Abstract
Paratuberculosis (Johne’s disease) is a fatal disease of ruminants for which no effective treatment is available. Presently, no drugs against Mycobacterium avium subsp. paratuberculosis (M. paratuberculosis), the causative agent of Johne’s disease, are approved for use in livestock. Additionally, M. paratuberculosis has been linked to a human chronic granulomatous ileitis (Crohn’s disease). To assist in the evaluation of antimicrobial agents with potential activity against M. paratuberculosis, we have developed a firefly luciferase-based assay for the determination of drug susceptibilities. The microorganism used was M. paratuberculosis K-10(pYUB180), a clinical isolate carrying a plasmid with the firefly luciferase gene. The MICs determined by the broth macrodilution method were as follows: amikacin, 2 μg/ml; Bay y 3118, 0.015 μg/ml; clarithromycin, 1.25 μg/ml; d-cycloserine, 25 μg/ml; ethambutol, 20 μg/ml; and rifabutin, 0.5 μg/ml. The strain was resistant to isoniazid and kanamycin. The results obtained by the luciferase assay were identical or fell within 1 doubling dilution. These results suggest that a combination of amikacin, clarithromycin, and rifabutin may be the most efficacious therapy for the treatment of M. paratuberculosis infections and that the use of fluoroquinolone class of antibiotics deserves further consideration. We demonstrate that the luciferase drug susceptibility assay is reliable for M. paratuberculosis and gives results within 7 days, whereas the broth macrodilution method requires 14 days.
Paratuberculosis (Johne’s disease) is an incurable, fatal disease of domestic and wild ruminants. Mycobacterium avium subsp. paratuberculosis (M. paratuberculosis) is the etiologic agent of this disease, which is manifested by chronic diarrhea and weight loss. After months of diarrhea and wasting, the affected animals either die or are culled (9, 11). In the United States, the prevalence of M. paratuberculosis infection in dairy and beef cattle herds has reached 34% in certain areas (12, 13) and causes millions of dollars in lost revenue annually (35). Furthermore, M. paratuberculosis has been tentatively linked to Crohn’s disease, a chronic granulomatous ileitis of humans. This disease mimics other mycobacterial infections in both animals and humans (31). Evidence supporting the possibility that M. paratuberculosis is the etiologic agent of Crohn’s disease include culture of this organism from intestinal tissue (11) and amplification of the subspecies-specific IS900 sequence of M. paratuberculosis from biopsy specimens by PCR (11, 21).
Currently, treatment of paratuberculosis in cattle is limited to the extralabel use of therapeutic agents (29, 30), and no antibiotic treatment is recommended for clinical cases of Crohn’s disease. Even with a prolonged drug regimen, paratuberculosis in cattle is invariably fatal. A significant problem hindering studies of the antimicrobial susceptibilities of this organism is the long generation time of M. paratuberculosis and the tendency of certain antibiotics to degrade during the evaluation period. Therefore, the objective of this study was to develop a new assay that tests the drug susceptibilities of M. paratuberculosis and that can be used to identify and screen a very large number of compounds in less time. This technology will facilitate the discovery of more powerful and less toxic drugs than those currently available for the treatment of these diseases. It is hoped that the development of this method, which is amenable to high-throughput screens, will allow the identification of compounds which will eventually result in shorter and more effective treatments for Johne’s disease and possibly Crohn’s disease.
Recently, methods for the assessment of the antimicrobial susceptibilities of mycobacteria have used the luciferase gene from Photinus pyralis, the American firefly; these methods have been described elsewhere (1, 14, 15, 19, 20). This gene has been introduced by transformation and phage infection into slowly growing mycobacterial species, including M. paratuberculosis (14, 17, 20). The ability of an antimicrobial agent to inhibit the growth of these strains can then be measured by determining the decrease in bioluminescence. Here, we report on the development of a firefly luciferase-based method for determination of the drug susceptibilities of M. paratuberculosis. For the development of this assay, we tested prototype drugs from various classes of antimicrobial agents. Since there is no standardized method for determination of the drug susceptibilities of mycobacteria except Mycobacterium tuberculosis, we compared the luciferase-based assay with a previously reported broth macrodilution assay used to determine the drug susceptibilities of M. paratuberculosis (7).
MATERIALS AND METHODS
Bacterial strains and growth conditions.
