Abstract
Background
Recent data suggest that glial cells may be involved in the analgesic effects and abuse liability of opioids. Preclinical studies have demonstrated that mu-opioid-receptor-selective agonists, such as oxycodone, activate glia and increase the release of cytokines, causing a suppression of opioid-induced analgesic effects. Preclinical studies also show that certain medications, such as the broad-spectrum tetracycline antibiotic minocycline, inhibit opioid-induced glial activation and thereby enhance the analgesic effects of opioids. Importantly, minocycline reduces the rewarding effects of opioids at the same doses that it enhances opioid-induced analgesia.
Aims
The purpose of the present study was to assess the effects of acute administration of minocycline on the subjective, physiological, and analgesic effects of oxycodone in human research volunteers.
Design
This study was a within-subject, randomized, double-blind outpatient study. Participants completed five separate sessions in which they received 0, 100, or 200 mg minocycline (MINO) simultaneously with either 0 or 40 mg oxycodone (OXY). The subjective, physiological, and analgesic effects of OXY were measured before and repeatedly after drug administration.
Settings and Participants
Participants were between 21 and 45 years of age, non-treatment seeking, non-dependent recreational opioid users (N=12). This study was conducted between 2013-2014 at the New York State Psychiatric Institute in New York, NY.
Findings
MINO 100 and 200 mg were safe and well-tolerated in combination with OXY 40 mg. MINO 200 mg administered with OXY 40 mg attenuated OXY-induced positive subjective effects such as “Good Effect" and "Liking" compared to OXY alone. MINO did not alter the physiological or analgesic effects of OXY.
Conclusions
MINO may attenuate the abuse liability of mu-opioid-receptor-selective agonists.
Keywords: Microglia, Opioid, Minocycline, Abuse Potential
1. Introduction
Glia are activated in response to a variety of stimuli (e.g., injury, disease, etc.) to release proinflammatory cytokines that create or exacerbate pain (Watkins et al 2005). Interestingly, preclinical studies suggest that chronic morphine administration also results in glial activation and cytokine release, which in turn may suppress narcotic analgesic effects (Chao et al. 1994). This may explain why increasing doses of opioids are often needed for relief of chronic pain leading to increased risk of tolerance and dependence and an increase in pain sensitivity known as opioid-induced hyperalgesia. It is unclear how the type, frequency or pattern of opioid use in humans contributes to the neuroinflammation that may lead to this paradoxical phenomenon. One preclinical study examined the neuroinflammatory effects of morphine and fentanyl in male rats and found that each drug induces neuroinflammation differently (more pronounced inflammation with fentanyl) through activation in the dorsal raphe nucleus through Toll-like receptor 4 (TLR4) in astrocytes and opioid receptors in neurons resulting in analgesia from opioids as well as fentanyl-induced hyperalgesia with repeated administrations (Carranza-Aguilar et al. 2020). This study also reported that MINO was effective in delaying tolerance to morphine and preventing fentanyl-induced hyperalgesia. As such, medications that reduce glial activation have been investigated as a means of improving the clinical utility of opioids.
The antibiotic minocycline (MINO) is a highly lipid soluble tetracycline with excellent penetration of the blood brain barrier (BBB) compared to other potent antibiotics (Cunha 2000). Achievement of peak serum concentration of MINO is superior to other tetracyclines with a serum half-life of approximately 16 hours, as well as higher concentrations of MINO in all tissues compared to serum concentration within one hour of dosing. MINO provides neuroprotection by rapidly penetrating the BBB, creating high therapeutic levels in the cerebrospinal fluid (Macdonald et al. 1973), and inhibiting activation of p38 mitogen-activated protein kinase (MAPK) in microglia, attenuating mRNA expression of tumor necrosis factor-alpha (TNF-alpha) and TNF-alpha converting enzyme, preventing excitotoxin-induced release of nitric oxide (NO) metabolites and IL-1 beta, and reducing mRNA induction of interleukin-1-beta-converting enzyme (Piao et al. 2006, Ledeboer et al. 2005, Tikka et al. 2001, Yrjanheikki et al. 1998). Preclinical studies have shown that inhibiting opioid-induced glial cell activation with MINO enhances narcotic analgesic effects (Ghazvini et al. 2015, Nazemi et al. 2015, Xiao-Peng et al. 2013, Hutchinson et al. 2008). These data suggest that MINO may significantly increase the peak magnitude and duration of opioid-induced analgesia (Hutchinson et al. 2008). In addition to enhancing opioid-induced analgesia, MINO has also been shown to significantly reduce morphine-induced conditioned place preference in rodents, an indicator of attenuated rewarding effects (Hutchinson et al. 2008).
A previous study conducted in humans demonstrated that compared to placebo, MINO attenuated the positive subjective effects of oral dextroamphetamine in non-dependent healthy volunteers (Sofuoglu et al. 2011) and attenuated subjective ratings of craving for cigarettes following intravenous nicotine administration (Sofuoglu et al. 2009). However, the ability of MINO to alter the abuse liability of opioids in non-opioid-dependent humans has not been assessed. Therefore, this double-blind, within-subject outpatient study was designed to assess whether the acute administration of oral MINO alters the subjective, physiological, analgesic, and cognitive effects of oxycodone (OXY), a commonly misused prescription opioid. These data may support investigation of glial modulation as a new target system for reducing the abuse liability of opioids.
2. Materials and Methods
2.1. Participants
After a telephone interview, eligible volunteers were scheduled for in-person screening at the New York State Psychiatric Institute. Initial screening visits included completion of questionnaires on drug use, general health, and medical history. Medical and psychiatric evaluations were performed with laboratory analyses (blood chemistry panel, complete blood count, liver profile, thyroid function test, and HCG levels in women). A qualitative urine drug toxicology test for opiates, benzodiazepines, methadone, buprenorphine, amphetamine, methamphetamine, phencyclidine, tetrahydrocannabinol (THC), cocaine and oxycodone was performed at each screening visit. Marijuana smokers had to have a quantitative urine analysis of THC value < 300 ng/ml at time of screening. If the value was greater than 300 ng/ml THC at the time of screening, it suggested heavier cannabis use and excluded the individual from the study (Moyer et al. 1987). Participants also received an electrocardiogram, tuberculosis test, and chest x-ray.
Participants were men and non-pregnant/non-lactating women who were healthy according to physical examinations and medical histories, not physically dependent on opioids as determined by clinical interviews and the pattern of opioid positive urine samples during screening, and not seeking treatment for their opioid use. These participants were between 21 and 45 years of age and using opioids for recreational purposes in amounts similar to or greater than administered in the present study, without physical dependence. Physical dependence was determined by clinical interview and urine toxicology and, if needed, a naloxone challenge test (Wang et al., 1974; Zilm and Sellers, 1978) was to be performed if participants consistently tested positive for opioids. However, in this study a naloxone challenge was not necessary for any of the participants based on the clinical presentation and patterns of urine drug screen results. Participants were excluded if they were seeking treatment for drug use, dependent on substances other than nicotine or caffeine, had a major neurological or Axis I psychiatric disorder (DSM-IV), were currently pregnant or breastfeeding, did not use an effective method of birth control, were currently receiving prescriptions for narcotic analgesics by a physician, had a history of any autoimmune disease, or were hypersensitive to study drugs (e.g tetracycline derivative antibiotics). Any participants with a current or recent risk of violence, or who were on parole/probation were also excluded.
Participants were compensated $15 for each screening visit, for up to 5 visits, and $25 for one computerized training session prior to initiating the study. Participants were paid $35 per study day with a $35 per day bonus for completion of the study. All participants signed an informed consent document describing the study in detail, along with its potential risks. At the end of the study, or during the study if requested, participants were offered referrals for treatment of their drug use. All study procedures were approved by the Institutional Review Board of the New York State Psychiatric Institute.
2.2. General Procedures
Five separate laboratory sessions were conducted on non-consecutive days to evaluate the effects of MINO on OXY-induced responses. Sessions were randomized and double-blinded. For safety, the nurses and physicians were not blinded to the MINO and OXY doses. Qualitative urinalysis for drugs of abuse was conducted prior to each outpatient session and the session was cancelled if the urine sample was positive for any substance, except THC. MINO and OXY were administered simultaneously at the time of dosing because the time to peak serum levels of both medications was approximately the same (Ordóñez et al. 2007, Brogden et al. 1975). The subjective, physiological, and analgesic effects of OXY 40 mg were tested in combination with each MINO dose (0, 100, 200 mg). The effects of MINO 0 mg in combination with OXY 0 mg, as well MINO 200 mg in combination with OXY 0 mg were tested as control conditions. The OXY 0 mg and MINO 100 mg condition was not tested due to limited pilot funds.
Laboratory sessions began between 1000 and 1030 hr and took approximately 360 minutes to complete. During the first 40 min of each session, vital signs monitoring was initiated, and baseline assessments of pupil diameter, subjective effects, performance effects, and analgesic effects were obtained. Participants received MINO (0, 100, or 200 mg) and OXY (0 or 40 mg) simultaneously orally at 0 min. Data were collected at the 15, 30, 60 90, 120, 150, 180, 240, and 300-minute time points post drug administration (Table 1; some endpoints were collected at fewer time points).
