%) | 0 (0.0%) | 0.40 |
| Degree | |||
| Occlusive | 19 (33.3%) | 4 (66.7%) | 0.11 |
| Nonocclusive | 31 (54.4%) | 1 (16.7%) | 0.08 |
| Extent of portal venous system occlusions | |||
| None | 38 (66.7%) | 4 (66.7%) | 1.00 |
| Splenic and/or mesenteric vein | 14 (24.6%) | 1 (16.7%) | 0.85 |
|
| |||
| Most recent pre-transplant imaging, N (%) | |||
| None | 2 (0.3%) | 0 (0.0%) | 0.72 |
| MRI | 478 (79.7%) | 26 (65.0%) | 0.03 |
| CT | 55 (9.2%) | 8 (20.0%) | 0.08 |
| Ultrasound | 12 (2.0%) | 6 (15.0%) | 0.003 |
| Unknown | 53 (8.9%) | 0 (0.0%) | 0.05 |
|
| |||
| Days from imaging to transplant, Median (IQR) | 43 (21–74) | 34 (15–79) | 0.40 |
|
| |||
| Indication for TIPS, N (%) | N/A | N/A | |
| Ascites | 18 (45.0%) | ||
| Variceal bleeding | 10 (25.0%) | ||
| Ascites and bleeding | 3 (7.5%) | ||
| Unknown | 9 (22.5%) | ||
|
| |||
| Pre-TIPS hepatic imaging | N/A | N/A | |
| MRI, N (%) | 8 (20.0%) | ||
| CT, N (%) | 9 (22.5%) | ||
| Ultrasound, N (%) | 3 (7.5%) | ||
| Unknown, N (%) | 20 (50.0%) | ||
| Days between imaging and TIPS placement, Median (IQR) | 54 (10–126) | ||
| Malignancy identified on imaging, N (%) | 1 (2.5%) | ||
|
| |||
| Days between TIPS and transplant, Median (IQR) | N/A | 674 (309–1414) | N/A |
|
| |||
| Pre-transplant imaging | |||
| Number of visualized lesions, N (%) | |||
| 0 | 64 (10.7%) | 19 (47.5%) | <0.001 |
| 1 | 303 (50.5%) | 13 (32.5%) | 0.03 |
| 2 | 152 (25.4%) | 4 (10.0%) | 0.03 |
| 3 or more | 79 (13.2%) | 4 (10.0%) | 0.56 |
| Unknown | 2 (0.3%) | 0 (0.0%) | 0.72 |
| Incidence of indeterminate lesions, N (%) | 190 (31.7%) | 14 (35.0%) | 0.66 |
| Diameter of dominant lesion (mm)**, Median (IQR) | 22 (17–31) | 25 (22–28) | 0.24 |
| Total diameter (mm) of all lesions, Median (IQR)*** | 28 (17–43) | 20 (0.0–40) | 0.009 |
|
| |||
| Explant evaluation | |||
| Number of lesions, Median (IQR) | 2 (1–3) | 2 (1–4) | 0.67 |
| Total diameter (mm) of all lesions, Median (IQR) | 35 (23–56) | 30 (18–53) | 0.18 |
| Occult malignancy, N (%) | 257 (43.1%) | 32 (80.0%) | <0.001 |
| Single occult lesion | 143 (23.9%) | 18 (45.0%) | 0.003 |
| More than one occult lesion | 115 (19.2%) | 14 (35.0%) | 0.02 |
| Outside Milan criteria, N (%) | 166 (28.3%) | 14 (35.0%) | 0.37 |
| Differentiation, N (%) | |||
| Well-differentiated | 102 (17.1%) | 6 (15.0%) | 0.73 |
| Moderately differentiated | 301 (50.3%) | 26 (65.0%) | 0.07 |
| Poorly differentiated | 90 (15.0%) | 6 (15.0%) | 0.99 |
| Invasion, N (%) | |||
| Micro lympho-vascular invasion | 305 (50.9%) | 22 (55.0%) | 0.62 |
| Major vascular invasion | 26 (4.4%) | 3 (7.5%) | 0.79 |
| Hepatocholangiocarcinoma | 20 (3.3%) | 3 (7.5%) | 0.67 |
|
| |||
| Ischemic time, Median (IQR) | |||
| Warm ischemia (minutes) | 54 (48–59) | 55 (50–59) | 0.29 |
| Cold ischemia (hours) | 306 (249–368) | 287 (238–349) | 0.29 |
AFP = alpha-fetoprotein. CT = computerized tomography. IQR = interquartile range. MELD = Model for End-Stage Liver Disease. MRI = magnetic resonance imaging. N= number. RFA = radiofrequency ablation. TACE = trans-arterial chemoembolization.
Patients who underwent multiple therapies are repeated in each applicable row.
Diameter of dominant lesion on the last pre-transplant image in patients with at least one suspected lesion.
Total diameter of all lesions includes patients with no reported malignancy on preoperative imaging.
Most subjects underwent MRI (85.9%) or CT (10.7%) as the last imaging modality a median of 42 days (IQR: 21–74) prior to transplant. In all study patients and in the subgroup of patients with visualized pre-transplant malignancy, the median number of lesions on pre-transplant imaging was 1 (IQR: 1–2). Although TIPS patients were more likely to have undergone imaging with ultrasound (15.0% vs. 2.0%, p=0.003), 14 of the 19 (73.7%) TIPS patients with unidentified pre-transplant HCC had undergone CT or MRI evaluation prior to transplant, similar to the 39 of 64 (60.9%, p=0.26) non-TIPS patients fitting the same criteria. Patients with TIPS were more likely to not have HCC identified on that imaging (47.5% vs. 10.7%, p<0.001) and their highest pre-transplant AFP was notably lower (10 ng/mL [IQR: 5–34] vs. 18 ng/mL [IQR: 7–83], p=0.02). For those with known pre-transplant malignancy, the median diameter of the dominant lesion on imaging was not statistically significantly different for patients with or without TIPS. The level of missingness was 5% or less for all variables used in final regression models with the exception of the type of pre-transplant imaging in the non-TIPS group (8.2%), the occlusive nature of PVTs (12.8%), and the presence of PVT extension into splenic and mesenteric vessels (9.5%).
