STRUCTURED ABSTRACT
Background
The incidence of and risk factors for late-onset kidney failure among survivors over the very long-term remains understudied.
Materials and Methods
25,530 childhood cancer survivors (median follow-up 22.3 years, interquartile range 17.4–28.8) diagnosed between 1970–1999 and 5,045 siblings from the Childhood Cancer Survivor Study were assessed for self-reported late-onset kidney failure, defined as dialysis, renal transplantation, or death attributable to kidney disease. Piecewise exponential models evaluated associations between risk factors and the rate of late-onset kidney failure.
Results
206 survivors and 10 siblings developed late-onset kidney failure, a 35-year cumulative incidence of 1.7% (95%CI=1.4–1.9) and 0.2% (95%CI=0.1–0.4) respectively, corresponding to an adjusted rate ratio [RR] 4.9 (95%CI=2.6–9.2). High kidney dose from radiotherapy (≥15Gy; RR=4.0, 95%CI=2.1–7.4), exposure to high-dose anthracycline (≥250mg/m2; RR=1.6, 95%CI=1.0–2.6) or any ifosfamide chemotherapy (RR=2.6, 95%CI=1.2–5.7), and nephrectomy (RR=1.9, 95%CI=1.0–3.4) were independently associated with elevated risk for late-onset kidney failure among survivors. Survivors who developed hypertension, particularly in the context of prior nephrectomy (RR=14.4, 95%CI=7.1–29.4 hypertension with prior nephrectomy; RR=5.9, 95%CI=3.3–10.5 hypertension without prior nephrectomy), or diabetes (RR=2.2, 95%CI=1.2–4.2) were also at elevated risk for late-onset kidney failure.
Conclusions
Survivors of childhood cancer are at increased risk for late-onset kidney failure. Kidney dose from radiotherapy ≥15Gy, high-dose anthracycline, any ifosfamide, and nephrectomy were associated with increased risk of late-onset kidney failure among survivors. Successful diagnosis and management of modifiable risk factors such as diabetes and hypertension may mitigate risk for late-onset kidney failure. The association of late-onset kidney failure with anthracycline chemotherapy represents a novel finding that warrants further study.
Keywords: Childhood cancer, survivorship, late-onset kidney failure, nephrectomy
BACKGROUND
With continued improvements in cancer therapies, over 85% of pediatric cancer patients will become long-term survivors.1 Because survivors are known to be at increased risk for the development of late-onset chronic health conditions related to their cancer therapy, an improved understanding of the incidence of these conditions and the treatments that place survivors at elevated risk, are of vital importance.2,3 Kidney injury is a common complication of cancer treatments in children. Treatment with platinum agents, ifosfamide, abdominal radiotherapy, or nephrectomy are associated with acute kidney injury and are recognized as potential risk factors for long-term kidney dysfunction among survivors.4–6 However, reports with extended follow-up in a large diverse survivor population are limited.
Progressive renal insufficiency affects over 13% of US adults aged 45–64 and is usually a clinically silent condition.7 In the context of progression to dialysis-dependence, kidney failure (also known as end-stage renal disease or end-stage kidney disease) imparts a disproportionate financial and workforce burden on healthcare systems and carries a mortality rate of 165 per 1000 patient-years.8 The reported prevalence of kidney conditions among survivors of childhood cancer is highly variable, depending upon the study group, treatment exposures, reported outcome and follow-up duration.4,9 It is estimated that 0.5% of all childhood cancer survivors develop life-threatening or disabling kidney failure after mean follow-up of 17 years from diagnosis.2 Among survivors of unilateral, non-syndromic Wilms tumor, the cumulative incidence of chronic dialysis-dependence or renal transplantation, is approximately 1% after 20 years.10,11 Unfortunately, few studies elaborate on this risk in a large cohort of survivors beyond 20 years from diagnosis.
The Childhood Cancer Survivor Study (CCSS) cohort provides a unique opportunity to build upon knowledge generated from previous studies by offering one of the largest childhood cancer survivor cohorts with detailed exposure and long-term follow-up. The purpose of this study is to characterize the cumulative incidence of kidney failure among a survivor population now in in early to mid-adulthood and to identify the risk factors associated with development of kidney failure.
MATERIALS AND METHODS
Study design and participants
The CCSS is a multi-institutional, retrospective cohort with prospective follow-up of five-year survivors of childhood cancer treated at one of 31 institutions in North America and includes a comparison group of nearest-age siblings selected by random sampling. Study methods and participant accrual in the CCSS have been reported previously.12,13 This study included 25,530 childhood cancer survivors and 5,045 siblings participating in the CCSS. All survivors were diagnosed with cancer before age 21 years and between 1970 and 1999. The institutional review board for each participating institution approved the CCSS protocol and participants provided informed consent.
Outcome measures
The primary outcome was self-reported late-onset kidney failure, defined as any grade four (life-threatening; defined as initiation of dialysis or renal transplantation) or grade five (fatal) renal condition based on the Common Terminology Criteria for Adverse Events (CTCAE), first occurring five or more years after primary cancer diagnosis.2,14 Survivors entered the cohort five years after their cancer diagnosis and siblings entered on the same dates as their corresponding survivors.
Procedures
Chemotherapy, radiotherapy and surgical data associated with original cancer treatment was systematically abstracted from the medical records of participating institutions. Cumulative chemotherapy exposure was evaluated as follows: anthracycline dose (none, 0.1–249, ≥250 mg/m2), cisplatin equivalent dose (none, 0.1–499, ≥500 mg/m2 with the cumulative dose of carboplatin weighted by 0.25),15,16 ifosfamide dose (none, 0.1–59, ≥60 g/m2), and intravenous methotrexate dose (none, 0.1–3999, ≥4000 mg/m2). Survivors who underwent unilateral nephrectomy as treatment for their primary cancer were identified based on International Classification of Diseases, Ninth Revision, Clinical Modification procedure codes abstracted from the participating institutions’ medical records. Radiotherapy records were centrally abstracted for details necessary for dose reconstruction, including prescription(s), date(s), and treatment field parameters, (i.e. dose, orientation, beam energy, field size, weighting, field blocking, and anatomical borders or field central axis coordinates). Each individual’s radiotherapy was then reconstructed on a computational phantom scaled to their age at the time of radiotherapy and mean doses to the right and left kidneys were calculated separately.17–19 The radiation variable included in the analysis was the lower dose of the two mean doses (right or left; divided categorically into: none, 0.1–9.9, 10–14.9, ≥15 Gy). For survivors who underwent unilateral nephrectomy, the dose to the remaining kidney was used.