Table 1.
Session Events
| Time (minutes) |
Event |
|---|---|
| −60 | Urine toxicology |
| −40 | Baseline vital signs/Pulse oximetry (every 5 minutes) Pupil photo/Subjective effects battery/Performance battery/Cold Pressor Test |
| 0 | Dose Administration |
| 15 | Pupil photo/Subjective effects battery |
| 30 | Pupil photo/Subjective effects battery/Cold Pressor Test |
| 60 | Pupil photo/Subjective effects battery/Performance battery/Cold Pressor Test |
| 90 | Pupil photo/Subjective effects battery |
| 120 | Pupil photo/Subjective effects battery/Performance battery/Cold Pressor Test |
| 150 | Pupil photo/Subjective effects battery |
| 180 | Pupil photo/Subjective effects battery/Performance battery/Cold Pressor Test |
| 240 | Pupil photo/Subjective effects battery/Performance battery/Cold Pressor Test |
| 300 | Pupil photo/Subjective effects battery/Performance battery/Cold Pressor Test |
2.3. Apparatus
Participants were seated in a comfortable chair in front of a Macintosh computer and a response manipulandum (‘mouse’) was used for the completion of tasks and questionnaires presented on the screen. Experimenters continuously monitored all computer activities, vital signs, and behaviors. A separate “control” computer was used for recording vital signs, data collection, and to control the participants’ computer.
2.4. Measures
2.4.1. Subjective Measures.
Twenty-six questions on a visual analog scale (VAS) were used to assess subjective and self-perceived physiological effects. For each question, participants made a mark on a 100-mm line, anchored at one end with ‘not at all’ and at the other with ‘extremely.’ The first 18 questions were labeled ‘I feel…’ ‘Stimulated,’ ‘Anxious,’ ‘Depressed,’ ‘Sedated,’ ‘Energetic,’ ‘High,’ ‘Focused,’ ‘Calm,’ ‘Able to Concentrate,’ ‘Alert,’ ‘Tired,’ ‘Talkative,’ ‘Self-confident,’ ‘Social,’ ‘Irritable,’ ‘Confused,’ ‘Good Effect,’ and ‘BadEffect.’ The next four questions assessed drug craving and were labeled ‘I Want… ‘ Opioids,’ ‘Cocaine,’ ‘Alcohol,’ and ‘Tobacco.’ The next three questions asked about the quality and potency of the drug and how much they liked the drug that they had received. The final question asked the participant to indicate on a scale from $0 to $25 how much they would pay for the drug received. Due to an error in the computer program, we were only able to analyze 15 of the 26 subjective VAS questions (‘Alert,’ ‘Anxious,’ ‘Bad Effect,’ ‘Depressed,’ ‘Good Effect,’ ‘High,’ ‘Irritable,’ ‘Liking,’ ‘Potent,’ ‘Sedated,’ ‘Stimulated,’ ‘Want Alcohol,’ ‘Want Cocaine,’ ‘Want Opioids,’ and ‘Want Tobacco’).
2.4.2. Physiological Effects.
Vital signs monitors (Criticare Poet Plus 8100 vital signs monitor, Critical Systems Inc., Waukesha, WI) were used to collect data on respiration (respiratory rate, SpO2 (an estimate of arterial oxygen saturation), and end tidal CO2 (expired carbon dioxide levels)) and cardiovascular function (heart rate, systolic pressure, and diastolic pressure). A blood pressure cuff recorded blood pressure and heart rate every 5 min throughout the sessions. A soft sensor attached to a pulse oximeter was placed on a finger of the nondominant hand to measure arterial oxygen saturation continuously throughout the session. Participants breathed room air throughout the sessions, but supplemental oxygen was available in case of clinically significant respiratory suppression. A NeurOptics™ Pupillometer was used at pre-specified time points to measure changes in pupil diameter under ambient lighting conditions.
2.4.3. Pain Assessments.
Participants’ ratings of pain during the cold pressor test (described below) were measured with a 15-item Short-form McGill Pain Questionnaire (Clinical Pain MPQ; Melzack 1987) and the Pain Intensity/Bothersomeness Scales. This assessment tool has been validated in clinical trials involving chronic pain patients (Dworkin et al. 2005; Kerns et al. 1985, Melzack 1987). Along with the MPQ, participants rated their current level of pain using the Pain Intensity/Bothersome Scales (‘Not at all' (0) to ‘Extremely' (10)) after immersion of the hand in cold (4°C) water during the CPT.
2.4.4. Cold Pressor Test (CPT).
The analgesic effects of oxycodone were evaluated with the CPT, a commonly used and well-established experimental model for producing pain in humans (Zacny et al. 1996) and reliably detects the analgesic effects of opioid medications (Comer et al. 2010; Jones et al. 2011). Crushed ice is added to a cold tank and warm water is placed in a warm tank. The temperature is maintained at 4°C in the cold tank and 37°C in the warm tank. Each participant is asked first to immerse the hand in the warm tank for 2 minutes (to equalize baseline skin temperature across participants). Next, they are asked to immerse the same hand in the cold tank for up to 3 minutes. Standard instructions were read to each participant before administration of the CPT. During and immediately after the cold-water immersion, subjective ratings of pain were measured using the MPQ and the Pain Intensity/Bothersome Scales (see above). Objective dependent measures included: pain threshold (time in seconds to the first report of pain) and pain tolerance (time in seconds to withdraw hand from the water). Previous investigations (Conley et al., 1997; Jones et al., 2011; Zacny et al., 1996) including those by the current investigators have shown that multiple tests can be carried out within the same day with little or no between-test differences.
2.4.5. Performance Tasks.
The performance battery consisted of two tasks: a 3-min digit symbol substitution task (DSST), and a 10-min divided attention task (DAT). Custom-made software was used for these performance tasks; see Comer et al. (1999) for details.
2.4.6. Side Effects.
Adverse events (AEs) were assessed each session day throughout the study using a modified version of the Systematic Assessment for Treatment Emergent Effects questionnaire (SAFTEE; Guy et al. 1986; Rabkin and Markowitz 1986). AEs were coded from a list of possible events and the severity (mild, moderate, or severe), potential causes (study drug, concurrent drug, concurrent illness, other known cause, or uncertain cause), action(s) taken (none, decreased dose, symptomatic therapy, study drug discontinued, and hospitalization), and outcome (recovered, abated with decreased dosage, ongoing, and under treatment, death) were recorded.
2.5. Drugs
Oxycodone HCl (Oxycodone Immediate Release (IR); 20 mg; KVK-Tech Newtown, PA) was administered in oral doses of 0 and 40 mg. Minocycline (Minocin®; 100 mg; Cardinal Health, Dublin, OH) was administered in oral doses of 0, 100, and 200 mg. Both medications were over-encapsulated and administered in identical size 00 capsules (manufactured by Capsugel) in order to maintain a dosing blind. Lactose monohydrate powder (Spectrum Chemicals, Gardena, CA) was used as the excipient/filler in the blinding of both the oxycodone and minocycline tablets. Two tablets of Oxycodone 20 mg were placed into one size 00 capsule to make one 40 mg capsule. Lactose was used to fill size 00 capsules in order to create placebo tablets or partially fill the over-encapsulated oxycodone and minocycline tablets. Three capsules that were identical in appearance were administered during each laboratory session. The following 5 dose combinations consisting of 3 capsules for each dose combination were administered:
OXY 0 mg + MINO 0 mg (3 capsules containing lactose (0 mg) each);
OXY 0 mg + MINO 200 mg (1 capsule containing lactose (0 mg) + 2 capsules containing 100 mg MINO per capsule);
OXY 40 mg + MINO 0 mg (1 capsule containing 40 mg oxycodone + 2 capsules containing lactose (0 mg) per capsule);
OXY 40 mg + MINO 100 mg (1 capsule containing 40 mg oxycodone + 1 capsule containing 100 mg minocycline + 1 capsule containing lactose (0 mg));
OXY 40 mg + MINO 200 mg (1 capsule containing 40 mg oxycodone + 2 capsules containing 100 mg minocycline per capsule).
2.6. Statistical Analyses
Demographic variables and adverse effects were summarized descriptively. Pearson's correlation was employed to measure the strength and direction of the relationship between demographic variables and the outcome measures of interest. Repeated-measures Analysis of Variance (ANOVA) was used to assess differences among the OXY (0 or 40 mg) and MINO (0, 100, or 200 mg) dose conditions. Both peak [or trough (i.e., maximum or minimum drug effect throughout the session, respectively)] and time-course of drug effects were analyzed for all relevant variables. Because this was an unbalanced study, a fully factorial design could not be used, thus, planned comparisons were performed between each of the OXY and MINO dose conditions to determine where significant differences exist. To reduce Type-I error due to multiple comparisons, the statistical significance level of α was set at <0.01. However, due to the exploratory nature of this study (i.e., the first clinical laboratory assessment of the interaction between OXY and MINO), an α >0.01 and <0.05 is used to indicate group differences that are approaching or “trending” towards statistical significance. All data analyses were performed using SPSS version 15 (SPSS I 2006) and SuperANOVA (Gagnon et al. 1990). All figures were created using GraphPad Prism 6 for Mac (GraphPad Software 2014). The analysis was not pre-registered and the results should be considered exploratory.
