Abstract
Alcohol misuse is associated with sleep disturbance and cognitive dysfunction. However, the neural processes inter-relating the severity of alcohol use, sleep disturbance and cognitive performance remain under-investigated. We addressed this issue with a dataset of 964 subjects (504 women) curated from the Human Connectome Project. Participants were assessed with the Pittsburgh Sleep Quality Index (PSQI) and fMRI while identifying relational dimension pictures and matching dimension pictures (as a control) in alternating blocks. Imaging data were analyzed with published routines and the results were evaluated at a corrected threshold. Subjects showed lower accuracy rate and longer reaction time (RT) in relational than control blocks. The difference in RT between the two blocks (RTRel-Con) was driven primarily by the RT and correlated positively with performance accuracy of relational trials, suggesting that a more cautious response (i.e., longer RTRel-Con) improved accuracy. The severity of alcohol use, identified from principal component analysis of drinking metrics, was positively correlated with sleep disturbance. Further, whole-brain regression identified activity of the superior colliculus (SC) during relational vs. control blocks in positive and negative correlation with RTRel-Con and PSQI score, respectively. Mediation and path analyses demonstrated a significant model: more severe alcohol use → greater sleep → disturbance diminished SC activity → impaired performance. These findings support the influences of alcohol misuse on sleep and suggest neural correlates that mediate the relationship between sleep disturbance and altered sustained attention in young adults.
Keywords: alcohol use disorder, sleep quality, attention, superior colliculus, thalamus
1. Introduction
1.1. Alcohol misuse and sleep disturbance
Alcohol misuse is known to be associated with sleep disturbance (Chakravorty et al., 2016; He et al., 2019). Heavy alcohol use and alcohol use disorder (AUD) have been linked to subjective reports of insomnia and polysomnographic findings of sleep disturbance (Arnedt et al., 2011; Crum et al., 2004; Dorrian et al., 2017; Haario et al., 2013). Epidemiologic studies showed that frequent alcohol consumption is associated with insomnia symptoms in both young (Miller et al., 2017) and older adults (Canham et al., 2015; Wang et al., 2016). The association between insomnia and heavy drinking is bi-directional; that is, heavy drinking predicts future insomnia symptoms and conversely insomnia increases the risk of heavy drinking (Haario et al., 2013). Heavy alcohol consumption has been linked to abnormal circadian, such as core body temperature, rhythm (Danel et al., 2001) and salivary melatonin levels (Rupp et al., 2007). Other studies associated alcohol consumption with later bedtime in adolescents (Hasler et al., 2017) and college students (Adan, 1994; Onyper et al., 2012), and with short night sleep duration (< 6 hours) (Chakravorty et al., 2016) and breathing-related sleep disorders (Kolla et al., 2018). Thus, a substantial literature has associated sleep disturbance with alcohol misuse.
1.2. Influence of sleep disturbance on attention
Sleep serves a multitude of physiological functions, including enhancement of immune defense (Besedovsky et al., 2012) and clearance of brain metabolites (Xie et al., 2013). Sleep is integral to cognitive functioning (Brownlow et al., 2020; Lowe et al., 2017; Scullin and Bliwise, 2015); for instance, sleep promotes memory encoding and consolidation (Stickgold, 2005). Poorer sleep, as characterized by questionnaire (e.g., Pittsburgh Sleep Quality Index; PSQI) assessment, is associated with impairment in executive function (Benitez and Gunstad, 2012), working memory (Fortier-Brochu and Morin, 2014; Shekleton et al., 2014), inhibitory control (Fortier-Brochu and Morin, 2014; Haimov et al., 2008), and sustained attention (Altena et al., 2008; Varkevisser and Kerkhof, 2005). Sustained attention showed circadian and homeostatic variations (Valdez, 2019), and sleep restriction impaired sustained attention (Shenfield et al., 2020), which is required to meet a wide array of cognitive challenges. For instance, in adolescents, sleep of a shorter than recommended duration and lack of recovery sleep for 1 to 7 nights can have deleterious effects on a wide range of cognitive functions including alertness (Lo et al., 2016) and visual attention (Agostini et al., 2017; Louca and Short, 2014). Poor sleep quality as evaluated by actigraphy is associated with inferior performance on tasks of working memory (Steenari et al., 2003) and executive functioning (Kuula et al., 2015).
The two most widely studied cognitive domains in sleep deprivation research are working memory and attention (Alhola and Polo-Kantola, 2007). Working memory requires sustained attention (Baddeley et al., 1999), which is impaired by sleep deprivation and restored after sleep (Hudson et al., 2020). Using a within-subject design with rested wakefulness and sleep deprivation counterbalanced across participants, a study reported that sleep deprivation resulted in lower inferior frontal and inferior occipital cortical activities bilaterally during visual contrast discrimination (Chee and Tan, 2010). Further, sleep deprivation attenuated brain activation independently of task difficulty, suggesting a non-specific effect on cognition, most likely a loss of sustained attention/vigilance. Another study of within-subject effects showed higher ventrolateral thalamic activity during visual attention when subjects were sleep-deprived overnight, potentially suggesting a compensatory process (Portas et al., 1998). Tomasi and colleagues likewise reported higher thalamic activation after sleep deprivation than during rested wakefulness in an attention task, and thalamic activity increased with task difficulty only during rested wakefulness (Tomasi et al., 2009). Total sleep deprivation was associated with longer reaction time (RT) and higher activity in frontal and posterior midline regions of the default mode network in the Psychomotor Vigilance Test (Drummond et al., 2005). Sleep deprivation altered perceptual sensitivity and the effects of interstimulus interval on RT in the Psychomotor Vigilance Test (Yang et al., 2018). Other studies suggest inter-subject variation in the effects of sleep deprivation on attention (Krause et al., 2017) and the potential roles of heritability (Kuna et al., 2012) and frontoparietal network activity (Cui et al., 2015) in accounting for the individual differences. Not surprisingly, impairment in attention and other cognitive processes have also been noted for clinical conditions that manifest sleep disturbance, including chronic obstructive pulmonary disease, obstructive sleep apnea, and primary insomnia (Fortier-Brochu et al., 2012; Olaithe et al., 2018; Stranks and Crowe, 2016; Torres-Sánchez et al., 2015).
1.3. Alcohol consumption and attention
These studies together demonstrate the influences of sleep disturbance on cognitive performance, particularly in behavioral tasks that require sustained attention. An extensive literature characterizes how alcohol misuse influences cognitive processing (Le Berre et al., 2017; Spear, 2018). For instance, individuals with alcohol use disorders relative to healthy controls showed decreased activation of parietal and prefrontal cortices during visual attention (Zehra et al., 2019). Acute alcohol intoxication altered electroencephalographic dynamics in visual control processing (Schiller et al., 2021) and event-related potentials during task switching (Wolff et al., 2018). Alcohol hangover was associated with deficits in selective attention (Devenney et al., 2019). On the other hand, the studies have not assessed sleep disturbance and it remains unclear whether or how sleep disruption may play a role in manifesting cognitive dysfunction. No work to our knowledge has investigated the neural processes inter-linking alcohol misuse, sleep disturbance and cognitive dysfunction.
1.4. The present study
The present study addressed this issue with clinical and imaging data of the Human Connectome Project (HCP), where the alcohol consumption and sleep disturbance measures were available from assessment with the Semi-Structured Assessment and the Genetics of Alcoholism and PSQI, respectively. We focused on the relational task, which queries individuals’ sustained attention during visual pattern matching, and broadly hypothesized that, across individuals, more severe alcohol use would lead to sleep disturbance and in turn influence the neural processes and performance in the relational task. We employed mediation and path analyses to characterize the inter-relationships of these clinical, neural and behavioral variables. Identifying the neural substrates relating the severity of alcohol use, sleep disturbance, and cognitive performance would advance both sleep and addiction medicine.
2. Materials and Methods
2.1. Dataset
We have obtained permission from the HCP to use both the Open and Restricted Access data for the current study. We employed the 1200 Subjects Release (S1200) data set, which includes 3T MR imaging and behavioral data collected of the Relational task from 1039 subjects. A total of 964 subjects were included in the study, after the exclusion of 75 subjects who had head movements greater than 2mm in translation or 2 degrees in rotation or for whom the images failed in registration to the template. All subjects were physically healthy with no severe neurodevelopmental, neuropsychiatric or neurologic disorders. Participants provided written informed consent and all aspects of the study, including subject recruitment, experimental procedures were conducted according to a protocol in accordance with the Declaration of Helsinki and approved by the Washington University Institutional Review Board (IRB #201204036; title: “Mapping the Human Connectome: Structure, Function and Heritability”).