Additional abstracted variables included: decade of diagnosis (1970–79, 1980–89, 1990–99), attained age, sex, race/ethnicity, self-reported history of genitourinary conditions, and hypertension or diabetes requiring medications prior to the primary endpoint. Subsequent malignant neoplasms (SMN) occurring five years or more after primary cancer diagnosis were also included. SMNs were identified via self- or next-of-kin proxy report or death certificate and confirmed by pathology report, death certificate, medical records, or both.20 SMNs were categorized as: none, non-renal SMN (excluding non-melanoma skin cancers), or renal SMN.
Statistical analysis
Descriptive statistics were tabulated for survivors who did and did not develop late-onset kidney failure. Cumulative incidence of late-onset kidney failure was estimated for survivors and siblings, overall and by cancer diagnosis, with death classified as a competing risk event and the follow-up starting at 5 years from the cancer diagnosis and ending with the earliest of development of the primary outcome, death, or most recent questionnaire completion (censoring). Kidney failure occurring prior to the cohort entry at 5 years from cancer diagnosis (n=126 survivors and no siblings) were included in the cumulative incidence as prevalent cases at cohort entry. Piecewise exponential regression models estimated adjusted rate ratios (RR) of late-onset kidney failure (excluding kidney failure that occurred before cohort entry) between survivors and siblings overall and among survivors by primary cancer diagnosis controlling for attained age, sex, and race/ethnicity.
Piecewise exponential models were also used to evaluate the rate of late-onset kidney failure among survivors in association with the following covariates: age at primary cancer diagnosis, known genitourinary condition, unilateral nephrectomy, cumulative anthracycline dose, platinum agent dose (cisplatin equivalent dose), cumulative IV methotrexate dose, cumulative ifosfamide dose, and mean kidney dose from radiotherapy, adjusting for time-varying attained age during follow up as natural cubic splines. These models were also adjusted for development of diabetes, hypertension, or SMN (occurring prior to kidney failure) as time-dependent variables. We additionally tested a priori hypothesized interactions between hypertension and nephrectomy, hypertension and kidney radiation, diabetes and nephrectomy, diabetes and kidney radiation.
To further investigate the association between exposure to anthracycline chemotherapy and late-onset kidney failure specifically, a sensitivity analysis among survivors who did not undergo nephrectomy or radiotherapy to the kidney, the two strongest treatment risk factors, was performed utilizing the same piecewise exponential model so that the anthracycline and late-onset kidney failure association could be evaluated without the complex dependency among the treatment variables. Additionally, a time-dependent covariate indicating the occurrence of heart failure was added to the model to assess whether the late-onset kidney failure association with anthracycline, a cardiotoxic chemotherapy agent, is mediated by, or independent of, the development of this cardiac condition.
Statistical analyses were conducted with R Statistical Software (version 3.5.2) and SAS version 9.4 (SAS Institute, Cary, NC). All statistical inferences were two-sided and p-values <0.05 were considered statistically significant.
RESULTS
A total of 25,530 survivors and 5,045 siblings were included in the analysis (Table 1). Median age at cancer diagnosis among survivors was 6.1 years (interquartile range [IQR], 3.0–12.4) and 6.7 years (IQR 3.0–13.2) for the sibling population. Median follow-up time was 22.4 (IQR 17.4–28.8) years for survivors and 27.8 (IQR 21.1–34.8) years for siblings.
Table 1.
Demographic and treatment characteristics of childhood cancer survivors and siblings who did and did not develop late onset kidney failure
| Characteristic | Late kidney failure | |||