Occult HCC
45.6% of all patients exhibited occult disease on explant pathology, which was far more likely in patients with TIPS than without (80.0% vs. 43.1%, p<0.001, Table 1 and Figure 1). By univariate analysis, TIPS was associated with occult disease with an odds ratio [OR] of 5.22 (p<0.001, Table 2). The presence of PVT (OR 2.02, p=0.007) and the presence of at least one indeterminate lesion on pre-transplant imaging (OR 1.47, p=0.02) also correlated with occult disease. When excluding TIPS patients, occult disease was more common in patients with partial or occlusive PVT than in those without (54.5% vs. 40.7%, p=0.02), with an occult malignancy incidence of 57.9% in patients with complete PVT (N=11). Among patients with an indeterminate lesion and TIPS or PVT, the incidences of occult disease increased to 92.9% and 60.0%, respectively.
Figure 1.
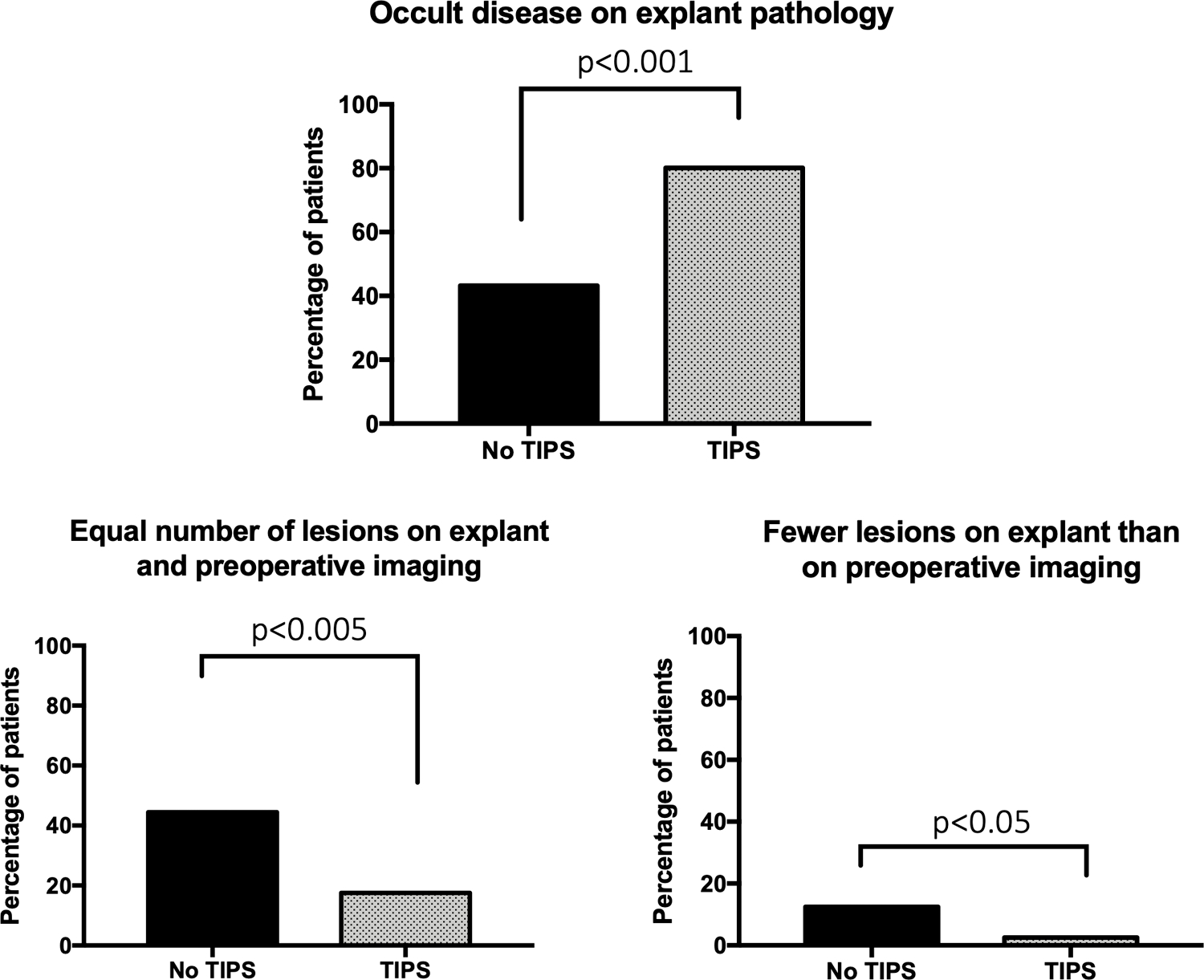
Differences between expected and actual tumor burden on explant by TIPS status. TIPS patients were statistically significantly more likely to have occult disease on explant pathology and less likely to have equal or fewer lesions than expected based on pre-transplant imaging.
Table 2.