Participants were evaluated with the Pittsburgh Sleep Quality Index (Buysse et al., 1989), which contains 19 self-rated questions. The 19 self-rated items are combined to 7 component scores, each of which was scored from 0 to 3 with “0” and “3” each indicating no and severe difficulty with sleep. Thus, individuals ranged from 0 to 21 in PSQI score, with a higher score representing worse sleep quality.
The HCP data comprised 15 inter-related drinking metrics to assess alcohol use behavior (Supplementary Table S1). We performed a principal component analysis on the 15 measures. Four principal components showed an eigenvalue > 1, with the first principal component (PC1) accounting for 49.15% and the other components each accounting for 9.66%, 8.89% and 7.17% of the variance. All drinking metrics loaded substantially on the PC1, and we used the PC1 to represent drinking severity in current study. Note that six of the 15 measures were reversed score so a higher PC1 weight indicated greater severity of alcohol use.
Table 1 shows the demographics, clinical and behavioral measures of the participants.
Table 1.
Demographics, clinical and behavioral measures of the participants
| Characteristic | Men (n = 460) | Women (n = 504) | t | p value |
|---|---|---|---|---|
| Age, Years | 27.9 ± 3.7 | 29.4 ± 3.6 | −6.58 | <0.001 |
| Education, Years | 14.9 ± 1.7 | 15.1 ± 1.8 | −1.66 | 0.098 |
| PSQI score | 4.6 ± 2.5 | 4.8 ± 2.9 | −1.92 | 0.055 |
| Drinking PC1 | 0.3 ± 1.0 | −0.3 ± 0.8 | 9.78 | <0.001 |
| ARRel-Con, % | −21.1 ± 16.5 | −21.2 ± 16.9 | −0.05 | 0.960 |
| RTRel-Con, ms | 494 ± 307 | 503 ± 308 | −0.27 | 0.789 |
Note: ARRel-con, the difference in accuracy rate between relational and control blocks. Age and years of education were controlled for in the two-sample t test of PSQI score, drinking PC1 weight, ARRel-con and RTRel-con.
2.2. Imaging protocol and relational task for fMRI
Imaging protocols are described in previous studies (Li et al., 2020a, b) and in Supplementary Methods.
Each participant completed two runs each of six blocks (three relation and three control) of the relational task (Barch et al., 2013; Smith et al., 2007) in a fixed order (first run: relation, control, control, relation, control, relation; second run: relation, control, relation, control, control, relation). Details of relational task are described in Supplementary Methods.
2.3. Imaging Data Modeling and Statistics
We followed published routines (Zhang et al., 2019; Zhornitsky et al., 2019) in data preprocessing and model constructing, as also described in Supplementary Methods.
We constructed for each individual subject the statistical contrast “relational vs. control.” In group analyses, we conducted a one-sample t test of the contrast “relational vs. control” to identify regional responses and a two-sample t test of the same contrast to identify sex differences between men and women with age and years of education as covariates. We also conducted voxel-wise regressions of the contrast (relational - control) against the differences in accuracy rate (relational - control; ARRel-Con), differences in reaction time (relational - control; RTRel-Con) of correct trials, PSQI score, and drinking PC1 weight, all with age, sex and years of education as covariates. We evaluated the results at an uncorrected voxel p < 0.001, in combination with cluster p < 0.05, corrected for FWE of multiple comparisons, on the basis of Gaussian random field theory, as implemented in SPM. We identified brain regions using the Data Processing & Analysis of Brain Imaging toolbox (DPABI) (Yan et al., 2016) and an atlas (Duvernoy, 2009), if the peak was not identified by the DPABI.
Functional regions of interest (ROIs) were defined based on clusters obtained from whole-brain analysis. In ROI analysis, we used MarsBar (http://marsbar.sourceforge.net/) to derive for each individual subject the activity (β’s averaged across voxels) for the ROIs.
2.4. Mediation and Path Analyses
We performed mediation analyses following published routines (MacKinnon et al., 2007; Wager et al., 2008), as detailed in the Supplementary Methods and our previous work (Zhang et al., 2019; Zhornitsky et al., 2019), to evaluate the relationships between neural markers, PSQI score and RT across all subjects (see Results).
Following up on mediation analysis and the findings of a significant correlation between PC1 of alcohol use severity and PSQI score (see Results), we performed path analyses (Supplementary Methods) to examine the interrelationship between alcohol use severity and PSQI score, superior colliculus activity and RT of correct trials.
3. Results
3.1. Drinking severity, PSQI score, and task performance
Men showed higher drinking severity PC1 than women (t = 9.78, p < 0.001); men and women did not differ significantly in PSQI score (Table 1).
For AR, an ANOVA (sex × block) showed a significant block main effect (F = 1021.6, p < 0.001) but not sex main effect (F = 0.4, p = 0.517) or sex × stimulus interaction (F = 0.0, p = 0.953) with age and years of education as covariates. In post-hoc analyses both men’s and women’s AR was higher for the control than relational blocks (p’s < 0.001) (Supplementary Figure S1A).
For RT, an ANOVA (sex × block) showed a significant block main (F = 1100.0, p < 0.001) but not sex main (F = 0.0, p = 0.933) or sex × block interaction (F = 0.1, p = 0.768) effect with age and years of education as covariates. Both men and women (p’s < 0.001) showed longer RT during relational than control trials (Supplementary Figure S1B).
For all subjects, we computed the difference in AR and RT during relational vs. control blocks, ARRel-Con and RTRel-Con, respectively. We performed pairwise regression among the four variables: drinking severity PC1, PSQI score, ARRel-Con and RTRel-Con for men and women separately with age and years of education as covariates and tested for slope differences between the sexes. None of the six regressions showed a significant difference in slope between men and women (all p’s > 0.285; Supplementary Table S2). Thus, we combined men and women in all subsequent analyses. Across all subjects, the PSQI score was correlated significantly with PC1 (r = 0.10, p = 0.002; Figure 1A). None of the other regressions yielded significant correlations (all p’s > 0.074).
Figure 1.
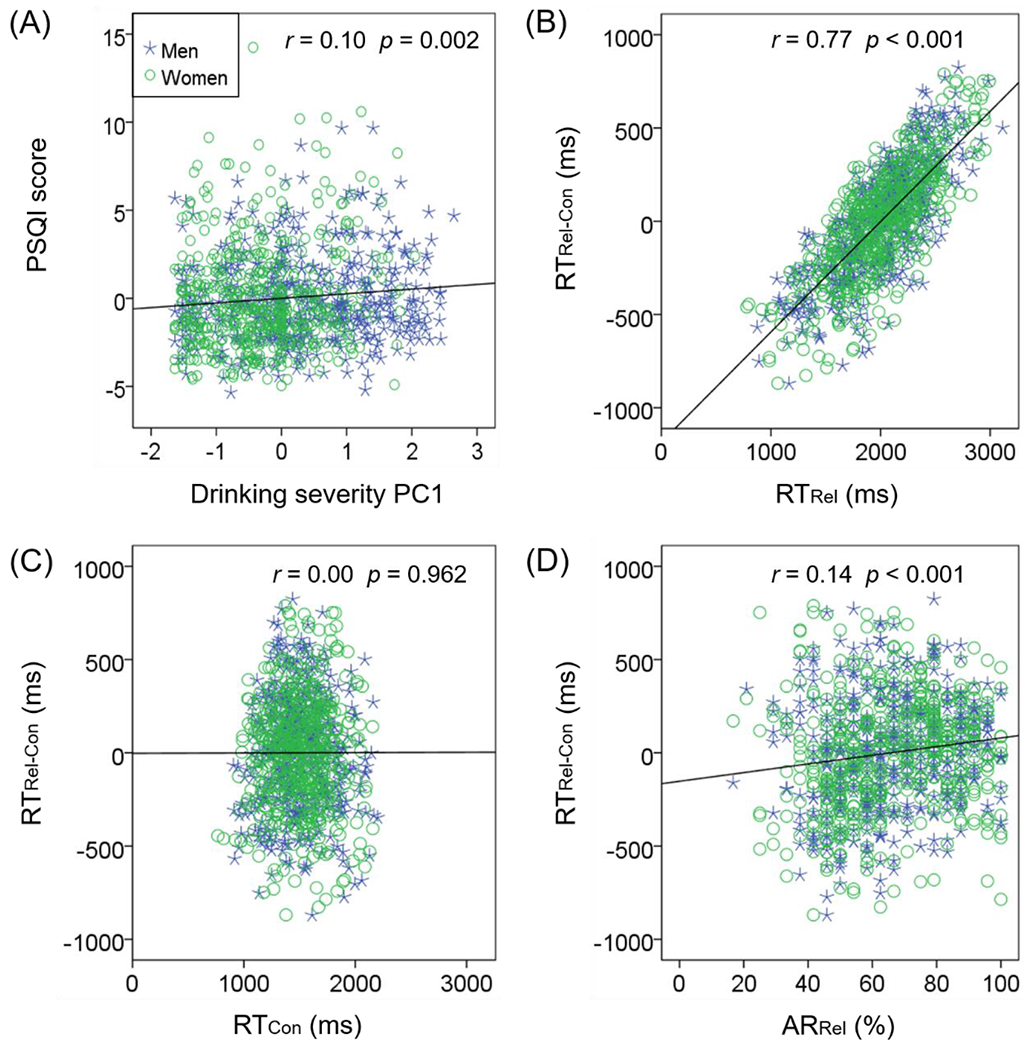
Scatter plots of the correlation between clinical and relational task performance variables. Data points are shown for men (blue asterisk) and women (green circle) separately. Men and women did not differ in the slopes of regressions; thus, the regression lines (black) show the correlations across all subjects. (A) PSQI score and drinking PC1 were positively correlated; RTRel-Con were positively correlated with RTRel (B) but not with RTCon (C); RTRel-Con was positively correlated with ARRel (D). Note that residuals are plotted here with age, sex, and years of education accounted for in the regressions.