|---|---|---|---|---|
| Survivors | Siblings | |||
| No kidney failure n=25324 | Kidney failure n=206 | No kidney failure n=5035 | Kidney failure n=10 | |
| Demographic | ||||
| Female sex | 11784 (46) | 95 (46) | 2639 (52) | 3 (30) |
| Race/ethnicity | ||||
| Non-Hispanic white | 20560 (80) | 167 (81) | 4361 (87) | 8 (80) |
| Non-Hispanic black | 1599 (6) | 19 (10) | 148 (3) | 1 (10) |
| Hispanic/Latino | 2011 (9) | 12 (5) | 215 (4) | 0 (0) |
| Other | 1154 (5) | 8 (4) | 311 (6) | 1 (10) |
| Age at diagnosis (y) | ||||
| 0–3 | 8222 (35) | 74 (35) | ||
| 4–9 | 7557 (32) | 58 (31) | ||
| 5–14 | 5367 (19) | 33 (15) | ||
| ≥15 | 4178 (14) | 41 (19) | ||
| Decade of diagnosis | ||||
| 1970–79 | 6536 (22) | 64 (29) | ||
| 1980–89 | 9903 (37) | 87 (42) | ||
| 1990–99 | 8885 (41) | 55 (29) | ||
| Primary cancer diagnosis | ||||
| Acute lymphoblastic leukemia | 6542 (36) | 42 (27) | ||
| Acute myeloid leukemia | 911 (3) | 8 (4) | ||
| Other leukemia | 323 (1) | 6 (3) | ||
| Central nervous system tumor | 4465 (15) | 17 (8) | ||
| Hodgkin lymphoma | 3087 (11) | 17 (8) | ||
| Non-Hodgkin lymphoma | 2065 (7) | 18 (8) | ||
| Wilms tumor | 2204 (8) | 46 (21) | ||
| Neuroblastoma | 1922 (7) | 19 (8) | ||
| Soft tissue sarcoma | 1744 (6) | 10 (4) | ||
| Ewing sarcoma | 2061 (7) | 23 (10) | ||
| Osteosarcoma | 727 (2) | 7 (3) | ||
| Other bone cancer | 1233 (4) | 15 (7) | ||
| Treatment exposures | 101 (0) | 1 (0) | ||
| Anthracycline dose (mg/m2) | ||||
| None | 11984 (49) | 70 (39) | ||
| 0.1–249 | 6676 (36) | 57 (39) | ||
| ≥250 | 3686 (15) | 41 (22) | ||
| Cisplatin equivalent dose (mg/m2) | ||||
| None | 20487 (91) | 153 (86) | ||
| 0.1–499 | 1378 (5) | 13 (7) | ||
| ≥500 | 1060 (4) | 14 (7) | ||
| Ifosfamide dose (g/m2) | ||||
| None | 21936 (95) | 165 (91) | ||
| 0.1–59 | 856 (3) | 13 (6) | ||
| ≥60 | 294 (1) | 5 (3) | ||
| Methotrexate dose (IV, mg/m2) | ||||
| None | 17818 (73) | 145 (82) | ||
| 0.1–3999 | 2325 (11) | 14 (9) | ||
| ≥4000 | 2567 (16) | 13 (9) | ||
| Kidney dose from radiotherapy (Gy) | ||||
| None | 10295 (50) | 73 (42) | ||
| 0.1–9.9 | 10932 (44) | 70 (39) | ||
| 10–14.9 | 914 (4) | 17 (10) | ||
| ≥15 | 367 (1) | 18 (9) | ||
| Unilateral nephrectomy | 1956 (7) | 43 (21) | ||
| Medical comorbidities * | ||||
| Known genitourinary condition | 542 (2) | 9 (5) | 10 (0) | 0 (0) |
| Diabetes | 1021 (4) | 21 (10) | 109 (2) | 1 (10) |
| Hypertension | 2792 (10) | 77 (36) | 461 (9) | 3 (30) |
| Subsequent malignant neoplasm (SMN) * | ||||
| None | 24013 (95) | 192 (93) | ||
| Non-renal SMN | 1267 (5) | 10 (5) | ||
| Renal SMN | 44 (0) | 4 (2) | ||
Percentages are column percentages
reported to have occurred prior to the primary endpoint
Analyses, including reported percentages and means/medians, were weighted to account for undersampling of acute lymphoblastic leukemia survivors (1987–1999), with a weight of 1.21 for age 0 or 11–20 years at diagnosis, and a weight of 3.63 for those aged 1–10 years
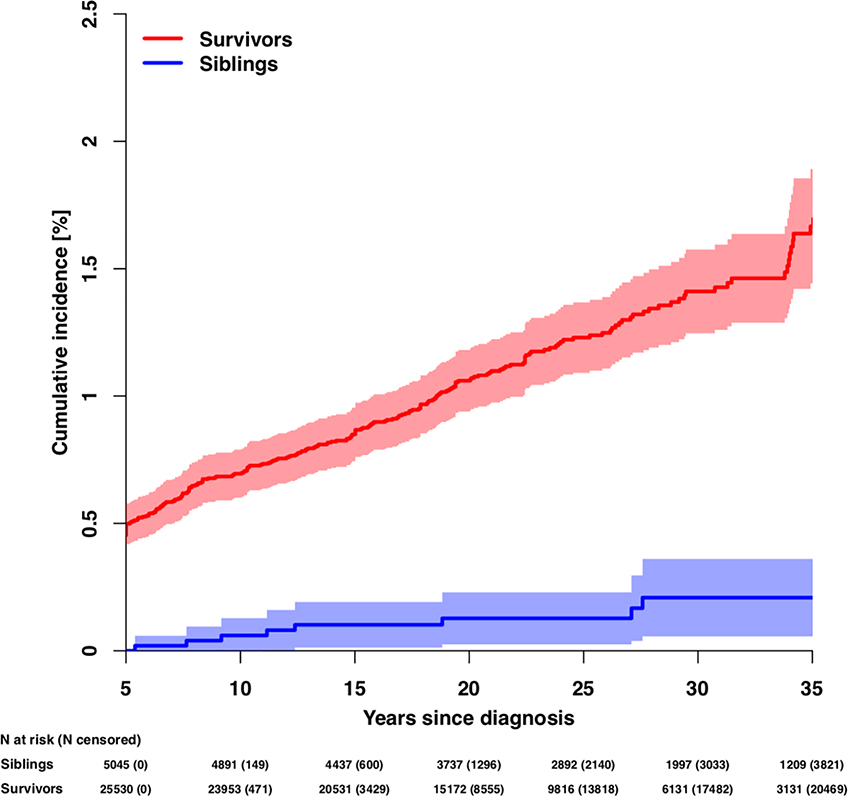
A total of 206 survivors and 10 siblings developed late-onset kidney failure. The median age of first occurrence of kidney failure for survivors and siblings was 22.5 (IQR 16.1–29.5) and 30.0 (IQR 25.5–39.5) years, respectively. The cumulative incidence of late-onset kidney failure at 35 years after primary cancer diagnosis was 1.7% (95% confidence interval [CI]=1.4–1.9) and 0.2% (95%CI=0.1–0.4) for survivors and siblings, respectively (p<0.001; Figure 1, Table 2). The 35-year cumulative incidences of late-onset kidney failure stratified by primary cancer diagnosis are shown in Table 2. The cumulative incidences of late-onset kidney failure by selected treatment exposures are displayed in Figure 2 and Supplementary Table 1.
Figure 1.
Cumulative incidence of late-onset kidney failure among survivors and siblings
Note: The number of participants at risk (number censored) at each 5-year interval post-diagnosis is listed below the x-axis. The number censored does not include those who experienced a competing risk event (death).
Table 2.
