Abstract
Hyperbaric oxygen (HBO) treatment of animals or ocular lenses in culture recapitulates many molecular changes observed in human age-related nuclear cataract. The guinea pig HBO model has been one of the best examples of such treatment leading to dose-dependent development of lens nuclear opacities. In this study, complimentary mass spectrometry methods were employed to examine protein truncation after HBO treatment of aged guinea pigs. Quantitative liquid chromatography-mass spectrometry (LC-MS) analysis of the membrane fraction of guinea pig lenses showed statistically significant increases in aquaporin-0 (AQP0) C-terminal truncation, consistent with previous reports of accelerated loss of membrane and cytoskeletal proteins. In addition, imaging mass spectrometry (IMS) analysis spatially mapped the acceleration of age-related αA-crystallin truncation in the lens nucleus. The truncation sites in αA-crystallin closely match those observed in human lenses with age. Taken together, our results suggest that HBO accelerates the normal lens aging process and leads to nuclear cataract.
Keywords: aquaporin-0, mass spectrometry, alpha crystallin, protein truncation, lens, hyperbaric oxygen, nuclear cataract, αA66-80 peptide
1. Introduction
Human age-related nuclear cataract (ARNC) is the most prevalent form of cataract and the leading cause of blindness worldwide. Multiple molecular mechanisms have been proposed to cause age-related lens opacities including oxidation (Truscott, 2005), protein aggregation, and multilamellar membrane formation (Costello et al., 2012). Unfortunately, very few animal models exist to recapitulate the exact ARNC phenotype (Truscott and Zhu, 2010). To date, the hyperbaric oxygen (HBO) treatment of aged guinea pigs has yielded features most similar to the molecular features of ARNC (Giblin et al., 1995; Simpanya et al., 2005). While human patients treated extensively with HBO develop myopia and nuclear cataract (Palmquist et al., 1984), guinea pigs treated in a similar manner develop myopia, increased lens nuclear light scatter (NLS) and loss of lens optical quality (Bantseev et al., 2004). All HBO-induced effects in the guinea pig lenses, including crystallin insolubilization and loss of reduced glutathione (GSH), occur only in the lens nucleus, not the cortex, due to the relatively low antioxidant activity present in the central region of the lens (Giblin, 2000).
Molecular effects detected in the HBO guinea pig lenses include: increased protein S-thiolation and protein disulfide (Giblin et al., 2013; Gosselin et al., 2007), protein aggregation (Simpanya et al., 2005), lipid oxidation (Borchman et al., 2000) and accelerated loss of cytoskeletal and membrane proteins through truncation (Padgaonkar et al., 1999). Such features are also detected in aged human lenses (Grey and Schey, 2009). Still, the molecular mechanisms that lead to lens opacification are not completely defined.
Mass spectrometry methods have had a major impact on lens and cataract research by providing the molecular specificity and sensitivity to detect age-related changes to lens proteins. Liquid chromatography-tandem mass spectrometry (LC-MS/MS) has been the driving tool behind proteomics research and has been used to determine the exact sites of protein posttranslational modifications in aged human lenses (Hains and Truscott, 2010; Hooi and Truscott, 2011; Lampi et al., 2014; Wilmarth et al., 2006). Since its development in 1997 (Caprioli et al., 1997), imaging mass spectrometry (IMS) has provided spatial localization of proteins and their modified forms directly from tissues. Indeed, age-related truncation of human lens crystallins (Grey and Schey, 2009) as well as phosphorylated crystallins have been localized (Han and Schey, 2006). Specialized methods have been developed to spatially localize PTMs on membrane proteins (Grey et al., 2009) and analysis of human lens APQ0 defined discrete regions of age-related truncation and deamidation (Wenke et al., 2015).
In this study, lenses from HBO treated guinea pigs were subjected to LC-MS/MS and IMS analysis to determine the extent and location of AQP0 and crystallin modification, respectively. The results suggest that HBO treatment accelerates the normal aging process of protein truncation occurring in the central region of the lens leading to loss of transparency.
2. Materials and methods
2.1. Animals
This study used male Hartley “retired breeder” guinea pigs, initially 18 months old, obtained from either Kuiper Rabbit Ranch (Indianapolis, IN, U.S.A.) or Hilltop Lab Animals (Scottdale, PA, U.S.A.) The animals were held for 1–2 weeks prior to treatment with HBO. Guinea pigs with cortical or nuclear lens opacities, as determined via slit lamp biomicroscopy, were excluded from the investigation. All work conformed to the US Department of Agriculture standards and the ARVO statement for the use of animals in ophthalmic and vision research, and was approved by the Oakland University Institutional Animal Care and Use Committee.
2.2. Treatment of guinea pigs with HBO
The treatment of guinea pigs with HBO in a 114 cm long, 46 cm wide pressure vessel (Amron, Escondido, CA, U.S.A.) has been described in detail previously (Simpanya et al., 2005). The animals were treated with 2.5 atm absolute of 100% O2 (USP Grade Medical Gas; Praxair, Danbury, CT) for 2.5 hrs, three times per week, on alternate days, for a total of either 15, 30 or 43 treatments over a 5, 10 or 15 week period, respectively (the peptide IMS study) or 81 or 84 treatments over a 7 month period (the AQP0 LC-MS/MS study). Aged-matched untreated guinea pigs were used as controls. A 15-week HBO-exposure has been shown previously to produce only a slight increase in lens nuclear light scattering (NLS) compared to controls (Padgaonkar et al., 1999), whereas a 7-month exposure results in a more substantial increase in NLS, particularly in the very center of the lens nucleus (Simpanya et al., 2005).
2.4. Preparation of lens membrane fraction for LC-MS/MS Analysis
Control and O2-treated animals were killed by CO2 asphyxiation, the eyes enucleated, and lenses removed by posterior approach. The lenses were frozen immediately and stored in liquid N2 until used. Control and HBO treated guinea pig lenses were vortexed in homogenizing buffer (25 mM Tris, 150 mM NaCl, 5 mM EDTA, 1 mM PMSF) to separate the cortex and the nucleus regions. The isolated nucleus accounted for about 50% of the total lens volume. The urea insoluble (UI) fraction of lens cortex and nucleus was prepared using a modification of that was previously employed to remove soluble proteins and non-AQP0 membrane components from the sample (Padgaonkar et al., 1999). Briefly, the samples were homogenized and centrifuged at 30,000g for 20 min. The pellets were washed with 4 M urea in homogenizing buffer followed by 8 M urea. For each wash, the samples were centrifuged at 30,000 g for 20 min to remove the supernatant. The pellets were then suspended in 8M urea containing 10 mM DTT at 56°C for 45 min to reduce disulfide bonds and the free thiol groups were then alkylated by adding iodoacetamide to 50 mM. The samples were incubated at 25°C in the dark for 45 min. The samples were then centrifuged at 30,000 g for 20 min and the supernatant was discarded. The pellets were washed with water followed by a 0.1 M NaOH wash and a final water wash. The remaining pellets were digested by trypsin (enzyme/protein ratio 1:20) in 10% acetonitrile in 50 mM Tris buffer, pH 8.0 at 37°C overnight. The samples were diluted to 0.25 μg/μL using 0.1% formic acid and readied for LC-MS/MS analysis.