Univariate and multivariable logistic regressions to evaluate factors associated with occult disease in all patients. Pseudo R-squared = 0.05 for multivariable regression.
| Univariate | Multivariable | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Independent variables | Odds ratio | 95% CI | P-value | Odds ratio | 95% CI | P-value |
|
| ||||||
| Age at transplant | 1.00 | 0.98–1.02 | 0.99 | -- | -- | -- |
|
| ||||||
| Female gender | 0.77 | 0.52–1.12 | 0.18 | -- | -- | -- |
|
| ||||||
| Etiology of liver disease | -- | -- | -- | |||
| Hepatitis C | 0.76 | 0.54–1.06 | 0.11 | |||
| Hepatitis B | 0.86 | 0.48–1.53 | 0.60 | |||
| Alcohol | 1.45 | 0.80–2.64 | 0.22 | |||
|
| ||||||
| Biochemical MELD score at time of transplant | 1.05 | 1.03–1.07 | <0.001 | 1.02 | 1.00–1.05 | 0.048 |
|
| ||||||
| Most recent AFP prior to transplant | 1.00 | 1.00–1.00 | 0.40 | -- | -- | -- |
|
| ||||||
| Days on waitlist | 1.00 | 1.00–1.00 | 0.93 | -- | -- | -- |
|
| ||||||
| Received pre-transplant treatment | ||||||
| TACE | 0.57 | 0.42–0.78 | <0.001 | -- | -- | |
| RFA | 0.78 | 0.50–1.21 | 0.27 | -- | ||
| Resection | 1.26 | 0.46–3.46 | 0.65 | |||
| Radioembolization | 0.39 | 0.10–1.47 | 0.16 | |||
| Percutaneous ethanol injection | 2.57 | 0.24–27.12 | 0.43 | |||
| Number of treatments | 0.79 | 0.67–0.92 | 0.003 | |||
|
| ||||||
| Most recent pre-transplant imaging | ||||||
| MRI | Ref. | -- | -- | Ref. | -- | -- |
| CT | 1.98 | 1.18–3.31 | 0.01 | 1.82 | 1.07–3.11 | 0.03 |
| Ultrasound | 6.72 | 1.93–23.31 | 0.003 | 4.15 | 1.11–15.48 | 0.03 |
|
| ||||||
| Findings on preoperative imaging | ||||||
| PVT | 2.02 | 1.22–3.39 | 0.007 | 1.97 | 1.14–3.40 | 0.02 |
| TIPS | 5.22 | 2.37–11.49 | <0.001 | 4.16 | 1.84–9.40 | <0.001 |
| Indeterminate lesion(s) | 1.47 | 0.87–1.48 | 0.02 | 1.50 | 1.02–2.18 | 0.04 |
|
| ||||||
| Days from imaging to transplant | 1.00 | 1.00–1.00 | 0.16 | -- | -- | -- |
AFP = alpha-fetoprotein. CI = confidence interval. CT = computerized tomography. MELD = Model for End-Stage Liver Disease. MRI = magnetic resonance imaging. PVT = portal venous thrombus. RFA = radiofrequency ablation. TACE = trans-arterial chemoembolization. TIPS = transjugular intrahepatic portosystemic shunt.
The remaining factors associated with occult disease on univariate analysis included biochemical MELD at the time of transplant (OR 1.05, p<0.001) and the use of CT (OR 1.98, p=0.01) or ultrasound (OR 6.72, p=0.003) for pre-transplant imaging, rather than MRI. As such, these factors were incorporated into the multivariable model, along with other factors that were distinct between TIPS subgroups and potentially explanatory of the outcome such as pre-transplant AFP and pre-transplant HCC therapy. LR revealed that TIPS presence was independently associated with occult HCC (OR 4.16, p<0.001). Evaluation with CT (OR 1.82, p=0.03) or ultrasound (OR 4.15, p=0.03) rather than MRI, higher biochemical MELD (OR 1.02, p=0.048), the presence of PVT (OR 1.97, p=0.02) on imaging or explant, and the presence of at least one indeterminate lesion on imaging (OR 1.50, p=0.04) were significant in the final regression (Table 2). The regression was also performed with the number of indeterminate lesions as a continuous variable, which remained statistically significantly associated with the outcome of occult disease (OR 1.40, p=0.04).
Although occult disease was more common in TIPS patients, the total tumor burden on explant pathology was not different between groups, as measured by the number of lesions, total summed diameter of all lesions, percentage of explants exceeding Milan criteria, degree of differentiation, and presence of micro lympho-vascular or major vascular invasion (Table 1). By linear regression, TIPS status was also not associated with greater total summed diameter of lesions on explant minus summed diameter of lesions on imaging when considering patients with the same number of lesions identified on explant pathology.
Propensity score matching included variables such as time between imaging and transplantation, time on the waitlist, biochemical MELD score, pre-transplant therapy, the presence of PVT, pre-transplant AFP, and the last imaging obtained before transplant. No variables were statistically significantly different between groups after matching. The presence of TIPS remained independently associated with occult disease between matched cohorts (Coeff. 0.26, p=0.004).
Sensitivity analysis
Given that ultrasound imaging is inferior to axial imaging in identifying lesions and that TIPS patients were more likely to have received this modality, occult disease was evaluated excluding those patients whose last pre-transplant imaging was ultrasound or an unknown modality. Occult disease remained more common in patients with TIPS (79.4% vs. 40.9%, p<0.001). In the multivariable regression, TIPS remained statistically significantly associated with occult disease (OR 3.68, p=0.02). In addition, given the implications of alternate pathology and the nearly statistically significant increase in occult disease for patients with hepatocholangiocarcinoma (69.6% vs. 45.0%, p=0.056), the regression was performed excluding this pathology. TIPS remained associated with occult disease (OR 4.01, p=0.001).