To better understand the source of the RT difference between the relational vs. control blocks, RTRel-Con, we observed that RTRel-Con was positively correlated with RTRel (r = 0.77, p < 0.001; Figure 1B) but not with RTCon (r = 0.00, p = 0.962; Figure 1C). Further, RTRel-Con was positively correlated with ARRel (r = 0.14, p < 0.001; Figure 1D), suggesting that overall, a slower response during relational vs. control blocks, likely reflecting sustained attention and cautious responding, facilitates performance accuracy.
3.2. Brain activations to relational vs. control blocks
We conducted a two-sample t test to compare men and women with age and years of education as covariates on the contrast of relational vs. control blocks, and no clusters showed sex differences at uncorrected voxel p < 0.001, in combination with cluster p < 0.05, corrected for FWE. We thus examined the regional responses for the entire cohort a one-sample t test. Supplementary Figure S2 shows the results. Relational vs. control blocks involved higher activation in bilateral superior/inferior frontal cortex, bilateral supplementary motor area, bilateral insula, bilateral inferior parietal cortex, bilateral precuneus. Conversely, control vs. relational involved higher activation in the bilateral orbitofrontal cortex, anterior, middle and posterior cingulate, bilateral calcarine, bilateral superior temporal cortex and bilateral hippocampus. Many of these brain regions were contiguous to form larger clusters, as summarized in Supplementary Table S3.
3.3. Brain activations in correlation with PSQI score, PC1, ARRel-Con, and RTRel-Con
A cluster encompassing the right pulvinar and superior colliculus (SC; x = 6, y = −26, z = −6, volume = 568 mm3, T = 4.37) showed activities during relation vs. control blocks in negative correlation with PSQI (Figure 2A), with age, sex and years of education as covariates. No clusters showed positive correlation with PSQI score at the same threshold.
Figure 2.
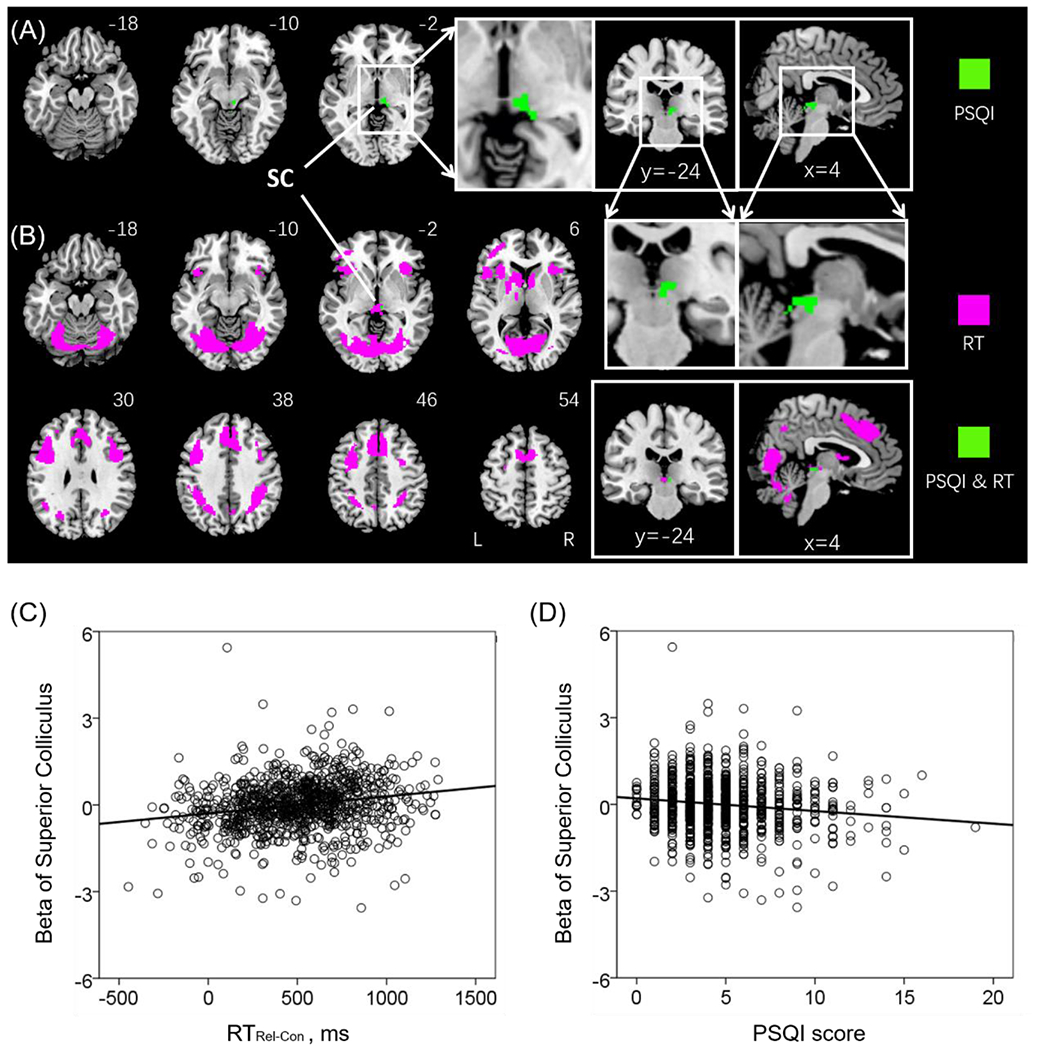
(A) One cluster comprising the pulvinar and superior colliculus (SC) showed negative correlation with PSQI (highlighted in green). (B) A number of clusters showed positive correlation with RTRel-Con (magenta); and one cluster, located in the SC, showed positive correlation with RTRel-Con and negative correlation with PSQI (green). Linear regression of (C) beta estimates of the SC vs. the difference in RT between relational and control blocks or RTRel-Con (D) beta estimates of SC vs. the PSQI score. Note that the scatter plots show the residuals, after age, sex, and years of education were accounted for. Clusters are overlaid on a T1 structural image in neurological orientation: right = right. The inset shows sagittal/coronal sections to highlight the cluster in the SC.
Figure 2B shows regional activations to the same contrast in correlation with RTRel-Con of correct trials across all subjects with sex, age and years of education as covariates (clusters summarized in Table 2). RTRel-Con was positively correlated with activation in bilateral fusiform, supplementary motor area cortex, insula, precuneus, caudate, middle occipital cortex and the SC. No clusters showed significant negative correlation with RTRel-Con at the same threshold.
Table 2.
Clusters showing correlation with RTRel-con
| Region | Cluster | Peak | Cluster | MNI coordinate (mm) | ||
|---|---|---|---|---|---|---|
|
| ||||||
| size (k) | Voxel (T) | FWE P-value | X | Y | Z | |
| Positive | ||||||
| Fusiform_R | 6923 | 18.50 | 0.000 | 26 | −78 | −10 |
| Supp_Motor_Area_L | 1673 | 13.70 | 0.000 | 0 | 16 | 50 |
| Insula_L | 3151 | 11.09 | 0.000 | −32 | 24 | −6 |
| Occipital_Mid_R | 674 | 10.67 | 0.000 | 30 | −76 | 16 |
| Insula_R | 1371 | 9.56 | 0.000 | 34 | 24 | −2 |
| Precuneus_R | 111 | 8.30 | 0.000 | 4 | −62 | 42 |
| Caudate_L | 806 | 8.26 | 0.000 | −14 | 6 | 14 |
| Superior colliculus | 78 | 7.56 | 0.000 | −2 | −28 | −2 |
Note: R: right; L: left.
