Abstract
Background:
Posttraumatic Stress Disorder (PTSD) often co-occurs with increased alcohol consumption (AC) and alcohol use disorder (AUD), however it is unknown whether the same etiologic influences underlying PTSD-AUD comorbidity are those underlying PTSD and AC.
Methods:
This study used large-scale genome wide association study (GWAS) data to test if PTSD and drinks per week [DPW]/AUD are causally related to one another, and if so, if PTSD precedes DPW/AUD and/or vice versa, using Mendelian Randomization on European ancestry GWAS summary statistics from the Psychiatric Genomics Consortia (PGC; PTSD), GWAS & Sequencing Consortium of Alcohol and Nicotine Use (GSCAN; DPW), and Million Veteran Program (MVP; AUD).
Results:
PTSD exerted a potentially causal effect on AUD (beta= 0.039, se= 0.014, p= 0.005), but not on DPW (beta= 0.002, se= 0.003, p= 0.414). Additionally, neither DPW (beta= 0.019, se= 0.041, p= 0.637) nor AUD (beta= 8.87×10−4, se= 0.001, p= 0.441) exerted a causal effect on PTSD.
Conclusions:
These findings are consistent with the self-medication model, in which individuals misuse alcohol as a way of coping with aversive trauma-related symptoms. These findings extend latent and molecular findings of shared and correlated risk between PTSD and alcohol phenotypes. Given the health behaviors associated with these phenotypes, these findings are important in that they suggest groups on which to prioritize prevention efforts. Further, they provide a rationale for future pre-clinical and clinical studies examining the biological mechanisms by which PTSD may impact AUD.
Keywords: Mendelian Randomization, Posttraumatic Stress Disorder, Alcohol Consumption, Alcohol Use Disorder
Introduction
Traumatic events are common, with approximately 70% of the general population being exposed to at least one trauma in their lifetime, and 30.5% exposed to four or more traumatic events (Benjet et al., 2016). Traumatic event exposure is a transdiagnostic risk factor, associated with a range of problematic outcomes, including but not limited to posttraumatic stress disorder (PTSD), the signature trauma-related disorder (Breslau, 2009). PTSD tends to co-occur with increased alcohol consumption (AC) and alcohol use disorder (AUD; Jakupcak et al., 2010, Vlahov et al., 2002, Debell et al., 2014). Indeed, more than half of males and about one-third of females with PTSD meet criteria for AUD (Kessler et al., 1995). Additionally, more than half of those seeking treatment for AUD meet criteria for PTSD (Simpson et al., 2012). The co-occurrence of PTSD and AC/AUD is associated with higher impairment (Blanco et al., 2013), suicidal ideation (Rojas et al., 2014), and poorer treatment prognosis (Blanco et al., 2013), making efforts to understand its etiology clinically important.
There are multiple non-mutually exclusive hypotheses about why these conditions co-occur. The self-medication model suggests that individuals consume or misuse alcohol in order to alleviate their aversive trauma-related symptoms (Stewart, 1996, Read et al., 2012). In contrast, the risky behavior model posits that individuals who use substances are at increased risk for trauma exposure and in turn PTSD (Windle, 1994). Finally, it is possible that there are pleiotropic (i.e., genetic) or shared environmental risk factors for both conditions (i.e., shared risk model) (Read, 2014). Despite the extensive literature surrounding these hypotheses, the etiologic relations underlying these co-occurring conditions remains unclear.
PTSD, AC and AUD are all moderately heritable (Stein et al., 2002, Knopik et al., 2004, Kaprio et al., 1987), with twin studies suggesting evidence for correlated genetic risks shared between PTSD-AC and PTSD-AUD (Xian et al., 2000, McLeod et al., 2001a). Until recently genome-wide association studies (GWAS) have yielded a limited number of genome-wide significant variants associated with AC (e.g., Clarke et al., 2017, Kranzler et al., 2019, Liu et al., 2019), problems (AUDIT-P; Sanchez-Roige et al., 2019), alcohol dependence (AD) or problematic alcohol use (e.g., Gelernter et al., 2014, Walters et al., 2018, Zhou et al., 2020), and PTSD (e.g., Nievergelt et al., 2019, Gelernter et al., 2019). However, recent large GWAS metanalyses for AC and problematic alcohol use have yielded 99 (Liu et al., 2019) and 29 (Zhou et al., 2020) robustly associated variants, respectively. Few studies to date have examined the genetic correlation between PTSD and AUD using molecular genetic techniques such as linkage disequilibrium score regression (Bulik-Sullivan et al., 2015), but recent research by our group found a moderate, significant correlation between PTSD and AD (rg=.35, Sheerin et al., 2020). To our knowledge, studies have yet to estimate genetic correlations between PTSD and AC. Major Depressive Disorder (MDD), is a disorder with high comorbidity and latent genetic overlap with PTSD (Sartor et al., 2012, Kessler et al., 1995). Recent work examining molecular genetic correlations between MDD and AD and alcohol quantity found positive significant genetic correlations between both pairs of phenotypes, with the correlation between MDD and AD being larger than the correlation between MDD and alcohol quantity (Polimanti et al., 2019).