Late-onset kidney failure among survivors of childhood cancer, overall and by primary cancer diagnosis
| 35-year cumulative incidence (95% confidence interval) |
Rate ratio* (95% confidence interval) |
P | |
|---|---|---|---|
| Siblings | 0.2 (0.1 – 0.4) | Ref. | - |
| Survivors (overall) | 1.7 (1.4 – 1.9) | 4.9 (2.6 – 9.2) | <0.001 |
| Survivors by diagnosis | |||
| Central nervous system tumor | 0.6 (0.3 – 0.8) | Ref. | - |
| ALL | 1.2 (0.9 – 1.5) | 1.5 (0.8 – 2.7) | 0.22 |
| AML | 2.0 (1.0 – 3.0) | 2.2 (1.0 – 5.2) | 0.060 |
| Hodgkin lymphoma | 0.9 (0.5 – 1.4) | 1.2 (0.6 – 2.4) | 0.64 |
| Non-Hodgkin lymphoma | 3.6 (2.2 – 4.9) | 2.0 (1.0 – 3.8) | 0.043 |
| Wilms tumor | 3.8 (2.8 – 4.8) | 4.7 (2.6 – 8.4) | <0.001 |
| Neuroblastoma | 2.1 (1.1 – 3.2) | 2.5 (1.2 – 4.9) | 0.010 |
| Soft tissue sarcoma | 1.0 (0.3 – 1.6) | 1.2 (0.6 – 2.7) | 0.63 |
| Ewing sarcoma | 1.7 (0.7 – 2.8) | 2.5 (1.0 – 5.9) | 0.047 |
| Osteosarcoma | 2.7 (1.3 – 4.1) | 2.6 (1.3 – 5.4) | 0.008 |
adjusted for attained age, sex and race/ethnicity
Figure 2.
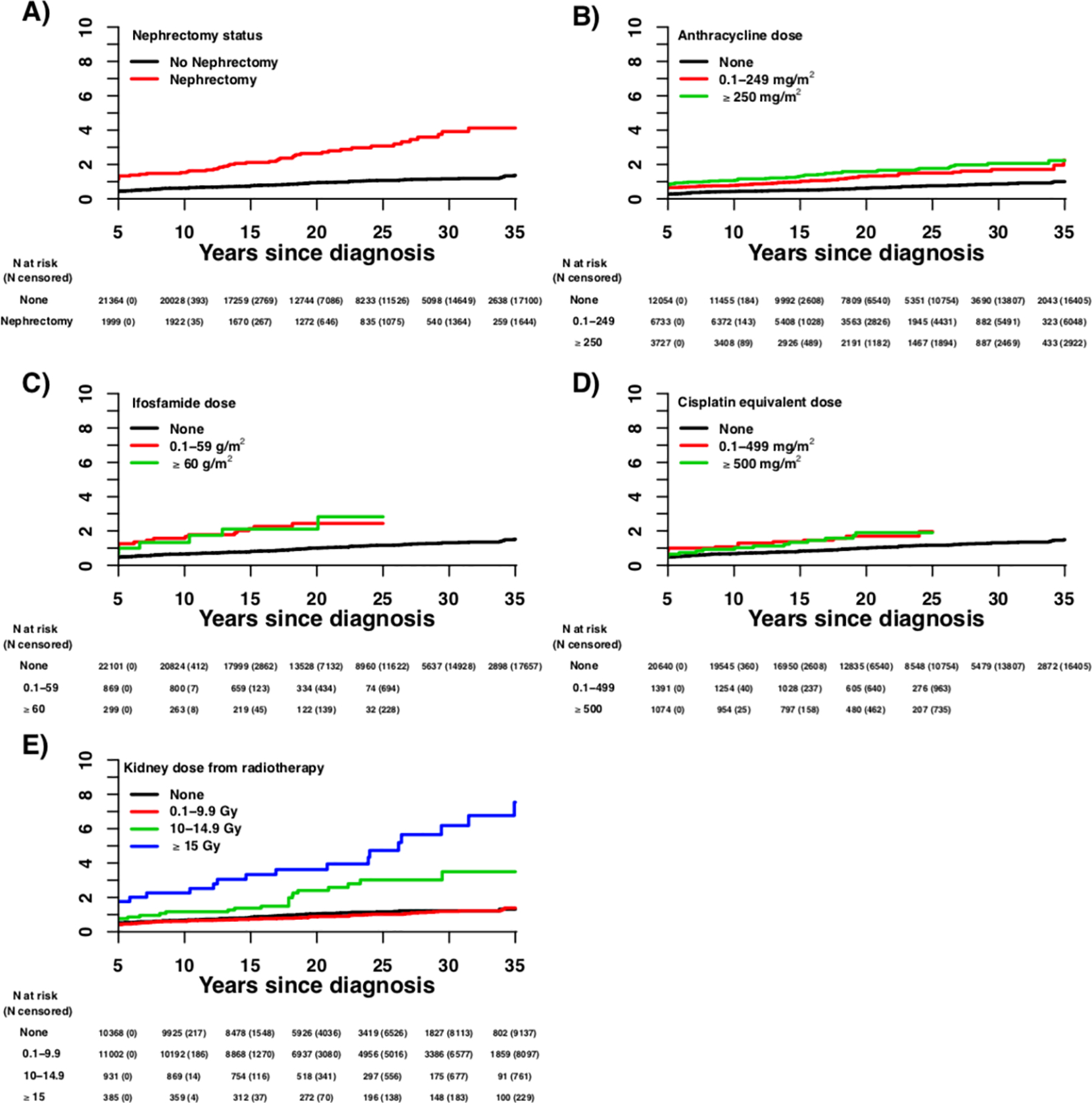
Cumulative incidence of late-onset kidney failure among survivors by (A) nephrectomy status, (B) anthracycline dose, (C) ifosfamide dose, and (D) cisplatin equivalent dose, and (E) kidney dose from radiotherapy
Note: The number of participants at risk (number censored) at each 5-year interval post-diagnosis is listed below the x-axis. The number censored does not include those who experienced a competing risk event (death).