The SC showed activations both in positive correlation with RTRel-Con and in negative correlation with PSQI score. We computed the β estimates of the SC and visualized the correlation in Pearson regressions in Figure 2C (r = 0.21, p < 0.001) and 2D (r = −0.14, p < 0.001).
Figure 3A shows the regional activations in positive correlation with PC1. Summarized in Table 3, the clusters with activities correlated with PC1 involved left inferior frontal, left precentral, left lingual cortex, bilateral supplementary motor area, and bilateral middle cingulum. Figure 3B shows the regional activations in positive correlation with RTRel-Con again in order to highlight the clusters in positive correlation with both PC1 and RTRel-Con, with age, sex and years of education as covariates. We computed the average β estimates of these clusters and visualized the correlations in Pearson regressions in Figure 3C (RTRel-Con: r = 0.42, p < 0.001) and 3D (PC1: r = 0.19, p < 0.001).
Figure 3.
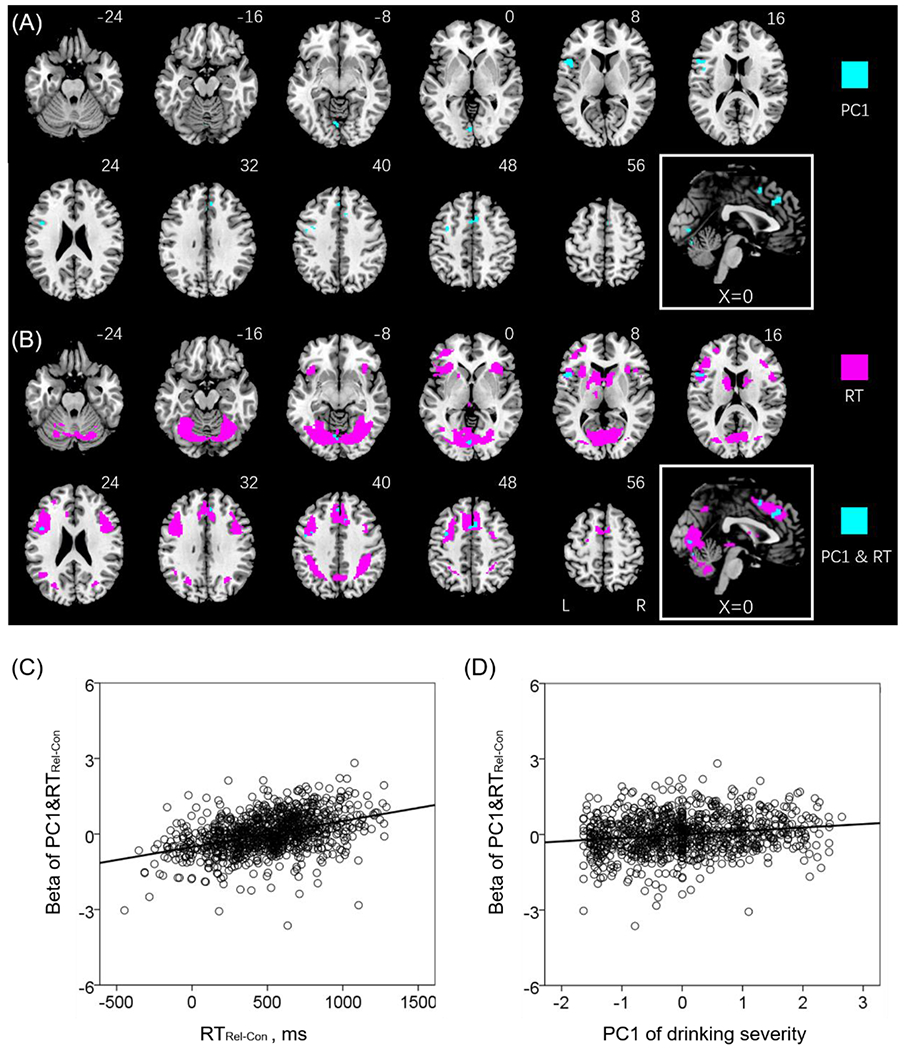
A number of clusters showed activities in positive correlation with PC1 (A), highlighted in cyan, and with RTRel-Con (B), highlighted in magenta. The voxels showing positive correlation both with RTRel-Con and PC1 were also highlighted in cyan in (B). Linear regression of (C) beta estimates of the overlapping voxels (PC1&RT) vs. the difference in RT between relational and control blocks (RTRel-Con) (D) beta estimates of PC1&RT vs. PC1. Note that the scatter plots show the residuals, after age, sex, and years of education were accounted for. Clusters are overlaid on a T1 structural image in neurological orientation: right = right. The inset shows a mid-sagittal section to highlight the clusters in the middle cingulum and supplementary motor area cortex.
Table 3.
Clusters showing activity in correlation with PC1.
| Region | Cluster size (k) | Peak Voxel (T) | Cluster FWE P-value | MNI coordinate (mm) |
||
|---|---|---|---|---|---|---|
| X | Y | Z | ||||
| Positive | ||||||
| Frontal_Inf_Oper_L | 204 | 5.01 | 0.000 | −50 | 14 | 8 |
| Precentral _L | 76 | 4.55 | 0.007 | −34 | 0 | 44 |
| Supp_Motor_Area_R | 109 | 4.10 | 0.001 | 8 | 12 | 50 |
| Lingual_L | 76 | 4.05 | 0.007 | −4 | −80 | 2 |
| Cingulum_Mid_L | 56 | 3.97 | 0.032 | 0 | 30 | 36 |
Note: R: right; L: left.
Whole brain linear regression of the contrast “relational — control” against ARRel-Con across all subjects, with sex, age and years of education as covariates, showed that ARRel-Con was positively correlated with activation of bilateral middle cingulum, left putamen and supramarginal gyrus (Supplementary Figure S3; clusters summarized in Supplementary Table S4). None of the clusters showed overlaps with those obtained with regression vs. the PSQI score or vs. PC1.
3.4. Mediation and path analyses
The shared regional activities of SC may represent the neural substrates interlinking sleep disturbance and RT in the relational task. Thus, we performed mediation analyses to examine the inter-relationship between activities of SC, PSQI score and RTRel-Con. For the sake of completeness, we evaluated all 6 models, although the models with activities of SC as dependent variables were conceptually unlikely. The results showed the model PSQI → activities of SC → RTRel-Con with the best fit (c - c’ = −3.61, p < 0.001; Figure 4A). Supplementary Table S5 shows the statistics of all other models. Further, PC1 was positively correlated with PSQI (r = 0.10, p = 0.002) across all subjects. We performed path analyses to examine the inter-relationship between alcohol use severity (PC1), sleep disturbance (PSQI), activities of SC and RT of correct trials, and the model PC1 → PSQI → activities of SC → RTRel-Con showed a good fit (Figure 4B; Fit indices: RMSEA = 0.04 [90% CI: 0.02 0.07], χ2/df = 2.65, SRMR = 0.03, and CFI = 0.91). Other path models and fit statistics are shown in Supplementary Figure S4 and Supplementary Table S6.
Figure 4.
