Abstract
Background.
Contralateral axillary nodal metastases (CAM) is classified as stage IV disease, although many centers treat CAM with curative intent. We hypothesized that patients with CAM, treated with multimodality therapy, would have improved overall survival (OS) versus patients with distant metastatic disease (M1) and similar OS to those with locally advanced breast cancer (LABC).
Methods.
Using the NCDB (2004–2016), we categorized adult patients with node-positive breast cancer into three study groups: LABC, CAM, and M1. Kaplan-Meier curves were used to visualize the unadjusted OS. Cox proportional hazards models were used to estimate the association of study group with OS.
Results.
A total of 94,487 patients were identified: 122 with CAM, 12,325 with LABC, and 82,040 with M1 (median follow-up 63.6 months). LABC and CAM patients had similar histology and rates of chemotherapy and endocrine therapy receipt. However, the CAM group had significantly larger tumors, more estrogen-receptor expression, higher T-stage, and more mastectomies than the LABC group. Compared with M1 patients, CAM patients were more likely to have grade 3 and cT4 tumors. Patients with CAM and LABC had similar 5-year unadjusted OS and significantly improved OS vs M1 patients. After adjustment, LABC and CAM patients continued to have similar OS and better OS vs M1 patients.
Conclusions.
CAM patients who receive multi-modal therapy with curative intent may have OS more comparable to LABC patients than M1 patients. Out data support a reevaluation of whether CAM should remain classified as M1, as N3 may better reflect disease prognosis and treatment goals.
Contralateral axillary metastasis (CAM) is an uncommon event in breast cancer, defined as the identification of tumor deposits in the nodes of the contralateral axilla.1–14 This may occur as: (1) a synchronous event with an initial locally advanced breast cancer (LABC), (2) a metachronous event in isolation, (3) a synchronous event with an in-breast tumor recurrence and presumed aberrant lymphatic drainage, and lastly as (4) a metachronous event with a local recurrence. Contemporary studies report frequencies ranging from 0.8 to 6%, seen more commonly with recurrent disease than primary diagnoses.1–10 The American Joint Committee on Cancer (AJCC) classifies CAM as stage IV disease and considers it to be a “distant event,” potentially occurring from circulating tumor cells.15 However, a growing body of evidence supports that CAM may be more appropriately classified as N3 disease, with some potentially cured with aggressive multimodal therapy. Thus, the presentation with CAM in the absence of other distant metastases represents a therapeutic dilemma in breast cancer.
Evidence of prolonged survival among patients with CAM managed with aggressive treatment is accumulating. Unfortunately, because CAM is infrequently encountered, current data are limited to single-institution, retrospective reviews. Most series report that patients with CAM often undergo complete axillary lymph node dissection (ALND) for management of the contralateral axilla in addition to receiving systemic therapy.1,6–10 Furthermore, about half of patients are being managed with radiation to the contralateral axilla.1,6–10 These findings suggest treatment with curative intent in a patient population that is otherwise considered metastatic, and is consistent with a trend towards managing CAM as a progressive locoregional process, and not as Stage IV disease.
The purpose of this study was twofold: (1) to describe the tumor characteristics of patients with CAM being managed with multimodal therapy using a large national database; and (2) to characterize the survival of breast cancer patients with CAM when treated with curative intent. We hypothesized that patients with CAM, without other sites of distant metastatic disease, who were treated with multi-modal therapy would have improved overall survival (OS) compared with patients with distant metastatic (M1) disease.
METHODS
Adult patients (age ≥18 years) diagnosed with breast cancer were identified in the National Cancer Data Base (NCDB) from 2004 to 2016. Patients diagnosed in 2016 were excluded due to lack of survival data. Patients were categorized into three study groups: (1) locally advanced breast cancer (LABC), (2) isolated contralateral axillary metastases (CAM), and (3) metastatic disease (M1) (Fig. 1). The LABC group included patients with stage cN2b/3a-c, M0 unilateral disease, who completed both definitive breast surgery and radiation. The CAM group was defined as patients with cM1 disease where the metastatic site was classified in the NCDB as “distant lymph nodes”, which includes cervical, contralateral or bilateral axillary and/or internal mammary nodes, or “other” distant lymph nodes, and no other metastatic sites were present. All patients in the CAM group also had to have completed definitive breast surgery and radiation, in addition to resection of the “distant lymph nodes.” The metastatic (M1) group consisted of patients who were classified as cM1 with disease at any sites other than or in addition to distant lymph nodes. Patients who had multiple metastatic sites, including distant lymph nodes, were included in the M1 group. Breast cancer patients who could not be categorized into one of these three groups were excluded. Notably, the NCDB is limited to initial diagnoses, does not include recurrent disease, and therefore these study cohorts were all primary cancers.
FIG. 1.