Although genetic correlations are useful for understanding the magnitude of the genetic association between phenotypes, they do not test or explicate the underlying mechanisms linking the constructs tested (i.e., causal effects vs. shared genetic vulnerabilities). Mendelian Randomization (MR; Davey Smith and Hemani, 2014) is one approach that can help clarify whether one phenotype is causally related to the other (or vice versa) via vertical or mediated pleiotropy or whether some other factor influences both variables leading to their association (via horizontal pleiotropic mechanisms or other unmeasured risks). Considering the two-sample MR framework where information regarding genetic associations are derived from two independent datasets (one for the exposure and one for the outcome; Hartwig et al., 2016), the vertical pleiotropy scenario assumes that the genetic variants are associated with the outcome only through their association with the exposure. Conversely, the horizontal pleiotropy scenario is characterized by an instrumental variable including genetic variants that are associated with the outcome independently from their effect on the exposure. Assuming that there is no horizontal pleiotropy, MR can be applied to test causal hypotheses between phenotypes using genetic variables (e.g., single nucleotide polymorphisms/SNPs) as instruments. In essence, the instruments (i.e., for GWAS MR studies SNPs) act as the independent variable (i.e., randomization) in the randomized controlled trials (RCTs; e.g., Ravera et al., 2018).
MR has been applied to the question of whether the association between MD and AD is consistent with a genetically mediated causal impact. Specifically, one study using this method (albeit with only two SNPs) failed to find a causal effect of AC on MDD (Wium-Andersen et al., 2015). A more recent study found evidence that MDD exerts a causal impact on AD but not on AUD (Polimanti et al., 2019). The causal effect of MDD on AD was supported by the absence of a bidirectional effect (i.e., MDD instrumental variable showed an effect on AD while AD instrumental variation did not affect MDD), heterogeneity within the genetic instrument, and the violations of the instrumental variable assumptions. Conversely, the relationship between MDD and AC was bidirectional and with evidence of heterogeneity and violations of the instrumental variable assumptions. Therefore, it is important to test the causal link between PTSD and multiple alcohol phenotypes (i.e., AC and AUD). Finding a causal link between PTSD and AC/AUD (or vice versa) would have potentially important translational implications, in particular about how to prioritize time with providers within the broader medical system (e.g., primary care). For example, if PTSD precedes AC/AUD (i.e., self-medication model) and medical providers come across patients with PTSD, but only have a short time to spend with these patients, they could prioritize referring for treatment for PTSD, as well as AUD prevention services (e.g., harm reduction). Alternatively, if AC/AUD precedes PTSD (i.e., risky behavior model), medical providers could prioritize providing/referring for AUD, as well as treatments that might help reduce risk for trauma exposure, and/or PTSD if trauma-exposed (e.g., Prolonged Exposure).
Expanding on the latent and molecular studies finding genetic correlations between PTSD-AC and PTSD-AUD, the current study examines the potential causal relations linking PTSD and AC (here, drinks per week/DPW), and PTSD and AUD among individuals of European ancestry (EA). This study leverages GWAS summary statistics for PTSD, DPW, and AUD from the Psychiatric Genomic Consortium (PGC) PTSD Workgroup, GWAS & Sequencing Consortium of Alcohol and Nicotine use (GSCAN), and Million Veteran Program (MVP), respectively. In addition to examining whether PTSD and DPW/AUD are causally related to one another, and whether there is heterogeneity in these effects, we also tested whether there may be other unmeasured variables that are leading to the association between constructs.