3. Results
3.1. Participants
Seventeen participants signed study consent and 12 participants completed the study. Four participants began the study but did not complete it. All four discontinued voluntarily or were lost to contact. One participant who signed study consent was lost to contact prior to starting the study. Demographic and drug use information for the 12 completers can be found in Table 2. On average, volunteers were 35±3 years of age. All participants had used prescription opioids recreationally within the previous 6 months with an average use of 1.4 days/week, equivalent to approximately 6 days/month.
Table 2.
Demographic characteristics of research participants (N= 12).
| Parameter | N (%) | |
|---|---|---|
| Gender | ||
| Male | 10 (83) | |
| Female | 2 (17) | |
| Race | ||
| Caucasian | 4 (33) | |
| African American | 5 (42) | |
| Hispanic/Latino | 2 (17) | |
| Other | 1 (8) | |
| Age (years, mean ± SEM) | 35.6 ± 2.8 | |
| Weight (pounds, mean± SEM) | 189.2 ± 8.8 | |
| Body Mass Index (BMI) (mean ± SEM) | 27.0 ± 1.3 | |
| Education (years, mean ± SEM) | 13.3 ± 0.7 | |
| Substance Use History | N (%) | Mean Frequency of Use (days/week, mean ± SEM) |
| Prescription Opioid | 12 (100) | 1.4 ± 0.4 |
| Heroin | 1 (8) | 1.5 |
| Cocaine | 3 (25) | 0.4 ± 0.3 |
| Sedatives/benzodiazepines | 3 (25) | 0.5 ± 0.5 |
| Marijuana | 7 (58) | 2.6 ± 0.9 |
| Tobacco | 5 (42) | 7.0 ± 0.0 |
| Alcohol | 10 (83) | 1.0 ± 0.3 |
| Hallucinogens | 1 (8) | 0.0 ± 0.0 |
SEM = Standard error of the mean
3.2. Physiological Effects
Figure 1 illustrates the physiological effects of MINO in combination with OXY. The top panel shows that OXY 40 mg combined with MINO 0 mg (F1,11 =66.079, P<0.001), 100 mg (F1,11 =88.298, P<0.001), or 200 mg (F1,11 =80.089, P<0.001) produced significant pupillary constriction compared to OXY 0 mg conditions. MINO did not produce pupillary constriction in combination with OXY 0 mg and it did not affect the degree of pupillary constriction in combination with OXY 40 mg.
Figure 1.
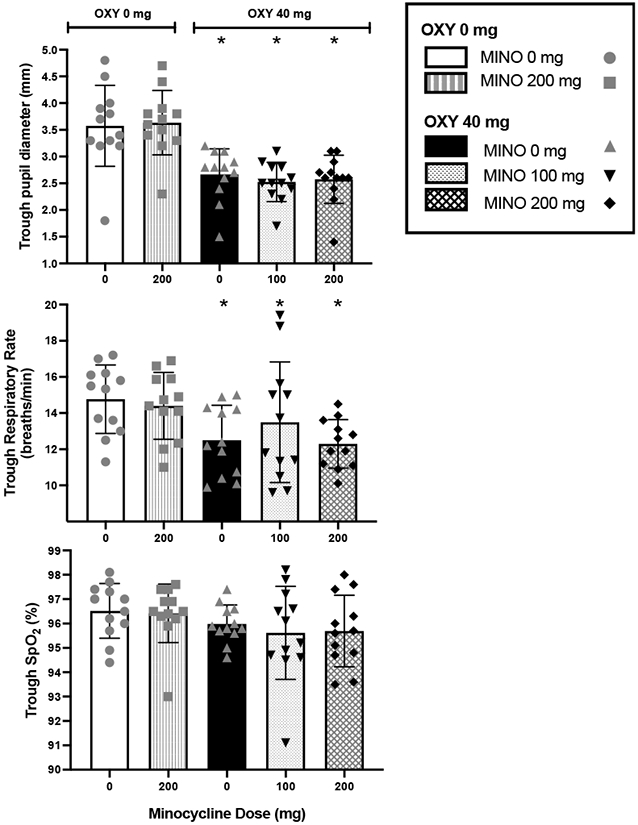
Physiological effects (individual data points, ± the standard error of the mean (S.E.M.)) of oxycodone 0 or 40 mg in combination with minocycline 0, 100, or 200 mg.
* indicates significance (P<0.01) from oxycodone 0 mg combined with minocycline 0 mg.
OXY 40 mg combined with MINO 0 and 200 mg produced decreases in respiratory rate (MINO 0 mg: F1,11 =19.412, P<0.001; MINO 200 mg: F1,11 =23.154, P<0.001) compared to OXY 0 mg conditions. While the combinations of OXY 40 mg + MINO 0 mg and OXY 40 mg + MINO 200 mg resulted in equal levels of respiratory depression, OXY 40 mg + MINO 100 mg produced a non-significant blunting of the respiratory depression compared to OXY 40 mg + MINO 200 mg (F1,11 =5.337, P<0.05) (Figure 1, middle panel). The respiratory rate when MINO 200 mg was administered in combination with OXY 0 mg did not differ from the respiratory rate when OXY 0 mg was administered in combination with MINO 0 mg.
MINO 200 mg alone did not significantly increase peak expired CO2 in comparison to placebo (vs MINO 0 mg + OXY 0 mg: F1,11 =1.22, P=0.275; data not shown). OXY 40 mg combined with MINO 0 mg significantly increased peak expired CO2 in comparison to the placebo condition (vs MINO 0 mg + OXY 0 mg: F1,11 =7.63, P<0.01), but not in comparison to MINO 200 mg alone (F1,11 =2.748 P=0.104; data not shown). OXY 40 mg combined with MINO 100 mg did not significantly differ from OXY 40 mg combined with MINO 0 (F1,11 =0.754, P=0.389; data not shown). OXY 40 mg combined with MINO 200 mg produced the most robust effect, significantly greater than all the other dose combinations (P’s<0.0001 - 0.01; data not shown).
A planned contrast of the three OXY 40 mg conditions compared to the two OXY 0 mg conditions revealed a significant decrease in arterial oxygen saturation (%SpO2; F1,11 =18.930, P<0.001). There were no significant changes in blood pressure and heart rate with MINO in the OXY 40 mg or OXY 0 mg conditions.
3.3. Subjective Effects
Figure 2 illustrates the time-course (left panels) and average peak ratings (right panels) with individual data points for three of the subjective effect questions - “I feel a good effect,” “I liked the choice” and “I feel high” (Figure 2, top, middle, and bottom panels, respectively). Participants reported higher average peak ratings of subjective effects when they received OXY 40 mg compared to OXY 0 mg in combination with all doses of MINO for “I feel a good effect” (top right panel, MINO 0 mg: F1,11 = 25.475, P<0.001, MINO 100 mg: F1,11 =16.166, P<0.001 , MINO 200 mg: F1,11 =10.291, P<0.005), “I liked the choice” (middle right panel, MINO 0 mg: F1,11 =18.464, P<0.001, MINO 100 mg: F1,11 =9.450, P<0.005, MINO 200 mg: F1,11 =7.502, P<0.01), and “I feel high” (bottom right panel; MINO 0 mg: F1,11 =54.595, P<0.001, MINO 100 mg: F1,11 =55.687, P<0.001, MINO 200 mg: F1,11 =44.271, P<0.001). Peak drug effects occurred between 1- and 2-hours post-drug administration (left panels).
Figure 2.

Comparisons of subjective effects as time-course (left panels) and individual data points with average peak ratings (± S.E.M.; right panels) of oxycodone 0 or 40 mg combined with minocycline 0, 100, or 200 mg.
Darkened data points signify significance (P<0.01) from oxycodone 40 mg combined with minocycline 0 mg.
* indicates significance from oxycodone 0 mg combined with minocycline 0 mg.
When OXY 40 mg was combined with MINO 100 mg, the average ratings of “I feel a good effect” decreased significantly at the 240- (F1,11 =13.801, P<0.001) and 300-minute (F1,11 =12.237, P<0.001) time points compared to OXY 40 mg alone (Figure 2, top left panel). Further, trends for blunting of “I feel a good effect” was seen with the combination of OXY 40 mg + MINO 200 mg compared to the OXY 40 mg + MINO 0 mg condition at the 60- (F1,11 =4.639, P<0.05), 90- (F1,11 =5.689, P<0.05), 240-, (F1,11 =4.154, P<0.05) and 300-minute (F1,11 =5.085, P<0.05) time points, although none of these effects were significant at P<0.01. Lastly, MINO 200 mg in combination with OXY 40 mg delayed the peak rating of “Good Effect” by 60 minutes compared to OXY 40 mg in combination with MINO 0 mg.