2.5. Capillary LC/MS analysis
Trypsin digested samples were separated by one-dimensional liquid chromatography on a fused silica capillary column (250 mm × 100 μm) packed with Phenomenex Jupiter resin (3 μm mean particle size, 300 Å pore size) using the following gradient at a flow rate of 0.5 μL/min: 0–70 min: 2–35% ACN (0.1% formic acid), 70–75min: 35–95% ACN (0.1% formic acid). The eluate from the analytical column was directly infused into a Velos Pro linear ion trap mass spectrometer (Thermo Fisher Scientific, San Jose, CA) for analysis using a data-dependent acquisition method with one precursor scan event to identify the top 15 most abundant ions in each MS scan, which were then selected for fragmentation. Dynamic exclusion (exclude after 1 spectrum, release after 15 sec, and exclusion list size of 300) was enabled. Normalized collision energy was 35%. For quantifying the level of AQP0 truncation, the instrument was operated in full MS/targeted MS2 mode and only signals matching C-terminal AQP0 peptides (both tryptic and non-tryptic peptides) were selected for fragmentation.
The data were searched by TagRecon algorithm (Dasari et al., 2010) against a Uniprot guinea pig database (April 04, 2013) with a static modification of carbamidomethylation of cysteine residues and variable modifications of oxidation of methionine and deamination of asparagine. Trypsin specificity was used with a maximum of two missed cleavage sites. The search results were filtered by IDPicker (Ma et al., 2009) by controlling protein FDR to less than 1%. For quantifying the level of truncation, the resulting raw files were imported into Skyline (MacLean et al., 2010) for peak-picking and quantitation was based on MS2 fragment ion intensities. Fragment ions that were used for quantification are listed in Supplemental Table 1. The normalized abundance was then calculated as the ratio of the total peak area from all product ions selected to the total peak area from all product ions of the fully tryptic AQP0 peptide 239–259. The results are presented as mean ± standard deviation (SD) of 4 independent experiments from four different lenses. Statistical analysis was done by student t-test at p < 0.05.
2.6. Top down LC-MS/MS
Identification of αA-crystallin peptide signals detected in the MALDI imaging data was made using a published method of top down LC‐MS/MS analysis (Schey et al., 2013, Anderson, 2020 #15). Guinea pig lens tissue (25 month control) sectioned at 20 micron were thaw mounted onto glass microscope slides (Tanner Scientific, Sarasota, FL, USA) with the intention that the nuclear region would not adhere as observed previously (Anderson et al., 2015) and could be easily removed with a clean razor blade. Lens nuclear tissue fragments were gently removed from the glass slide and placed with an Eppendorf tube. After an initial extraction with 20% acetonitrile, 15 μL of 30% acetonitrile/1% formic acid were added to the tube. After vortexing, 5 μL were removed and diluted to 10% acetonitrile/0.3% formic acid before being pressure loaded onto a trapping column (360 μm Å~ 150 μm id) self‐packed with C8 material (15 μm material, 300A Grace Vydac). Proteins were eluted from the trap column onto an analytical column (360 μm Å~ 100 μm id, 8 or 20 cm in length of C8 stationary phase material) and into an LTQ Orbitrap Velos mass spectrometer (Thermo Scientific, San Jose, CA) using a gradient of 98% solvent A (99.9% water/0.1% formic acid) to 60% solvent B (99.9% acetonitrile/0.1% formic acid) in 50 minutes. Eluted proteins were mass analyzed and tandem mass spectra were acquired using collision‐induced dissociation (CID) and electron transfer dissociation (ETD), sequentially, using a top seven data‐dependent method. Precursor ions were selected with a 2.5 Da window and CID was carried out with a normalized collision energy of 35 and an activation time of 10 ms. ETD was carried out with a 115 ms activation time. Dynamic exclusion was enabled with a repeat count of 4 and a repeat duration of 15 seconds. MS/MS spectra were manually interpreted based on predicted and measured molecular weights for the αA-crystallin truncation products. C- and z- product ion masses were compared with theoretical ETD fragmentation generated ion using ProteinProspector (http://prospector.ucsf.edu/prospector/mshome.htm) to validate assignments.
2.7. Mass spectrometry imaging
Dissected guinea pig lenses were sectioned at 20 μm thickness at a temperature of −20 °C in a Leica CM3050S cryostat. Sections were then adhered by applying gentle pressure to a pre-cooled (−20°C) electron microscopy ultra-thin carbon conductive adhesive tab (carbon filler conductive adhesive with a nonconductive cloth core material, total thickness 160 μm, 12 mm diameter, presented as small circular tabs attached to sheets) (Electron Microscopy Sciences, Hatfield, PA, USA) held with forceps to prevent heat transfer from fingers thawing the tissue. Sections were transferred to a pre-cooled −80 °C lyophilizing vessel and freeze-dried for at least three hours to ensure the samples did not thaw. The lyophilized sections were mounted onto gold-coated MALDI target plates before matrix deposition.
Matrix deposition
Matrix was deposited onto the samples using a Portrait 630 acoustic spotter (Labcyte Inc., Sunnyvale, CA, USA). The matrix composition was 20 mg/mL sinapinic acid (Sigma Aldrich, St. Louis, MO, USA) in 50:49.9:0.1 acetonitrile:water:trifluoroacetic acid. Matrix was applied in a 200 μm array with 40 passes of one droplet per pass. The instrument minimum repeat time was set to 120 seconds for the first five passes to prevent spots merging on the tissue. Time was then reduced to 90 seconds for the next 10 passes and 70 seconds for the remaining passes.
Data Acquisition
Data were acquired in linear mode using a Bruker AutoFlex Speed III instrument (Bruker Daltonics, Billerica, MA, USA) equipped with a Smartbeam II 1 KHz, 355 nm laser with 500 shots per pixel, a 200 μm step size, and a mass range of 4000–33000 Da. Images were generated using FlexImaging 5.0 and normalized to the total ion count; the relative intensity scales were adjusted for each m/z value to improve image quality. The instrument was calibrated using a mixture of insulin, cytochrome C, apomyoglobin and trypsinogen prior to data acquisition.
3. Results
3.1. LC-MS/MS analysis of AQP0 truncation
AQP0 in cortical and nuclear lens regions was analyzed by LC-MS/MS to determine the extent of modification upon HBO treatment. Expected N- and C-terminal tryptic peptides of guinea pig AQP0 were separated as expected and as seen in the base peak chromatogram of Figure 1. Specific AQP0 peptide signals are shown in selected ion chromatograms (Figure 1) and indicate strong signal for this abundant lens membrane protein. The observed peptides are consistent with the previously reported sequence of guinea pig AQP0 (Han et al., 2004) and the base peak chromatogram is dominated by AQP0 peptide signals. With the exception of large hydrophobic peptides corresponding to transmembrane regions, a majority of expected tryptic peptides were detected providing 53% sequence coverage. Deamidation was observed at residues Asn 245 and Asn 246 via high resolution mass spectrometry using a Q Exactive orbitrap instrument (Thermo Scientific, San Jose, CA) (data not shown). At least one of the residues is deamidated in the cortex sample and the non-deamidated forms of Asn245 and Asn246 are barely detectable in the nucleus sample (data not shown). The mass range selected in Figure 1 includes the signals for both one and two deamidations. Truncation sites in AQP0 were identified by searching the raw data using TagRecon with semi-tryptic specificity. Truncation at residues E250, G251, G253, E257 and T260 was detected. Figure 2 shows the selected ion chromatograms for some truncated C-terminal tryptic peptides in HBO-treated lens cortex and nucleus. The level of truncation is low in the cortex region as evidenced by the noise level in the chromatograms for lower abundance peptides, but truncation levels significantly increase in the nucleus region.
Figure 1: LC-MS analysis of guinea pig lens AQP0.