Occult HCC in patients with single-suspected lesion
316 patients underwent transplant for a single lesion identified on pre-transplant imaging (Table 3). Patients with TIPS within this subgroup continued to exhibit a higher biochemical MELD than their counterparts without TIPS (14 [IQR:13–17] vs. 12 [IQR:9–16], p=0.04). However, the other baseline differences that existed in the entire cohort between TIPS groups, such as pre-transplant tumor interventions and imaging modalities, were negated. The diameter of the lesion by pre-transplant imaging and pre-transplant AFP values were not statistically significantly different between groups.
Table 3.
Imaging and pathologic characteristics for patients who each exhibited a single identifiable lesion on pre-transplant imaging (N=316).
| No TIPS (n=303) |
TIPS (n=13) |
P value | |
|---|---|---|---|
|
| |||
| Age at transplant, Median (IQR) | 58.6 (53.7–64.3) | 59.5 (53.0–65.0) | 0.90 |
|
| |||
| Male gender, N (%) | 240 (79.2%) | 12 (92.3%) | 0.25 |
|
| |||
| Etiology, N (%) | |||
| Hepatitis C | 220 (72.6%) | 10 (76.9%) | 0.73 |
| Hepatitis B | 25 (8.3%) | 0 (0.0%) | 0.28 |
| Alcohol | 19 (6.3%) | 1 (7.7%) | 0.85 |
| Autoimmune | 7 (2.3%) | 0 (0.0%) | 0.58 |
| Non-alcoholic steatohepatitis | 7 (2.3%) | 0 (0.0%) | 0.58 |
| Cryptogenic/other | 18 (5.9%) | 1 (7.7%) | 0.80 |
|
| |||
| MELD | |||
| Allocation, Median (IQR) | 28 (24–29) | 27 (22–29) | 0.63 |
| Biochemical, Median (IQR) | 12 (9–16) | 14 (13–17) | 0.04 |
| Exception granted, N (%) | 273 (90.4%) | 10 (76.9%) | 0.12 |
|
| |||
| AFP (ng/mL), Median (IQR) | |||
| At time of listing | 12 (5–43) | 6.0 (3–35) | 0.36 |
| Highest prior to transplant | 16 (6–71) | 34 (10–84) | 0.38 |
| Most recent prior to transplant | 9.0 (4–27) | 17 (6–41) | 0.24 |
|
| |||
| Days on waitlist, Median (IQR) | 215 (82–404) | 349 (147–724) | 0.08 |
|
| |||
| Pre-transplant treatment, N (%) | |||
| None | 61 (20.3%) | 5 (38.5%) | 0.12 |
| TACE | 209 (69.0%) | 6 (46.2%) | 0.08 |
| RFA | 48 (15.8%) | 3 (23.1%) | 0.49 |
| Resection | 5 (1.7%) | 0 (0.0%) | 0.64 |
| Radioembolization | 0 (0.0%) | 0 (0.0%) | 0.99 |
| Percutaneous ethanol injection | 0 (0.0%) | 0 (0.0%) | 0.99 |
| Number of treatments | 1 (1–1) | 1 (0–1) | 0.06 |
|
| |||
| Most recent pre-transplant imaging, N (%) | |||
| MRI | 252 (83.4%) | 11 (84.6%) | 0.92 |
| CT | 27 (8.9%) | 1 (7.7%) | 0.88 |
| Ultrasound | 4 (1.3%) | 1 (7.7%) | 0.34 |
|
| |||
| Days from imaging to transplant, Median (IQR) | 46 (26–74) | 54 (34–90) | 0.66 |
|
| |||
| Pre-transplant imaging | |||
| Diameter of lesion (mm), Median (IQR) | 22 (16–33) | 24 (20–38) | 0.29 |
|
| |||
| Explant evaluation | |||
| Number of lesions, Median (IQR) | 1 (1–2) | 2 (1–3) | 0.02 |
| Diameter (mm) of dominant lesion, Median (IQR) | 25 (18–35) | 29 (25–35) | 0.36 |
| Occult disease | 104 (34.3%) | 8 (61.5%) | 0.049 |
| Outside Milan criteria, N (%) | 51 (16.8%) | 7 (53.9%) | <0.001 |
| Four or more lesions, N (%) | 18 (5.9%) | 3 (23.1%) | 0.02 |
AFP = alpha-fetoprotein. CT = computerized tomography. IQR = interquartile range. MELD = Model for End-Stage Liver Disease. MRI = magnetic resonance imaging. N= number. RFA = radiofrequency ablation. TACE = trans-arterial chemoembolization.
Occult disease in this subgroup remained more frequent in patients with TIPS (61.5% vs. 34.3%, p=0.049). Moreover, the presence of extensive occult disease (four or more lesions) was more likely in TIPS patients (23.1% vs. 5.9%, p=0.02), who accordingly exceeded Milan criteria on explant pathology with greater frequency (53.9% vs. 16.8%, p<0.001). In multivariable regression models, TIPS status was independently associated with a greater number of occult lesions (Coefficient 1.14, p=0.003, Supplemental Table 1) and extension beyond Milan criteria on explant pathology (OR 13.47; p=0.001, Supplemental Table 2).
Survival analysis
HCC recurrence was identified in 92 patients (14.4%). DFS was 94.9% at 1 year and 84.9% at 5 years in all study patients. Patients outside of Milan criteria on explant pathology experienced a shorter DFS of 87.6% at 1 year and 68.5% at 5 years. Factors independently associated with shorter DFS on multivariable analysis included poor histologic differentiation (hazard ratio [HR] 2.02, p=0.01), major vascular invasion on explant pathology (HR 2.43, p=0.01), positive surgical margin (HR 2.71, p=0.046), and exceeding Milan criteria on explant pathology (HR 2.97, p<0.001) (Supplemental Table 3). Among patients with a single identified lesion on pre-transplant imaging, DFS was 94.9% at 1 year and 86.5% at 5 years (Figure 2). Findings on explant pathology that were associated with shorter DFS in this subgroup included micro lympho-vascular invasion (HR 4.10, p<0.001) and exceeding Milan criteria (HR 3.21, p=0.001) (Supplemental Table 3). TIPS status was not independently associated with DFS and TIPS patients were not more likely to have local or distant recurrent disease.