MINO 100 mg in combination with OXY 40 mg reduced subjective ratings of “I liked the choice” at the 90- (F1,11 =8.267, P<0.005), 120- (F1,11 =6.311, P<0.05), 180- (F1,11 =12.761, P<0.001) time points in comparison to OXY 40 mg in combination with MINO 0 mg. In addition, MINO 200 mg reduced average ratings of “I liked the choice” produced by OXY 40 mg as early as 60 minutes post-dose administration (F1,11=8.520, P<0.005), with significance at the 90-minute (F1,11=15.487, P<0.001) time point. Overall, there was a dose-dependent decrease in peak ratings for “I liked the choice” with increased doses of MINO in combination with OXY 40 mg, though this effect was not statistically significant (Figure 2, right middle panel).
Participants reported significantly greater feelings of “Potency” and “High” after administration of all OXY 40 mg conditions (MINO 0 mg: F1,11 =31.845, P<0.001, MINO 100 mg: F1,11 =30.552, P< 0.001, MINO 200 mg: F1,11=25.161, P<0.001) compared to OXY 0 mg + MINO 0 mg. MINO, however, did not alter time point or peak ratings of “Potent” or “High” (Figure 2, left and right lower panel).
Table 3 shows ratings of “Bad Effect” as a function of MINO and OXY doses. All OXY 40 mg conditions produced increased ratings of “Bad Effect” compared to OXY 0 mg. Common side effects of opioid medications were reported by the subjects on the SAFTEE, such as drowsiness, nausea, and vomiting. MINO did not significantly alter ratings of “Bad Effect.” There was a trend for increased ratings of “Anxious” with OXY 40 mg + MINO 100 mg compared to OXY 40 mg + MINO 0 mg (F1,11 =5.17, P<0.05). Trends for increased ratings of “Depressed” also occurred with OXY 40 mg + MINO 100 mg (F1,11 =6.851, P<0.05) and OXY 40 mg + MINO 200 mg (F1,11 =4.618, P<0.05) compared to OXY 0 mg + MINO 200 mg, but these effects were not statistically significant.
Table 3.
Peak Subjective Effects of Oral Minocycline in Combination with Oral Oxycodone (N=12 unless otherwise indicated). Values represent means (standard errors of the mean) from peak comparisons.
| Oxycodone (0 mg) | Oxycodone (40 mg) | ||||
|---|---|---|---|---|---|
| Minocycline Dose (mg, oral) | |||||
| 0 mg | 200 mg | 0 mg | 100 mg | 200 mg | |
| VAS Subjective Efects | |||||
| Bad effect | 11.9 (8.4) | 8.4 (5.1) | 35.2 (11.0)a*b* | 33.8 (11.0)a*b* | 31.1 (9.9)cb* |
| Good effect | 10.2 (6.2) | 11.8 (4.8) | 59.3 (9.3)a*b* | 49.3 (10.1)a*b* | 41.4 (10.1)a*b* |
| High | 15.6 (7.5) | 8.4 (3.1) | 65.8 (8.3)a*b* | 66.3 (7.9)a*b* | 60.8 (7.2)a*b* |
| Liking | 12.1 (7.9) | 17.1 (6.1) | 54.8 (10.3)a*b* | 42.7 (10.5)a*d | 39.3 (9.6)a*d |
| Potent | 11.6 (6.6) | 13.2 (4.7) | 60.3 (11.1)a*b* | 59.3 (11.0)a*b* | 54.9 (9.7)a*b* |
| VAS Craving Effects | |||||
| Want alcohol (N=10) | 12.3 (9.8) | 3.0 (1.6) | 19.9 (9.7) | 15.4 (7.4) | 12.7 (8.6) |
| Want cocaine (N=3) | 24.3 (15.6) | 5.0 (3.2) | 31.0 (14.1) | 13.3 (7.3) | 24.0 (18.2) |
| Want opioid (N=12) | 9.4 (6.4) | 6.3 (4.4) e | 15.3 (9.2) | 9.2 (7.7) | 8.8 (8.3) |
| Want tobacco (N=5) | 63.0 (17.9) | 27.8 (17.8)c | 41.2 (22.9) | 48.6 (17.0) | 38.8 (21.4) |
| VAS Cognitive Effects | |||||
| Alert | 49.3 (9.4) | 43.5 (7.5) | 43.0 (7.1) | 54.7 (5.7) | 41.2 (7.0) |
| Anxious | 18.9 (7.7) | 11.2 (7.1) | 7.1 (3.6)c | 19.7 (8.1)e | 10.2 (3.3) |
| Depressed | 11.9 (5.9) | 5.3 (3.0) | 11.1 (7.0) | 18.8 (10.3)d | 16.4 (8.7)d |
| Irritable | 21.0 (9.5) | 15.5 (5.4) | 23.8 (9.9) | 24.8 (10.0) | 18.9 (5.4) |
| Sedated | 18.7 (9.5) | 16.8 (8.7) | 61.0 (9.3)a*b* | 64.0 (8.8)a*b* | 57.9 (8.5)a*b* |
| Stimulated | 11.8 (4.6) | 14.2 (5.2) | 33.1 (9.5)cd | 24.5 (6.2) | 31.8 (9.4)cd |
| CPT Analgesic Effects | |||||
| MPQ Sum | 34.6 (4.0) | 34.4 (4.0) | 33.4 (4.3) | 34.9 (4.0) | 31.9 (3.4) |
| How bothersome | 8.6 (0.6) | 8.8 (0.6) | 8.0 (0.8) | 9.1 (0.5) | 7.7 (0.9)f |
| Pain Intensity | 8.6 (0.6) | 8.8 (0.6) | 8.2 (0.8) | 9.1 (0.4) | 7.8 (0.9)f |
| Latency to feel pain | 43.6 (18.6) | 53.0 (19.4) | 66.1 (20.5) | 76.8 (19.4)c | 56.8 (18.1) |
| Latency to withdraw | 60.5 (20.5) | 61.5 (21.0) | 89.5 (21.1)cd | 93.8 (19.8)a*b* | 89.1 (21.0)cd |
represents significant difference (p<0.01) from oxycodone 0 mg + minocycline 0 mg.
represents significant difference (p<0.01) from oxycodone 0 mg + minocycline 200 mg.
represents difference (p<0.05) from oxycodone 0 mg + minocycline 0 mg.
represents difference (p<0.05) from oxycodone 0 mg + minocycline 200 mg.
represents difference (p<0.05) from oxycodone 40 mg + minocycline 0 mg.
represents difference (p<0.05) from oxycodone 40 mg + minocycline 100 mg.
VAS = Visual Analog Scale, CPT = Cold Pressor Test
Craving.
Table 3 shows subjective ratings of drug craving. In the OXY 0 mg conditions, MINO 200 mg showed a trend for decreased ratings of “Want Tobacco” (F1,11 =5.966, P<0.05) in the 5 participants who smoked tobacco. There was also a trend for decreased ratings of “Want Opioids” in the OXY 0 mg + MINO 200 mg conditions compared to OXY 40 mg + MINO 0 mg (F1,11 =5.627, P<0.05), though this effect was not statistically significant. MINO did not significantly affect ratings of “Want Alcohol” in participants who reported drinking alcohol (N=10) and “Want Cocaine” among the cocaine users (N=3).
3.4. Analgesic Effects
Figure 3 shows the time course (left panels) and peak latency (right panels) to first report pain (top panel) and the latency to withdraw (bottom panel) the hand from the cold water during each dose condition. Analyses revealed no significant effect of OXY or MINO on latency to feel pain. However, active OXY increased the latency to withdraw the hand from cold water (OXY 0 mg + MINO 0 mg vs: OXY 40 + MINO 0 mg (P<0.01), OXY 40 + MINO 100 mg (P<0.01), OXY 40 + MINO 200 mg (P<0.05)). No significant moderating effects of MINO were found on this objective measure. Figure 4 shows that active OXY reduced subjective pain “Intensity” (top panels) and “Bothersomeness” (lower panels) during cold water immersion. These differences did not approach statistical significance and no moderating effect of MINO was found in the peak comparisons. In the time course analysis, there was an increase in analgesic effect (P<0.05, painful; P<0.01, bothersome) at the 180-min time-point after administration of OXY 40 mg + MINO 200 mg compared to OXY 40 mg alone. No main effects of OXY, MINO or OXY+MINO interaction was found on the MPQ. Analgesic effects as a function of oxycodone and minocycline dose conditions can be found in Table 3.
Figure 3.

Time course (left panel) and peak (right panel) ratings (individual data points , ± S.E.M.) of pain threshold (latency to feel pain) and pain tolerance (latency to withdraw) as a function of oxycodone 0 or 40 mg combined with minocycline 0, 100, or 200 mg.
Darkened data points represent significance (P<0.01) from oxycodone 40 mg combined with minocycline 0 mg.
* indicates significance (P<0.01) from oxycodone 0 mg combined with minocycline 0 mg.
Figure 4.

Time course (left panel) and trough (right panel) subjective ratings (individual data points, ± S.E.M.) of the intensity and bothersomeness of pain as assessed immediately after the hand was withdrawn from the cold water.
Darkened data points represent significance (P<0.01) from oxycodone 40 mg combined with minocycline 0 mg.