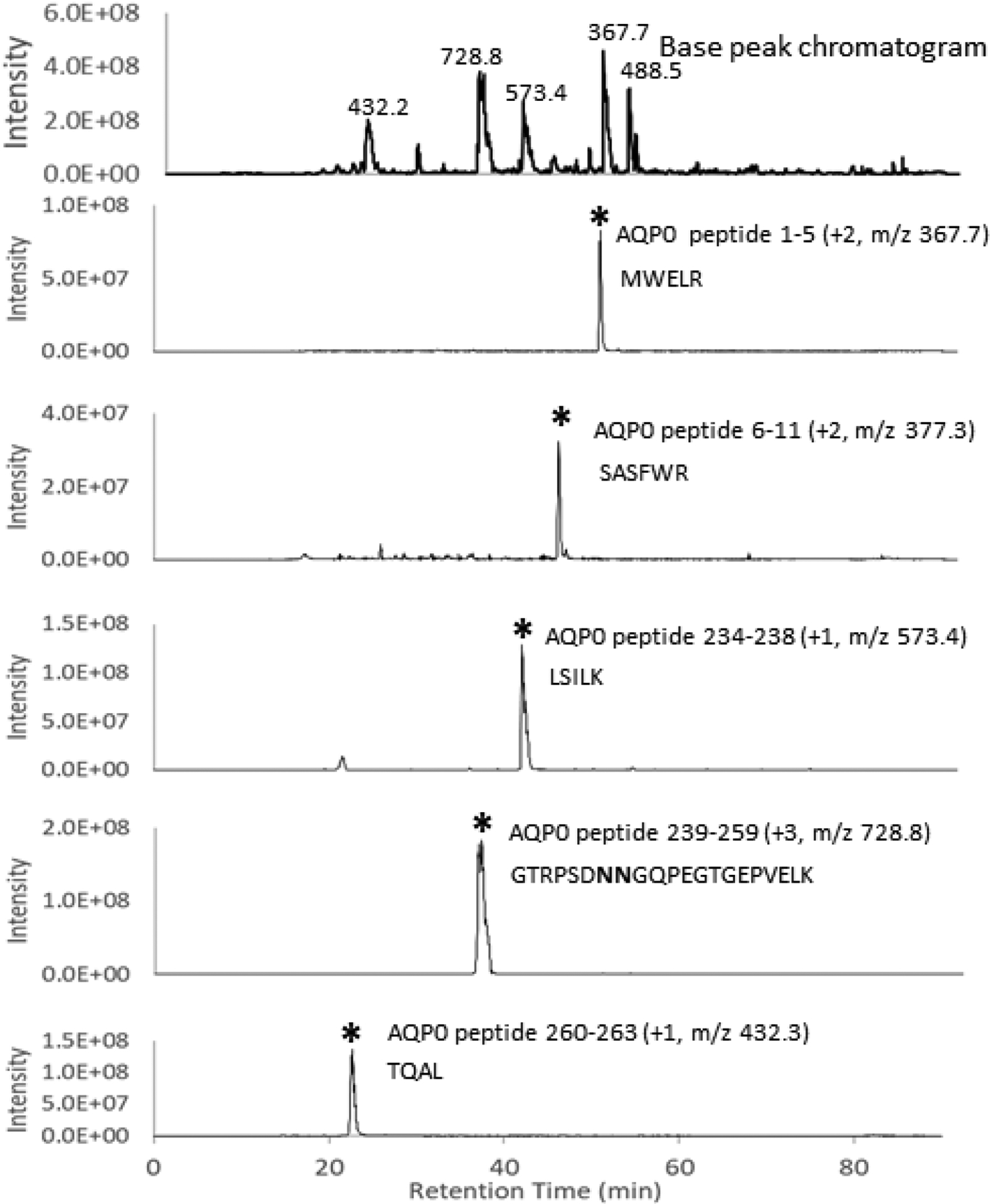
The base peak chromatogram of a lens tryptic digest of the membrane fraction from a control 25-month-old guinea pig lens cortex is shown in the top panel where m/z values of ions observed in the the major base peaks are labeled. Selected ion chromatograms (SICs) for expected N- and C-terminal tryptic peptides are shown in the lower panels. Each SIC is accompanied by the AQP0 residue numbers of the selected peptide, the observed ion charge state, the m/z of the plotted signal, and the peptide sequence. Asterisks indicate the peak of interest. Bold N in the peptide sequence indicates Asp due to partial or complete deamidation.
Figure 2: LC-MS analysis of C-terminal truncation products of guinea pig lens AQP0.
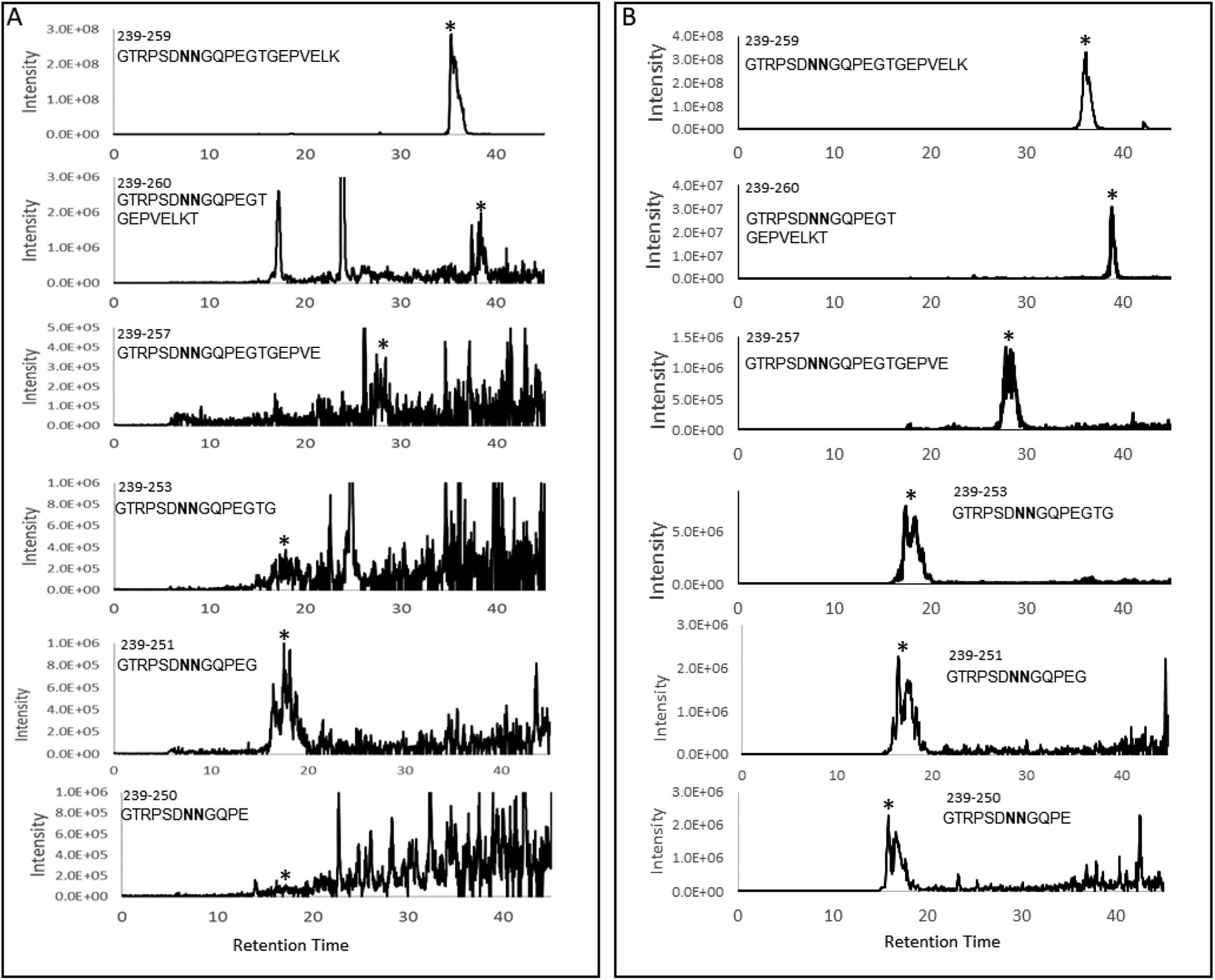
Selected ion chromatograms for truncated C-terminal tryptic peptides in the cortex (A) and nucleus (B) regions of an 81x HBO-treated lens from a 25 month old animal (sequences are indicated next to the eluted peaks). Asterisks indicate the peaks of interest. Bold N in the peptide sequence indicates partial or complete deamidation.