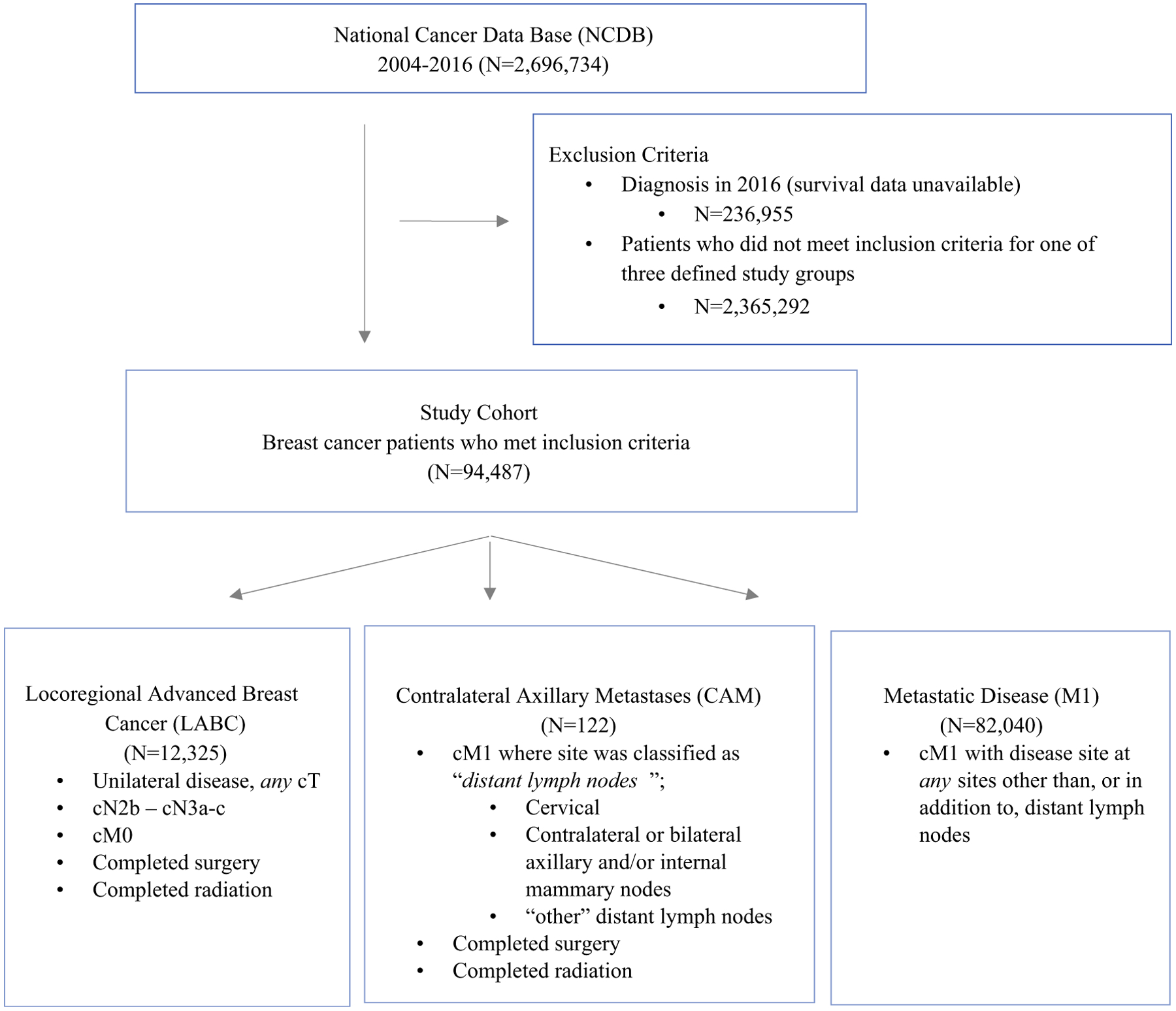
Patient Flow Diagram (NCDB 2016 dataset)
Patient characteristics were summarized by N (%) for categorical variables and median (interquartile range, IQR) for continuous variables for all patients and according to study group: LABC, CAM, M1. Groups were compared using the chi-square or Fisher’s exact test for categorical variables and the analysis of variance (ANOVA) for continuous variables, as appropriate. A Kaplan-Meier curve was used to visualize unadjusted OS. Median survival time plus 5- and 10-year survival rates were estimated using the Kaplan-Meier method, and the log-rank test was used to test for differences between groups. A Cox proportional hazards model was used to estimate the association of study group with OS after adjustment for known covariates, including age, year of diagnosis, gender, race/ethnicity, insurance status, income level, education level, facility type and location, distance traveled to treating institution, Charlson/Deyo comorbidity score, histology, cT stage, surgery type, receipt of chemotherapy, and endocrine therapy, and hazard ratios (HRs), and 95% confidence intervals (CIs) are reported. Study-group pair-wise HRs also were estimated from this adjusted model and are reported. To account for the correlation of patients treated at the same facility, a robust sandwich covariance estimator was used in the adjusted survival model.
Only patients with available data for all covariates were included in each model, and effective sample sizes are reported for each table/figure. A p value < 0.05 was considered statistically significant. No adjustments were made for multiple comparisons. All statistical analyses were conducted using SAS, version 9.4 (SAS Institute, Cary, NC) or R, version 3.5.1 (R Foundation for Statistical Computing, Vienna, Austria). Due to use of de-identified data, our institutional review board granted the study exempt status.
RESULTS
A total of 94,487 patients meeting the inclusion criteria were identified: 122 with CAM, 12,325 with LABC, and 82,040 with M1 disease (Fig. 1). Patient demographics and tumor characteristics are shown in Table 1 stratified by study group. Progressively advanced disease (LABC -> CAM -> M1) was associated with older age (median ages 55, 57, and 62, respectively, p < 0.001). Compared with patients with LABC, CAM patients had a larger tumor size (median 3.95 vs. 3.70 cm), higher frequency of grade 3 tumors (grade 3, 63.1% vs. 59.0%), more estrogen-receptor (ER)-positive tumors (68.9% vs. 59.9%), and lower rates of triple-negative disease (15.9% vs. 23.8%). In addition, the CAM group had markedly higher T-stage, both in the initial clinical classification (cT4, 41.0% vs. 22.4%) and at final pathologic assignment (pT4, 15.6% vs. 7.2%).
TABLE 1.