M. paratuberculosis K-10(pYUB180) was grown in Middlebrook 7H9 broth with 0.05% Tween 80 and 0.5 μg of mycobactin J (Allied Monitor, Fayette, Mo.) per ml at 37°C as described previously (17). The construction of the Escherichia coli-Mycobacterium spp. shuttle plasmid pYUB180 has been described previously (20). This plasmid contains both the firefly luciferase gene downstream from the Mycobacterium bovis BCG hsp60 promoter (Phsp60) and a kanamycin resistance gene as a selectable marker. All starter cultures used to inoculate test cultures were grown in the presence of 50 μg of kanamycin per ml to an optical density at 600 nm of 0.3 to 0.4. To create an inoculum free of cellular clumping, 50 ml of cell culture was sonicated for 30 s with a Vibra-Cell model VC600 disrupter (Sonics and Materials, Inc., Danbury, Conn.), passed through a 27-gauge needle three times, vortexed on high for 30 s, and allowed to sit for a minimum of 5 min. The top 5 ml was then used for the inoculation of cultures for MIC assays. Cell viability, as determined by BacLight Bacterial Viability staining (Molecular Probes, Eugene, Oreg.), demonstrated that 85% of the bacteria were viable after being subjected to this procedure. A total of 50 μl of this prepared culture (ca. 7.5 × 106 CFU) was then inoculated into each flask containing an antibiotic, and the cells were grown at 37°C.
Antimicrobial agents.
Amikacin, d-cycloserine, ethambutol, kanamycin, and isoniazid (all from Sigma Chemical Co., St. Louis, Mo.) and Bay y 3118 (generously donated by L. E. Bermudez and L. S. Young, Kuzell Institute, San Francisco, Calif.) were prepared in sterile deionized water. Rifabutin (Pharmacia Inc., Columbus, Ohio) and clarithromycin (Abbott Laboratories, Abbott Park, Ill.) were prepared in dimethyl sulfoxide (Fisher Scientific, St. Louis, Mo.). All further dilutions of each antibiotic were prepared in growth medium. The ranges of concentrations tested were as follows: amikacin, 0.5 to 64 μg/ml; Bay y 3118, 0.008 to 100 μg/ml; clarithromycin, 0.625 to 80 μg/ml; d-cycloserine, 0.8 to 100 μg/ml; ethambutol, 1.25 to 160 μg/ml; isoniazid, 1.95 to 250 μg/ml; kanamycin, 1 to 128 μg/ml; and rifabutin, 0.03 to 4 μg/ml.
Broth macrodilution assays.
Susceptibility testing was performed in triplicate for each antimicrobial agent by following the guidelines of the National Committee for Clinical Laboratory Standards (22) with a total of eight twofold drug dilutions in 10 ml of complete Middlebrook 7H9 broth with 0.05% Tween 80 and 0.5 μg of mycobactin J per ml. Each culture was grown in sterile 25-cm2 tissue culture flasks, and for each antibiotic three flasks without antimicrobial agent were included as controls. The MIC was defined as the minimum concentration of the antimicrobial agent which inhibited growth at day 14.
Luminescence assays.
Assays for bioluminescence were performed by transferring 100 μl of each growing culture into a separate 75-mm polystyrene test tube (Sarstedt, Newton, N.C.) containing 250 μl of complete Middlebrook 7H9 broth without Tween 80. A total of 100 μl of 1 mM luciferin (Sigma) with 0.45 M sodium citrate (pH 5.0) was automatically injected into each tube, and the bioluminescence was measured for 20 s without preset delay with an AutoLumat LB953 luminometer (Wallac Instruments, Gaithersburg, Md.). Bioluminescence was expressed as the number of relative light units (RLUs) detected in the measurement period. RLUs were obtained by dividing the number of photoelectrons detected by 10, as specified by the manufacturer. The initial (day 0) RLUs were measured within 30 min of inoculation. Other RLU outputs were measured after 3, 7, and 14 days of incubation.
Definitions, calculations, and interpretation.
The raw data that were obtained were normalized by determining the initial and final ratios of test and control RLUs for each antibiotic as described previously (27). Briefly, the initial light output ratio was calculated as the ratio of test RLUs to control RLUs on day 0. The final ratio was calculated as the ratio of test RLUs to control RLUs and was determined individually for days 3, 7, and 14. Results for each time point were then expressed as the percent relative change in bioluminescence, which was calculated as follows: (final ratio/initial ratio) × 100. The MIC was defined as the lowest drug concentration that gave a relative change in bioluminescence of ≤1.0% + 0.5%. At these values, the bioluminescence of the sample is extinguished to less than 1% of the original light output at day 0.
RESULTS
Testing of susceptibility to selected antimycobacterial agents by luciferase assays.