Figure 2.
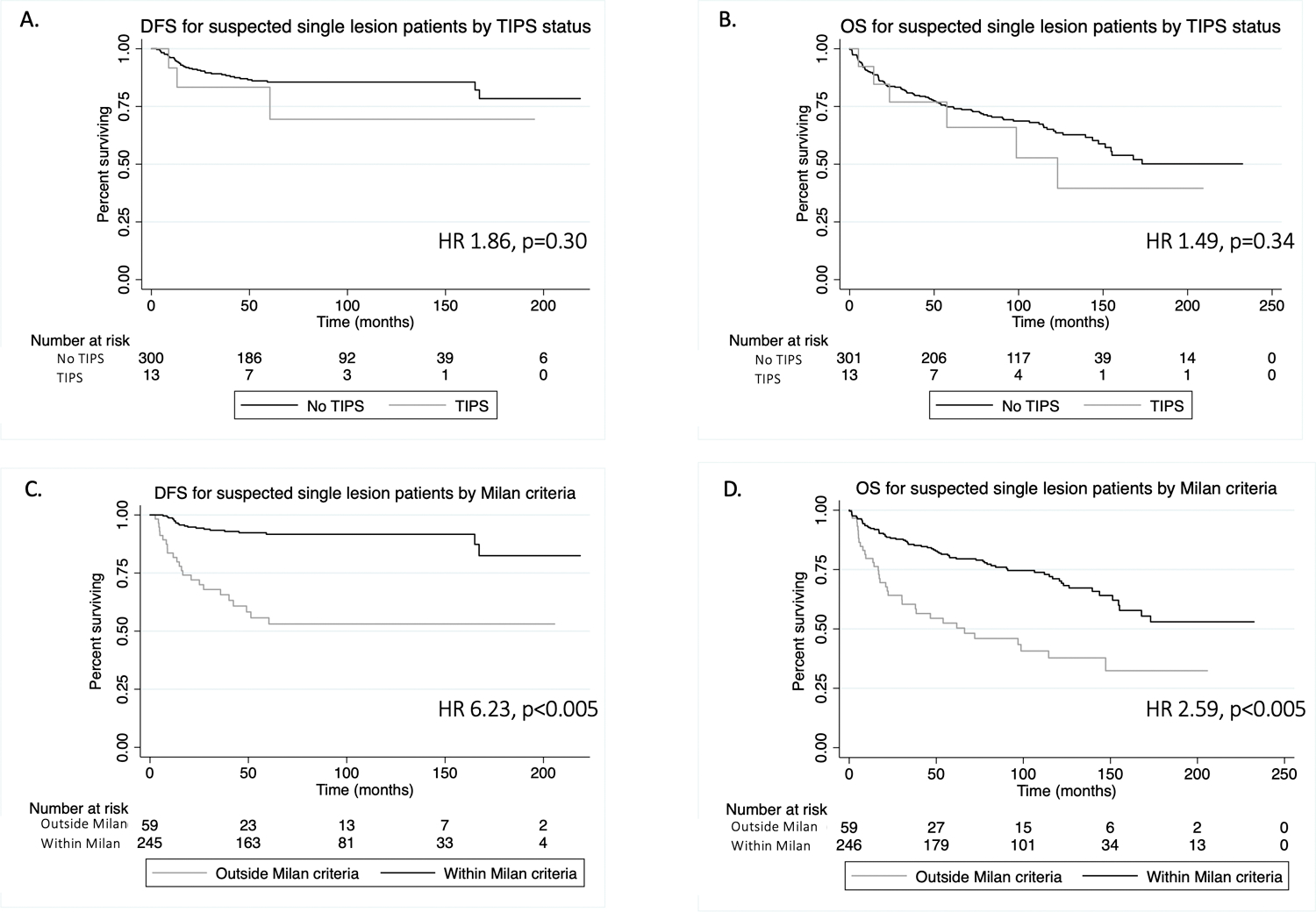
Kaplan-Meier survival analysis of disease-free survival (DFS) and overall survival (OS) for patients with a single lesion identified on pre-transplant imaging. Figures A and B demonstrate DFS (A) and OS (B) by TIPS status. Figures C and D demonstrate DFS (C) and OS (D) by Milan criteria on explant pathology.
240 patients (37.5%) died. Median OS was 15.6 years (1-year: 90.1%; 5-year: 72.5%) in all patients. Patients outside Milan criteria on explant pathology had a shorter OS of 86.1% at 1 year and 62.4% at 5 years compared to 91.7% and 76.6%, respectively, for those within Milan criteria. Factors associated with reduced OS included patient age (HR 1.02, p=0.02), poor histologic differentiation (HR 1.51, p=0.01), and recurrence (OR 4.42, p<0.001) (Supplemental Table 4). Increased OS was associated with more recent year of transplant (HR 0.96, p=0.02). For patients with a single lesion identified on pre-transplant imaging, OS was 90.1% at 1 year and 74.4% at 5 years (Figure 2). Factors associated with reduced OS in this subgroup included recurrence (HR 4.65, p<0.001) and poor histologic differentiation (HR 1.76 p=0.02) (Supplemental Table 4). When recurrence was removed from the multivariable models, explant Milan status became statistically significantly associated with OS (HR 2.72, p<0.001); the other factors remained consistent. TIPS status was not independently associated with OS.