3.2. Quantification of AQP0 C-terminal truncation
The signals of the truncated peptides in the cortex samples were very weak as shown in Figure 2, therefore quantification based on the precursor ion signal is not reliable. Further, the observed signals for the same m/z are split, most likely due to isomerization of Asp and deamidated Asn residues. To increase the signal-to-noise ratio, the MS/MS data were analyzed. In this method, the peak areas of selected fragment ion signals were used for quantification. The selected ion chromatograms for the b10 ion from different truncated peptides can be found in Supplemental Figure 1. To quantify the levels of truncation in control and HBO-treated lenses, the total peak areas of selected fragment ions from each truncated peptide were normalized by the total peak areas of selected fragment ions from C-terminal tryptic peptide 239–259. The signal for this expected (non-truncated) tryptic peptide is detected in all samples and represents the amount of untruncated AQP0 in each sample. The results can be found in Figure 3A and Figure 3B. The relative levels of truncation in the lens cortex of HBO treated animals did not show statistically significant differences compared to the levels of truncation in the control lenses (Figure 3A); however, truncation in the lens nucleus of HBO treated animals increased compared with the control lenses (Figure 3B). The difference reached statistical significance for truncation at residues E250, G253, E257 and T260.
Figure 3: Quantitative analysis of AQP0 C-terminal truncation in control and HBO treated guinea pig lenses.
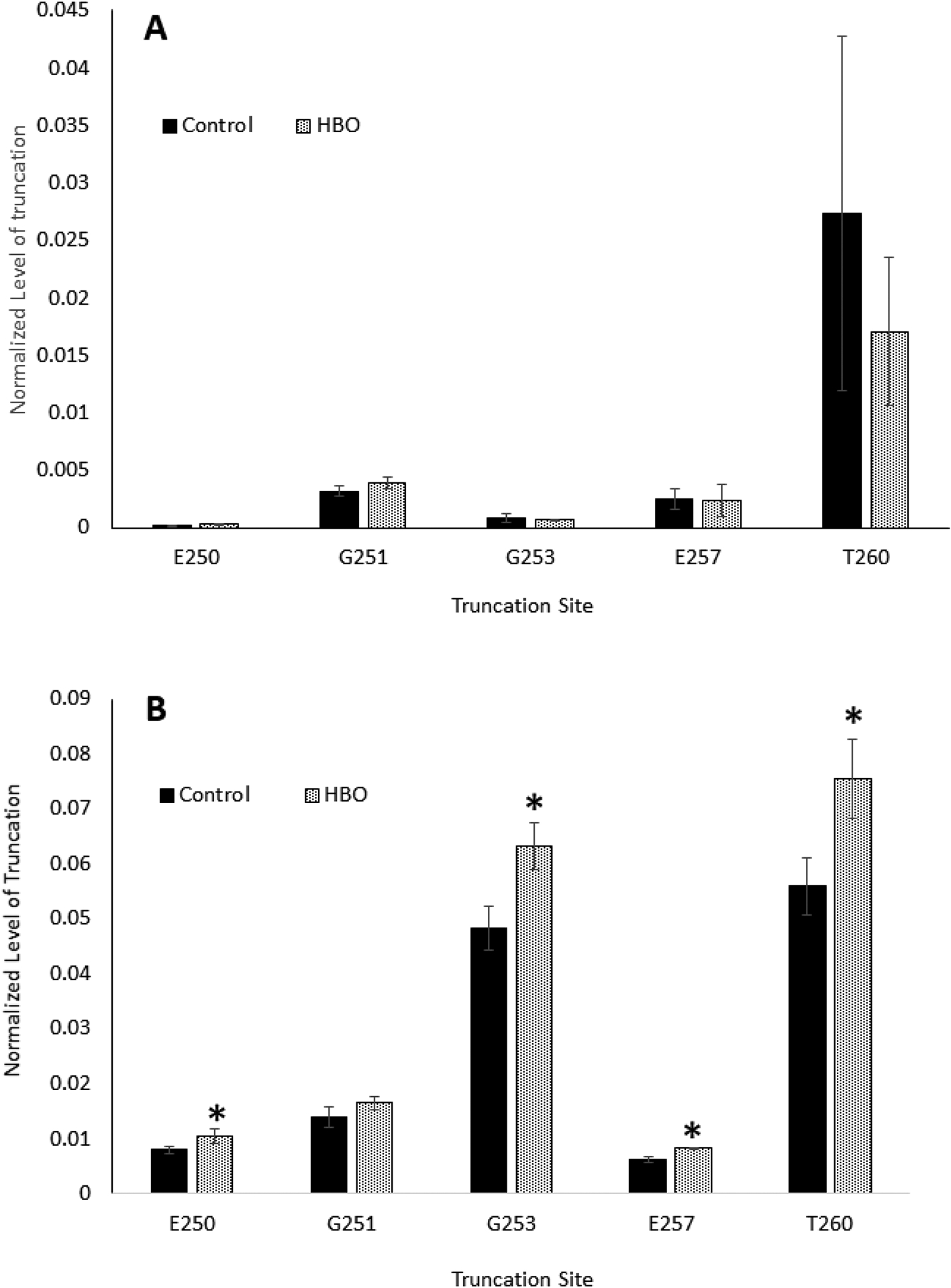
A: Samples from cortex; B: samples from nucleus (note the different vertical axis scales in the two figures). Percent truncation at 5 different C-terminal amino acid residues is shown. Animals were treated with HBO either 81 or 84 times over a 7-month period (25-months at time of analysis). The y-axis indicates the ratio of the truncated peptide signal to the corresponding non-truncated peptide signal of AQP0 tryptic peptide containing residues 239–259. Results are expressed as means +/− S.D. n=4 * indicates a statistically significant difference (p<0.05).
3.8. Imaging mass spectrometry of crystallin peptides
The results shown in Figure 3 demonstrate that treatment of guinea pigs with HBO (81–84 times over a 7-month period) induces C-terminal truncation of lens nuclear AQP0. In addition, we have previously shown using MALDI-TOF MS analysis of lens urea soluble supernatants that a similar treatment of guinea pigs with HBO produced a significant accumulation of an 1848 Da fragment of αA-crystallin, αA66–80, in the lens nucleus, compared to controls (Raju et al., 2015). Thus, it appears that an elevated level of O2 is somehow able to induce fragmentation of proteins in the center of the lens. The purpose of the next experiment was to use imaging mass spectrometry to investigate the spatial localization of crystallin truncation products in lenses of guinea pigs treated with HBO using imaging mass spectrometry.