Patient and tumor characteristics, National Cancer Data Base, 2004–2016
| All patients (N = 94,487) | Locoregional advanced (N = 12,325) | CAM (N = 122) | M1 (N = 82,040) | p | |
|---|---|---|---|---|---|
| Age – median (IQR) | 61 (51–71) | 55 (46–64) | 57 (48–63) | 62 (52–73) | < 0.001 |
| Gender | < 0.001 | ||||
| Female | 93,224 (98.7%) | 12,223 (99.2%) | 121 (99.2%) | 80,880 (98.6%) | |
| Race/ethnicity | < 0.001 | ||||
| Non-Hispanic black | 15,204 (16.1%) | 2,051 (16.6%) | 24 (19.7%) | 13,129 (16%) | |
| Non-Hispanic white | 65,473 (69.3%) | 8,170 (66.3%) | 86 (70.5%) | 57,217 (69.7%) | |
| Hispanic | 5,259 (5.6%) | 981 (8%) | 8 (6.6%) | 4,270 (5.2%) | |
| Other | 3,058 (3.2%) | 483 (3.9%) | 1 (0.8%) | 2,574 (3.1%) | |
| Charlson/Deyo comorbidity score | < 0.001 | ||||
| 0 | 7,6412 (80.9%) | 10,568 (85.7%) | 104 (85.2%) | 65,740 (80.1%) | |
| 1 | 13,135 (13.9%) | 1,441 (11.7%) | 14 (11.5%) | 11,680 (14.2%) | |
| >2 | 4,940 (5.2%) | 316 (2.6%) | 4 (3.3%) | 4,620 (5.6%) | |
| Tumor size (cm) – median (IQR) | 4 (2.4–6) | 3.7 (2.4–6) | 3.95 (2.5–6.05) | 4 (2.4–6) | < 0.001 |
| Histology | < 0.001 | ||||
| Ductal | 60,884 (64.4%) | 9,333 (75.7%) | 98 (80.3%) | 51,453 (62.7%) | |
| Lobular | 15,257 (16.1%) | 2,051 (16.6%) | 10 (8.2%) | 13,196 (16.1%) | |
| Other | 18,346 (19.4%) | 941 (7.6%) | 14 (11.5%) | 17,391 (21.2%) | |
| Grade | < 0.001 | ||||
| 1 | 5,299 (5.6%) | 478 (3.9%) | 0 (0%) | 4,821 (5.9%) | |
| 2 | 28,528 (30.2%) | 3,465 (28.1%) | 32 (26.2%) | 25,031 (30.5%) | |
| 3 | 36,998 (39.2%) | 7,273 (59%) | 77 (63.1%) | 29,648 (36.1%) | |
| ER status | < 0.001 | ||||
| ER+ | 63,073 (66.8%) | 7,386 (59.9%) | 84 (68.9%) | 55,603 (67.8%) | |
| ER− | 22,972 (24.3%) | 4,756 (38.6%) | 37 (30.3%) | 18,179 (22.2%) | |
| PR status | < 0.001 | ||||
| PR+ | 50,026 (52.9%) | 5,881 (47.7%) | 56 (45.9%) | 44,089 (53.7%) | |
| PR− | 34,893 (36.9%) | 6,223 (50.5%) | 64 (52.5%) | 28,606 (34.9%) | |
| HER2 status * | < 0.001 | ||||
| HER2+ | 13,041 (22.2%) | 2,121 (26.5%) | 23 (28%) | 10,897 (21.5%) | |
| HER2− | 38,084 (64.9%) | 5,486 (68.6%) | 56 (68.3%) | 32,542 (64.3%) | |
| Tumor subtype * | < 0.001 | ||||
| HER2+ | 13,041 (22.2%) | 2,121 (26.5%) | 23 (28%) | 10,897 (21.5%) | |
| HR+/HER2− | 30,093 (51.3%) | 3,577 (44.7%) | 43 (52.4%) | 26,473 (52.3%) | |
| Triple-negative | 7,767 (13.2%) | 1,902 (23.8%) | 13 (15.9%) | 5,852 (11.6%) | |
| Clinical T-stage | < 0.001 | ||||
| cT0/IS | 1,611 (1.7%) | 102 (0.8%) | 2 (1.6%) | 1,507 (1.8%) | |
| cT1 | 11,061 (11.7%) | 1,657 (13.4%) | 15 (12.3%) | 9,389 (11.4%) | |
| cT2 | 24,223 (25.6%) | 4,637 (37.6%) | 34 (27.9%) | 19,552 (23.8%) | |
| cT3 | 12,966 (13.7%) | 2,873 (23.3%) | 11 (9%) | 10,082 (12.3%) | |
| cT4 | 27,924 (29.6%) | 2,757 (22.4%) | 50 (41%) | 25,117 (30.6%) | |
| cTX | 15,957 (16.9%) | 290 (2.4%) | 9 (7.4%) | 15,658 (19.1%) | |
| Clinical N-stage | ‡ | ||||
| cN0 | 18,672 (19.8%) | 0 (0%) | 29 (23.8%) | 18,643 (22.7%) | |
| cN1 | 28,342 (30%) | 0 (0%) | 40 (32.8%) | 28,302 (34.5%) | |
| cN2 | 10,012 (10.6%) | 825 (6.7%) | 20 (16.4%) | 9,167 (11.2%) | |
| cN3 | 20,088 (21.3%) | 11,500 (93.3%) | 28 (23%) | 8,560 (10.4%) | |
| cNX | 16,641 (17.6%) | 0 (0%) | 5 (4.1%) | 16,636 (20.3%) | |
| Pathologic T-stage | < 0.001 | ||||
| pT0/IS | 2,621 (2.8%) | 1,622 (13.2%) | 13 (10.7%) | 986 (1.2%) | |
| pT1 | 6,170 (6.5%) | 2,467 (20%) | 28 (23%) | 3,675 (4.5%) | |