When compared with siblings and adjusted for age, sex, and race/ethnicity, survivors were more likely to develop late-onset kidney failure (adjusted rate ratio [RR]=4.9, 95%CI=2.6–9.2; Table 2). Among survivors, the rate ratios of late-onset kidney failure (central nervous system tumor as referent) were greatest among survivors of Wilms tumor (RR=4.7, 95%CI=2.6–8.4), osteosarcoma (RR=2.6, 95%CI=1.3–5.4), Ewing sarcoma (RR=2.5, 95%CI=1.0–5.9), neuroblastoma (RR=2.5, 95%CI=1.2–4.9), and non-Hodgkin lymphoma (RR=2.0, 95%CI=1.0–3.8).
Sex, race/ethnicity, and age at diagnosis were not associated with late-onset kidney failure in multivariable analysis (Table 3). Development of diabetes (RR=2.2, 95%CI=1.2–4.2) prior to the primary outcome was associated with an increased risk for late-onset kidney failure. Hypertension elevated late-onset kidney failure rates differently with nephrectomy (RR of late-onset kidney failure by hypertension without nephrectomy = 5.9, 95%CI=3.3–10.5 vs. 14.4, 95%CI=7.1–29.4 with nephrectomy, p=0.034 for interaction). No interaction between diabetes and either kidney radiotherapy or nephrectomy status was observed.
Table 3.
Risk factors for late onset kidney failure among survivors of childhood cancer
| Rate ratio* (95% confidence interval) |
P | |
|---|---|---|
| Demographic variables | ||
| Male sex (vs. female) | 1.3 (0.9 – 1.9) | 0.097 |
| Race/Ethnicity | ||
| Non-Hispanic white | Ref. | - |
| Non-Hispanic black | 1.8 (0.9 – 3.5) | 0.084 |
| Hispanic/Latino | 0.8 (0.4 – 1.6) | 0.53 |
| Other | 1.2 (0.5 – 2.5) | 0.70 |
| Age at initial cancer diagnosis (y) | ||
| 0–3 | Ref. | - |
| 4–9 | 1.4 (0.9 – 2.0) | 0.13 |
| 5–14 | 0.8 (0.5 – 1.5) | 0.56 |
| ≥15 | 1.7 (0.9 – 3.3) | 0.085 |
| Medical comorbidities # | ||
| Known genitourinary condition (vs. none) | 1.7 (0.7 – 4.1) | 0.27 |
| Diabetes (vs. none) | 2.2 (1.2 – 4.2) | 0.012 |
| Hypertension (vs. none), by nephrectomy status | ||
| Hypertension and no nephrectomy | 5.9 (3.3 – 10.5) | <.001 |
| Hypertension and prior nephrectomy | 14.4 (7.1 – 29.4) | <.001 |
| Treatment exposures | ||
| Chemotherapy | ||
| Anthracycline dose (mg/m2) | ||
| None | Ref. | - |
| 0.1–249 | 1.5 (1.0 – 2.3) | 0.058 |
| ≥250 | 1.6 (1.0 – 2.6) | 0.045 |
| Cisplatin equivalent dose (mg/m2) | ||
| None | Ref. | - |
| 0.1–499 | 1.6 (0.8 – 2.9) | 0.18 |
| ≥500 | 1.5 (0.7 – 3.0) | 0.26 |
| Ifosfamide dose (g/m2) | ||
| None | Ref. | - |
| 0.1–59 | 2.4 (1.3 – 4.6) | 0.008 |
| ≥60 | 3.0 (1.0 – 9.2) | 0.059 |
| Methotrexate dose (IV, mg/m2) | ||
| None | Ref. | - |
| 0.1–3999 | 0.6 (0.3 – 1.4) | 0.28 |
| ≥4000 | 0.6 (0.3 – 1.2) | 0.15 |
| Radiotherapy | ||
| Kidney dose from radiotherapy (Gy) | ||
| None | Ref. | - |
| 0.1–9.9 | 0.8 (0.5 – 1.3) | 0.38 |
| 10–14.9 | 1.6 (0.8 – 3.3) | 0.16 |
| ≥15 | 4.0 (2.1 – 7.4) | <0.001 |
| Surgery | ||
| Unilateral nephrectomy (vs. none) | 1.9 (1.0 – 3.4) | 0.040 |
| Subsequent malignant neoplasm (SMN) | ||
| None | Ref. | - |
| Non-renal SMN | 1.3 (0.5 – 3.3) | 0.58 |
| Renal SMN | 15.1 (4.2 – 55.0) | <0.001 |
additionally, adjusted for attained age
reported to have occurred prior to the primary endpoint
Among chemotherapy agents, exposure to high anthracycline dose (≥250mg/m2, RR=1.6, 95%CI-1.0–2.6) was independently associated with development of late-onset kidney failure. A sensitivity analysis excluding survivors who underwent nephrectomy or kidney radiotherapy showed a persistent, independent association between high anthracycline dose and late-onset kidney failure (≥250mg/m2, RR=4.8, 95%CI-1.9–12.2, not shown). The anthracycline and late-onset kidney failure association was not altered by the inclusion of indicators of coronary artery disease or congestive heart failure prior to the primary outcome. Exposure to any ifosfamide was associated with the rate of late-onset kidney failure (RR=2.6, 95%CI=1.2–5.7, Supplemental Table 2). When examined by dose range, there appeared to be a dose-related relationship associated with Ifosfamide, though this did not achieve statistical significance for the high-dose category (0.1–59 g/m2, RR=2.4, 95%CI-1.3–4.6; ≥60g/m2, RR=3.0, 95%CI-1.0–9.2). Additionally, survivors who received platinum agent chemotherapy had a 50% higher rate of late-onset kidney failure, however, this association did not achieve statistical significance when examined by dose-range or as a binary variable. Exposure to the combination of any ifosfamide plus any platinum agent chemotherapy was associated with a significantly elevated rate of late renal failure among survivors (RR=3.8, 95%CI=1.8–8.0, Supplemental Table 2).