Discussion:
Milan criteria incorporate the size and number of HCC lesions detected on pre-transplant imaging and are utilized to identify liver transplant candidates who are best suited to have a successful outcome. Clinicians therefore rely on accurate imaging to determine appropriate candidacy for transplant. This study demonstrates that the presence of a TIPS at the time of pre-transplant imaging is independently associated with a greater incidence of occult malignancy on explant pathology.
We sought to explore potential mechanisms behind the increased occult disease in TIPS patients. We considered that pathologic evaluation may have been altered based on the presence of TIPS, however all explants were sectioned and evaluated using an established internal protocol that involved removal of each TIPS and consistent, thin sectioning of all explants, minimizing bias in the pathologic assessment. Another consideration is that TIPS patients exhibited higher biochemical MELD scores, which could represent more progressive or long-standing cirrhosis and therefore a higher propensity to develop malignancy. However, TIPS patients had no greater burden of malignancy on explant pathology than patients without TIPS in terms of the size and number of lesions, the presence of tumor burden exceeding Milan criteria, and tumor differentiation and vascular invasion. This similarity supports the theory that the entire degree of occult disease cannot be attributed to increased severity of liver disease predisposing to malignancy. The equivalence of tumor burden between TIPS groups is consistent with prior histologic evaluations of liver explants, including a retrospective analysis of histopathologic data from 214 patients (68 patients with TIPS) (21). Further, biochemical MELD was included in our multivariable models and propensity score matching and TIPS status remained independently associated with occult disease, suggesting an alternative underlying mechanism.
Another difference between TIPS subgroups worth considering is the lower rate of pre-transplant TACE in TIPS patients, which persisted even when excluding patients with no known pre-transplant malignancy. Reduced pre-transplant treatment may have contributed to some of the occult disease burden, although this factor was incorporated into the multivariable model and propensity score matching, and TIPS status remained independently associated with occult disease. In addition, among patients with a single lesion identified prior to transplant, TIPS subgroups were similar in terms of pre-transplant interventions (Table 3), but TIPS remained independently associated with occult disease, the presence of four or more lesions, and extension beyond Milan criteria on explant pathology.
As TIPS patients exhibited fewer lesions on pre-transplant imaging but an equal burden of malignancy on explant pathology compared to patients without TIPS, we hypothesize that the increased burden of occult disease is due to an inability to visualize tumors on imaging among those with TIPS. Although TIPS patients were more likely to have undergone ultrasound imaging and less likely to have received an MRI as the last pre-transplant imaging, most TIPS patients (73.7%) who did not have any lesions identified on pre-transplant imaging had undergone CT or MRI evaluation, which was not dissimilar to the rate in non-TIPS patients. As such, when patients who underwent ultrasound evaluation were excluded from the regression, TIPS status remained independently associated with occult disease. This association suggests that even axial imaging is insufficient to adequately diagnose pre-transplant HCC in patients with TIPS. TIPS were not associated with an increase in summed diameter of lesions on explant minus summed diameter of lesions on imaging when comparing patients with the same number of explant lesions, suggesting that TIPS impact the identification of lesions more than they underestimate the size of identified lesions. Prior studies have not demonstrated a higher incidence of occult malignancy in TIPS patients, but diagnoses have been made based on imaging modalities (22alit, which we now suggest may be of decreased utility in these patients.
One theory behind this mechanism is that the nodularity in cirrhotic TIPS patients impacts the capacity to identify lesions by imaging. Degree of cirrhosis between TIPS groups can be evaluated in future studies. Another theory is that alterations in the contribution of portal venous and hepatic arterial flow (10,14) change the characteristics that typically inform the diagnosis of HCC. To further explore this idea, we investigated the role of PVT, as occlusion of the portal vein should cause a similar reduction in portal venous contribution to the hepatic parenchyma. Indeed, occult disease was statistically significantly more common in patients with any degree of PVT in our study. Individual factors of PVTs such as their occlusive nature and location within the portal vein were not associated with occult disease in our study, which may be a factor of small sample size or may imply that any degree of flow alteration can impact diagnostic accuracy. Further studies in this area may be warranted.
We also noted that a prior study identified more Liver Imaging Reporting and Data System (LI-RADS) 3 lesions in some TIPS patients (23), and we considered that these atypical lesions could corroborate our findings if ultimately found to be malignant on explant pathology. Although LI-RADS categorization was not available during the majority of our study timeframe, we did confirm that the presence and number of indeterminate lesions on imaging were independently associated with occult disease. Strikingly, 92.9% of TIPS patients and 60.0% of PVT patients with indeterminate lesions on pre-transplant imaging exhibited occult disease on explant pathology, suggesting that the presence of altered perfusion may have reduced the ability to definitively characterize these lesions as HCC. Another factor that may have contributed to poor visualization is susceptibility artifact from TIPS, limiting evaluation of the liver parenchyma immediately adjacent to the stent. Finally, shunting away from the liver parenchyma (due to a TIPS or PVT) could predispose the liver to develop confluent fibrosis, producing a signal intensity that obscures malignancy.
The relevance of occult disease is its impact on recurrence and survival. Overall, TIPS status was not independently associated with shorter DFS or OS, which is consistent with the fact that patients with and without TIPS had similar extents of malignancy on explant pathology. However, in the low-risk group of patients with single suspected lesions based on pre-transplant imaging, TIPS patients were statistically significantly more likely to exceed Milan criteria. Being outside Milan criteria on explant pathology was independently associated with reduced DFS in the multivariable model. Milan criteria was also independently associated with reduced OS when recurrence was removed from the model, suggesting an effect of Milan status on mortality through recurrence.