Lenses of two-month old untreated animals were found to have nearly zero levels of six crystallin peptides ranging in molecular weight from 6,065 to 12,181 Da (Figure 4). The low intensity signals in the young lens appear as green and blue pixels in the images. In contrast, lenses of 22-month-old untreated animals contained substantial amounts of each of the six peptides, located primarily in the lens nucleus, as indicates by spatially localized yellow and red pixels. These peptides observed by IMS have been identified by matching measured molecular weights with predicted truncation product molecular weights: αA1–50 (6,065 Da), αA1–58 (6996), αA1–65 (7,721 Da), αA1–78 (9289), αA1–80 (9,577 Da) and αA1–101 (11,978 Da) (Table 1). In addition, three peptides, αA1–101, A1–80, and A1–78 were sequenced from 22-month control tissue extracts using top-down tandem mass spectrometry to confirm their sequence assignments (Supplemental Figures 2–4). Remarkably, 22-month-old guinea pigs that had previously received 43 treatments with HBO over a 15-week period showed significantly lower levels of each of the truncated αA-crystallin peptides in their lens nuclei (Fig. 4) as indicated by fewer high intensity, red pixels compared to the age-matched control lenses. We hypothesized that the truncation observed in the lens nucleus of the control 22-month-old animals is due to normal aging while HBO treatment accelerated the truncation products resulting in further truncation of the observed signals and, thus a reduction in the observed levels of these products in the images.
Figure 4: Imaging mass spectrometry of lens crystallin peptides in guinea pigs treated with HBO.
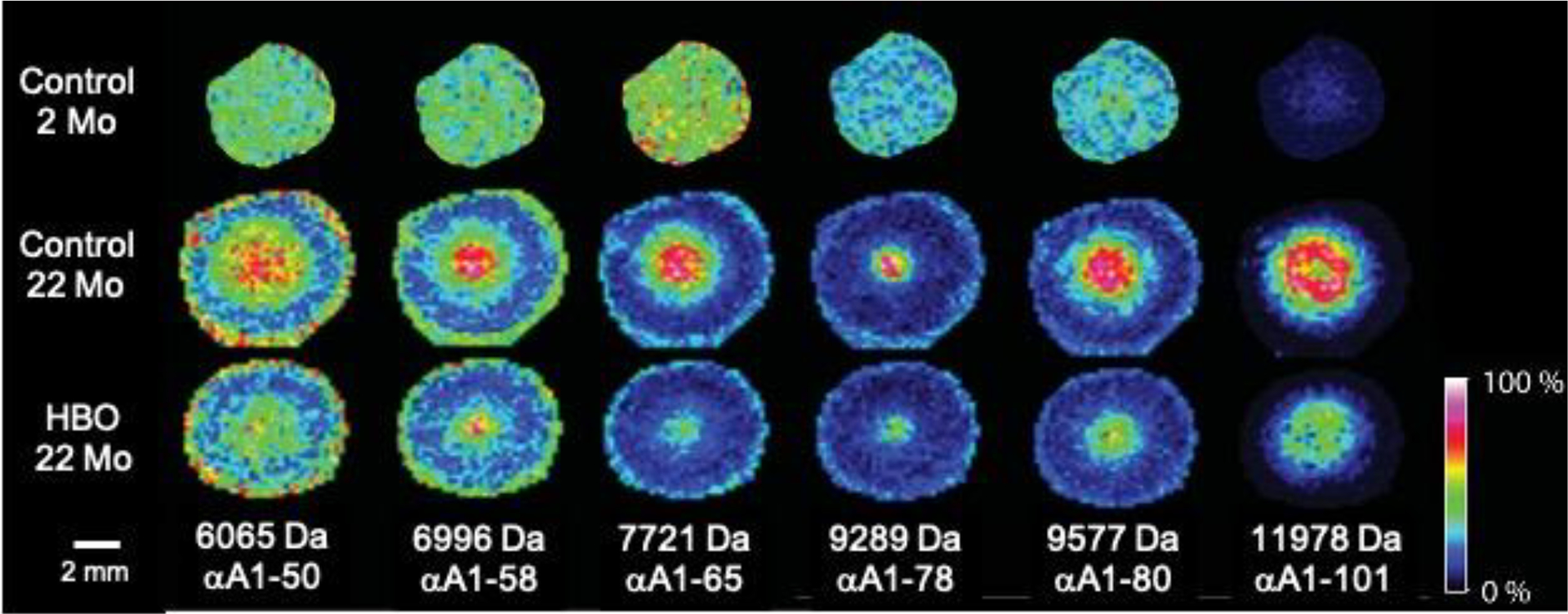
Spatial distribution of selected IMS signals that represent crystallin truncation peptides. Sequence assignments are shown. The ages of the animals were 22 months for HBO-treated, and either 2 or 22 months for untreated controls. Experimental animals were treated 43 times with HBO over a 15-week period. The results shown are representative of two experiments.
Table 1.
Measured and predicted masses of αA-crystallin truncation products.
| Truncation Product | Predicted m/z | Measured m/z |
|---|---|---|
| αA1–50 | 6066 | 6065 |
| αA1–58 | 6998 | 6996 |
| αA1–65 | 7727 | 7721 |
| αA1–78 | 9291 | 9289 |
| αA1–80 | 9575 | 9577 |
| αA1–101 | 11979 | 11978 |
To test this hypothesis, we examined lenses with fewer HBO treatments (15X and 30X). A comparison of lenses from animals treated 15, 30, and 43 times with HBO are shown in Figure 5. Note that with the fewest number of treatments, the signals for the truncated crystallins, with the exception of αA1–78, are higher in the nuclei of HBO treated animals compared to control animal lenses as evidenced by a larger number of yellow and red pixels in the nucleus region. In addition, with further HBO treatments, the truncation products are reduced in signal compared to controls, supporting our hypothesis of accelerated aging with HBO treatment. Specifically, at the 30X treatment stage, the truncation products are abundant in the control lenses, presumably due to increased age (5 weeks older) and less so in the HBO treated lenses. At the 43X HBO time point, the truncation products are at relatively low levels in both control and HBO treated lenses due to further modification of the peptides.
Figure 5: Imaging mass spectrometry of lens crystallin peptides in guinea pigs treated with HBO over time.