Kidney dose from radiotherapy ≥15Gy (RR=4.0, 95%CI=2.1–7.4) and unilateral nephrectomy (RR=1.9, 95%CI=1.0–3.4) were independently associated with late-onset kidney failure. Development of a renal SMN was significantly associated with progression to late-onset kidney failure (RR=15.1, 95%CI=4.2–55.0); there was no association with non-renal SMNs.
DISCUSSION
In this study, we report that nearly two percent of childhood cancer survivors will develop late-onset kidney failure by 35 years follow-up, as well as several novel findings that will impact the care of childhood cancer survivors moving forward. First, the association between diabetes or hypertension and the subsequent development of kidney failure identifies important modifiable targets to mitigate risk in survivors, particularly among those survivors with hypertension and a history of nephrectomy during treatment. Second, the association between anthracycline chemotherapy and late-onset kidney failure may represent an additional target for future treatment regimen modifications pending further validation. Third, this study corroborates previous reports implicating kidney dose from radiotherapy, unilateral nephrectomy, and ifosfamide as potential risk factors for late-onset kidney failure. To our knowledge, this study represents the largest cohort of childhood cancer survivors with the longest reported follow-up assessing risk for late-onset kidney failure in this vulnerable population.
Diabetes and hypertension are reported as the two most common medical diagnoses predisposing to kidney failure and both become more prevalent among survivors of childhood cancer survivors with age.3,8 Both conditions are treatable, making them important modifiable risk factors for kidney failure among survivors. The findings in the present study suggest that carrying a diagnosis of either diabetes or hypertension places survivors at elevated risk for late-onset kidney failure. Importantly, survivors who underwent nephrectomy during cancer treatment and subsequently developed hypertension had over two-fold higher rates of late-onset than those without prior nephrectomy (RR of late-onset kidney failure by developing hypertension is 14.4 vs. 5.9), suggesting synergistic, rather than simply additive effects. It is of the utmost importance that long-term survivors be screened for diabetes and hypertension based upon their risk profile as childhood cancer survivors and in accordance with society guidelines.
Anthracycline compounds are associated with long-term cardiotoxicity in up to 7% of childhood cancer survivors 30 years after treatment.21–23 The present study identifies a novel association between anthracycline treatment and late-onset kidney failure over the very-long term. Few small reports and animal studies have suggested a potential direct nephrotoxic effect from anthracyclines.24 We attempted a thorough investigation of the potential indirect mechanisms for late kidney failure after exposure to anthracyclines through sensitivity analyses, including assessment of indirect consequences from cardiotoxicity or the potential confounding effects of having undergone nephrectomy or kidney radiotherapy and still observed a persistent association between anthracycline chemotherapy and late-onset kidney failure. This may be explained by the association between anthracycline exposure and long-term risk for hypertension among survivors of childhood cancer.25 However, it remains possible that anthracycline exposure may be a surrogate for a different, unmeasured exposure or confounder, and further study is warranted to clarify the details of this association and to replicate the finding in other survivor populations.
Ifosfamide and platinum agents are considered nephrotoxic chemotherapeutics with risk for chronic glomerular and tubular toxicity.26–28 Ifosfamide causes dose-dependent acute and chronic glomerular and tubular damage in up to half of children after completion of treatment, though the effect on long-term kidney failure is less clear.29–31 Similarly, platinum agents may also cause dose-dependent acute and chronic glomerular and tubular damage affecting over 60% of children and as many as 90% of adults five years after treatment.9,32 The findings in the present study support prior reports regarding the association between ifosfamide dose and chronic kidney disease, while also further elaborating on the risk of more advanced kidney disease through the observation of increased risk for late-onset kidney failure in survivors who were treated with ifosfamide. Furthermore, an additive effect on rates of late kidney failure was observed among survivors who were exposed to both ifosfamide and platinum agent chemotherapy regimens, consistent with previous reports on chronic ifosfamide nephrotoxicity.33 The present study is limited in its ability to study all combinations of chemotherapy and other treatment exposures, but it will be important to evaluate the impact of multi-agent and multimodal treatment regimens, and not only the impact of individual treatments on risk for late kidney failure in future studies. Efforts should be taken to limit ifosfamide dose whenever feasible and those requiring cumulative doses greater that 60g/m2 should be carefully monitored and treated for other conditions that can contribute to compromised renal function.5
The present study also identified a 50% increase in risk of late-onset kidney failure after platinum agent chemotherapy, but the association did not achieve statistical significance. While it is likely that platinum agents contribute to chronic kidney dysfunction, the number of survivors who reached our primary outcome may have been insufficient to show the dose-response or statistical significance that would be expected based upon prior reports in childhood cancer survivors where changes in glomerular filtration rate was the primary outcome.27
Radiotherapy-induced kidney injury from abdominal radiotherapy is a known risk factor for long-term impairment of glomerular function in childhood cancer survivors and the Children’s Oncology Group Long Term Follow-Up Guidelines suggest that survivors who received ≥10Gy of abdominal radiotherapy be monitored for kidney toxicity.5,34 However, we report a novel association between kidney dose from radiotherapy and late-onset kidney failure. This is important because the prescribed dose to the abdomen is a less specific dose metric that does not account for radiotherapy field blocking, whereas the kidney dose would account for this. This finding should inform future guidelines to include kidney dose as a distinct risk factor from abdominal dose with a certain cut-off at kidney dose ≥15Gy, and ≥10Gy warranting initial evaluation for long-term kidney toxicity. Furthermore, these data may support utilization of a <10Gy kidney dose as a normal tissue constraint/optimization parameter for radiotherapy treatment planning whenever possible.