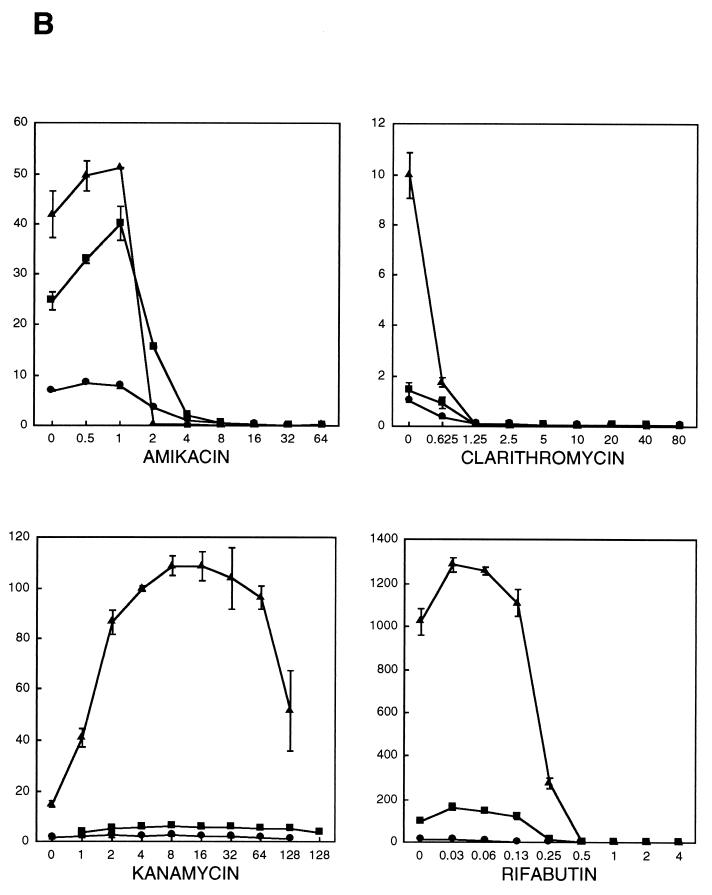
The changes in the bioluminescence of strain M. paratuberculosis K-10(pYUB180) in the presence of each antimicrobial agent are shown in Fig. 1. Each graph depicts the mean RLUs of three independent experiments for each concentration of antibiotic after 3, 7, and 14 days of incubation. Increases in RLUs could be observed by day 3 postinoculation in the presence of subinhibitory drug concentrations, thus reflecting an increase in the numbers of viable bacteria. This trend continued through day 14 postinoculation, with total RLUs increasing by 100- to 1,000-fold. For those antibiotics with a discernible MIC, RLUs declined to background levels and remained at this level for all higher drug concentrations.
FIG. 1.
Bioluminescence of M. paratuberculosis K-10(pYUB180) after incubation for 3 (circles), 7 (squares), and 14 (triangles) days in broth cultures containing different antimicrobial agents (A and B). Bioluminescence (RLU × 0.001; y axis) is plotted against the drug concentration (in micrograms per milliliter; x axis). The value for each time point is the mean of three independent experiments. Bars representing standard deviations are indicated. The mean bioluminescence of the cultures immediately after inoculation (day 0) was 340 ± 170.
The percent relative changes in bioluminescence for the critical dilutions of each type of antibiotic are presented in Table 1. Values of ≤1% + 0.5% (100-fold extinction of bioluminescence compared with the bioluminescence for controls containing no drug) were observed by day 7 for amikacin, Bay y 3118, clarithromycin, d-cycloserine, ethambutol, and rifabutin, with corresponding MICs of 16, 0.015, 1.25, 25, 20, and 0.5 μg/ml, respectively. For day 14, identical MICs could be determined for clarithromycin, d-cycloserine, and rifabutin. The MIC of amikacin was significantly lower (2 μg/ml), while the MICs of Bay y 3118 and ethambutol were 0.008 and 10 μg/ml, respectively, and were within 1 doubling dilution of the MIC determined by day 7. Since for kanamycin and isoniazid the percent relative change in bioluminescence remained well above 1% for all dilutions through day 14, no MIC could be determined for either of these antibiotics.
TABLE 1.
Percent relative changes in bioluminescence for critical concentrations of the antimicrobial agents tested
| Antibiotic type and antibiotic | Concn (μg/ml) | Relative change in biolumines-cence (%) on the following daya:
|
||
|---|---|---|---|---|
| 3 | 7 | 14 | ||
| Cell wall biosynthesis inhibitors | ||||
| d-Cycloserine | 12.5 | 32.1 ± 3.5 | 28.9 ± 2.8 | 9.2 ± 1.5 |
| 25bc | 2.3 ± 0.5 | 0.4 ± 0.1 | <0.2 | |
| 50 | <0.2 | <0.2 | <0.2 | |
| Ethambutol | 5 | 55 ± 10 | 24 ± 22 | 25.1 ± 5.8 |
| 10c | 18.4 ± 3.9 | 2.2 ± 0.5 | <0.2 | |
| 20b | 7.9 ± 1.9 | 1.1 ± 0.4 | <0.2 | |
| 40 | 5.8 ± 1.4 | 1.4 ± 0.4 | <0.2 | |
| Isoniazid | 62.5 | 63 ± 17 | 163 ± 49 | 97 ± 27 |
| 125 | 38 ± 10 | 93 ± 25 | 86 ± 22 | |
| 250 | 11.3 ± 2.9 | 11.5 ± 3.1 | 53 ± 13 | |
| DNA supercoiling inhib-itors, Bay y 3118 | 0.0 | 100 ± 19 | 100 ± 20 | 100 ± 19 |
| 0.008c | 39 ± 42 | 4.5 ± 5.7 | 0.7 ± 0.8 | |
| 0.015b | 24.2 ± 4.6 | 1.4 ± 0.6 | 0.5 ± 0.4 | |
| 0.03 | 13.5 ± 2.8 | 0.7 ± 0.6 | <0.2 | |
| Protein biosynthesis inhibitors | ||||
| Aminoglycosides | ||||
| Amikacin | 1 | 86.3 ± 5.3 | 126 ± 14 | 95 ± 11 |
| 2c | 52.0 ± 8.0 | 65 ± 11 | 0.4 ± 0.3 | |
| 4 | 15.4 ± 1.4 | 8.1 ± 0.9 | 0.6 ± 0.9 | |