3.5. Performance Effects
Table 4 compares the effects of OXY and MINO on performance of the DAT and DSST. The OXY 40 mg in combination with MINO 100 mg and MINO 200 mg conditions produced a significant increase in latency to identify the target compared to OXY 0 mg + MINO 0 mg (F1,11 =23.224, P< 0.001 and F1,11 =13.329, P<0.001 respectively), as indicated by the “Maximum speed” item on the DAT. There was a significant increase in the tracking distance between the cursor and moving stimulus of the OXY 40 mg + MINO 200 mg condition compared to OXY 0 mg + MINO 0 mg (F1,11 =9.716, P<0.01) and compared to OXY 0 mg + MINO 200 mg (F1,11 =12.075, P =0.001) during the DAT. False alarms showed a trend for being higher in the OXY 40 mg + MINO 200 mg condition compared to the OXY 0 mg + MINO 200 mg (F1,11 =4.367, P<0.05). There were no significant differences between dosing conditions in the number of hits and misses in the DAT.
Table 4.
Select performance measure for the Divided Attention Task (DAT) and Digit-Symbol Substitution Task (DSST) as a function of oral oxycodone and minocycline dose. Values represent the mean performance post-drug (standard error) from peak comparisons.
| Oxycodone (0 mg) | Oxycodone (40 mg) | ||||
|---|---|---|---|---|---|
| Minocycline Dose (mg, oral) | |||||
| 0 mg | 200 mg | 0 mg | 100 mg | 200 mg | |
| DAT | |||||
| Number of False Alarms | 3.2 (1.1) | 2.8 (0.9) | 3.2 (0.9) | 4.9 (1.5) | 5.2 (1.1)d |
| Number of Hits | 18.8 (0.4) | 19.1 (0.3) | 18.8 (0.5) | 19.3 (0.3) | 18.9 (0.5) |
| Number of Misses | 0.8 (0.3) | 0.9 (0.4) | 1.5 (0.4) | 1.0 (0.3) | 1.8 (0.9) |
| Maximum Speed | 6.4 (0.8) | 6.0 (0.5) | 4.6 (0.4)a*d | 3.7 (0.6)a*b* | 4.3 (0.5)a*b* |
| Tracking Distance (m) | 15.6 (3.0) | 14.4 (1.7) | 21.4 (2.2)d | 20.5 (2.9) | 25.3 (1.9)a*b* |
| DSST | |||||
| Total Number Attempted | 76.2 (3.1)f | 73.8 (3.4) | 75.4 (5.4) | 67.1 (2.6)c | 68.4 (3.9) |
| Total Number Correct | 72.3 (5.1)f | 70.2 (3.6) | 73.0 (5.0)f | 62.0 (4.3)ce | 64.7 (4.8) |
represents significant difference (p<0.01) from oxycodone 0 mg + minocycline 0 mg.
represents significant difference (p<0.01) from oxycodone 0 mg + minocycline 200 mg.
represents difference (p<0.05) from oxycodone 0 mg + minocycline 0 mg.
represents difference (p<0.05) from oxycodone 0 mg + minocycline 200 mg.
represents difference (p<0.05) from oxycodone 40 mg + minocycline 0 mg.
represents difference (p<0.05) from oxycodone 40 mg + minocycline 100 mg.
DAT = Divided attention task, DSST= Digit symbol substitution task
Concerning the DSST, there were no significant differences among the various dose conditions but several trends. For example, there was a non-significant decrease in the number of patterns attempted in the OXY 40 mg + MINO 100 mg condition compared to the OXY 0 mg + MINO 0 mg condition (F1,11 = 4.215, P < 0.05). These two dose conditions similarly differed on the total number of correctly identified patterns (F1,11 = 4.658, P < 0.05). The total number of correctly identified patterns was lower following the administration of OXY 40 mg + MINO 100 mg in comparison to OXY 40 mg + MINO 0 mg (F1,11 = 5.364, P < 0.05).
3.6. Relationship Between Body Weight, Recreational Opioid Use and Primary Outcome Measures
To assess whether the participants’ opioid use characteristics were associated with the primary pharmacodynamic effects of oxycodone, the investigators assessed the association between prescription (Rx) opioid use and response to oxycodone alone (OXY 40mg + MINO 0mg). Prescription opioid use was quantified in terms of: years of Rx opioid use (#Years), the number of Rx opioid pills used per occasion/day (#Pills), and number of Rx opioid uses per week (#Days/Week). Because 41% of our sample could not specify the exact Rx opioid they regularly used and/or the mg amount, use in terms of morphine mg equivalence could not be calculated. Peak VAS ratings of “liking” (i.e., positive subjective effect) were not significantly associated with #Years (r=−0.11, P=0.73), #Pills (r=−0.29, P=0.39), or #Days/Week (r=−0.28, P=0.93). The DSST total correct (i.e., cognitive effects) was not significantly associated with #Years (r=−0.43, P=0.16), #Pills (r=0.54, P=0.07), or #Days/Week (r=−0.09, P=0.78). Likewise, MPQ summary score (i.e., analgesic effects) was not significantly associated with: #Years (r=0.04, P=0.90), #Pills (r=0.02, P=0.94), or Days/Week (r=−0.17, P=0.62). Finally, participants’ body weight was not associated with drug “liking” (r=0.05, P=0.88), DSST total correct (r=−0.22, P=0.49), or MPQ summary score (r=−0.08, P=0.81).
To assess whether the participants’ opioid use characteristics were associated with the interaction between oxycodone and minocycline, the association between prescription opioid use and the percent difference between oxycodone alone was calculated (OXY 40mg + MINO 0mg) and oxycodone plus minocycline (OXY 40 mg + MINO 200 mg). Percent shift in peak VAS rating of “liking” was not significantly associated with #Years (r=−0.25, P=0.43), #Pills (r=−0.009, P=0.97), or #Days/Week (r=−0.02, P=0.95). Percent shift in DSST total correct was not significantly associated with #Years (r=−0.43, P=0.16), #Pills (r=0.24, P=0.45), or #Days/Week (r=0.02, P=0.96). Finally, percent shift in MPQ summary score was not significantly associated with: #Years (r=0.01, P=0.97), #Pills (r=−0.38, P=0.22, or #Days/Week (r=−0.51, P=0.11). Finally, participants’ body weight was not associated with percent shift in drug “liking” (r=−0.31, P=0.33), DSST total correct (r=−0.22, P=0.42), or MPQ summary score (r=−0.27, P=0.39).
3.7. Adverse Events
OXY and MINO were generally well tolerated, although all of the 12 participants from whom SAFTEE data were collected reported at least one adverse event (AE). The most common treatment-related AEs were ‘general disorders’ (e.g., dizziness, drowsiness, itching, lightheaded, nausea, vomiting), with most having occurred in 8 or fewer participants during sessions when they received OXY 40 mg. All reported AEs resolved within 24h.
4. Discussion
The present data suggest that acute administration of MINO may attenuate OXY-induced positive subjective responses in non-dependent recreational opioid users. The most compelling findings were blunting of “I feel a good effect” in the time-course data and significantly reduced subjective ratings of “I liked the choice” with OXY 40 mg + MINO 200 mg. FDA guidance on measures of abuse potential prioritize assessment of drug “liking” in trials such as these (FDA 2017). On this measure MINO (200 mg) produced a “medium” attenuation (Cohen’s D effect size = 0.47) in OXY’s positive subjective effects (Kelley and Preacher, 2012). Averaged across all three measures of positive subjective effects (i.e., liking, good effect, and high) MINO’s effect constituted a “medium” Cohen’s D effect size of 0.41. These results were consistent with the conclusions drawn in preclinical studies examining the attenuating effects of MINO on morphine-induced conditioned place preference (Hutchinson et al. 2008). It is also notable that the time course of dampened subjective responses (Figure 2, right panels) corresponds to the rapid (1-4 hour) time to reach peak serum concentration of MINO and penetration of MINO through the BBB. The attenuation of subjective effects of OXY cannot be otherwise explained by MINO-induced side effects as there were no adverse events or side effects observed in the MINO 200 mg alone condition relative to placebo. There is no evidence to suggest that MINO increases anxiety, depression, or “Bad Effects” when administered alone, although ratings of anxiety increased with MINO 100 mg in combination with OXY compared to OXY alone and depression increased with MINO 200 mg in combination with OXY compared to MINO 200 mg alone. While minocycline is being studied as an adjunctive anti-depressant and anxiolytic treatment (Husain et al. 2015, Vogt et al. 2015, Dean et al. 2014), its efficacy in reducing mood symptoms has not been elucidated. Our study showed the opposite effect – an increase in anxiety and depression with MINO in combination with OXY, as compared to OXY alone. This may be because our subjects did not have clinical depression or anxiety and were not opioid dependent. There have been bodies of research focused on the comorbidity of mood and anxiety disorders in the opioid dependent population (Rogers et al. 2021). Additionally, studies have shown that chronic MINO treatment (not acute) exerts an antidepressant effect and inhibits neuroinflammation in preclinical data (Yang et al. 2020) and is effective as an augmentation treatment to antidepressants in clinical data (Nettis et al. 2021). The present study focused on a population that may not have levels of inflammation comparable to those with chronic depression, anxiety, and/or substance use disorders. Therefore, MINO may prove more effective as an antidepressant or anxiolytic medication in a population with opioid physical dependence and a concomitant mood/anxiety disorder as the neuroinflammation may be more severe. Instead of improving mood and anxiety in this study examining recreational, non-dependent opioid users, MINO may have reduced the pleasurable effects of OXY by attenuating its subjective effects. The attenuation of OXY-induced positive subjective effects may be better explained by the ability of MINO to alter dopamine neurotransmission. A preclinical study examined the effects of MINO on extracellular dopamine levels in the nucleus accumbens after administration of methamphetamine and found that MINO significantly attenuated the methamphetamine-induced increase in extracellular dopamine (DA) levels within the nucleus accumbens (Arezoomandan and Haghparast 2015, Fujita et al. 2012). This may provide an explanation for the reduction of OXY-induced rewarding effects, as well as “Bad effects” in humans.