| pT2 | 8,863 (9.4%) | 2,948 (23.9%) | 30 (24.6%) | 5,885 (7.2%) | |
| pT3 | 4,484 (4.7%) | 1,599 (13%) | 14 (11.5%) | 2,871 (3.5%) | |
| pT4 | 5,497 (5.8%) | 886 (7.2%) | 19 (15.6%) | 4,592 (5.6%) | |
| pTX | 4,7181 (49.9%) | 2,506 (20.3%) | 15 (12.3%) | 44,660 (54.4%) | |
| Pathologic N-stage | < 0.001 | ||||
| pN0 | 6,100 (6.5%) | 2,220 (18%) | 37 (30.3%) | 3,843 (4.7%) | |
| pN1 | 6,957 (7.4%) | 1,463 (11.9%) | 22 (18%) | 5,472 (6.7%) | |
| pN2 | 5,017 (5.3%) | 1,302 (10.6%) | 20 (16.4%) | 3,695 (4.5%) | |
| pN3 | 7,659 (8.1%) | 4,714 (38.2%) | 19 (15.6%) | 2,926 (3.6%) | |
| pNX | 48,815 (51.7%) | 2,326 (18.9%) | 18 (14.8%) | 46,471 (56.6%) |
Variable used in study group definition
HER2 status and tumor subtype reported for patients diagnosed 2010 and afterData presented as N (%) unless otherwise specified. Percentages may not add up to 100 due to rounding or missing values
IQR interquartile range; ER estrogen receptor; PR progesterone receptor; HER2 human epidermal growth factor receptor 2
Consistent with a higher T-stage, the CAM group underwent mastectomy with higher frequency (84.4% vs. 78.2%) compared with the LABC group (Table 2). The LABC and CAM groups had nearly identical rates of adjuvant systemic therapy (chemotherapy: 94.6% vs. 95.1%, and endocrine therapy: 55.9% vs. 55.7%).
TABLE 2.
Treatment, adjuvant therapy, National Cancer Data Base, 2004–2016
| All patients (N = 94,487) | Locoregional advanced (N = 12,325) | CAM (N = 122) | M1 (N = 82,040) | p | |
|---|---|---|---|---|---|
| Surgery type | ‡ | ||||
| Lumpectomy | 9,230 (9.8%) | 2,690 (21.8%) | 19 (15.6%) | 6,521 (7.9%) | |
| Mastectomy | 24,112 (25.5%) | 9,635 (78.2%) | 103 (84.4%) | 14,374 (17.5%) | |
| No surgery | 60,640 (64.2%) | 0 (0%) | 0 (0%) | 60,640 (73.9%) | |
| Other | 505 (0.5%) | 0 (0%) | 0 (0%) | 505 (0.6%) | |
| Lymph node surgery | |||||
| # LNs examined – median (IQR) | 11 (3 – 17) | 15 (10 – 21) | 10 (5 – 14.5) | 7 (1 – 14) | < 0.001 |
| # Positive LNs – median (IQR) | 3 (1 – 10) | 9 (1 – 14) | 3 (1 – 8) | 2 (1 – 6) | < 0.001 |
| Receipt of chemotherapy | 53,248 (56.4%) | 11,658 (94.6%) | 116 (95.1%) | 41,474 (50.6%) | < 0.001 |
| Receipt of endocrine therapy | 47,427 (50.2%) | 6,884 (55.9%) | 68 (55.7%) | 40,475 (49.3%) | < 0.001 |
| Receipt of radiation therapy | 39,168 (41.5%) | 12,325 (100%) | 122 (100%) | 26,721 (32.6%) | ‡ |
Variable used in study group definition
Data presented as N (%) unless otherwise specified. Percentages may not add up to 100 due to rounding or missing values
Median follow-up was 63.6 months (95% confidence interval CI] 63.0–64.2) for the entire study cohort. While LABC patients had a slightly longer median OS than CAM (102.1 vs. 83.2 months), this difference did not achieve significance; both LABC and CAM groups had significantly longer OS than the M1 group (median 24.7 months). Five-year unadjusted OS for patients with LABC and CAM was 64.7% (95% CI 63.7–65.7) versus 58.2% (95% CI 46.7–68.1), which were significantly improved compared with M1 patients [21.6% (95% CI 21.3–22.0)] (Fig. 2). After adjustment for multiple covariates, OS in patients with LABC and CAM were comparable and significantly better than that of M1 patients (hazard ratio [HR] 0.48, 95% CI 0.46–0.50 and HR 0.44, 95% CI 0.33–0.61, compared with reference M1) (Table 3).
FIG. 2.