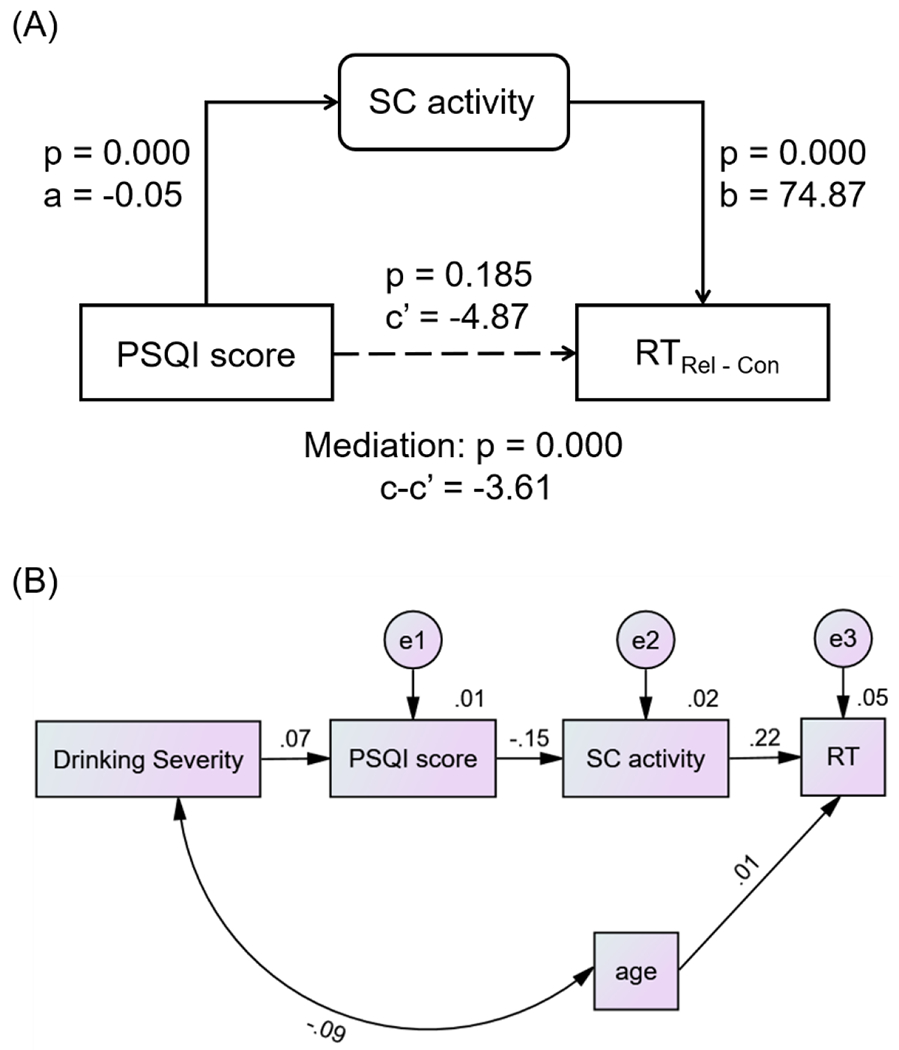
(A) Mediation model to show the inter-relationship of PSQI score, SC activity and RTRel-Con. SC activity completely medicated the relationship between sleep disturbance and reaction time in the relational task. (B) Path model to show the inter-relationship of drinking severity (PC1), PSQI, SC activity and RTRel-Con.
Figure 3B shows the regional activation both in positive correlation with PC1 and RTRel-Con (highlighted in cyan), with age, sex and years of education as covariates. The shared regional activities may represent the neural substrates interlinking drinking severity and RTRel-Con in the relational task. However, in the mediation analyses to examine the inter-relationship between the shared correlates, PC1 and RTRel-Con, no models showed a significant fit (all p’s > 0.097).
4. Discussion
Relational vs. control visual stimulus matching required sustained attention and involved higher activity in bilateral superior/inferior frontal cortex, supplementary motor area, insula, precuneus and inferior parietal cortex. Conversely, control vs. relational trials involved higher activity in the bilateral superior temporal cortex, hippocampus and calcarine. The findings mirror those reported in earlier imaging studies (Barch et al., 2013; Smith et al., 2007; Tomasi and Volkow, 2019). We identified regional brain responses in correlation with individual differences in RT and sleep quality. The SC demonstrated activities during relational vs. control blocks each in negative and positive correlation with PSQI score and RTRel-Con. Mediation and path analyses showed that SC activity mediated the relationship between drinking severity, sleep disturbance and attentional performance. Together, these findings support the influences of heavier drinking on sleep and suggest the neural substrates interrelating alcohol consumption, sleep disturbance and attentional dysfunction.
4.1. The superior colliculus, visual attention and sleep disturbance
The SC is a dome-shaped subcortical laminar structure in the mammalian midbrain, with the superficial layers receiving visual inputs directly from the retina in a topological map (Chan et al., 2011; Olivé et al., 2018). Cortical and subcortical regions involved in eye movement control project directly or indirectly to the SC (Merker, 2013). Together with the pulvinar, a thalamic nucleus critical for visual processing and attentional control (Bridge et al., 2016; Kastner et al., 2020) and projecting to the SC (May, 2006), the SC may be involved with directing eye movements between the stimuli during the relational task (Hu et al., 2019; Robinson and McClurkin, 1989).
Preclinical studies investigated SC activities in sleep. Sleep is regulated by circadian mechanisms, and in the absence of a circadian clock, overall sleep-wake rhythmicity is preserved and remains synchronized to the external light-dark cycle (Lupi et al., 2008). Neuronal activities of the SC are not eliminated during sleep (Cohen and Castro-Alamancos, 2010). Melanopsin-dependent sleep regulation was correlated with the activation of sleep-promoting neurons in the ventrolateral preoptic area and the SC (Lupi et al., 2008). A rodent study showed that dark and light signals affect sleep-wake behaviors through distinct pathways comprising the SC (Zhornitsky et al., 2019), and the regulatory effects appear independent from cortical visual processes (Miller et al., 1998). Thus, sleep disturbance may directly affect SC activities.
In human imaging, the SC responds to visual stimulation (Singh et al., 2018), and plays a key role in supporting spatial attention (Awh and Jonides, 2001; Jerde et al., 2012; Kasai and Isa, 2016; Katyal and Ress, 2013; Schneider and Kastner, 2009), and eye movement (Crapse et al., 2018; Grimaldi et al., 2018), target selection for goal-directed motor responses (Krauzlis et al., 2013; Merker, 2007), spatial working memory (Rahmati et al., 2020), and decision making (Basso et al., 2021; Basso and May, 2017). With its projections to brain stem motor nuclei, the SC supports innate behaviors (Furigo et al., 2010) and engages automatic attention to salient stimuli (Liddell et al., 2005; Mares et al., 2016; Nguyen et al., 2014; Rosa Salva et al., 2015; Wei et al., 2015). The SC is regulated “top-down” by cortical regions (Merker, 2013) in shifting from covert to overt attention (Sato et al., 2016) and integrating endogenous with externally drive attention (Katyal and Ress, 2014; Menon and Uddin, 2010; Mysore and Knudsen, 2014; Xuan et al., 2016). Studies in macaque monkeys showed that representations of visual priority emerge more rapidly in the SC than in primary visual cortex (White et al., 2017). Thus, the current findings of diminished SC activation during sustained attention in relation to sleep disturbance are consistent with this body of literature.
As with the SC, activation of the pulvinar was also negatively correlated with PSQI score. The pulvinar is the largest thalamic nucleus and supports cross-modal sensory processing and integration (Barron et al., 2015; Bourgeois et al., 2020) and other attentional processes (Bourgeois et al., 2020; Fiebelkorn and Kastner, 2020; Fiebelkorn et al., 2019; Gattass et al., 2018; Jaramillo et al., 2019; Stitt et al., 2018). The pulvinar is activated by the SC as well as directly by retinal inputs before visual signals reach cortical structures (Bridge et al., 2016; Diano et al., 2017). The most prominent ascending visual pathway to the inferior pulvinar passes through the superior colliculus (Bridge et al., 2016). Another study reported neurons in the visual pulvinar that relay signals from the SC to the medial temporal cortex for higher-order processing (Berman and Wurtz, 2010, 2011). Given the extensive roles of the SC and pulvinar in attentional control, it is likely that sleep disturbance will lead to this subcortical circuit dysfunction more broadly than described here for the visual relational task.
4.2. Alcohol misuse and cortical activation during visual relational processing
Although alcohol use severity did not appear to directly impact the performance in the relational task, the dorsomedial prefrontal cortex in the supplementary motor area (SMA) and pre-SMA, left inferior frontal cortex, and visual cortex showed higher activation in association with more severe alcohol use and prolonged RT. These regional activities were not related to the PSQI score, suggesting that they may reflect direct influences of chronic alcohol consumption on the brain. The higher activities may also indicate a functional compensatory process in these young adult participants.
4.3. Limitations and conclusions of the study
Some limitations should be noted for the study. First, the findings of mediation and path analyses only suggest directional influences. Whether or how alcohol consumption and sleep disturbance influence attentional performance via the SC, pulvinar and other cortical structures needs be verified experimentally. Second, although alcohol use severity varied significantly across individuals, the HCP data represent largely a non-clinical sample. Thus, how the current findings extend to addicted individuals needs to be examined. It is possible, for instance, that the direct effects of chronic alcohol consumption on the brain would be more severe as to mask the mediating roles of sleep disturbance. Third, PSQI scores varied across individuals and correlated positively with drinking severity; however, more objective measures of sleep quality may refine the current findings.
In conclusion, the current study extend the literature by establishing the neural processes relating alcohol misuse, sleep disturbance and attentional performance. In this population of young adult drinkers, the superior colliculus may play a crucial role in manifesting the effects of alcohol-related sleep disruption on visual attention deficits.
Supplementary Material
Funding Support and Acknowledgement
The current study is supported by National Key Research and Development Program of China No.2020YFC2007300, No. 2020YFC2007301 (XT) and NIH grants DA051922, AG072893 and AA021449 (C-SRL). Data were provided by the Human Connectome Project, WU-Minn Consortium (Principal Investigators: David Van Essen and Kamil Ugurbil; 1U54MH091657) funded by the 16 NIH Institutes and Centers that support the NIH Blueprint for Neuroscience Research; and by the McDonnell Center for Systems Neuroscience at Washington University.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competing interests
The authors declare that they have no competing interests.