MINO overall did not significantly alter the physiological effects of OXY. In the preclinical studies, MINO was reported to attenuate, in a dose-dependent manner, morphine-induced suppression of tidal volume, minute volume, inspiratory force and expiratory force (Hutchinson et al. 2008). In our study, we found a slight reversal of respiratory depression, as measured by respiratory rate, with the MINO 100 mg + OXY 40 mg condition. However, this effect was small with no change in peak expired CO2 level and it did not occur with MINO 200 mg + OXY 40 mg. The clinical significance of this finding requires further investigation. Though there was a statistically significant increase in expired CO2 of the MINO 200 mg + OXY 40 mg condition, the variability between all conditions is negligible and clinically insignificant.
An interesting finding was that MINO decreased wanting of tobacco in smokers in the OXY 0 mg condition. Previous studies suggest that MINO may decrease use of tobacco in smokers and have a greater reduction in craving for cigarettes following smoking under MINO treatment compared to placebo (Sofuoglu et al. 2009, Agrawal et al. 2011). The mechanism of action is unknown, but the findings from our study support these data and suggest the potential utility of MINO as a treatment for nicotine cravings.
Our findings were inconsistent with studies that have shown cognitive improvement with MINO. Again, this may be due to the minimal levels of neuroinflammation in this study population. Preclinical studies indicate that MINO improves cognitive deficits induced by neuroinflammation (Hou et al. 2016) and sleep deprivation (Wadhwa et al. 2017). Additionally, clinical studies have shown MINO to enhance cognitive performance, specifically speed of performance in a response inhibition task (Sofuoglu et al. 2011), and improve performance in social decision-making tasks, suggesting MINO as a potential treatment strategy in patients needing cognitive remediation (Sofuoglu et al. 2013, Kato et al. 2012, Watabe et al. 2012). Previous studies have shown relatively few impairments in task performance with opioid administration in opioid-dependent individuals (Comer et al. 1999, Zacny 1995, Foltin and Fischman 1995, Pickworth et al. 1993, Preston et al. 1989, 1992, Strain et al. 1992), however in this study the non-dependent opioid users may be more susceptible to the cognitive impairing effects of opioids compared to individuals who are dependent on opioids. Extended MINO dosing may be a potential treatment strategy to address impaired decision making and increased impulsivity associated with opioid use disorder (Sofuoglu et al. 2013, Kato et al. 2012, Watabe et al. 2012, Sofuoglu et al. 2011). Future studies are warranted to better understand the cognitive effects of MINO in individuals with opioid use disorders.
MINO produced no significant effects on oxycodone-induced analgesia. There are mixed findings regarding the effects of minocycline on pain in humans. Some studies indicate improved pain or attenuated hyperalgesia (Samour et al. 2017) while others conclude that there are no significant effects on pain (Arout et al. 2018, Curtin et al. 2017, Vanelderen et al. 2015). Differences in drug potency and administration protocols may contribute to these divergent results. In preclinical studies however, minocycline has been shown to enhance opioid analgesia (Akgűn et al. 2019, Hutchinson et al. 2008) and prevent hyperalgesia (Mika et al. 2007). A potential mechanism of the effect in preclinical models may be through the inhibition of p38 mitogen-activated protein kinase (MAPK), a key regulator of the expression of proinflammatory cytokines and other mediators such as COX-2 (Kumar et al. 2003). Highly selective p38 MAPK inhibitors, such as MINO, can be very potent in decreasing pain responses, as well as reducing tolerance to morphine (Hameed et al. 2010, Cui et al. 2008, Piao et al. 2006, Ledeboer et al. 2005, Schafers et al. 2003 and Svensson et al. 2003). Repeated administration of MINO intraperitoneally or intrathecally demonstrated analgesia in preclinical studies for the treatment of neuropathic pain (Mika et al. 2009). It is unclear why the present results were inconsistent with previous human and animal studies. The difficulty in translating research from rodent glia to human immune function has been noted in a previous study (Smith and Dragunow 2014). In the current study, orally administered minocycline treatment did not affect the threshold or tolerance to pain assessed with the CPT in individuals who were not dependent on opioids. A similar study examining experimental pain and addiction-related outcomes with a 15-day treatment regimen of MINO 200 mg in opioid-dependent individuals showed similar findings with no significant effect of MINO on pain severity when compared to placebo (Arout et al. 2018). Also, some of the participants in this study were non-heavy cannabis users so residual cannabis may have produced analgesic effects on its own. The interaction between opioids, cannabis and MINO is unknown and should be considered for future studies. Additionally, the present study consisted of predominantly male completers and a limited number of females. Sex differences are well demonstrated in immune responsiveness and analgesia. Unfortunately, studies examining gender as an independent variable are limited. Preclinical studies have shown that females experience increased inflammation and hyperalgesia in response to stress compared to males (Schwarz and Bilbo 2012, Garcia-Segura and Melcangi 2006, Marriott and Huet-Hudson 2006, Cook et al. 2005). Females demonstrate greater release of pro-inflammatory cytokines than males and may be more susceptible to over-activation and chronic long-term effects of inflammation (LaPrairie and Murphy 2007, Streit et al. 2004). One review suggests that gender differences in the glial activation of TLR4 as well as sex differences in gonadal steroids may mediate the varied responses to morphine-induced analgesia and inflammation found between men and women (Doyle et al. 2017). Therefore, it should be considered that MINO may have a different analgesic response in a study focused on females with opioid use disorders.
The present study had several limitations. First, due to limited funds, we could not run all of the conditions required to have a fully factorial design. If a sixth condition of 100 mg MINO + OXY 0 mg were added, a two-factor repeated measures ANOVA could have been used and would have reduced the need to rely on planned contrasts. Furthermore, it is possible that we would have observed different effects if subjects were maintained on OXY and/or MINO rather than the acute dosing procedures used in this study. Dosing of MINO and OXY was not adjusted to body weight, which may have some impact on the volume of distribution of both MINO and OXY as well as outcome measures. We had a small sample size of predominantly male, non-dependent opioid users which precluded examination of sex differences in responses. Also, due to limited funding this study was conducted on an outpatient basis and it is possible that other factors, such as cannabis use, could have affected the results.
5. Conclusions
Microglial inhibitors, such as MINO, have been shown to significantly attenuate measures of the abuse liability of opioids while enhancing their analgesic effects in preclinical studies (Hutchinson et al. 2008). The present study is the first to demonstrate the dose-dependent effects of MINO on oxycodone-induced subjective responses in non-opioid-dependent humans. This study provides evidence that supports further investigation of MINO in opioid use disorders.
Highlights.
Microglial inhibitors, such as minocycline (MINO), may be involved in attenuating the abuse liability of oral oxycodone (OXY).
Oral MINO 100 mg and 200 mg was safe and well-tolerated in combination with oral OXY 40 mg.
MINO attenuated OXY-induced positive subjective effects compared to OXY alone.
MINO did not alter physiologic or analgesic effects of OXY.
Acknowledgements
We gratefully acknowledge the support of the National Institute on Drug Abuse in the form of research grants to SDC (R01 DA16759) and HDK (P50 DA09236). In addition, we thank Jeanne Manubay, MD, Maria Sullivan, MD, PhD, Janet Murray, RN, and Claudia Tindall, RN, for their medical assistance, as well as Verena Metz, PhD, Jessica Fogel, BA, Rachel Luba, BA, Brian Wade, BA, and Andrew Segoshi, BA for their technical assistance. Part of the data were presented at the annual meeting of the Experimental Biology Conference in Boston, Massachusetts, April 2013 and the annual meeting of the College on Problems of Drug Dependence in San Diego, California, 2013. The experiment complies with the current laws of the U.S.
Funding:
This research was funded by the National Institute on Drug Abuse in the form of research grants to SDC (R01 DA16759) and HDK (P50 DA09236).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Declaration
Dr. Comer has no competing interests in relation to the work described. Dr. Mogali, Dr. Askalsky, Ms. Madera and Dr. Jones declare no potential conflicts of interest. This study was an original concept. The authors listed have full control of all primary data and agree to allow the journal to review their data if requested.