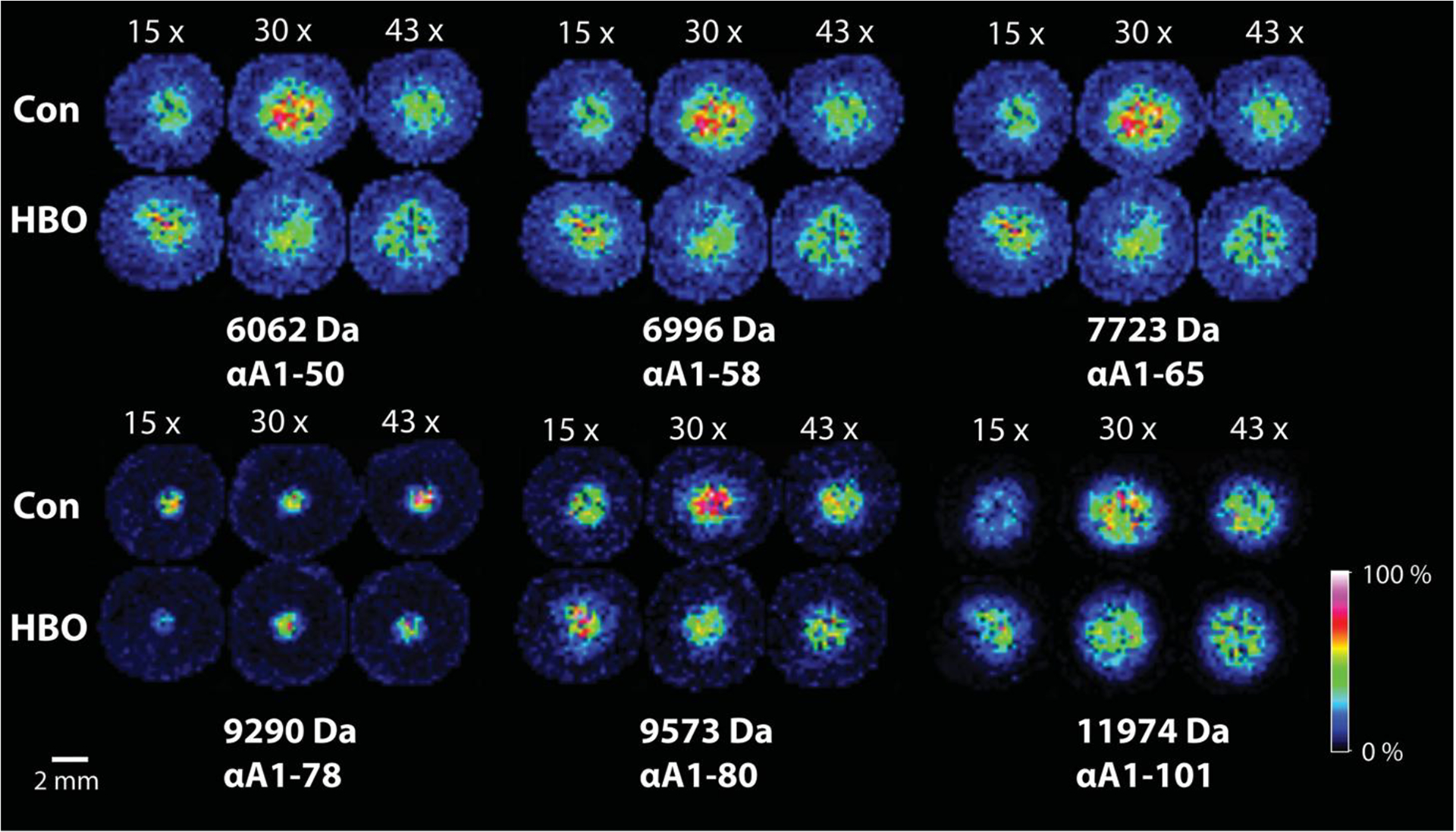
Spatial distribution of selected IMS signals in lenses as a function of 15X, 30X, or 43X treatments with HBO (19, 20.5 and 22 months of age, respectively). Sequence assignments are shown.
4. Discussion
Using complimentary mass spectrometry methods, an HBO treatment-associated increase in lens protein truncation was detected for both lens αA-crystallin and AQP0. HBO treatment induced protein truncation at the same sites as those observed in control, untreated lenses suggesting an acceleration of the aging phenotype. The accelerated truncation of crystallins and AQP0 is consistent with early studies of HBO treated animals where accelerated loss of cytoskeletal proteins and AQP0 was reported (Padgaonkar et al., 1999). Interestingly, increased AQP0 truncation with HBO treatment occurs only in the lens nucleus where the age-related opacity occurs (Giblin et al., 1995). IMS results spatially localize a similar increase in αA-crystallin truncation to the lens nucleus with HBO treatment.
The sites of AQP0 truncation reported in this study vary from Gly to Glu to Thr and differ from those reported in human lenses (Asp243, Asn246, and Asn259)(Ball et al., 2004). A number of non-enzymatic truncation mechanisms have been reported including truncation at Asn/Asp residues (Voorter et al., 1988; Wang et al., 2019) and Ser residues (Lyons et al., 2016; Su et al., 2012; Takemoto, 1995) and human AQP0 truncation is believed to occur via non-enzymatic mechanisms. In fact, when an AQP0 C-terminal synthetic peptide was incubated in phosphate buffer, spontaneous cleavage at Asn residues was observed (Ball et al., 2004). When the guinea pig C-terminal peptide was incubated in buffer, no cleavage was observed (Michael Friedrich & Roger Truscott, personal communication). How HBO treatment leads to increased truncation remains unknown. If the underlying mechanism of truncation is non-enzymatic, how HBO treatment would enhance such cleavage is unclear given that oxidative processes have not been invoked to explain lens protein truncation. However, it is of interest that in previous work conducted by the authors (Giblin et al., 2006), it was found that a 5-month exposure of guinea pigs to UVA light also produced a significant increase in truncation of lens nuclear (but not cortical) AQP0 residues G250 and G253, compared to age-matched controls, as was found in this study with HBO-treatment. This suggests that the oxidant H2O2 may somehow be connected with the observed truncation since both oxygen and UVA light are known to generate peroxide in the lens nucleus via metal-catalyzed oxidation of GSH and ascorbate, or interaction with NADPH, respectively (Cunningham et al., 1985; Giblin, 2000; Linetsky and Ortwerth, 1995; Simpanya et al., 2008). A 7-month treatment with HBO and a 5-month exposure to UVA light each produced increased lens NLS in the guinea pig. Another possible enzymatic mechanism for truncation is cleavage via calpain. Treatment of rat lens AQP0 with m-calpain resulted in enhanced cleavage at Asp244, but this only partially mimicked truncation at Lys238 and Gly239 observed in untreated lenses and in selenite-induced cataract lenses (Schey et al., 1999). It cannot be ruled out that calpain isoform Lp82, which has been detected in the guinea pig lens (Nakamura et al., 2000), may have contributed to the observed truncation. LP82 becomes activated at a lower concentration than m-calpain (Ueda et al., 2001), but decreases in activity with maturation of the lens (Shearer et al., 1998). Increases in calcium that would enhance calpain activity have not been observed in lenses of HBO-treated guinea pigs (Padgaonkar et al., 1999), mimicking the absence of any calcium increase occurring during formation of human ARNC (Duncan and Bushell, 1975).
The truncation sites observed for αA-crystallin include: Gln50, Asp58, Arg65, Lys78, Phe80, and Asn101. Note that truncation at Gln, Asp, and Asn are commonly observed in long lived proteins presumably via succinimide or glutaramide intermediates (Voorter et al., 1988; Wang et al., 2019). Interestingly, Gln50, Asp58, Lys78 and Phe80 residues are N-terminal to serine residues that are known to undergo truncation (N-terminal to Ser) and these sites are identical to those sites observed in truncated human αA-crystallin (Lyons et al., 2016; Su et al., 2012; Takemoto, 1995). All sites observed, except Lys78, are major sites of truncation in human αA-crystallin (Grey et al., 2009).A previous study identified more than 25 small crystallin fragments <3.5 kDa present in old human lenses and human cataracts, existing primarily in the nucleus (Santhoshkumar et al., 2008). One of the fragments, αA66–80 peptide, was shown to bind to both human and guinea pig αA-crystallin, producing crystallin aggregation and precipitation (Raju et al., 2015; Santhoshkumar et al., 2011). In addition, mass spectrometry analysis of the urea-soluble fraction of lenses of HBO-treated guinea pigs showed a significant increase in the level of αA66–80 peptide compared to age-matched controls (Raju et al., 2015). In this current IMS study, although αA66–80 peptide was not detected in either control or experimental guinea pig lenses, likely due to low overall abundance or ion suppression from other small crystallin peptides, ample amounts of the precursor of αA66–80, αA1–80 peptide, as well as a truncation product of αA1–80, αA1–65, were detected in 22 month-old control lenses, but to a much lesser extent in 22 month-old lenses of HBO-treated animals (Fig. 4). Additional future experiments could combine classical LC-MS/MS analysis of lens peptides at various HBO treatment levels to elucidate the fate of larger truncation products observed in the current study. We speculate that increased levels of oxygen in the lens nucleus somehow induces truncation of the αA1–80 peptide to produce deleterious αA66–80 peptide which binds to αA-crystallin, causing crystallin aggregation and increased lens NLS known to be associated with the HBO model for human ARNC. Thus, the HBO model may be a good model to explore the relationship of oxidation an lens protein truncation.