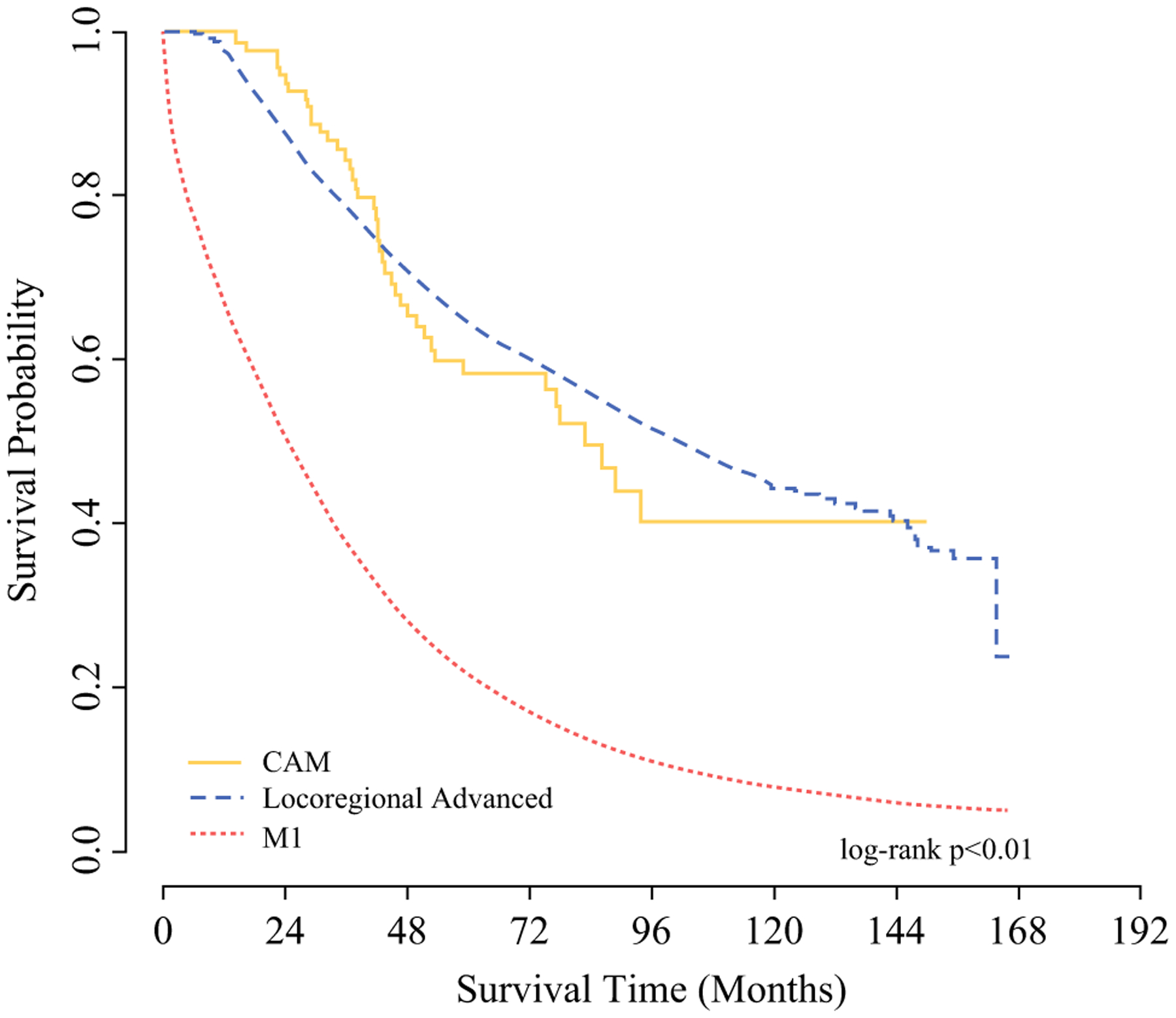
Kaplan-Meier curve showing unadjusted overall survival for breast cancer patients with LABC, CAM, and M1 disease (N = 94,484)
TABLE 3.
Adjusted overall survival (N = 81,659)
| Covariate | Hazard ratio (95% CI) | p | Overall p |
|---|---|---|---|
| Study group | < 0.001 | ||
| M1 | REF | ||
| CAM | 0.444 (0.326–0.605) | < 0.001 | |
| Locoregional advanced | 0.48 (0.463–0.498) | < 0.001 | |
| Age (yr) | 1.011 (1.01–1.012) | < 0.001 | < 0.001 |
| Year of diagnosis | 0.995 (0.991–0.998) | 0.006 | 0.006 |
| Gender | 0.09 | ||
| Female | REF | ||
| Male | 1.076 (0.988–1.171) | 0.09 | |
| Race/ethnicity | < 0.001 | ||
| Non-Hispanic white | REF | ||
| Non-Hispanic black | 1.131 (1.099–1.164) | < 0.001 | |
| Hispanic | 0.812 (0.765–0.862) | < 0.001 | |
| Other | 0.901 (0.848–0.958) | < 0.001 | |
| Insurance status | < 0.001 | ||
| Private | REF | ||
| Government | 1.19 (1.163–1.218) | < 0.001 | |
| Not insured | 1.274 (1.21–1.342) | < 0.001 | |
| Income level | < 0.001 | ||
| <$48,000 | REF | ||
| ≥$48,000 | 0.937 (0.912–0.962) | < 0.001 | |
| Education level | 0.47 | ||
| ≤87% High school graduation rate | REF | ||
| >87% High school graduation rate | 0.99 (0.965–1.017) | 0.47 | |
| Facility type | < 0.001 | ||
| Academic | REF | ||
| Community | 1.149 (1.091–1.21) | < 0.001 | |
| Comprehensive | 1.124 (1.081–1.169) | < 0.001 | |
| Integrated network | 1.087 (1.025–1.154) | 0.006 | |
| Facility location | 0.006 | ||
| South | REF | ||
| Midwest | 1.042 (1.001–1.084) | 0.05 | |
| Northeast | 0.96 (0.913–1.01) | 0.11 | |
| West | 0.975 (0.93–1.022) | 0.29 | |
| Distance traveled to treating institution | 1 (1–1) | 0.002 | 0.002 |
| Charlson/Deyo Comorbidity score | < 0.001 | ||
| 0 | REF | ||
| 1 | 1.259 (1.222–1.298) | < 0.001 | |
| ≥2 | 1.629 (1.555–1.706) | < 0.001 | |
| Histology | < 0.001 | ||
| Ductal | REF | ||
| Lobular | 1.022 (0.996–1.048) | 0.10 | |
| Other | 1.164 (1.132–1.196) | < 0.001 | |
| Clinical T-stage | < 0.001 | ||
| cT4 | REF | ||
| cT3 | 0.904 (0.877–0.932) | < 0.001 | |
| cT2 | 0.807 (0.786–0.829) | < 0.001 | |
| cT1 | 0.75 (0.726–0.776) | < 0.001 | |
| cT0/IS | 0.679 (0.63–0.733) | < 0.001 | |
| cTX | 0.873 (0.846–0.9) | < 0.001 | |
| Surgery | <0.001 | ||
| Mastectomy | REF | ||
| Lumpectomy | 1.017 (0.978–1.057) | 0.40 | |
| No surgery | 1.625 (1.581–1.67) | <0.001 | |
| Other | 0.945 (0.8–1.116) | 0.50 | |
| Receipt of chemotherapy | 0.65 (0.632–0.668) | <0.001 | <0.001 |
| Receipt of endocrine therapy | 0.463 (0.451–0.475) | <0.001 | <0.001 |
DISCUSSION
We present, for the first time, an analysis of patients in a large national database showing that breast cancer patients with isolated CAM have similar survival to patients with LABC (cN2b-N3a-c) when they are treated with curative intent. The current approach to patients with stage IV breast cancer focuses on systemic therapy and symptom palliation rather than cure.15,17 However, patients with CAM have been shown to have significantly prolonged survival compared with other stage IV patients when they are treated with curative intent.6,8,9,14 A recent meta-analysis by Caswell-Jin reported a median survival of 38 months in patients with recurrent metastatic breast cancer, and 31 months in patients with de novo stage IV disease.16 While this is slightly longer than the 24.7 months that we reported here, our study reveals CAM patients had a median OS of 83.2 months, which was significantly longer than that of patients with stage IV disease and similar to patients with LABC (102.1 months). In their systematic review of the CAM literature, Moossdorff et al. reported an OS of 82.5% with a median follow up of 50.3 months, which is similar to patients with locoregional disease, and divergent from those with metastatic disease.14 These results build on an accumulating body of evidence that patients with CAM have the potential for prolonged survival similar to that of N3 patients if treated with multimodal therapy including surgery.6–10,14
Contralateral axillary metastases can occur in both the primary and recurrent breast cancer setting. Previous studies have demonstrated that aberrant lymphatic drainage occurs in 18% to 51% of recurrent breast cancers compared with just 15% of primary cancers.4,12,13 In the case of primary disease such as the NCDB population presented herein, de novo atypical lymphatic drainage patterns or bulky ipsilateral tumor may lead to the appearance of CAM.13 In addition, dermal and lymphatic involvement by tumor are more commonly seen in cases of CAM.6,7 This is the population represented by our study, as the NCDB only captures initial breast cancer cases.