Declarations of interest: None
References
- Arout CA, Waters AJ, MacLean RR, Compton P, Sofuoglu M (2019) Minocycline does not affect experimental pain or addiction-related outcomes in opioid maintained patients. Psychopharmacology 236, 2857. 10.1007/s00213-018-5146-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agrawal RG, Hewetson A, George CM, Syapin PJ, Bergeson SE (2011) Minocycline reduces ethanol drinking. Brain Behav Immun 25:S165–169. doi: 10.1016/j.bbi.2011.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akgűn E, Lunzer MM, Portoghese P (2019) Combined glia inhibition and opioid receptor agonism afford highly potent analgesics without tolerance. ACS Chemical Neuroscience 10 (4), 2004–2011. doi: 10.1021/acschemneuro.8b00323. [DOI] [PubMed] [Google Scholar]
- Arezoomandan R, Haghparast A (2015) Administration of the glial cell modulator, minocycline, in the nucleus accumbens attenuated the maintenance and reinstatement of morphine-seeking behavior. Can J Physiol Pharmacol 94:257–264. [DOI] [PubMed] [Google Scholar]
- Brogden RN, Speight TM, Avery GS (1975) Minocycline: A review of its antibacterial and pharmacokinetic properties and therapeutic use. Drugs 9:251–291. [DOI] [PubMed] [Google Scholar]
- Carranza-Aguilar CJ, Hernández-Mendoza A, Mejias-Aponte C, Rice KC, Morales M, González-Espinosa C, Cruz SL (2020) Morphine and Fentanyl Repeated Administration Induces Different Levels of NLRP3-Dependent Pyroptosis in the Dorsal Raphe Nucleus of Male Rats via Cell-Specific Activation of TLR4 and Opioid Receptors. Cell Mol Neurobiol. September 14. doi: 10.1007/s10571-020-00957-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao CC, Gekker G, Sheng WS, Hu S, Tsang M, Peterson PK (1994) Priming effect of morphine on the production of tumor necrosis factor-alpha by microglia: implications in respiratory burst activity and human immunodeficiency virus-1 expression. J Pharmacol Exp Ther 269:198–203. [PubMed] [Google Scholar]
- Comer SD, Collins ED, MacArthur RB, Fischman MW (1999) Comparison of intravenous and intranasal heroin self-administration by morphine-maintained humans. Psychopharmacology 143:327–338. [DOI] [PubMed] [Google Scholar]
- Comer SD, Sullivan MA, Vosburg SK, Kowalcyzk WJ, Houser J (2010) Abuse liability of oxycodone as a function of pain and drug use history. Drug Alcohol Depend 109:130–138. doi: 10.1016/j.drugalcdep.2009.12.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conley KM, Toledano AY, Apfelbaum JL, Zacny JP. (1997) Modulating effects of a cold water stimulus on opioid effects in volunteers. Psychopharmacology (Berl). 131(4):313–320. [DOI] [PubMed] [Google Scholar]
- Cook CD, Nickerson MD. (2005) Nociceptive sensitivity and opioid antinociception and antihyperalgesia in Freund's adjuvant-induced arthritic male and female rats. The Journal of pharmacology and experimental therapeutics. 313(1):449–459. [DOI] [PubMed] [Google Scholar]
- Cui Y, Liao XX, Liu W, Guo RX, Wu ZZ, Zhao CM, Chen PX, Feng JQ (2008) A novel role of minocycline: attenuating morphine antinociceptive tolerance by inhibition of p38 MAPK in the activated spinal microglia. Brain Behav Immun 22:114–123. [DOI] [PubMed] [Google Scholar]
- Cunha BA (2000) Minocycline versus Doxycycline in the Treatment of Lyme Neuroborreliosis. Clinical Infectious Diseases. January;30(1):237–8. doi: 10.1086/313604. [DOI] [PubMed] [Google Scholar]
- Curtin CM, Kenney D, Suarez P, Hentz VR, Hernandez-Boussard T, Mackey s, Carroll IR (2007) A double-blind placebo randomized controlled trial of minocycline to reduce pain after carapl tunnel and trigger finger release. J Hand Surg 42: 166–174. [DOI] [PubMed] [Google Scholar]
- Dean OM, Maes M, Ashton M, Berk L, Kanchanatawan B, Sughondhabirom A, Tangwongchai S, Ng C, Dowling N, Malhi GS, Berk M (2014) Protocol and rationale-the efficacy of minocycline as an adjunctive treatment for major depressive disorder: a double blind, randomized, placebo controlled trial. Clin Psychopharmacol Neurosci 12:180–188. doi: 10.9758/cpn.2014.12.3.180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle HH, Murphy AZ (2017) Sex differences in innate immunity and its impact on opioid pharmacology. J Neurosci Res 95(1-2):487–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dworkin RH, Turk DC, Farrar JT et al. (2005) Core outcome measures for chronic pain clinical trials: IMMPACT recommendations. Pain 113:9–19. [DOI] [PubMed] [Google Scholar]
- Foltin RW, Fischman MW (1995) Interaction of buprenorphine with cocaine-morphine combinations. Exp Clin Psychopharmacol 3:261–269. [Google Scholar]
- Fujita Y, Kunitachi S, Iyo M, Hashimoto K (2012) The antibiotic minocycline prevents methamphetamine-induced rewarding effects in mice. Pharmacol Biochem Behav 101:303–306. doi: 10.1016/j.pbb.2012.01.005 [DOI] [PubMed] [Google Scholar]
- Gagnon J, Roth JM, Carroll M, Haycock KA, Plamondon J, Feldman DS, et al. (1990) Superanova accessible general linear modeling. Yale J Biol Med 63:191–192. [Google Scholar]
- Ghazvini H, Rezayof A, Ghasemzadeh Z, Zarrindast MR (2015) μ-Opioid and N-methyl-D-aspartate receptors in the amygdala contribute to minocycline-induced potentiation of morphine analgesia in rats. Behav Pharmacol 26:383–392. doi: 10.1097/FBP.0000000000000126 [DOI] [PubMed] [Google Scholar]
- GraphPad Software, Inc. Prism 6 for Mac (2014). La Jolla, CA. [Google Scholar]
- Guy W, Wilson WH, Brooking B, Manov G, Fjetland O (1986) Reliability and validity of SAFTEE: preliminary analyses. Psychopharmacol Bull 22:397–401. [PubMed] [Google Scholar]
- Hameed H, Hameed M, Christo PJ (2010) The Effect of Morphine on Glial Cells as a Potential Therapeutic Target for Pharmacological Development of Analgesic Drugs. Curr Pain Headache Rep 14:96–104. doi: 10.1007/s11916-010-0093-y. [DOI] [PubMed] [Google Scholar]
- Hou Y, Xie G, Liu X, Li G, Jia C, Xu J, Wang B (2016) Minocycline protects against lipopolysaccharide-induced cognitive impairment in mice. Psychopharmacology 233:905–916. [DOI] [PubMed] [Google Scholar]
- Husain MI, Chaudry IB, Rahman RR, Hamirani MM, Qurashi I, Khoso AB, Deakin JFW, Husain N, Young AH (2015) Minocycline as an adjunct for treatment-resistant depressive symptoms:study protocol for a pilot randomised controlled trial. Trials 16:410. doi: 10.1186/s13063-015-0933-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson MR, Northcutt AL, Chao LW, Kearney JJ, Zhang Y, Berkelhammer DL, Loram LC, Rozeske RR, Bland ST, Maier SF, Gleeson TT, Watkins LR (2008) Minocycline suppresses morphine-induced respiratory depression, suppresses morphine-induced reward, and enhances systemic morphine-induced analgesia. Brain Behav Immun 22:1248–1256. doi: 10.1016/j.bbi.2008.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones JD, Sullivan MA, Manubay J, Vosburg SK, Comer SD (2011) The subjective, reinforcing, and analgesic effects of oxycodone in patients with chronic, nonmalignant pain who are maintained on sublingual buprenorphine/naloxone. Neuropsychopharmacology 36:411–422. doi: 10.1038/npp.2010.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato TA, Watabe M, Tsuboi S, Ishikawa K, Hashiya K, Monji A, Utsumi H, Kanba S (2012) Minocycline modulates human social decision-making: possible impact of microglia on personality-oriented social behaviors. PLoS One 7:e40461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley Ken; Preacher Kristopher J. (2012). "On Effect Size". Psychological Methods. 17 (2): 137–152. [DOI] [PubMed] [Google Scholar]
- Kerns RD, Turk DC, Rudy TE (1985) The West Haven–Yale Multidimensional Pain Inventory (WHYMPI). Pain 23:345–356. [DOI] [PubMed] [Google Scholar]
- Kumar SJ, Boehm J, Lee JC (2003) p38 MAP kinases: key signaling molecules as therapeutic targets for inflammatory diseases. Nat Rev Drug Discov 2:717–726. [DOI] [PubMed] [Google Scholar]