| 8 | 7.2 ± 1.3 | 1.9 ± 0.3 | 0.2 ± 0.2 | |
| 16b | 3.0 ± 1.3 | 0.5 ± 0.1 | 0.2 ± 0.1 | |
| Kanamycin | 32 | 91 ± 32 | 91 ± 31 | 429 ± 150 |
| 64 | 83 ± 29 | 99 ± 34 | 453 ± 151 | |
| 128 | 56 ± 19 | 68 ± 25 | 220 ± 100 | |
| Macrolide, clarithro-mycin | 0.625 | 34.7 ± 7.2 | 68 ± 23 | 18.3 ± 3.2 |
| 1.25bc | 5.2 ± 3.8 | 1.1 ± 2.0 | 0.2 ± 0.3 | |
| 2.5 | 6.1 ± 1.8 | 0.4 ± 0.3 | <0.2 | |
| Transcription inhibitors | ||||
| Rifabutin | 0.25 | 8.5 ± 2.7 | 11.7 ± 2.6 | 30.7 ± 6.7 |
| 0.5bc | 6.0 ± 1.6 | 0.5 ± 0.3 | 0.2 ± 0.2 | |
| 1 | 5.1 ± 1.6 | 0.2 ± 0.1 | <0.2 | |
Boldface type indicates a relative change in luminescence of ≤1.0% + 0.5%.
MIC for day 7.
MIC for day 14.
Comparison of the luciferase and broth macrodilution assays.
The antimicrobial activities determined by the bioluminescence assay at day 7 or 14 were closely correlated with those determined by the broth macrodilution assay (determined at day 14; Table 2). The MICs obtained by the luciferase assay at day 7 and by the broth microdilution assay were identical for Bay y 3118, clarithromycin, d-cycloserine, ethambutol, and rifabutin but differed by 2 doubling dilutions for amikacin. At day 14, the results of the luciferase assay were identical to those of the broth macrodilution assay for amikacin, clarithromycin, d-cycloserine, and rifabutin, but the results differed by 1 doubling dilution for Bay y 3118 and ethambutol. Strain K-10(pYUB180) was resistant to isoniazid and kanamycin, and no MIC within the concentration range tested could be determined by any of the methods.
TABLE 2.
Comparison of broth macrodilution and bioluminescence MICsa
| Drug | Drug concn range (μg/ml) | Broth macro-dilution MIC (μg/ml) | Luciferase assay MIC (μg/ml)
|
|
|---|---|---|---|---|
| Day 7 | Day 14 | |||
| d-Cycloserine | 0.8–100 | 25 | 25 | 25 |
| Ethambutol | 1.25–160 | 20 | 20 | 10 |
| Isoniazid | 1.95–250 | ≥250 | ≥250 | ≥250 |
| Bay y 3118 | 0.008–1.0 | 0.015 | 0.015 | 0.008 |
| Amikacin | 0.5–64 | 2 | 16 | 2 |
| Kanamycin | 1–128 | ≥128 | ≥128 | ≥128 |
| Clarithromycin | 0.625–80 | 1.25 | 1.25 | 1.25 |
| Rifabutin | 0.03–4 | 0.5 | 0.5 | 0.5 |
MICs were determined as described in Materials and Methods.
To determine if an increase in the antibiotic concentrations would inhibit bioluminescence without affecting the total numbers of viable bacteria, the CFUs over an entire 8-dilution range were measured for a representative drug (amikacin). At subinhibitory concentrations of antibiotic, no changes from control values of either RLUs or CFUs were found (data not shown). In the presence of the drug at the MIC, simultaneous 100-fold decreases in both RLUs and CFUs were observed, with this correlation being maintained for the higher drug concentrations. Therefore, decreasing RLUs reflected a corresponding reduction in the number of viable bacteria. The activity of a representative antibiotic against the wild-type strain M. paratuberculosis K-10 was tested (amikacin) by the broth macrodilution method to confirm that antimicrobial sensitivity was not influenced by plasmid pYUB180. The MICs for K-10 and K-10(pYUB180) were identical (2 μg/ml) (data not shown). The activity of kanamycin against strain K-10 was also tested by the broth macrodilution method to confirm that the kanamycin-resistant phenotype was conferred by pYUB180. The resulting MIC of 1.25 μg/ml (data not shown) showed that the wild-type strain of M. paratuberculosis K-10 was sensitive to kanamycin.