Unilateral nephrectomy is also acknowledged as a risk factor for long-term kidney dysfunction.26,27,35 Among pediatric renal tumor survivors, 8% have been reported to develop chronic kidney disease.35 Long-term follow-up from the NWTSG report the 20-year cumulative incidence of kidney failure between 0.7% and 1.3% for those with unilateral disease and up to 15% for bilateral disease.10,11 Herein, we observed an estimated 35-year cumulative incidence of late-onset kidney failure among survivors who underwent therapy-related nephrectomy for their primary cancer of 4.1%, a figure that builds upon prior reports by including a larger and more diverse population of survivors and suggests that risk for late-onset kidney failure continues to increase among survivors as follow-up is extended. Importantly, this observation is heavily influenced by the subsequent development of hypertension.
There are important limitations to consider in this study. First, given that outcomes are self-reported, the possibility for misclassification exists and more precise clinical data needed to identify survivors developing less severe forms of late kidney disease are unavailable. Second, the CCSS does not collect data on all variables that may contribute to late-onset kidney failure, therefore we are unable to assess the impact of other potential risk factors such as supportive treatments (e.g. nephrotoxic antibiotic regimens, diuresis, tumor lysis syndrome), hemodynamic perturbations from critical illness, or other nephrotoxic therapies. Third, although over 1,500 non-Hispanic Black survivors were evaluated, the number is limited relative to non-Hispanic White survivors and may have precluded our ability to detect an association between race and late-onset kidney failure. Finally, competing risk events such as late mortality, known to occur at accelerated rates among childhood cancer survivors, can be statistically accounted for as we do herein, but may still cause the estimation of incidence of late-onset kidney failure that would otherwise develop in this population to be underestimated.
In conclusion, among a large cohort of childhood cancer survivors, the estimated incidence of late-onset kidney failure at up to 35 years following cancer diagnosis is 1.7%, a nearly five-fold increased risk relative to sibling controls. Notably, survivors who developed diabetes or hypertension were at greater risk for late-onset kidney failure, particularly those who developed hypertension in the context of a prior nephrectomy. In addition, the present study identifies a novel association between anthracycline chemotherapy and late-onset kidney failure that has not been previously shown in survivors of childhood cancer, a finding that warrants further investigation. Clinicians caring for childhood cancer survivors should be aware of these risk factors and perform active surveillance for kidney dysfunction according to long-term follow-up guidelines with annual blood pressure monitoring and baseline blood urea nitrogen, creatinine and electrolyte laboratory tests at entry to long-term follow-up and as clinically indicated for at-risk survivors. The findings in this study may suggest that survivors exposed to high-dose anthracycline chemotherapy be considered for close surveillance and additional screening tests as well.5,6
Supplementary Material
Supplementary Table 2. Alternative model of risk factors for late onset kidney failure among survivors of childhood cancer, including combination ifosfamide and platinum agent chemotherapy
Supplementary Table 1. 35-year cumulative incidence of late kidney failure among survivors of childhood cancer, by treatment exposures
HIGHLIGHTS.
1.7% of childhood cancer survivors develop late-onset kidney failure after 35 years
≥15Gy kidney dose of radiotherapy ifosfamide anthracycline or nephrectomy risk factors
Diabetes and hypertension are potentially modifiable factors to mitigate risk
Acknowledgments
Funding information
This work was supported by the US National Cancer Institute [Grant number CA55727], US Cancer Center Support (CORE) [Grant number CA21765] to St. Jude Children’s Research Hospital, and the American Lebanese Syrian Associated Charities.
Footnotes
Competing Interests
Authors have no competing financial or non-financial interests in relation to the present work.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Howlader N, Noone AM, Krapcho M, et al. SEER Cancer Statistics Review, 1975–2017, National Cancer Institute. Bethesda, MD, https://seer.cancer.gov/csr/1975_2017/, based on November 2019 SEER data submission, posted to the SEER web site, April2020. [Google Scholar]
- 2.Oeffinger KC, Mertens AC, Sklar CA, et al. Chronic health conditions in adult survivors of childhood cancer. N Engl J Med. 2006; 355:1572–1582. [DOI] [PubMed] [Google Scholar]
- 3.Bhakta N, Liu Q, Ness KK, et al. The cumulative burden of surviving childhood cancer: an initial report from the St Jude Lifetime Cohort Study (SJLIFE). Lancet. 2017; 290:2569–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kooijmans ECM, Bökenkamp A, Tjahjadi NS, et al. Early and late adverse renal effects after potentially nephrotoxic treatment for childhood cancer. Cochrane Database Syst Rev. 2019; 3(3):CD008944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Children’s Oncology Group (COG) (2018) Long-term follow-up guidelines for survivors of childhood, adolescent, and young adult cancers. http://www.survivorshipguidelines.org/ Accessed April 2020.