- LaPrairie JL, Murphy AZ. Female rats are more vulnerable to the long-term consequences of neonatal inflammatory injury. Pain 132Suppl. 2007;1:S124–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledeboer A, Sloane EM, Milligan ED, Frank MG, Mahony JH, Maier SF, Watkins LR (2005) Minocycline attenuates mechanical allodynia and proinflammatory cytokine expression in rat models of pain facilitation. Pain 115:71–83. [DOI] [PubMed] [Google Scholar]
- Macdonald H, Kelly RG, Allen S, Noble JF, Kanegis LA (1973) Pharmacokinetic studies on minocycline in man, Clin Pharmacol Ther, vol. 14 (pg. 852–61). [DOI] [PubMed] [Google Scholar]
- Marriott I, Huet-Hudson YM. Sexual dimorphism in innate immune responses to infectious organisms. Immunol Res. 2006;34(3):177–192. [DOI] [PubMed] [Google Scholar]
- Melzack R (1987) The short-form McGill Pain Questionnaire. Pain 30:191–197. [DOI] [PubMed] [Google Scholar]
- Mika J, Wawrzczak-Bargiela A, Osikowicz M, Makuch W, Przewlocka B (2009) Attenuation of morphine tolerance by minocycline and pentoxifylline in naive and neuropathic mice. Brain Behav Immun 23:75–84. doi: 10.1016/j.bbi.2008.07.005 [DOI] [PubMed] [Google Scholar]
- Moyer TP, Palmen MA, Johnson P, Charlson JR, Ellefson PJ (1987) Marijuana testing-how good is it? Mayo Clin Proc 62:413–417. [DOI] [PubMed] [Google Scholar]
- Nazemi S, Manaheji H, Zaringhalam J, Sadeghi M, Haghparast A (2012) Post-injury repeated administrations of minocycline improve the antinociceptive effect of morphine in chronic constriction injury model of neuropathic pain in rat. Pharmacol Biochem Behav 102:520–525. doi: 10.1016/j.pbb.2012.07.001 [DOI] [PubMed] [Google Scholar]
- Ordóñnez Gallego A, González Barón M, Espinosa Arranz E (2007) Oxycodone: a pharmacological and clinical review. Clin Transl Oncol 9:298–307. [DOI] [PubMed] [Google Scholar]
- Piao ZG, Cho M, Park CK, Hong JP, Choi SY, Lee SJ, Lee S, Park K, Kim JS, Oh SB (2006) Activation of glia and microglial p38 MAPK in medullary dorsal horn contributes to tactile hypersensitivity following trigeminal sensory nerve injury. Pain 121:219–231. [DOI] [PubMed] [Google Scholar]
- Pickworth WB, Johnson RE, Holicky BA, Cone EJ (1993) Subjective and physiologic effects of intravenous buprenorphine in humans. Clin Pharmacol Ther 53:570–576. [DOI] [PubMed] [Google Scholar]
- Preston KL, Bigelow GE, Bickel WK, Liebson IA (1989) Drug discrimination in human postaddicts: agonist-antagonist opioids. J Pharmacol Exp Ther 250:184–196. [PubMed] [Google Scholar]
- Preston KL, Liebson IA, Bigelow GE (1992) Discrimination of agonist-antagonist opioids in humans trained on a two-choice saline-hydromorphone discrimination. J Pharmacol Exp Ther 261:62–71. [PubMed] [Google Scholar]
- Rabkin JG, Markowitz JS (1986) Side effect assessment with SAFTEE: pilot study of the instrument. Psychopharmacol Bull 22:389–396. [PubMed] [Google Scholar]
- Samour MS, Nagi SS, Shortland PJ, Mahns DA (2017) Minocycline prevents muscular pain hypersensitivity and cutaneous allodynia produced by repeated intramuscular injections of hypertonic saline in healthy human participants. J Pain 18:994–1005. [DOI] [PubMed] [Google Scholar]
- Schafers M, Svensson CI, Sommer C, Sorkin LS (2003) Tumor necrosis factor-alpha induces mechanical allodynia after spinal nerve ligation by activation of p38 MAPK in primary sensory neurons. JNeurosci 23:2517–2521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz JM, Bilbo SD. Sex, glia, and development: interactions in health and disease. Hormones and behavior. 2012;62(3):243–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AM, Dragunow M (2014) The human side of microglia. Trends Neurosci 37:125–135. [DOI] [PubMed] [Google Scholar]
- Sofuoglu M, DeVito EE, Waters AJ, Carroll KM (2013) Cognitive enhancement as a treatment for drug addictions. Neuropharmacology 64:452–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofuoglu M, Mooney M, Kosten T, Waters A, Hashimoto K (2011) Minocycline attenuates subjective rewarding effects of dextroamphetamine in humans. Psychopharmacology 213:61–68. doi: 10.1007/s00213-010-2014-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofuoglu M, Waters AJ, Mooney M, O’Malley SS (2009) Minocycline reduced craving for cigarettes but did not affect smoking or intravenous nicotine responses in humans. Pharmacol Biochem Behav 92:135–140. doi: 10.1016/j.pbb.2008.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SPSS I SPSS 15.0.0 for Windows (2006) Pearson-Prentice Hall: Chicago, IL. [Google Scholar]
- Strain EC, Preston KL, Liebson IA, Bigelow GE (1992) Acute effects of buprenorphine, hydromorphone and naloxone in methadone-maintained volunteers. J Pharmacol Exp Ther 261:985–993. [PubMed] [Google Scholar]
- Streit WJ, Mrak RE, Griffin WS. Microglia and neuroinflammation: a pathological perspective. Journal of nenroinflammation. 2004;1(1):14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svensson CI, Marsala M, Westerlund A, Calcutt NA, Campana WM, Freshwater JD, Catalano R, Feng Y, Protter AA, Scott B, Yaksh TL (2003) Activation of p38 mitogen-activated protein kinase in spinal microglia is a critical link in inflammation-induced spinal pain processing. J Neurochem 86:1534–1544. [DOI] [PubMed] [Google Scholar]
- Tikka T, Fiebich BL, Goldsteins G, Keinanen R, Koistinaho J (2001) Minocycline, a tetracycline derivative, is neuroprotective against excitotoxicity by inhibiting activation and proliferation of microglia. JNeurosci 21:2580–2588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanelderen P, van Zundert J, Kozicz T, Puylaert M, de Vooght P, Mestrum R, Heylen R, Roubos E, Vissers K (2015) Effect of minocycline on lumbar radicular neuropathic pain: a randomized, placebo-controlled, double-blind clinical trial with amitriptyline as a comparator. Anesthesiology 122:399–406. [DOI] [PubMed] [Google Scholar]
- Vogt MA, Mallien AS, Pfeiffer N, Inta I, Gass P, Inta D (2016) Minocycline does not evoke anxiolytic and antidepressant-like effects in C57BL/6 mice. Behav Brain Res 301:96–101. doi: 10.1016/j.bbr.2015.12.015. [DOI] [PubMed] [Google Scholar]
- Wadhwa M, Prabhakar A, Ray K, Roy K, Kumari P, Jha PK, Kishore K, Kumar S, Panjwani U (2017) Inhibiting the microglia activation improves the spatial memory and adult neurogenesis rat hippocampus during 48 h of sleep deprivation. J Neuroinflammation 14:222. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Wang RI, Wiesen RL, Lamid S, Roh BL. (1974) Rating the presence and severity of opiate dependence. Clin Pharmacol Therapeu 16(4): 653–658. [DOI] [PubMed] [Google Scholar]
- Watabe M, Kato TA, Monji A, Horikawa H, Kanba S (2012) Does Minocycline, an antibiotic with inhibitory effects on microglial activation, sharpen a sense of trust in social interaction? Psychopharmacology 220:551–557. [DOI] [PubMed] [Google Scholar]
- Watkins LR, Hutchinson MR, Johnston IN, Maier SF (2005) Glia: novel counter-regulators of opioid analgesia. Trends Neurosci 28:661–669. [DOI] [PubMed] [Google Scholar]
- Xiao-Peng M, Chen L, Wang W, Wu D, Wang LY, Zhang T, Zhang H, Li YQ (2013) Combination of tramadol with minocycline exerted synergistic effects on a rat model of nerve injury-induced neuropathic pain. Neurosignals 21:184–196. [DOI] [PubMed] [Google Scholar]
- Yrjanheikki J, Keinanen R, Pellikka M, Hokfelt T, Koistinaho J (1998) Tetracyclines inhibit microglial activation and are neuroprotective in global brain ischemia. Proc Natl Acad Sci USA 95:15769–15774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zacny JP (1995) A review of the effects of opioids on psychomotor and cognitive functioning in humans. Exp Clin Psychopharmacol 3:432–466. [Google Scholar]
- Zacny JP, McKay MA, Toledano AY, Marks S, Young CJ, Klock PA, Apfelbaum JL (1996) The effects of a cold-water immersion stressor on the reinforcing and subjective effects of fentanyl in healthy volunteers. Drug Alcohol Depend 42:133–142. [DOI] [PubMed] [Google Scholar]
- Zilm DH, Sellers EM. (1978) The quantitative assessment of physical dependence on opiates, Drug Alcohol Depend 3:419–428. [DOI] [PubMed] [Google Scholar]