A unique concern in this study is that of sample bias, as patients are often identified as TIPS candidates due to a lack of suspected malignancy. Given that this series was comprised of patients who ultimately exhibited HCC on explant pathology, TIPS patients may have been more likely to be labeled as having occult malignancy. However, the relationship between TIPS and occult disease remained even when excluding patients with no known malignancy prior to transplant. We also did not capture the timing of pre-transplant therapies in order to assess patients who underwent interventions between the last pre-transplant imaging and transplant, which may have been higher in patients without TIPS given the more frequent use of pre-transplant therapy in this group. We do not suspect that this would significantly impact the findings, as TIPS patients had relatively lower pre-transplant AFP despite differences in intervention rates and treated lesions were still captured as malignancies in this study. We also do not suspect this would be a large portion of the population given that 85.8% of the population underwent only 2 or fewer pre-transplant interventions and the median time between diagnosis and transplant (240 days) was more than five times longer than the median time between last imaging and transplant (42 days). Other limitations of this study include its retrospective nature and the use of single institution data, which may reduce the generalizability of the findings. The results will need to be further evaluated in large multicenter studies, however the data support a hypothesis that has mechanistic plausibility. The contemporary relevance of these findings may also be reduced by the fact that imaging standards and assessment have evolved over time, however the same criteria were applied to patients with and without TIPS and the year of transplant was included in all regression models, reducing the probability that methodology impacted the association between TIPS and occult disease. We opted not to perform a retrospective review of the imaging to apply contemporary standards given that this would introduce bias in this study of patients with known HCC.
In an era with limited organ supply to meet the demand for liver transplantation, organs are directed toward patients with the highest likelihood of survival based on eligibility criteria. This study suggests that TIPS patients are more likely to have a greater burden of occult malignancy on explant pathology than expected based on pre-transplant imaging, especially when an indeterminate lesion is present. When those patients are outside Milan criteria on explant pathology, the recurrence rates are higher. These findings suggest that there is a need to aggressively surveil patients with TIPS, which may include using adjusted diagnostic imaging criteria to incorporate altered flow characteristics associated with TIPS and a higher index of suspicion involving more liberal use of tissue biopsy in order to appropriately identify and manage malignancy.
Supplementary Material
Supplemental Table 1. Univariate and multivariable linear regressions to evaluate factors associated with a greater number of lesions on explant pathology in patients with a single suspected lesion based on pre-transplant imaging. R-squared = 0.07 for multivariable regression.
Supplemental Table 2. Univariate and multivariable logistic regressions to evaluate factors associated with exceeding Milan criteria on explant pathology in patients with a single suspected lesion based on pre-transplant imaging. Pseudo R-squared = 0.24 for multivariable regression.
Supplemental Table 3. Univariate and multivariable Cox survival analyses for disease-free survival in all patients (top of cell) and in patients with a single lesion visualized on pre-transplant imaging (bottom of cell). Harrell’s C statistic = 0.75 (all patients) and 0.83 (suspected single lesion patients).
Supplemental Table 4. Univariate and multivariable Cox survival analyses for overall survival in all patients (top of cell) and in patients with a single lesion on pre-transplant imaging (bottom of cell). Harrell’s C statistic = 0.68 (all patients) and 0.67 (suspected single lesion patients).
Grants and financial support:
Research reported in this publication was partially supported by the National Center for Advancing Translational Sciences of the National Institutes of Health under awards UL1TR001878 and TL1TR001880. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Abbreviations:
- AFP
alpha-fetoprotein
- CT
computerized tomography
- DFS
disease-free survival
- HCC
hepatocellular carcinoma
- IQR
interquartile range
- LR
logistic regression
- MELD
Model for End Stage Liver Disease
- MRI
magnetic resonance imaging
- N
number
- OS
overall survival
- PVT
portal vein thrombosis
- RFA
radiofrequency ablation
- TACE
trans-arterial chemoembolization
- T
tesla
- TIPS
transjugular intrahepatic portosystemic shunt(s)
Footnotes
Conflicts of interest: None
References
- 1.Llovet JM, Bustamante J, Castells A, Vilana R, Ayuso M del C, Sala M, et al. Natural history of untreated nonsurgical hepatocellular carcinoma: rationale for the design and evaluation of therapeutic trials. Hepatol Baltim Md. 1999January;29(1):62–7. [DOI] [PubMed] [Google Scholar]
- 2.Elwir S, Lake J. Current Status of Liver Allocation in the United States. Gastroenterol Hepatol. 2016March;12(3):166–70. [PMC free article] [PubMed] [Google Scholar]
- 3.Mazzaferro V, Regalia E, Doci R, Andreola S, Pulvirenti A, Bozzetti F, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996March14;334(11):693–9. [DOI] [PubMed] [Google Scholar]
- 4.Heimbach JK, Kulik LM, Finn RS, Sirlin CB, Abecassis MM, Roberts LR, et al. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatol Baltim Md. 2018;67(1):358–80. [DOI] [PubMed] [Google Scholar]