5. Conclusions
It is clear from the current study that HBO treatment accelerates the natural aging process that includes protein truncation. The mechanism of oxidation-induced truncation remains unknown and may differ between αA-crystallin and AQP0; however, our data are consistent with the notion that the HBO guinea pig model recapitulates human ARNC.
Supplementary Material
Acknowledgements
We thank Cliff Snitgen, Janet Schofding and Mitun Chablani in the Oakland University Biomedical Research Support Facility for professional care of the guinea pigs. Li-Ren Lin MD, conducted slit lamp examination of the eyes of the animals. Oakland University students Aparna Bhat, Melodie Denstadt, Michael Lupe, Brijesh Patel, Pavan Vempaty, Nathanial Whitcomb and Jasper Yung treated the guinea pigs with HBO. Victor Leverenz isolated the lenses and the lens nuclear and cortical sections. Vanita Padgaonkar, PhD, and Francis Simpanya, PhD, isolated AQP0.
Funding
This work was supported in part by NIH grant EY02027 (FJG), EY013462 (KLS), EY024258 (KLS), and EY008126.
Footnotes
Competing Interests
The authors declare no competing interests.
References
- Anderson DM, Floyd KA, Barnes S, Clark JM, Clark JI, Mchaourab H and Schey KL, 2015. A method to prevent protein delocalization in imaging mass spectrometry of non-adherent tissues: application to small vertebrate lens imaging. Anal Bioanal Chem. 407, 2311–2320. 10.1007/s00216-015-8489-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball LE, Garland DL, Crouch RK and Schey KL, 2004. Post-translational modifications of aquaporin 0 (AQP0) in the normal human lens: spatial and temporal occurrence. Biochemistry. 43, 9856–9865. 10.1021/bi0496034 [DOI] [PubMed] [Google Scholar]
- Bantseev V, Oriowo OM, Giblin FJ, Leverenz VR, Trevithick JR and Sivak JG, 2004. Effect of hyperbaric oxygen on guinea pig lens optical quality and on the refractive state of the eye. Exp Eye Res. 78, 925–931. 10.1016/j.exer.2004.01.002 [DOI] [PubMed] [Google Scholar]
- Borchman D, Giblin FJ, Leverenz VR, Reddy VN, Lin LR, Yappert MC, Tang D and Li L, 2000. Impact of aging and hyperbaric oxygen in vivo on guinea pig lens lipids and nuclear light scatter. Invest Ophthalmol Vis Sci. 41, 3061–3073. [PubMed] [Google Scholar]
- Caprioli RM, Farmer TB and Gile J, 1997. Molecular imaging of biological samples: localization of peptides and proteins using MALDI-TOF MS. Anal Chem. 69, 4751–4760. 10.1021/ac970888i [DOI] [PubMed] [Google Scholar]
- Costello MJ, Burette A, Weber M, Metlapally S, Gilliland KO, Fowler WC, Mohamed A and Johnsen S, 2012. Electron tomography of fiber cell cytoplasm and dense cores of multilamellar bodies from human age-related nuclear cataracts. Exp Eye Res. 101, 72–81. 10.1016/j.exer.2012.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham ML, Johnson JS, Giovanazzi SM and Peak MJ, 1985. Photosensitized production of superoxide anion by monochromatic (290–405 nm) ultraviolet irradiation of NADH and NADPH coenzymes. Photochem Photobiol. 42, 125–128. 10.1111/j.1751-1097.1985.tb01549.x [DOI] [PubMed] [Google Scholar]
- Dasari S, Chambers MC, Slebos RJ, Zimmerman LJ, Ham AJ and Tabb DL, 2010. TagRecon: high-throughput mutation identification through sequence tagging. J Proteome Res. 9, 1716–1726. 10.1021/pr900850m [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan G and Bushell AR, 1975. Ion analyses of human cataractous lenses. Exp Eye Res. 20, 223–230. 10.1016/0014-4835(75)90136-0 [DOI] [PubMed] [Google Scholar]
- Giblin FJ, 2000. Glutathione: a vital lens antioxidant. J Ocul Pharmacol Ther. 16, 121–135. 10.1089/jop.2000.16.121 [DOI] [PubMed] [Google Scholar]
- Giblin FJ, David LL, Wilmarth PA, Leverenz VR and Simpanya MF, 2013. Shotgun proteomic analysis of S-thiolation sites of guinea pig lens nuclear crystallins following oxidative stress in vivo. Mol Vis. 19, 267–280. [PMC free article] [PubMed] [Google Scholar]
- Giblin FJ, Han J and Schey KL, 2006. Identification of sites of truncation of AQP0 induced by hyperbaric oxygen and UVA light in vivo. Invest Ophthalmol Vis Sci. 47, 4088. [Google Scholar]
- Giblin FJ, Padgaonkar VA, Leverenz VR, Lin LR, Lou MF, Unakar NJ, Dang L, Dickerson JE Jr. and Reddy VN, 1995. Nuclear light scattering, disulfide formation and membrane damage in lenses of older guinea pigs treated with hyperbaric oxygen. Exp Eye Res. 60, 219–235. 10.1016/s0014-4835(05)80105-8 [DOI] [PubMed] [Google Scholar]
- Gosselin ME, Kapustij CJ, Venkateswaran UD, Leverenz VR and Giblin FJ, 2007. Raman spectroscopic evidence for nuclear disulfide in isolated lenses of hyperbaric oxygen-treated guinea pigs. Exp Eye Res. 84, 493–499. 10.1016/j.exer.2006.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grey AC, Chaurand P, Caprioli RM and Schey KL, 2009. MALDI imaging mass spectrometry of integral membrane proteins from ocular lens and retinal tissue. J Proteome Res. 8, 3278–3283. 10.1021/pr800956y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grey AC and Schey KL, 2009. Age-related changes in the spatial distribution of human lens alpha-crystallin products by MALDI imaging mass spectrometry. Invest Ophthalmol Vis Sci. 50, 4319–4329. 10.1167/iovs.09-3522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hains PG and Truscott RJ, 2010. Age-dependent deamidation of lifelong proteins in the human lens. Invest Ophthalmol Vis Sci. 51, 3107–3114. 10.1167/iovs.09-4308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han J, Little M, David LL, Giblin FJ and Schey KL, 2004. Sequence and peptide map of guinea pig aquaporin 0. Mol Vis. 10, 215–222. [PubMed] [Google Scholar]
- Han J and Schey KL, 2006. MALDI tissue imaging of ocular lens alpha-crystallin. Invest Ophthalmol Vis Sci. 47, 2990–2996. 10.1167/iovs.05-1529 [DOI] [PubMed] [Google Scholar]
- Hooi MY and Truscott RJ, 2011. Racemisation and human cataract. D-Ser, D-Asp/Asn and D-Thr are higher in the lifelong proteins of cataract lenses than in age-matched normal lenses. Age (Dordr). 33, 131–141. 10.1007/s11357-010-9171-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lampi KJ, Wilmarth PA, Murray MR and David LL, 2014. Lens beta-crystallins: the role of deamidation and related modifications in aging and cataract. Prog Biophys Mol Biol. 115, 21–31. 10.1016/j.pbiomolbio.2014.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linetsky M and Ortwerth BJ, 1995. The generation of hydrogen peroxide by the UVA irradiation of human lens proteins. Photochem Photobiol. 62, 87–93. 10.1111/j.1751-1097.1995.tb05243.x [DOI] [PubMed] [Google Scholar]