Materials and Methods
Study Design
MR is employed to investigate potential causal relations between exposures and outcomes (e.g., PTSD and alcohol phenotypes) using genomic data (e.g., individual SNPs) as instrumental variables. In this way, MR is analogous to randomized controlled trials (RCTs) in that it is able to overcome the primary limitation of observational studies: unmeasured confounding variables (Evans & Davey Smith, 2015, Davies et al., 2018). Indeed, in cases where RCTs are impossible, for example when attempting to investigate the potential causal pathways between PTSD and DPW/AUD (Evans & Davey Smith, 2015), MR is a viable alternative. Within the MR framework, two sample MR (Lawlor, 2016, Hartwig et al., 2016) takes advantage of GWAS summary data for the two included phenotypes, using two independent samples. In addition to its convenience (i.e., not having to use datasets that have both the exposure and outcome measured, and not requiring raw genotypes), there are a number of benefits of two-sample MR, including increased statistical power and reduced concern about weak instrument bias (Burgess & Thompson, 2011). In the two-sample framework, the independence of exposure and outcome datasets reduces the inflation of type-1 error rates (over‐rejection of the null) due to the weak association of the instrumental variable with the exposure (Burgess et al., 2016).
Data Sources
PTSD summary statistics came from the PGC- PTSD Freeze 2.0 (n = 23,212 PTSD cases, 151,447 controls) EA participants (Nievergelt et al., 2019). Case status refers to lifetime (when possible) or current PTSD diagnosis. DPW statistics came from the GSCAN dataset (n = 537,187 EA participants1) (Liu et al., 2019). AUD summary statistics were derived from the recent problematic alcohol use GWAS meta-analysis (Zhou et al., 2020). Because of the inclusion of United Kingdom Biobank (UKB) and PGC datasets in GSCAN and PGC-PTSD GWAS, we selected the AUD GWAS statistics derived from the meta-analysis of MVP Phase 1 and Phase 2 data only to ensure independence of samples (n 267,391). There is no known overlap of MVP cohort with PGC and GSCAN samples. MVP GWAS statistics were accessed via dbGAP (study accession phs001672.v4.p1; Zhou et al., 2020). Notably, the PGC-PTSD (49.3% female) and GSCAN (52.2% female) samples described here were about half female, while the MVP sample was nearly all male (>92% male). As Two-Sample MR is sensitive to sample overlap, we excluded the PGC and UKB components of the Zhou et al study as several of those AUD/problematic alcohol use (PAU) samples were also in PTSD 2.0. Moreover, we excluded these overlapping samples from the AUD GWAS as the PTSD 2.0 sample size was less well-powered than the AUD GWAS.
Genetic Correlation
The Linkage Disequilibrium Score Regression (Bulik-Sullivan et al., 2015) method was used to test per-trait SNP-heritability (h2) and trait pair genetic correlation (rg) using 1000 Genomes Project Phase 3 Europeans as an external linkage disequilibrium (LD) reference panel. The major histocompatibility complex region (between 26Mb and 34Mb on chromosome six) was removed due to the complex LD structure at this locus.
Mendelian Randomization
The R package TwoSampleMR (Hemani et al., 2017) was used to estimate bidirectional causal associations between phenotypes based on two ways of defining SNPs associated with the exposure trait: (1) using all LD-independent SNPs, and (2) using all LD-independent SNPs with exposure association p-values either < 5×10−8 if several genome-wide significant loci exist (as was the case for DPW), or using p-values 5×10−5 if there were too few genome-wide significant loci. LD clumping was performed using r2 threshold of 0.001 within 10,000kb regions of the genome. To perform MR, SNPs were extracted from each exposure trait meeting p-value inclusion criteria and harmonized with the outcome variable to ensure consistent effect alleles. Because we were applying MR methods to weak genetic instruments (i.e., instruments including genetic variants that have association p-values with the exposure >5×10−8) using MR robust adjusted profile scores (MR-RAPS), we applied leave-one-out and heterogeneity tests to verify that the effects detected were not due to the presence of outliers within the genetic instruments tested. MR assumes that genetic instruments only affect the outcome through the exposure. If this assumption holds, variant effect estimates should be homogeneous between the two summary association statistics (Labrecque and Swanson, 2018). Heterogeneity tests formally evaluate this hypothesis and when significant may suggest pleiotropic mechanisms and/or the presence of a mediator variable that is not accounted for. Leave-one-out analyses determine whether any one SNP among the genetic instruments contributes a large effect on the causal estimate between exposure and outcome. If detected, these variants can bias the causal estimate away from the null. When identified in combination with a nominally significant causal estimate, outlier variants were eliminated considering 99%, 98%, 95%, 90%, and 80% confidence intervals of the effects included within the genetic instrument until heterogeneity was removed.