DISCUSSION
Presently, no drugs are approved for the treatment of Johne’s disease in livestock. Therefore, antibiotic therapy is limited to the extralabel use of standard antimicrobial agents. Various treatment regimens for the control of Johne’s disease have been proposed, and these have had various levels of success, but ultimately the disease is fatal to the animal. It is hoped that the development of the assay described here will allow the discovery of more effective and less toxic drugs. The most commonly prescribed treatment regimen consists of isoniazid in combination with rifabutin and/or ethambutol, followed by a daily dose of isoniazid for the duration of the animal’s life (18, 30). Although isoniazid is used to treat infections caused by M. tuberculosis and M. bovis (23), the results presented here indicate that isoniazid therapy may not be the most effective treatment for M. paratuberculosis infections. Further in vitro screening of antimicrobial agents is warranted.
Our data indicate that the luciferase-based MIC assay may be applied for this purpose. It has the advantage that it requires less time to obtain MIC data. Furthermore, the other method commonly used to determine M. paratuberculosis susceptibility is the BACTEC assay (33). However, this assay requires a dedicated and specialized piece of equipment. In contrast, the firefly luciferase assay can be performed with a less expensive instrument such as a regular liquid scintillation counter or a one-well luminometer.
An important consideration in mycobacterial drug susceptibility assays is the use of Tween 80. Van Boxtel et al. (33) observed that at concentrations of 0.1 to 1%, Tween 80 affected colony morphology and caused cells to be more susceptible to the tested drugs. A similar effect was observed with strains of the M. avium complex (36). However, in a study with strains of M. tuberculosis, M. bovis BCG, M. avium, and Mycobacterium intracellulare, it was shown that Tween 80 had a significant effect on the MIC of rifampin but did not alter the activities of the other antimicrobial agents tested (2). In general, the absence of Tween 80 leads to considerable cell clumping, introducing additional sources of variation for MIC determinations. In our experiments, we used Tween 80 at a concentration of 0.05% so as to minimize both clumping of cells and detergent-induced changes in drug susceptibility.
To demonstrate the applicability of the firefly luciferase assay in the determination of M. paratuberculosis drug susceptibility, we selected eight prototype drugs inhibiting different targets involved in basic bacterial processes. These processes included inhibitors of cell wall biosynthesis (d-cycloserine, isoniazid, and ethambutol), protein synthesis (clarithromycin, amikacin, and kanamycin), DNA supercoiling (Bay y 3118), and RNA synthesis (rifabutin, a structural analog of rifampin with higher levels of antimycobacterial activity). Amikacin, isoniazid, and rifampin have been suggested as therapeutic agents for Johne’s disease (34). d-Cycloserine, ethambutol, clarithromycin, and kanamycin are used as primary (ethambutol) or second-line antimycobacterial agents in human medicine to treat both M. tuberculosis and M. avium infections. Although Bay y 3118 has no therapeutic use for the treatment of mycobacterial diseases in animals or humans due to its phototoxicity (26), it was used as an example of the new types of fluoroquinolones that are being developed and that have extended activity against gram-positive organisms and high levels of inhibitory activity against mycobacteria (3, 4). Overall, M. paratuberculosis was sensitive to the majority of the antibiotics tested. The drug susceptibilities determined by the bioluminescence method were similar to those determined by the broth macrodilution method (Table 2). For most drugs, the MIC obtained on day 7 by the bioluminescence method was identical to the value obtained by the broth macrodilution method. Therefore, the bioluminescence assay provides a more rapid determination of a drug’s effectiveness against M. paratuberculosis.
Individually, the activities of the antibiotics tested here correlated well with those described in previous reports on the susceptibilities of mycobacteria to antimicrobial agents. Of the studies reporting on the susceptibility of mycobacteria to d-cycloserine, M. tuberculosis was susceptible to the drug at 25 to 75 μg/ml (25), whereas Mycobacterium sp. isolated from patients with Crohn’s disease were resistant to d-cycloserine at 5, 10, and 20 μg/ml (7). Our findings of a MIC of 25 μg/ml for M. paratuberculosis are in contrast to those of Thorel and coworkers (32), who reported that M. paratuberculosis strains are resistant to d-cycloserine at 30 to 50 μg/ml in Middlebrook 7H11 agar. The results from those researchers may not be comparable to our results due to methodological differences in drug susceptibility assays. Wallace et al. (34) demonstrated that for the M. avium complex, bacterial strains which are resistant to a single fixed drug concentration in agar dilutions are inhibited by lower concentrations of the same drug in broth culture.