References
- Adan ANA, 1994. Chronotype and personality factors in the daily consumption of alcohol and psychostimulants. Addiction 89(4), 455–462. [DOI] [PubMed] [Google Scholar]
- Agostini A, Carskadon MA, Dorrian J, Coussens S, Short MA, 2017. An experimental study of adolescent sleep restriction during a simulated school week: changes in phase, sleep staging, performance and sleepiness. Journal of Sleep Research 26(2), 227–235. [DOI] [PubMed] [Google Scholar]
- Alhola P, Polo-Kantola P, 2007. Sleep deprivation: Impact on cognitive performance. Neuropsychiatr Dis Treat 3(5), 553–567. [PMC free article] [PubMed] [Google Scholar]
- Altena E, Van Der Werf YD, Strijers RLM, Van Someren EJW, 2008. Sleep loss affects vigilance: effects of chronic insomnia and sleep therapy. Journal of Sleep Research 17(3), 335–343. [DOI] [PubMed] [Google Scholar]
- Arnedt JT, Rohsenow DJ, Almeida AB, Hunt SK, Gokhale M, Gottlieb DJ, Howland J, 2011. Sleep following alcohol intoxication in healthy, young adults: effects of sex and family history of alcoholism. Alcohol Clin Exp Res 35(5), 870–878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awh E, Jonides J, 2001. Overlapping mechanisms of attention and spatial working memory. Trends Cogn Sci 5(3), 119–126. [DOI] [PubMed] [Google Scholar]
- Baddeley A, Cocchini G, Della Sala S, Logie RH, Spinnler H, 1999. Working Memory and Vigilance: Evidence from Normal Aging and Alzheimer’s Disease. Brain and Cognition 41(1), 87–108. [DOI] [PubMed] [Google Scholar]
- Barch DM, Burgess GC, Harms MP, Petersen SE, Schlaggar BL, Corbetta M, Glasser MF, Curtiss S, Dixit S, Feldt C, Nolan D, Bryant E, Hartley T, Footer O, Bjork JM, Poldrack R, Smith S, Johansen-Berg H, Snyder AZ, Van Essen DC, Consortium WU-MH, 2013. Function in the human connectome: task-fMRI and individual differences in behavior. NeuroImage 80, 169–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barron DS, Eickhoff SB, Clos M, Fox PT, 2015. Human pulvinar functional organization and connectivity. Hum Brain Mapp 36(7), 2417–2431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basso MA, Bickford ME, Cang J, 2021. Unraveling circuits of visual perception and cognition through the superior colliculus. Neuron 109(6), 918–937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basso MA, May PJ, 2017. Circuits for Action and Cognition: A View from the Superior Colliculus. Annu Rev Vis Sci 3, 197–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benitez A, Gunstad J, 2012. Poor sleep quality diminishes cognitive functioning independent of depression and anxiety in healthy young adults. Clin Neuropsychol 26(2), 214–223. [DOI] [PubMed] [Google Scholar]
- Berman RA, Wurtz RH, 2010. Functional Identification of a Pulvinar Path from Superior Colliculus to Cortical Area MT. The Journal of Neuroscience 30(18), 6342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman RA, Wurtz RH, 2011. Signals conveyed in the pulvinar pathway from superior colliculus to cortical area MT. J Neurosci 31(2), 373–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besedovsky L, Lange T, Born J, 2012. Sleep and immune function. Pflugers Arch 463(1), 121–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourgeois A, Guedj C, Carrera E, Vuilleumier P, 2020. Pulvino-cortical interaction: An integrative role in the control of attention. Neuroscience & Biobehavioral Reviews 111, 104–113. [DOI] [PubMed] [Google Scholar]
- Bridge H, Leopold DA, Bourne JA, 2016. Adaptive Pulvinar Circuitry Supports Visual Cognition. Trends Cogn Sci 20(2), 146–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brownlow JA, Miller KE, Gehrman PR, 2020. Insomnia and Cognitive Performance. Sleep Med Clin 15(1), 71–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ, 1989. The Pittsburgh sleep quality index: A new instrument for psychiatric practice and research. Psychiatry Research 28(2), 193–213. [DOI] [PubMed] [Google Scholar]
- Canham SL, Kaufmann CN, Mauro PM, Mojtabai R, Spira AP, 2015. Binge drinking and insomnia in middle-aged and older adults: the Health and Retirement Study. Int J Geriatr Psychiatry 30(3), 284–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakravorty S, Chaudhary NS, Brower KJ, 2016. Alcohol Dependence and Its Relationship With Insomnia and Other Sleep Disorders. Alcohol Clin Exp Res 40(11), 2271–2282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan KC, Li J, Kau P, Zhou IY, Cheung MM, Lau C, Yang J, So K.-f., Wu EX, 2011. In vivo retinotopic mapping of superior colliculus using manganese-enhanced magnetic resonance imaging. NeuroImage 54(1), 389–395. [DOI] [PubMed] [Google Scholar]
- Chee MWL, Tan JC, 2010. Lapsing when sleep deprived: Neural activation characteristics of resistant and vulnerable individuals. NeuroImage 51(2), 835–843. [DOI] [PubMed] [Google Scholar]
- Cohen JD, Castro-Alamancos MA, 2010. Behavioral state dependency of neural activity and sensory (whisker) responses in superior colliculus. J Neurophysiol 104(3), 1661–1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crapse TB, Lau H, Basso MA, 2018. A Role for the Superior Colliculus in Decision Criteria. Neuron 97(1), 181–194. e186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crum RM, Ford DE, Storr CL, Chan Y-F, 2004. Association of Sleep Disturbance with Chronicity and Remission of Alcohol Dependence: Data from a Population-Based Prospective Study. Alcoholism: Clinical and Experimental Research 28(10), 1533–1540. [DOI] [PubMed] [Google Scholar]
- Cui J, Tkachenko O, Gogel H, Kipman M, Preer LA, Weber M, Divatia SC, Demers LA, Olson EA, Buchholz JL, Bark JS, Rosso IM, Rauch SL, Killgore WDS, 2015. Microstructure of frontoparietal connections predicts individual resistance to sleep deprivation. NeuroImage 106, 123–133. [DOI] [PubMed] [Google Scholar]
- Danel T, Libersa C, Touitou Y, 2001. The effect of alcohol consumption on the circadian control of human core body temperature is time dependent. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology 281(1), R52–R55. [DOI] [PubMed] [Google Scholar]
- Devenney LE, Coyle KB, Verster JC, 2019. Memory and attention during an alcohol hangover. Hum Psychopharmacol 34(4), e2701–e2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diano M, Celeghin A, Bagnis A, Tamietto M, 2017. Amygdala Response to Emotional Stimuli without Awareness: Facts and Interpretations. Front Psychol 7, 2029–2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorrian J, Heath G, Sargent C, Banks S, Coates A, 2017. Alcohol use in shiftworkers. Accident Analysis & Prevention 99, 395–400. [DOI] [PubMed] [Google Scholar]
- Drummond SPA, Bischoff-Grethe A, Dinges DF, Ayalon L, Meloy MJ, 2005. The Neural Basis of the Psychomotor Vigilance Task. Sleep 28(9), 1059–1068. [PubMed] [Google Scholar]
- Duvernoy HM, 2009. The Human Brain. Second Edition. Springer-Verlag, Wien/New York. [Google Scholar]
- Fiebelkorn IC, Kastner S, 2020. Functional Specialization in the Attention Network. Annual review of psychology 71, 221–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiebelkorn IC, Pinsk MA, Kastner S, 2019. The mediodorsal pulvinar coordinates the macaque fronto-parietal network during rhythmic spatial attention. Nat Commun 10(1), 215–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortier-Brochu É, Beaulieu-Bonneau S, Ivers H, Morin CM, 2012. Insomnia and daytime cognitive performance: A meta-analysis. Sleep Medicine Reviews 16(1), 83–94. [DOI] [PubMed] [Google Scholar]
- Fortier-Brochu E, Morin CM, 2014. Cognitive impairment in individuals with insomnia: clinical significance and correlates. Sleep 37(11), 1787–1798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furigo IC, de Oliveira WF, de Oliveira AR, Comoli E, Baldo MVC, Mota-Ortiz SR, Canteras NS, 2010. The role of the superior colliculus in predatory hunting. Neuroscience 165(1), 1–15. [DOI] [PubMed] [Google Scholar]
- Gattass R, Soares JGM, Lima B, 2018. The Role of the Pulvinar in Spatial Visual Attention, in: Gattass R, Soares JGM, Lima B (Eds.), The Pulvinar Thalamic Nucleus of Non-Human Primates: Architectonic and Functional Subdivisions. Springer International Publishing, Cham, pp. 57–60. [Google Scholar]