- 5.Aufhauser DD, Sadot E, Murken DR, Eddinger K, Hoteit M, Abt PL, et al. Incidence of Occult Intrahepatic Metastasis in Hepatocellular Carcinoma Treated With Transplantation Corresponds to Early Recurrence Rates After Partial Hepatectomy. Ann Surg. 2018May;267(5):922–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Civan JM. Liver Transplantation for HCC: The Milan Criteria. In: Doria C, editor. Contemporary Liver Transplantation: The Successful Liver Transplant Program [Internet]. Cham: Springer International Publishing; 2016. p. 1–19. Available from: 10.1007/978-3-319-05543-5_11-2 [DOI] [Google Scholar]
- 7.Kitzing YX, Ng BH, Kitzing B, Waugh R, Kench JG, Strasser SI, et al. Washout of hepatocellular carcinoma on portal venous phase of multidetector computed tomography in a pre-transplant population: Portal venous phase washout of HCCs. J Med Imaging Radiat Oncol. 2015December;59(6):673–80. [DOI] [PubMed] [Google Scholar]
- 8.Keussen I, Song H-Y, Bajc M, Cwikiel W. Changes in the Distribution of Hepatic Arterial Blood Flow Following TIPS with Uncovered Stent and Stent-Graft: An Experimental Study. Cardiovasc Intervent Radiol. 2002August;25(4):314–7. [DOI] [PubMed] [Google Scholar]
- 9.Radeleff B, Sommer C-M, Heye T, Lopez-Benitez R, Sauer P, Schmidt J, et al. Acute Increase in Hepatic Arterial Flow During TIPS Identified by Intravascular Flow Measurements. Cardiovasc Intervent Radiol. 2009January;32(1):32–7. [DOI] [PubMed] [Google Scholar]
- 10.Preibsch H, Spira D, Thaiss WM, Syha R, Nikolaou K, Ketelsen D, et al. Impact of transjugular intrahepatic portosystemic shunt implantation on liver perfusion measured by volume perfusion CT. Acta Radiol Stockh Swed 1987. 2017October;58(10):1167–73. [DOI] [PubMed] [Google Scholar]
- 11.Lautt WW. Mechanism and role of intrinsic regulation of hepatic arterial blood flow: hepatic arterial buffer response. Am J Physiol-Gastrointest Liver Physiol. 1985November1;249(5):G549–56. [DOI] [PubMed] [Google Scholar]
- 12.Sacerdoti D, Serianni G, Gaiani S, Bolognesi M, Bombonato G, Gatta A. Thrombosis of the portal venous system. J Ultrasound. 2007March;10(1):12–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hennedige T, Venkatesh SK. Imaging of hepatocellular carcinoma: diagnosis, staging and treatment monitoring. Cancer Imaging. 2012;12(3):530–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Umar NK, Badshah MB, Sandrasegaran K, Ghabril M, Agarwal S, Tann M, et al. The Presence of Portal Vein Thrombosis Alters the Classic Enhancement Associated with Diagnosis of Hepatocellular Carcinoma. Dig Dis Sci. 2015July;60(7):2196–200. [DOI] [PubMed] [Google Scholar]
- 15.Sarin SK, Philips CA, Kamath PS, Choudhury A, Maruyama H, Nery FG, et al. Toward a Comprehensive New Classification of Portal Vein Thrombosis in Patients With Cirrhosis. Gastroenterology. 2016October;151(4):574–577.e3. [DOI] [PubMed] [Google Scholar]
- 16.Jiang H-Y, Chen J, Xia C-C, Cao L-K, Duan T, Song B. Noninvasive imaging of hepatocellular carcinoma: From diagnosis to prognosis. World J Gastroenterol. 2018June14;24(22):2348–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dunkler D, Plischke M, Leffondré K, Heinze G. Augmented backward elimination: a pragmatic and purposeful way to develop statistical models. PloS One. 2014;9(11):e113677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maldonado G, Greenland S. Simulation study of confounder-selection strategies. Am J Epidemiol. 1993December1;138(11):923–36. [DOI] [PubMed] [Google Scholar]
- 19.Mickey RM, Greenland S. The impact of confounder selection criteria on effect estimation. Am J Epidemiol. 1989January;129(1):125–37. [DOI] [PubMed] [Google Scholar]
- 20.Garrido MM, Kelley AS, Paris J, Roza K, Meier DE, Morrison RS, et al. Methods for constructing and assessing propensity scores. Health Serv Res. 2014October;49(5):1701–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Borentain P, Garcia S, Gregoire E, Vidal V, Ananian P, Ressiot E, et al. Transjugular intrahepatic portosystemic shunt is a risk factor for liver dysplasia but not hepatocellular carcinoma: A retrospective study of explanted livers. Dig Liver Dis. 2015January;47(1):57–61. [DOI] [PubMed] [Google Scholar]
- 22.Hüsing-Kabar A, Meister T, Köhler M, Domschke W, Kabar I, Wilms C, et al. Is de novo hepatocellular carcinoma after transjugular intrahepatic portosystemic shunt increased? United Eur Gastroenterol J. 2018April;6(3):413–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wong K, Ozeki K, Kwong A, Patel BN, Kwo P. The effects of a transjugular intrahepatic portosystemic shunt on the diagnosis of hepatocellular cancer. PloS One. 2018;13(12):e0208233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.De Santis A, Iegri C, Kondili L, Riggio O, Salvatori FM, Catalano C, et al. Hepatocellular carcinoma in cirrhotic patients with transjugular intrahepatic portosystemic shunt: A retrospective case–control study. Dig Liver Dis. 2014August;46(8):726–30. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table 1. Univariate and multivariable linear regressions to evaluate factors associated with a greater number of lesions on explant pathology in patients with a single suspected lesion based on pre-transplant imaging. R-squared = 0.07 for multivariable regression.
Supplemental Table 2. Univariate and multivariable logistic regressions to evaluate factors associated with exceeding Milan criteria on explant pathology in patients with a single suspected lesion based on pre-transplant imaging. Pseudo R-squared = 0.24 for multivariable regression.
Supplemental Table 3. Univariate and multivariable Cox survival analyses for disease-free survival in all patients (top of cell) and in patients with a single lesion visualized on pre-transplant imaging (bottom of cell). Harrell’s C statistic = 0.75 (all patients) and 0.83 (suspected single lesion patients).
Supplemental Table 4. Univariate and multivariable Cox survival analyses for overall survival in all patients (top of cell) and in patients with a single lesion on pre-transplant imaging (bottom of cell). Harrell’s C statistic = 0.68 (all patients) and 0.67 (suspected single lesion patients).