- 6.Skinner R, Wallace WHB, Levitt GA (2005) United Kingdom Children’s Cancer Study Group Late Effects Group. Therapy based long term follow up practice statement. United Kingdom Children’s Cancer Study Group. http://www.cclg.org.uk/write/MediaUploads/Member%20area/Treatment%20guidelines/LTFU-full.pdf. Accessed April 2020. [Google Scholar]
- 7.Centers for Disease Control and Prevention. Chronic Kidney Disease in the United States, 2019. Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention; 2019. [Google Scholar]
- 8.Saran R, Robinson B, Abbott KC, et al. US Renal Data System 2019 Annual Data Report: epidemiology of kidney disease in the United States. Am J Kidney Dis. 2020; 75(1)(suppl 1):Svi–Svii. [DOI] [PubMed] [Google Scholar]
- 9.Skinner R Late renal toxicity of treatment for childhood malignancy: risk factors, long-term outcomes, and surveillance. Pediatr Nephrol. 2018; 33:215–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Breslow NE, Collins AJ, Ritchey ML, et al. End stage renal disease in patients with Wilms tumor: results from the National Wilms Tumor Study Group and the United States Renal Data System. J Urol. 2005; 174:1972–1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lange J, Peterson SM, Takashima JR, et al. Risk factors for end stage renal disease in non-WT1-syndromic Wilms tumor. J Urol. 2011; 186:378–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Robison LL, Armstrong GT, Boice JD, et al. The Childhood Cancer Survivor Study: a national Cancer Institute-supported resource for outcome and intervention research. J Clin Oncol. 2009; 27:2308–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leisenring WM, Mertens AC, Armstrong GT, et al. Chronic disease in the Childhood Cancer Survivor Study cohort: A review of published findings. J Clin Oncol. 2009; 27(14):2319–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gibson TM, Mostoufi-Moab S, Stratton KL, et al. Temporal patterns in the risk of chronic health conditions in survivors of childhood cancer diagnosed 1970–1999: a report from the Childhood Cancer Survivor Study cohort. Lancet Oncol. 2018; 19(12):1590–1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Travis LB, Holowaty EJ, Bergfeldt K, et al. Risk of leukemia after platinum-based chemotherapy for ovarian cancer. N Engl J Med. 1999; 340:351–357. [DOI] [PubMed] [Google Scholar]
- 16.Madenci AL, Dieffenbach BV, Liu Q, et al. Late-onset anorectal disease and psychosocial impact in survivors of childhood cancer: a report from the Childhood Cancer Survivor Study. Cancer. 2019; 125:3873–3881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gupta A, Shrestha S, Owens C, et al. Development of an age-scalable 3D computational phantom in DICOM standard for late effects studies of childhood cancer survivors. Biomed Phys Eng Express. 6 (2020) 065004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Howell RM, Smith SA, Weathers RE, Kry SF, Stovall M. Adaptations to a generalized radiation dose reconstruction methodology for use in epidemiologic studies: an update from the MD Anderson Late Effect Group. Radiat Res. 2019; 192(2):169–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stovall M, Weathers R, Kasper C, et al. Dose reconstruction for therapeutic and diagnostic radiation exposures: use in epidemiological studies. Radiat Res. 2006; 166:141–57. [DOI] [PubMed] [Google Scholar]
- 20.Turcotte LM, Liu Q, Yasui Y, et al. Temporal trends in treatment and subsequent neoplasm risk among 5-year survivors of childhood cancer, 1970–2015. JAMA. 2017; 317(8):814–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van der Pal HJ, van Dalen EC, van Delden E, et al. High risk of symptomatic cardiac events in childhood cancer survivors. J Clin Oncol. 2012; 30(13):1429–37. [DOI] [PubMed] [Google Scholar]
- 22.Mulrooney DA, Yeazel MW, Kawashima T, et al. Cardiac outcomes in a cohort of adult survivors of childhood and adolescent cancer: retrospective analysis of the Childhood Cancer Survivor Study cohort. BMJ. 2009; 339:b4606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mulrooney DA, Hyun G, Ness NK, et al. Major cardiac events for adult survivors of childhood cancer diagnosed between 1970 and 1999: report from the Childhood Cancer Survivor Study cohort. BMJ. 2020; 368: l6794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jhaveri KD, Shah HH, Calderon K, Campenot ES, Radhakrishnan J. Glomerular diseases seen with cancer and chemotherapy: a narrative review. Kidney Int. 2013; 84(1):34–44. [DOI] [PubMed] [Google Scholar]
- 25.Meacham LR, Chow EJ, Ness KK, et al. Cardiovascular risk factors in adult survivors of pediatric cancer- a report from the Childhood Cancer Survivor Study. Cancer Epidemiol Biomarkers Prev. 2010; 19(1): 170–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Knijnenburg SL, Jaspers MW, van der Pal HJ, et al. Renal dysfunction and elevated blood pressure in long-term childhood cancer survivors. Clin J Am Soc Nephrol. 2012; 7:1416–1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mulder RL, Knijnenburg SL, Geskus RB, et al. Glomerular function time trends in long-term survivors of childhood cancer: a longitudinal study. Cancer Epidemiol Biomarkers Prev. 2013; 22(10); 1736–46. [DOI] [PubMed] [Google Scholar]
- 28.McMahon KR, Harel-Sterling M, Pizzi M, Huynh L, Hessey E, Zappitelli M. Long-term renal follow-up of children treated with cisplatin, carboplatin, or ifosfamide: a pilot study. Pediatr Nephrol. 2018; 33:2311–2320. [DOI] [PubMed] [Google Scholar]
- 29.Skinner R, Cotterill SJ, Stevens MC. Risk factors for nephrotoxicity after ifosfamide treatment in children: a UKCCSG Late Effects Group study. United Kingdom Children’s Cancer Study Group. Br J Cancer. 2000; 82(10):1636–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Prasad VK, Lewis IJ, Aparicio SR, et al. Progressive glomerular toxicity of ifosfamide in children. Med Pediatr Oncol. 1996; 27((3):149–55. [DOI] [PubMed] [Google Scholar]
- 31.Skinner R, Pearson AD, English MW, et al. Risk factors for ifosfamide nephrotoxicity in children. Lancet. 1996; 348(9027):578–80. [DOI] [PubMed] [Google Scholar]
- 32.Latcha S, Jaimes EA, Patil S, Glezerman IG, Mehta S, Flombaum CD. Long-term renal outcomes after cisplatin treatment. Clin J Am Soc Nephrol. 2016; 11(7):1173–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jones DP, Spunt SL, Green D, Springate JE. Renal late effects in patients treated for cancer in childhood: a report from the Children’s Oncology Group. Pediatr Blood Cancer. 2008; 51:724–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dekkers IA, Blijdorp K, Cransberg K, et al. Long-term nephrotoxicity in adult survivors of childhood cancer. Clin J Am Soc Nephrol. 2013; 8:922–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schiavetti A, Altavista P, De Luca L, et al. Long-term renal function in unilateral non-syndromic renal tumor survivors treated according to International Society of Pediatric Oncology protocols. Pediatr Blood Cancer. 2015; 62:1637–1644. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 2. Alternative model of risk factors for late onset kidney failure among survivors of childhood cancer, including combination ifosfamide and platinum agent chemotherapy
Supplementary Table 1. 35-year cumulative incidence of late kidney failure among survivors of childhood cancer, by treatment exposures