- Lyons B, Kwan AH and Truscott RJ, 2016. Spontaneous cleavage of proteins at serine and threonine is facilitated by zinc. Aging Cell. 15, 237–244. 10.1111/acel.12428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma ZQ, Dasari S, Chambers MC, Litton MD, Sobecki SM, Zimmerman LJ, Halvey PJ, Schilling B, Drake PM, Gibson BW and Tabb DL, 2009. IDPicker 2.0: Improved protein assembly with high discrimination peptide identification filtering. J Proteome Res. 8, 3872–3881. 10.1021/pr900360j [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maclean B, Tomazela DM, Shulman N, Chambers M, Finney GL, Frewen B, Kern R, Tabb DL, Liebler DC and Maccoss MJ, 2010. Skyline: an open source document editor for creating and analyzing targeted proteomics experiments. Bioinformatics. 26, 966–968. 10.1093/bioinformatics/btq054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura Y, Fukiage C, Shih M, Ma H, David LL, Azuma M and Shearer TR, 2000. Contribution of calpain Lp82-induced proteolysis to experimental cataractogenesis in mice. Invest Ophthalmol Vis Sci. 41, 1460–1466. [PubMed] [Google Scholar]
- Padgaonkar VA, Lin LR, Leverenz VR, Rinke A, Reddy VN and Giblin FJ, 1999. Hyperbaric oxygen in vivo accelerates the loss of cytoskeletal proteins and MIP26 in guinea pig lens nucleus. Exp Eye Res. 68, 493–504. 10.1006/exer.1998.0630 [DOI] [PubMed] [Google Scholar]
- Palmquist BM, Philipson B and Barr PO, 1984. Nuclear cataract and myopia during hyperbaric oxygen therapy. Br J Ophthalmol. 68, 113–117. 10.1136/bjo.68.2.113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raju M, Mooney BP, Thakkar KM, Giblin FJ, Schey KL and Sharma KK, 2015. Role of alphaA-crystallin-derived alphaA66–80 peptide in guinea pig lens crystallin aggregation and insolubilization. Exp Eye Res. 132, 151–160. 10.1016/j.exer.2015.01.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santhoshkumar P, Raju M and Sharma KK, 2011. alphaA-crystallin peptide SDRDKFVIFLDVKHF accumulating in aging lens impairs the function of alpha-crystallin and induces lens protein aggregation. PLoS One. 6, e19291. 10.1371/journal.pone.0019291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santhoshkumar P, Udupa P, Murugesan R and Sharma KK, 2008. Significance of interactions of low molecular weight crystallin fragments in lens aging and cataract formation. J Biol Chem. 283, 8477–8485. 10.1074/jbc.M705876200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schey KL, Anderson DM and Rose KL, 2013. Spatially-directed protein identification from tissue sections by top-down LC-MS/MS with electron transfer dissociation. Anal Chem. 85, 6767–6774. 10.1021/ac400832w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schey KL, Fowler JG, Shearer TR and David L, 1999. Modifications to rat lens major intrinsic protein in selenite-induced cataract. Invest Ophthalmol Vis Sci. 40, 657–667. [PubMed] [Google Scholar]
- Shearer TR, Ma H, Shih M, Hata I, Fukiage C, Nakamura Y and Azuma M, 1998. Lp82 calpain during rat lens maturation and cataract formation. Curr Eye Res. 17, 1037–1043. 10.1076/ceyr.17.11.1037.5232 [DOI] [PubMed] [Google Scholar]
- Simpanya MF, Ansari RR, Leverenz V and Giblin FJ, 2008. Measurement of lens protein aggregation in vivo using dynamic light scattering in a guinea pig/UVA model for nuclear cataract. Photochem Photobiol. 84, 1589–1595. 10.1111/j.1751-1097.2008.00390.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpanya MF, Ansari RR, Suh KI, Leverenz VR and Giblin FJ, 2005. Aggregation of lens crystallins in an in vivo hyperbaric oxygen guinea pig model of nuclear cataract: dynamic light-scattering and HPLC analysis. Invest Ophthalmol Vis Sci. 46, 4641–4651. 10.1167/iovs.05-0843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su SP, Lyons B, Friedrich M, Mcarthur JD, Song X, Xavier D, Truscott RJ and Aquilina JA, 2012. Molecular signatures of long-lived proteins: autolytic cleavage adjacent to serine residues. Aging Cell. 11, 1125–1127. 10.1111/j.1474-9726.2012.00860.x [DOI] [PubMed] [Google Scholar]
- Takemoto LJ, 1995. Identification of the in vivo truncation sites at the C-terminal region of alpha-A crystallin from aged bovine and human lens. Curr Eye Res. 14, 837–841. 10.3109/02713689508995806 [DOI] [PubMed] [Google Scholar]
- Truscott RJ, 2005. Age-related nuclear cataract-oxidation is the key. Exp Eye Res. 80, 709–725. 10.1016/j.exer.2004.12.007 [DOI] [PubMed] [Google Scholar]
- Truscott RJ and Zhu X, 2010. Presbyopia and cataract: a question of heat and time. Prog Retin Eye Res. 29, 487–499. 10.1016/j.preteyeres.2010.05.002 [DOI] [PubMed] [Google Scholar]
- Ueda Y, Mccormack AL, Shearer TR and David LL, 2001. Purification and characterization of lens specific calpain (Lp82) from bovine lens. Exp Eye Res. 73, 625–637. 10.1006/exer.2001.1071 [DOI] [PubMed] [Google Scholar]
- Voorter CE, De Haard-Hoekman WA, Van Den Oetelaar PJ, Bloemendal H and De Jong WW, 1988. Spontaneous peptide bond cleavage in aging alpha-crystallin through a succinimide intermediate. J Biol Chem. 263, 19020–19023. [PubMed] [Google Scholar]
- Wang Z, Friedrich MG, Truscott RJW and Schey KL, 2019. Cleavage C-terminal to Asp leads to covalent crosslinking of long-lived human proteins. Biochim Biophys Acta Proteins Proteom. 1867, 831–839. 10.1016/j.bbapap.2019.06.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenke JL, Rose KL, Spraggins JM and Schey KL, 2015. MALDI Imaging Mass Spectrometry Spatially Maps Age-Related Deamidation and Truncation of Human Lens Aquaporin-0. Invest Ophthalmol Vis Sci. 56, 7398–7405. 10.1167/iovs.15-18117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilmarth PA, Tanner S, Dasari S, Nagalla SR, Riviere MA, Bafna V, Pevzner PA and David LL, 2006. Age-related changes in human crystallins determined from comparative analysis of post-translational modifications in young and aged lens: does deamidation contribute to crystallin insolubility? J Proteome Res. 5, 2554–2566. 10.1021/pr050473a [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