When CAM presents as a recurrence, there is evidence that physical disruption of lymphatics by prior axillary surgery and/or radiation causes lymphatic pathways to be rerouted toward supraclavicular, internal mammary, and other non-ipsilateral axillary nodal basins.3,11 In addition, dermal and chest wall involvement with the primary tumor is more commonly seen in cases of CAM.6,7 Although speculative, these observations suggest that CAM may originate from a locoregional process rather than true metastatic disease in the setting of both primary, and recurrent breast cancer.
We have also reported that patients with CAM have higher grade tumors than patients with LABC, which is similar to prior studies.1,8,9 The existing data are less consistent with regards to the relationship between CAM and tumor size. We have reported that tumor size in the CAM group was overall larger than in the LABC group, which is consistent with some of the previous data.1 The rate of ER expression among the CAM group (68.9%) was similar to that reported in previous studies.6,8 A notable outlier from other published studies, the report published by Chkheidze and colleagues reported an overall low rate of ER expression (25%) and high rate of triple-negative disease (67%).9 Our findings—a high overall rate of ER expression and a relatively low rate of triple-negative disease—support the conclusion that patients with CAM have less aggressive tumor biology, consistent with a progressive locoregional process rather than distant metastatic spread. We have noted that approximately 25% of patients in the cM1 group underwent surgery (Table 2). This likely represents patients undergoing palliative resections of their primary tumor and not for survival benefit or curative intent. This is supported by a study of patients with metastatic breast cancer published by Amann and colleagues where they report that 33.9% of their cohort underwent palliative resection of the primary tumor after the diagnosis of metastatic disease.26
The AJCC uses a tumor-node-metastasis (TNM) classification system for cancer reporting and prognostication.15 These TNM classifications are prognostic indicators for breast cancer and provide the foundation for determinants of additional pretreatment evaluation, surgical decision-making, and both neoadjuvant and adjuvant systemic therapy decision-making.23 The current manual for staging places patients with contralateral axillary lymph node involvement into the M1, stage IV group. This is similar to the ipsilateral supraclavicular nodal involvement in the 1997 AJCC Manual for Staging of Cancer.18 In 2001, MD Anderson Cancer Center reported a cohort of 70 patients with ipsilateral supraclavicular metastases but no evidence of other distant metastases.19 These patients were treated with combined-modality therapy, similar to patients with LABC, and were found to have no difference in OS as compared to Stage IIIB patients, and significantly improved over those with Stage IV breast cancer. While a small cohort, the MD Anderson data suggested a clinical scenario that indicates progressive locally advanced disease. These data contributed to the reclassification of ipsilateral supraclavicular nodal metastases as cN3c disease and has been supported in the literature since its reclassification.20–22
Limitations
There are a few limitations related to using the NCDB, including the absence of recurrence or breast-specific survival data. However, OS is considered a reasonable endpoint for patients with distant metastatic disease in particular, because these patients are most likely to die from their disease, rather than from other competing causes of mortality. It also is important to acknowledge that our study includes a lack of specificity in the definition of CAM based on the constraints of the NCDB, with contralateral lymph nodes being clustered with cervical nodes, contralateral internal mammary nodes, and “other” distant nodes. Because we required the CAM group to complete surgery, specifically with the resection of distant lymph nodes and radiation, we expect that this group is repre-entative of our target study population. This requirement would almost certainly exclude internal mammary nodes, and true distant lymph nodes (e.g., mesenteric lymph