- Grimaldi P, Cho SH, Lau H, Basso MA, 2018. Superior colliculus signals decisions rather than confidence: analysis of single neurons. J Neurophysiol 120(5), 2614–2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haario P, Rahkonen O, Laaksonen M, Lahelma E, Lallukka TEA, 2013. Bidirectional associations between insomnia symptoms and unhealthy behaviours. Journal of Sleep Research 22(1), 89–95. [DOI] [PubMed] [Google Scholar]
- Haimov I, Hanuka E, Horowitz Y, 2008. Chronic Insomnia and Cognitive Functioning Among Older Adults. Behavioral Sleep Medicine 6(1), 32–54. [DOI] [PubMed] [Google Scholar]
- Hasler BP, Franzen PL, de Zambotti M, Prouty D, Brown SA, Tapert SF, Pfefferbaum A, Pohl KM, Sullivan EV, De Beilis MD, Nagel BJ, Baker FC, Colrain IM, Clark DB, 2017. Eveningness and Later Sleep Timing Are Associated with Greater Risk for Alcohol and Marijuana Use in Adolescence: Initial Findings from the National Consortium on Alcohol and Neurodevelopment in Adolescence Study. Alcoholism: Clinical and Experimental Research 41(6), 1154–1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He S, Hasler BP, Chakravorty S, 2019. Alcohol and sleep-related problems. Curr Opin Psychol 30, 117–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu F, Kamigaki T, Zhang Z, Zhang S, Dan U, Dan Y, 2019. Prefrontal Corticotectal Neurons Enhance Visual Processing through the Superior Colliculus and Pulvinar Thalamus. Neuron 104(6), 1141–1152.e1144. [DOI] [PubMed] [Google Scholar]
- Hudson AN, Van Dongen HPA, Honn KA, 2020. Sleep deprivation, vigilant attention, and brain function: a review. Neuropsychopharmacology 45(1), 21–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaramillo J, Mejias JF, Wang X-J, 2019. Engagement of Pulvino-cortical Feedforward and Feedback Pathways in Cognitive Computations. Neuron 101(2), 321–336. e329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerde TA, Merriam EP, Riggall AC, Hedges JH, Curtis CE, 2012. Prioritized Maps of Space in Human Frontoparietal Cortex. The Journal of Neuroscience 32(48), 17382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasai M, Isa T, 2016. Imaging population dynamics of surround suppression in the superior colliculus. European Journal of Neuroscience 44(8), 2543–2556. [DOI] [PubMed] [Google Scholar]
- Kastner S, Fiebelkorn IC, Eradath MK, 2020. Dynamic pulvino-cortical interactions in the primate attention network. Current Opinion in Neurobiology 65, 10–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katyal S, Ress D, 2013. Attentional base response in intermediate layers of human superior colliculus measured using high-resolution fMRI. Journal of Vision 13(9), 224–224. [Google Scholar]
- Katyal S, Ress D, 2014. Endogenous attention signals evoked by threshold contrast detection in human superior colliculus. J Neurosci 34(3), 892–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolla BP, Foroughi M, Saeidifard F, Chakravorty S, Wang Z, Mansukhani MP, 2018. The impact of alcohol on breathing parameters during sleep: A systematic review and meta-analysis. Sleep Medicine Reviews 42, 59–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause AJ, Simon EB, Mander BA, Greer SM, Saletin JM, Goldstein-Piekarski AN, Walker MP, 2017. The sleep-deprived human brain. Nat Rev Neurosci 18(7), 404–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krauzlis RJ, Lovejoy LP, Zénon A, 2013. Superior colliculus and visual spatial attention. Annu Rev Neurosci 36, 165–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuna ST, Maislin G, Pack FM, Staley B, Hachadoorian R, Coccaro EF, Pack AI, 2012. Heritability of performance deficit accumulation during acute sleep deprivation in twins. Sleep 35(9), 1223–1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuula L, Pesonen A-K, Martikainen S, Kajantie E, Lahti J, Strandberg T, Tuovinen S, Heinonen K, Pyhälä R, Lahti M, Räikkönen K, 2015. Poor sleep and neurocognitive function in early adolescence. Sleep Medicine 16(10), 1207–1212. [DOI] [PubMed] [Google Scholar]
- Le Berre A-P, Fama R, Sullivan EV, 2017. Executive Functions, Memory, and Social Cognitive Deficits and Recovery in Chronic Alcoholism: A Critical Review to Inform Future Research. Alcohol Clin Exp Res 41(8), 1432–1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G, Zhang S, Le TM, Tang X, Li C-SR, 2020a. Neural responses to negative facial emotions: Sex differences in the correlates of individual anger and fear traits. NeuroImage 221, 117171–117171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G, Zhang S, Le TM, Tang X, Li C-SR, 2020b. Neural Responses to Reward in a Gambling Task: Sex Differences and Individual Variation in Reward-Driven Impulsivity. Cereb Cortex Commun 1(1), tgaa025–tgaa025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liddell BJ, Brown KJ, Kemp AH, Barton MJ, Das P, Peduto A, Gordon E, Williams LM, 2005. A direct brainstem-amygdala-cortical ‘alarm’ system for subliminal signals of fear. NeuroImage 24(1), 235–243. [DOI] [PubMed] [Google Scholar]
- Lo JC, Ong JL, Leong RLF, Cooley JJ, Chee MWL, 2016. Cognitive Performance, Sleepiness, and Mood in Partially Sleep Deprived Adolescents: The Need for Sleep Study. Sleep 39(3), 687–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louca M, Short MA, 2014. The effect of one night’s sleep deprivation on adolescent neurobehavioral performance. Sleep 37(11), 1799–1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe CJ, Safati A, Hall PA, 2017. The neurocognitive consequences of sleep restriction: A meta-analytic review. Neuroscience & Biobehavioral Reviews 80, 586–604. [DOI] [PubMed] [Google Scholar]
- Lupi D, Oster H, Thompson S, Foster RG, 2008. The acute light-induction of sleep is mediated by OPN4-based photoreception. Nature Neuroscience 11(9), 1068–1073. [DOI] [PubMed] [Google Scholar]
- MacKinnon DP, Fairchild AJ, Fritz MS, 2007. Mediation analysis. Annu Rev Psychol 58, 593–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mares I, Smith ML, Johnson MH, Senju A, 2016. Direct gaze facilitates rapid orienting to faces: Evidence from express saccades and saccadic potentials. Biol Psychol 121 (Pt A), 84–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May PJ, 2006. The mammalian superior colliculus: laminar structure and connections, in: Buttner-Ennever JA (Ed.) Progress in Brain Research. Elsevier, pp. 321–378. [DOI] [PubMed] [Google Scholar]
- Menon V, Uddin LQ, 2010. Saliency, switching, attention and control: a network model of insula function. Brain Struct Funct 214(5–6), 655–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merker B, 2007. Consciousness without a cerebral cortex: A challenge for neuroscience and medicine. Behavioral and Brain Sciences 30(1), 63–81. [DOI] [PubMed] [Google Scholar]
- Merker B, 2013. The efference cascade, consciousness, and its self: naturalizing the first person pivot of action control. Front Psychol 4, 501–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller AM, Obermeyer WH, Behan M, Benca RM, 1998. The superior colliculus-pretectum mediates the direct effects of light on sleep. Proc Natl Acad Sci USA 95(15), 8957–8962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller MB, DiBello AM, Carey KB, Borsari B, Pedersen ER, 2017. Insomnia severity as a mediator of the association between mental health symptoms and alcohol use in young adult veterans. Drug Alcohol Depend 177, 221–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mysore SP, Knudsen EI, 2014. Descending control of neural bias and selectivity in a spatial attention network: rules and mechanisms. Neuron 84(1), 214–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen M, Matsumoto J, Hori E, Maior R, Tomaz C, Tran A, Ono T, Nishijo H, 2014. Neuronal responses to face-like and facial stimuli in the monkey superior colliculus. Frontiers in Behavioral Neuroscience 8(85). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olaithe M, Bucks RS, Hillman DR, Eastwood PR, 2018. Cognitive deficits in obstructive sleep apnea: Insights from a meta-review and comparison with deficits observed in COPD, insomnia, and sleep deprivation. Sleep Medicine Reviews 38, 39–49. [DOI] [PubMed] [Google Scholar]