For ethambutol, our reported MICs of 10 μg/ml correlate well with previous findings of MICs of 5 μg/ml for M. avium and M. paratuberculosis (10, 15). Ethambutol is often prescribed in combination with isoniazid. Both M. tuberculosis and M. bovis are extremely sensitive to isoniazid, but non-M. tuberculosis mycobacteria are significantly more resistant to this drug. M. paratuberculosis K-10(pYUB180) was resistant to isoniazid at ≥250 μg/ml. In previous reports, animal isolates of M. paratuberculosis (8) and human isolates of Mycobacterium sp. (10) were resistant to isoniazid at the single concentration of 10 μg/ml. Similarly, Wallace et al. (34) demonstrated that isolates of Mycobacterium marinum, M. avium complex, Mycobacterium smegmatis, and Mycobacterium chelonae were resistant to isoniazid at 16 to >32 μg/ml, the highest concentrations tested. Further studies are needed to elucidate if the high level of resistance observed in our study is due to a strain variation or if it is typical for most strains of M. paratuberculosis.
As expected, strain K-10(pYUB180) was resistant to kanamycin due to the production of the 3′-aminoglycoside phosphotransferase encoded by the Tn903-derived aph gene on plasmid pYUB180. Amikacin is another deoxystraptamine aminoglycoside which is structurally similar to kanamycin, but it is a poor substrate for the 3′-aminoglycoside phosphotransferase enzyme. Our MIC of 2 μg/ml and previous in vitro antimicrobial susceptibility results of 0.5 to 5 μg/ml for M. paratuberculosis (7) and Mycobacterium sp. (10) indicate that it is highly effective against this mycobacterial species. Our results are in contrast to those of Cooksey et al. (15), who reported that the MIC of amikacin is >100 μg/ml for a recombinant strain of M. avium carrying the same aph gene.
For Bay y 3118, our data indicated that M. paratuberculosis was very sensitive, with an MIC of 0.015 μg/ml. This agrees with other reports demonstrating MICs of 0.06 to 4 μg/ml for M. tuberculosis (28), M. avium complex (4, 28), and non-M. tuberculosis mycobacterial species (3). Clarithromycin is indicated for the treatment of human infections involving the M. avium complex, usually in combination with amikacin, rifabutin, and ethambutol. The MIC of 1.25 μg/ml that we found is slightly higher than the previously reported MICs of 0.25 μg/ml for M. paratuberculosis (24) and 0.5 μg/ml for M. avium (15). The rifamycin family of antibiotics has largely been reserved for use in human medicine, so that its value as a treatment in veterinary medicine may be underestimated. We report the MIC to be 0.5 μg/ml, consistent with values of 0.06 to 0.5 μg/ml for M. avium (14, 15) and other mycobacterial species (10).
Multiple-drug therapy is usually indicated for the treatment of mycobacterial infections. In vivo results of combination therapy against experimentally induced M. paratuberculosis infections in animals are unavailable, but Fattorini et al. (16) recently reported that monotherapy with isoniazid was ineffective in clearing M. avium infections in beige mice and that the triple-drug combination of isoniazid-amikacin-clarithromycin was the most effective. Of the drugs tested in our study, amikacin, Bay y 3118, clarithromycin, and rifabutin had MICs of 2 μg/ml or lower. On the basis of these results, we recommend that these classes of antibiotics be subjected to in vivo tests of their activities against M. paratuberculosis infections.
ACKNOWLEDGMENTS
This research was supported by grant 95-37204-2148 from the U.S. Department of Agriculture NRI Competitive Grant Program, Cooperative State Research Project NEB 14-077, and Institute of Agriculture and Natural Resources (Agricultural Research Division) Interdisciplinary Research Program Project NEB 14-090.
We thank L. E. Bermudez and L. S. Young for providing compound Bay y 3118. We acknowledge L. E. Bermudez, J. D. Cirillo, G. E. Duhamel, and L. S. Young for critical review of the manuscript.