Results
Heritability
The observed-scale h2 estimates of PTSD, AUD, and DPW were all significantly different from zero (PTSD-2.0: h2-z = 5.43, p = 5.50×10−8; AUD: h2-z = 16.84, p = 1.17×10−63; DPW: h2-z = 15.11, p = 8.70×10−102) and were consistent with previously reported results. This demonstrates that these datasets are informative with respect to the genetic liability of traits investigated.
Effects of PTSD on AUD
Using the subset of 60 PTSD-2.0 SNPS with p-values less than 5×10−5, we detected a positive but non-significant effect of PTSD on AUD (MR-RAPS β = 0.017, 95% CI [-0.055,0.089], p = 0.657) in the presence of significant heterogeneity among the variants included in the PTSD genetic instrument (Q = 78.6, df = 58, p<1×10−30). We did not find evidence for horizontal pleiotropy (MR Egger intercept = −4.0×10−4, p = 0.918). After the removal of four outlier variants with effects outside the 95% confidence interval observed within the PTSD genetic instrument (Figure S1), the heterogeneity was removed (Q = 64.0, df = 54, p = 0.165) and the absence of horizontal pleiotropy persisted (MR Egger intercept = −0.003, p = 0.500). With 56 variants included in the PTSD genetic instrument, the causal estimate between PTSD and AUD remained non-significant (MR-RAPS β = 0.023 95% CI [-0.053,0.099], p = 0.550; Table S1). To investigate whether this non-significant effect was due to a lack of power, we tested a PTSD genetic instrument based on genome-wide LD-independent variants (n = 1,446 SNPs). In the absence of both heterogeneity (Q = 1,480, df = 1,444, p = 0.244) and horizontal pleiotropy (MR Egger intercept = 0.002, p = 0.07), we detected a significant positive causal effect of PTSD on AUD surviving Bonferroni multiple testing correction accounting for the number of the MR-RAPS methods applied (MR-RAPS using FALSE/L2 combination correction: β = 0.039, 95% CI [0.015,0.063], p = 0.005; see Figure 1). No significant over-dispersion was observed in the MR-RAPS test (pleiotropy variance = 7.02×10−6, 95% CI [-3.91×10−4,4.05×10−4], p= 0.946). Additionally, the effects estimated were consistent across different methods for MR-RAPS correction of over-dispersion and loss-of-function (Table S2).