- Olivé I, Densmore M, Harricharan S, Théberge J, McKinnon MC, Lanius R, 2018. Superior colliculus resting state networks in post-traumatic stress disorder and its dissociative subtype. Hum Brain Mapp 39(1), 563–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onyper SV, Thacher PV, Gilbert JW, Gradess SG, 2012. Class Start Times, Sleep, and Academic Performance in College: A Path Analysis. Chronobiology International 29(3), 318–335. [DOI] [PubMed] [Google Scholar]
- Portas CM, Rees G, Howseman AM, Josephs O, Turner R, Frith CD, 1998. A specific role for the thalamus in mediating the interaction of attention and arousal in humans. J Neurosci 18(21), 8979–8989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahmati M, DeSimone K, Curtis CE, Sreenivasan KK, 2020. Spatially Specific Working Memory Activity in the Human Superior Colliculus. J Neurosci 40(49), 9487–9495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson DL, McClurkin JW, 1989. The visual superior colliculus and pulvinar. Rev Oculomot Res 3, 337–360. [PubMed] [Google Scholar]
- Rosa Salva O, Mayer U, Vallortigara G, 2015. Roots of a social brain: Developmental models of emerging animacy-detection mechanisms. Neuroscience & Biobehavioral Reviews 50, 150–168. [DOI] [PubMed] [Google Scholar]
- Rupp TL, Acebo C, Carskadon MA, 2007. Evening Alcohol Suppresses Salivary Melatonin in Young Adults. Chronobiology International 24(3), 463–470. [DOI] [PubMed] [Google Scholar]
- Sato W, Kochiyama T, Uono S, Toichi M, 2016. Neural mechanisms underlying conscious and unconscious attentional shifts triggered by eye gaze. NeuroImage 124, 118–126. [DOI] [PubMed] [Google Scholar]
- Schiller B, Heinrichs M, Beste C, Stock A-K, 2021. Acute alcohol intoxication modulates the temporal dynamics of resting electroencephalography networks. Addiction Biology n/a(n/a), e13034. [DOI] [PubMed] [Google Scholar]
- Schneider KA, Kastner S, 2009. Effects of Sustained Spatial Attention in the Human Lateral Geniculate Nucleus and Superior Colliculus. The Journal of Neuroscience 29(6), 1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scullin MK, Bliwise DL, 2015. Sleep, cognition, and normal aging: integrating a half century of multidisciplinary research. Perspect Psychol Sci 10(1), 97–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shekleton JA, Flynn-Evans EE, Miller B, Epstein LJ, Kirsch D, Brogna LA, Burke LM, Bremer E, Murray JM, Gehrman P, Lockley SW, Rajaratnam SMW, 2014. Neurobehavioral performance impairment in insomnia: relationships with self-reported sleep and daytime functioning. Sleep 37(1), 107–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenfield L, Beanland V, Filtness A, Apthorp D, 2020. The impact of sleep loss on sustained and transient attention: an EEG study. PeerJ 8, e8960–e8960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh V, Pfeuffer J, Zhao T, Ress D, 2018. Evaluation of spiral acquisition variants for functional imaging of human superior colliculus at 3T field strength. Magn Reson Med 79(4), 1931–1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith R, Keramatian K, Christoff K, 2007. Localizing the rostrolateral prefrontal cortex at the individual level. NeuroImage 36(4), 1387–1396. [DOI] [PubMed] [Google Scholar]
- Spear LP, 2018. Effects of adolescent alcohol consumption on the brain and behaviour. Nature Reviews Neuroscience 19(4), 197–214. [DOI] [PubMed] [Google Scholar]
- Steenari M-R, Vuontela V, Paavonen EJ, Carlson S, FjÄLlberg M, Aronen ET, 2003. Working Memory and Sleep in 6- to 13-Year-Old Schoolchildren. Journal of the American Academy of Child & Adolescent Psychiatry 42(1), 85–92. [DOI] [PubMed] [Google Scholar]
- Stickgold R, 2005. Sleep-dependent memory consolidation. Nature 437(7063), 1272–1278. [DOI] [PubMed] [Google Scholar]
- Stitt I, Zhou ZC, Radtke-Schuller S, Fröhlich F, 2018. Arousal dependent modulation of thalamo-cortical functional interaction. Nat Commun 9(1), 2455–2455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stranks EK, Crowe SF, 2016. The Cognitive Effects of Obstructive Sleep Apnea: An Updated Meta-analysis. Archives of Clinical Neuropsychology 31(2), 186–193. [DOI] [PubMed] [Google Scholar]
- Tomasi D, Volkow ND, 2019. Association Between Brain Activation and Functional Connectivity. Cereb Cortex 29(5), 1984–1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasi D, Wang RL, Telang F, Boronikolas V, Jayne MC, Wang GJ, Fowler JS, Volkow ND, 2009. Impairment of attentional networks after 1 night of sleep deprivation. Cereb Cortex 19(1), 233–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres-Sánchez I, Rodríguez-Alzueta E, Cabrera-Martos I, López-Torres I, Moreno-Ramírez MP, Valenza MC, 2015. Cognitive impairment in COPD: a systematic review. J Bras Pneumol 41(2), 182–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdez P, 2019. Circadian Rhythms in Attention. Yale J Biol Med 92(1), 81–92. [PMC free article] [PubMed] [Google Scholar]
- Varkevisser M, Kerkhof GA, 2005. Chronic insomnia and performance in a 24–h constant routine study. Journal of Sleep Research 14(1), 49–59. [DOI] [PubMed] [Google Scholar]
- Wager TD, Davidson ML, Hughes BL, Lindquist MA, Ochsner KN, 2008. Prefrontal-subcortical pathways mediating successful emotion regulation. Neuron 59(6), 1037–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y-M, Chen H-G, Song M, Xu S-J, Yu L-L, Wang L, Wang R, Shi L, He J, Huang Y-Q, Sun H-Q, Pan C-Y, Wang X-Y, Lu L, 2016. Prevalence of insomnia and its risk factors in older individuals: a community-based study in four cities of Hebei Province, China. Sleep Medicine 19, 116–122. [DOI] [PubMed] [Google Scholar]
- Wei P, Liu N, Zhang Z, Liu X, Tang Y, He X, Wu B, Zhou Z, Liu Y,Li J, Zhang Y, Zhou X, Xu L, Chen L, Bi G, Hu X, Xu F, Wang L, 2015. Processing of visually evoked innate fear by a non-canonical thalamic pathway. Nat Commun 6, 6756–6756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White BJ, Kan JY, Levy R, Itti L, Munoz DP, 2017. Superior colliculus encodes visual saliency before the primary visual cortex. Proc Natl Acad Sci U S A 114(35), 9451–9456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff N, Gussek P, Stock A-K, Beste C, 2018. Effects of high-dose ethanol intoxication and hangover on cognitive flexibility. Addiction Biology 23(1), 503–514. [DOI] [PubMed] [Google Scholar]
- Xie L, Kang H, Xu Q, Chen MJ, Liao Y, Thiyagarajan M, O’Donnell J, Christensen DJ, Nicholson C, Iliff JJ, Takano T, Deane R, Nedergaard M, 2013. Sleep drives metabolite clearance from the adult brain. Science 342(6156), 373–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xuan B, Mackie M-A, Spagna A, Wu T, Tian Y, Hof PR, Fan J, 2016. The activation of interactive attentional networks. NeuroImage 129, 308–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan C-G, Wang X-D, Zuo X-N, Zang Y-F, 2016. DPABI: Data Processing & Analysis for (Resting-State) Brain Imaging. Neuroinformatics 14(3), 339–351. [DOI] [PubMed] [Google Scholar]
- Yang FN, Xu S, Chai Y, Basner M, Dinges DF, Rao H, 2018. Sleep deprivation enhances inter-stimulus interval effect on vigilant attention performance. Sleep 41(12), zsy189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zehra A, Lindgren E, Wiers CE, Freeman C, Miller G, Ramirez V, Shokri-Kojori E, Wang G-J, Talagala L, Tomasi D, Volkow ND, 2019. Neural correlates of visual attention in alcohol use disorder. Drug Alcohol Depend 194, 430–437. [DOI] [PubMed] [Google Scholar]
- Zhang S, Zhornitsky S, Le TM, Li C-SR, 2019. Hypothalamic Responses to Cocaine and Food Cues in Individuals with Cocaine Dependence. Int J Neuropsychopharmacol 22(12), 754–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhornitsky S, Zhang S, Ide JS, Chao HH, Wang W, Le TM, Leeman RF, Bi J, Krystal JH, Li C-SR, 2019. Alcohol Expectancy and Cerebral Responses to Cue-Elicited Craving in Adult Nondependent Drinkers. Biol Psychiatry Cogn Neurosci Neuroimaging 4(5), 493–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