REFERENCES
- 1.Arain T M, Resconi A E, Singh D C, Stover C K. Reporter gene technology to assess activity of antimycobacterial agents in macrophages. Antimicrob Agents Chemother. 1996;40:1542–1544. doi: 10.1128/aac.40.6.1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arain T M, Resconi A E, Hickey M J, Stover C K. Bioluminescence screening in vitro (Bio-Siv) assays for high-volume antimycobacterial drug discovery. Antimicrob Agents Chemother. 1996;40:1536–1541. doi: 10.1128/aac.40.6.1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bauernfeind A. Comparative in-vitro activities of the new quinolone, Bay y 3118, and ciprofloxacin, sparfloxacin, tosufloxacin, CI-960 and CI-990. J Antimicrob Chemother. 1993;31:505–522. doi: 10.1093/jac/31.4.505. [DOI] [PubMed] [Google Scholar]
- 4.Bermudez L E, Inderlied C B, Kolonoski P, Wu M, Barbara-Burnham L, Young L S. Activities of Bay Y 3118, levofloxacin, and ofloxacin alone or in combination with ethambutol against Mycobacterium avium complex in vitro, in human macrophages, and in beige mice. Antimicrob Agents Chemother. 1996;40:546–551. doi: 10.1128/aac.40.3.546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chiodini R J, Van Kruiningen H J, Merkal R S. Ruminant paratuberculosis (Johne’s disease): the current status and future prospects. Cornell Vet. 1984;74:218–262. [PubMed] [Google Scholar]
- 6.Chiodini R J, Van Kruiningen H J, Merkal R S, Thayer W R, Coutu J A. Characteristics of an unclassified Mycobacterium species isolated from patients with Crohn’s disease. J Clin Microbiol. 1984;20:966–971. doi: 10.1128/jcm.20.5.966-971.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chiodini R J, Van Kruiningen H J, Thayer W R, Coutu J A, Merkal R S. In vitro antimicrobial susceptibility of a Mycobacterium sp. isolated from patients with Crohn’s disease. Antimicrob Agents Chemother. 1984;26:930–932. doi: 10.1128/aac.26.6.930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chiodini R J. Biochemical characteristics of various strains of Mycobacterium paratuberculosis. Am J Vet Res. 1986;47:1442–1445. [PubMed] [Google Scholar]
- 9.Chiodini R J, Van Kruiningen H J. The prevalence of paratuberculosis in culled New England cattle. Cornell Vet. 1986;76:91–104. [PubMed] [Google Scholar]
- 10.Chiodini R J. Bactericidal activities of various antimicrobial agents against human and animal isolates of Mycobacterium paratuberculosis. Antimicrob Agents Chemother. 1990;34:366–367. doi: 10.1128/aac.34.2.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cocito C, Gilot P, Coene M, de Kesel M, Poupart P, Vannuffel P. Paratuberculosis. Clin Microbiol Rev. 1994;7:328–345. doi: 10.1128/cmr.7.3.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Collins M T, Sockett D C, Goodger W J. Herd prevalence, geographic distribution and risk factors for bovine paratuberculosis in Wisconsin. J Am Vet Med Assoc. 1992;187:323–329. [PubMed] [Google Scholar]
- 13.Collins M T, Sockett D C, Goodger W J, Conrad T A, Thomas C B, Carr D J. Herd prevalence and geographic distribution of, and risk factors for, bovine paratuberculosis in Wisconsin. J Am Vet Med Assoc. 1994;204:636–641. [PubMed] [Google Scholar]
- 14.Cooksey R C, Crawford J T, Jacobs W R, Jr, Shinnick T M. A rapid method for screening antimicrobial agents for activities against a strain of Mycobacterium tuberculosis expressing firefly luciferase. Antimicrob Agents Chemother. 1993;37:1348–1352. doi: 10.1128/aac.37.6.1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cooksey R C, Morlock G P, Beggs M, Crawford J T. Bioluminescence method to evaluate antimicrobial agents against Mycobacterium avium. Antimicrob Agents Chemother. 1995;39:754–756. doi: 10.1128/AAC.39.3.754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fattorini L, Xiao Y, Mattei M, Li Y, Iona E, Ricci M L, Thorensen O F, Creti R, Orefici G. Activities of isoniazid alone and in combination with other drugs against Mycobacterium avium infection in beige mice. Antimicrob Agents Chemother. 1998;42:712–714. doi: 10.1128/aac.42.3.712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Foley-Thomas E M, Whipple D L, Bermudez L E, Barletta R G. Phage infection, transfection and transformation of Mycobacterium avium complex and Mycobacterium paratuberculosis. Microbiology. 1995;141:1173–1181. doi: 10.1099/13500872-141-5-1173. [DOI] [PubMed] [Google Scholar]
- 18.Gezon H M, Bither H D, Gibbs H C, Acker E J, Hanson L A, Thompson J K, Jorgenson R D. Identification and control of paratuberculosis in a large goat herd. Am J Vet Res. 1988;49:1817–1823. [PubMed] [Google Scholar]