nodes), as these are rarely resected. Cervical lymph nodes are considerably less common than CAM.24 Although treatment of these lymph nodes varies and could include resection, they are more likely to be sampled by FNA or core biopsy, and unlikely to represent a significant portion of our study population.24 While CAM study group was defined in such a way as to highlight the benefit of aggressive treatment in these patients, the inclusion criteria also helped to eliminate other more common sites of metastatic disease such as internal mammary nodes, which would have confounded the results. Notably, the survival characteristics within the study group are comparable to those in CAM patients from other studies, suggesting a reasonably representative CAM group, and also similar to longstanding SEER data, supporting the validity of our approach.8,14,25 The authors acknowledge the potential for selection bias in how the study group was defined. Because radiation and surgery were included in the definition of the CAM study group, it is possible these patients had a tumor biology or underlying health status more amenable to aggressive treatment. It therefore may be true that the tumor biology of all CAM patients differs somewhat from our results. Finally, although this study represents the largest study of CAM to date, the sample size remains relatively small, reflecting the infrequent diagnosis of this clinical scenario.
CONCLUSIONS
Using a large national database, we showed that breast cancer patients with isolated CAM have similar survival to patients with LABC (cN2b-N3a-c) when they are treated with curative intent. Our study represents one of the largest series of CAM and one of the only studies that is not limited to cases from a single institution. Based on the comparable OS of CAM to other LABC patients when treated with curative intent, we submit that the AJCC consider reclassifying isolated CAM as N3 disease.
ACKNOWLEDGMENT
Dr. O. Fayanju is supported by the National Institutes of Health (NIH) under Award Number 1K08CA241390 (PI: Fayanju). Samantha Thomas had a consulting relationship with Abbvie, Inc. on work related to bioequivalence that ended in January 2019. This work was unrelated to this work.
DISCLOSURES
This work is also supported by the Duke Cancer Institute through NIH Grant P30CA014236 (PI: Kastan).
Footnotes
Presentation at American Society of Breast Surgeon’s Annual Meeting: Oral presentation at the 22nd Annual Meeting, Virtual Oral Presentation, Orlando, Florida, 2021
REFERENCES
- 1.Morcos B, Jaradat I, El-Ghanem M. Characteristics of and therapeutic options for contralateral axillary lymph node metastasis in breast cancer. EJSO. 2011;37:418–21. [DOI] [PubMed] [Google Scholar]
- 2.Agarwal A, Heron DE, Sumkin J, Falk J. Contralateral uptake and metastases in sentinel lymph node mapping for recurrent breast cancer. J Surg Oncol. 2005;92:4–8. [DOI] [PubMed] [Google Scholar]
- 3.Pasta V, Monteleone F, D’Orazi V, Del Vecchio L, Sottile D, Iacobelli S, Monti M. Typical and atypical lymphatic flows in breast carcinoma. Ann Ital Chir. 2015;86(4):311–6. [PubMed] [Google Scholar]
- 4.Van der Ploeg IM, Oldenburg HSA, Rutgers EJT, Baas-Vrancken Peters MTFD, Kroon BBR, Valdes-Olmos RA, Nieweg OE. Lymphatic drainage patterns for the treated breast. Ann Surg Oncol. 2010;17:1069–75. [DOI] [PubMed] [Google Scholar]
- 5.Jaffer S, Goldfarb AB, Gold JE, Szport A, Bleiweiss IJ. Contralateral axillary lymph node metastasis as a first evidence of locally recurrent breast carcinoma. Cancer. 1995;75(12):2875–8. [DOI] [PubMed] [Google Scholar]
- 6.Kiluk JV, Prowler V, Lee MC, Khakpour N, Laronga C, Cox CE. Contralateral axillary nodal involvement from invasive breast cancer. Breast. 2014;23:291–4. [DOI] [PubMed] [Google Scholar]
- 7.Guru SD, Loprinzi CL, Yan E, Hoskin TA, Jakub JW. Contralateral axillary metastases in breast cancer: Stage IV disease or a locoregional event? Am Surg. 2019;85(12):1391–6. [PubMed] [Google Scholar]
- 8.Magnoni F, Colleoini M, Mattar D, Corso G, Bagnardi V, Fras-soni S, Santomauro G, Jereczek-Fossa BA, Veronesi P, Galimberti V, Sacchini V, Intra M. Contralateral axillary lymph node metastases from breast carcinoma: Is it time to review TNM cancer staging. Ann Surg Oncol. 2020;27:4488–99. [DOI] [PubMed] [Google Scholar]
- 9.Chkheidze R, Sanders MAG, Haley B, Leitch AM, Sahoo S. Isolated contralateral axillary lymph node involvement in breast cancer represents a locally advanced disease not distant metastases. Clin Breast Cancer. 2017;18(4):298–304. [DOI] [PubMed] [Google Scholar]