- 19.Hickey M J, Arain T M, Shawar R M, Humble D J, Langhorne M H, Morgenroth J N, Stover C K. Luciferase in vivo expression technology: use of recombinant mycobacterial reporter strains to evaluate antimycobacterial activity in mice. Antimicrob Agents Chemother. 1996;40:400–407. doi: 10.1128/aac.40.2.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jacobs W R, Jr, Barletta R G, Udani R, Chan J, Kalkut G, Sosne G, Kieser T, Sarkis G J, Hatfull G F, Bloom B R. Rapid assessment of drug susceptibilities of Mycobacterium tuberculosis by means of luciferase reporter phages. Science. 1993;260:819–822. doi: 10.1126/science.8484123. [DOI] [PubMed] [Google Scholar]
- 21.Mishina D, Katsel P, Brown S T, Gilberts E C A M, Greenstein R J. On the etiology of Crohn disease. Proc Natl Acad Sci USA. 1996;93:9816–9820. doi: 10.1073/pnas.93.18.9816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.National Committee for Clinical Laboratory Standards. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. Approved standard M7-A3. Wayne, Pa: National Committee for Clinical Laboratory Standards; 1993. [Google Scholar]
- 23.Prescott J F, Baggot J D. Antimicrobial therapy in veterinary medicine. Ames: Iowa State University Press; 1993. pp. 288–289. [Google Scholar]
- 24.Rastogi N, Goh K S, Labrousse V. Activity of clarithromycin compared with those of other drugs against Mycobacterium paratuberculosis and further enhancement of its extracellular and intracellular activities by ethambutol. Antimicrob Agents Chemother. 1992;36:2843–2846. doi: 10.1128/aac.36.12.2843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rastogi N, Labrousse V, Goh J S. In vitro activities of fourteen antimicrobial agents against drug susceptible and resistant clinical isolates of Mycobacterium tuberculosis and comparative intracellular activities against the virulent H37Rv strain in human macrophages. Curr Microbiol. 1996;33:167–175. doi: 10.1007/s002849900095. [DOI] [PubMed] [Google Scholar]
- 26.Schmidt U, Schluter G. Studies on the mechanism of phototoxicity of Bay y 3118 and other quinolones. Adv Exp Med Biol. 1996;387:117–120. doi: 10.1007/978-1-4757-9480-9_16. [DOI] [PubMed] [Google Scholar]
- 27.Shawar R M, Humble D J, Van Dalfsen J M, Stover C K, Hickey M J, Steele S, Mitscher L A, Baker W. Rapid screening of natural products for antimycobacterial activity by using luciferase-expressing strains of Mycobacterium bovis BCG and Mycobacterium intracellulare. Antimicrob Agents Chemother. 1997;41:570–574. doi: 10.1128/aac.41.3.570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sirgel F A, Venter A, Heilmann H-D. Comparative in-vitro activity of Bay y 3118, a new quinolone, and ciprofloxacin against Mycobacterium tuberculosis and Mycobacterium avium complex. J Antimicrob Chemother. 1995;35:349–357. doi: 10.1093/jac/35.2.349. [DOI] [PubMed] [Google Scholar]
- 29.St.-Jean G, Jernigan A D. Treatment of Mycobacterium paratuberculosis infection in ruminants. Vet Clin N Am Food Anim Pract. 1991;7:793–804. doi: 10.1016/s0749-0720(15)31085-9. [DOI] [PubMed] [Google Scholar]
- 30.St.-Jean G. Treatment of clinical paratuberculosis in cattle. Vet Clin N Am Food Anim Pract. 1996;12:417–430. doi: 10.1016/s0749-0720(15)30414-x. [DOI] [PubMed] [Google Scholar]
- 31.Thompson D E. The role of mycobacteria in Crohn’s disease. J Med Microbiol. 1994;41:74–94. doi: 10.1099/00222615-41-2-74. [DOI] [PubMed] [Google Scholar]
- 32.Thorel M-F, Krichevsky M, Levy-Frebault V V. Numerical taxonomy of mycobactin-dependent mycobacteria, emended description of Mycobacterium avium, and description of Mycobacterium avium subsp. avium subsp. nov., Mycobacterium avium subsp. paratuberculosis subsp. nov., and Mycobacterium avium subsp. silvaticum subsp. nov. Int J Syst Bacteriol. 1990;40:254–260. doi: 10.1099/00207713-40-3-254. [DOI] [PubMed] [Google Scholar]
- 33.Van Boxtel R M, Lambrecht R S, Collins M T. Effects of colonial morphology and Tween 80 on antimicrobial susceptibility of Mycobacterium paratuberculosis. Antimicrob Agents Chemother. 1990;34:2300–2303. doi: 10.1128/aac.34.12.2300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wallace R J, Nash D R, Steele L C, Steingrube V. Susceptibility testing of slowly growing mycobacteria by a microdilution MIC method with 7H9 broth. J Clin Microbiol. 1986;24:976–981. doi: 10.1128/jcm.24.6.976-981.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wilson D J, Rossiter C A, Han H R, Sears P M. Financial effects of Mycobacterium paratuberculosis on mastitis, culling and milk production in clinically normal dairy cattle. In: Chiodini R J, Hines II E E, Collins M T, editors. Proceedings of the Fifth International Colloquium on Paratuberculosis. Madison, Wis: International Association for Paratuberculosis, Inc.; 1996. pp. 151–158. [Google Scholar]
- 36.Yamori S, Tsukamura M. Paradoxical effect of Tween 80 between the susceptibility to rifampicin and streptomycin and the susceptibility to ethambutol and sulfadimethoxine in the Mycobacterium avium-Mycobacterium intracellulare complex. Microbiol Immunol. 1991;35:921–926. doi: 10.1111/j.1348-0421.1991.tb02031.x. [DOI] [PubMed] [Google Scholar]