- 10.Wang W, Yuan P, Wang J, Ma F, Zhang P, Li Q, Xu B. Management of contralateral axillary lymph node metastasis from breast cancer: a clinical dilemma. Tumori. 2014;100:600–4. [DOI] [PubMed] [Google Scholar]
- 11.Maaskant-Braat AJG, Roumen RMH, Voogd AC, Pijpers R, Luiten EJT, Rutgers EJT, Nieuwenhuijzen GAP. Sentinel Node and Recurrent Breast Cancer (SNARB): results of a nationwide registration study. Ann Surg Oncol. 2013;20(2):620–6. [DOI] [PubMed] [Google Scholar]
- 12.Maaskant-Braat AJG, de Bruijn SZ, Woensdregt K, Pijpers H, Voogd AC, Nieuwenhuijzen GAP. Lymphatic mapping after breast surgery. Breast. 2012;21:444–8. [DOI] [PubMed] [Google Scholar]
- 13.Newman EA, Cimmino VM, Sabel MS, Diehl KM, Frey KA, Chang AE, Newman LA. Lymphatic mapping and sentinel lymph node biopsy for patients with local recurrence after breast-conservation therapy. Ann Surg Oncol. 2006;13(1):52–7. [DOI] [PubMed] [Google Scholar]
- 14.Moossdorff M, Vugts G, Maaskant-Braat AJG, Strobbe LJA, Voogd AC, Smidt ML, Nieuwenhuijzen GAP. Contralateral lymph node recurrence in breast cancer: Regional event rather than distant metastatic disease. A systematic review of the literature. EJSO. 2015;41:11280–336. [DOI] [PubMed] [Google Scholar]
- 15.Amin MB, Edge S, Greene F, Byrd DR, Brookland RK, Washington MK, Gershenwald JE, Compton CC, Hess KR, et al. (eds) AJCC Cancer Staging Manual (8th edition). Springer International Publishing: American Joint Commission on Cancer; [cited 2021 May 3]. 2017. [Google Scholar]
- 16.Caswell-Jin JL, Plevritis SK, Cadham CJ, Xu C, Stout NK, Sledge GW, Mandelblatt JS, Kurian AW. Change in survival in metastatic breast cancer with treatment advances: meta-analysis and systematic review. JNCI Cancer Spectrum. 2018; 2(4): pky062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huston TL, Pressman PI, Moore A, Vahdat L, Hoda SA, Kato M, et al. The presentation of contralateral axillary lymph node metastases from breast carcinoma: a clinical management dilemma. Breast. 2007;13:158–64. [DOI] [PubMed] [Google Scholar]
- 18.Fleming ID, Cooper JS, Henson DE, Hutter RVP, Kennedy BJ, Murphy GP, O’Sullivan B, Sobin LH, Yarbo JW (eds) AJCC cancer staging manual, 5th edn. Lippincott-Raven: American Joint Commission on Cancer; [cited 2021 May 3]. 1997. [Google Scholar]
- 19.Brito RA, Valero V, Buzdar AU, Booser DJ, Ames F, Strom E, Moss M, Theriault RL, Frye D, Kau SW, Asmar L, McNeese M, Singletary SE, Hortobagyi GN. Long-term results of combined-modality therapy for locally advanced breast cancer with ipsilateral supraclavicular metastases: The University of Texas M.D. Anderson Cancer Center experience. J Clin Oncol. 2001;19(3):628–33. [DOI] [PubMed] [Google Scholar]
- 20.Singletary SE, Allred C, Ashley P, Bassett LW, Berry D, Bland KI, Borgen PI, Clark G, et al. Revision of the American Joint Committee on Cancer staging system for breast cancer. J Clin Oncol. 2002;20(17):3628–36. [DOI] [PubMed] [Google Scholar]
- 21.Tamirisa NP, Ren Y, Campbell BM, Thomas SM, Fayanju OM, Plichta JK, Rosenberger LH, Force J, Hyslop T, Hwang ES, Greenup RA. Treatment patterns and outcomes of women with breast cancer and supraclavicular nodal metastases. Ann Surg Oncol. 2021;28(4):2146–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Olivotto IA, Chua B, Allan SJ, Speers CH, Chia S, Ragaz J. Long-term survival of patients with supraclavicular metastases at diagnosis of breast cancer. J Clin Oncol. 2003;21(5):851–4. [DOI] [PubMed] [Google Scholar]
- 23.National Comprehensive Cancer Network. Breast Cancer (Version 3.2021). Available at: https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf.2021.
- 24.Kim JS, Kim K, Shin KH, Kim JH, Ahn SD, Kim SS, Kim YB, Chang JS, Choi DH, Park W, Kim TH, Chun M, Cha J, Kim JH, Lee DS, Lee SY, Park HJ. Cervical lymph node involvement above the supraclavicular fossa in breast cancer: comparison with stage IIIC. J Breast Cancer. 2020;23(2):194–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.National Cancer Institute: Surveillance, Epidemiology, and End Results Program. Available at: https://seer.cancer.gov/statfacts/html/breast.html.Accessed10 April 2021.
- 26.Amann E, Huang DJ, Weber WP, Eppenberger-Castori S, Schmidd SM, Hess TH, Guth U. Disease-related surgery in patients with distant metastatic breast cancer. Eur J Surg Oncol. 2013;39(11):1192–8. [DOI] [PubMed] [Google Scholar]