Figure 1.
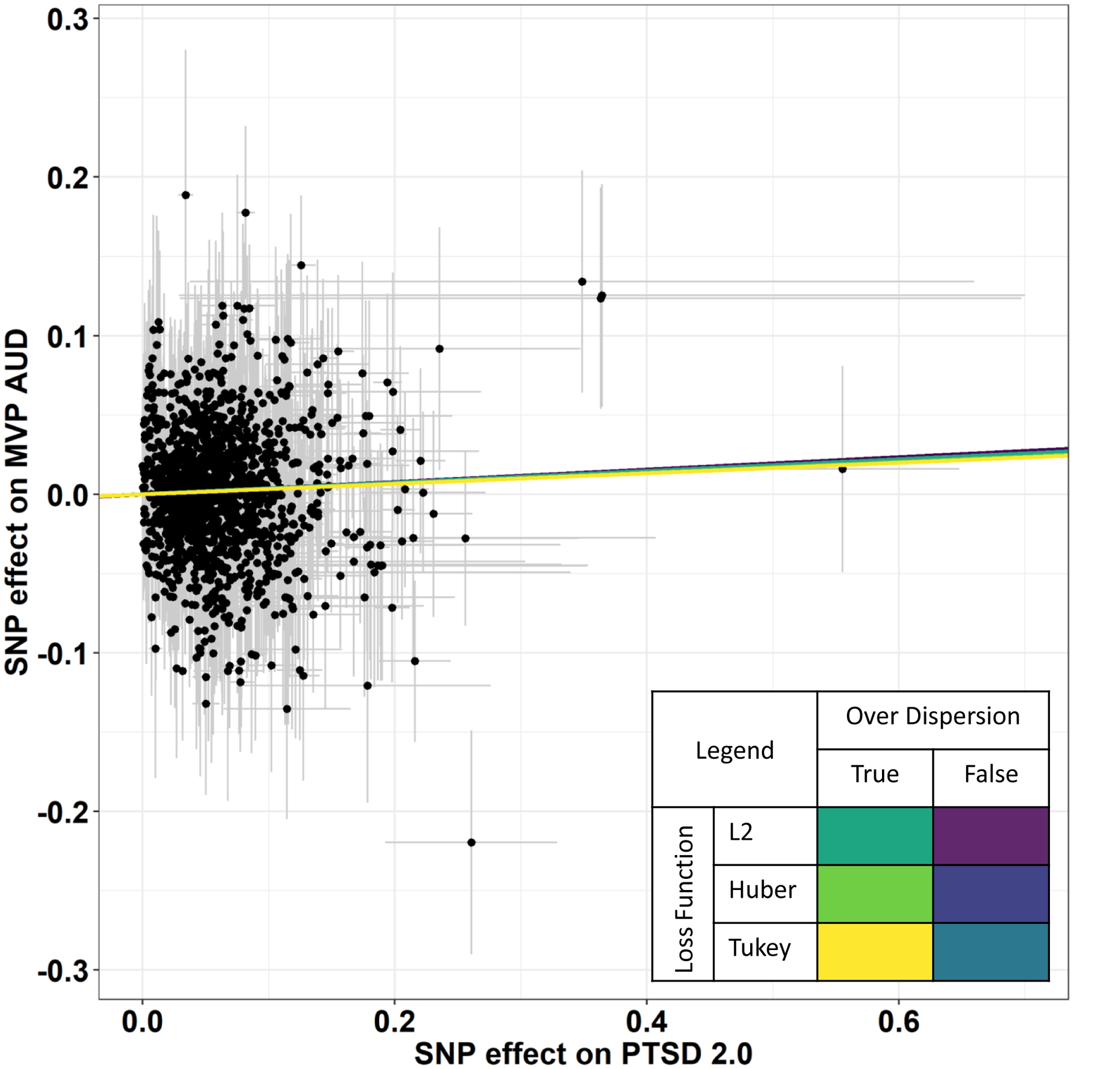
Significant causal effect of PTSD-2.0 on AUD using 1,446 LD-independent genetic instruments and multiple robust adjust profile over dispersion parameters (Table S4)
Effects of AUD on PTSD
Using a subset of 140 AUD-associated SNPS (p<5×10−5), we detected a non-significant effect of AUD on PTSD-2.0 (MR-RAPS β = 8.87×10−4, 95% CI [-0.001,0.003], p = 0.441) in the presence of genetic instrument heterogeneity (Q = 368,924, df = 138, p<1×10−30) but not horizontal pleiotropy (MR Egger intercept = −1.0×10−4, p = 0.501). Heterogeneity among the genetic instruments persisted and no significant causal effects were detected (Table S3). A similar scenario was observed when using a genetic instrument based on genome-wide LD-independent variants (n = 1,239 LD independent SNPs). Significant effect size heterogeneity was observed (Q = 1,244,529, df = 1,237, p<1×10−30) that could not be eliminated by removing SNPs with outlier effect estimates. At all confidence interval thresholds, horizontal pleiotropy among the genetic instruments was not detected and no significant effects were identified (Table S4). Additionally, MR-RAPS analysis showed significant over-dispersion within the genome-wide AUD genetic instrument (pleiotropy variance = 0.001, 95% CI [8.50×10−4,0.0012], p = 1.16×10−76).
Effects of PTSD on DPW
Using the subset of 132 PTSD-2.0 SNPs with p-values less than 5×10−5, we detected findings that were generally non-significant but inconsistent across methods (see Table S5). We found non-significant heterogeneity (Q = 150.99, df = 130, p = 0.101) and non-significant pleiotropy (MR Egger intercept = −0.002, p = 0.052). We next used 3,433 LD independent genetic instruments across the genome to test whether these inconsistent results were due to lack of power. In the presence of nominal heterogeneity (Q = 3,580, df = 3,431, p = 0.038) but no horizontal pleiotropy (MR Egger intercept = 1.16×10−4, p = 0.451), we detected a consistent, non-significant effect of PTSD-2.0 on DPW (MR-RAPS β = −0.002, 95% CI [-0.008,0.004], p = 0.414; Table S5).
Effects of DPW on PTSD
Using a subset of 6 DPW SNPs (p<5×10−8; as mentioned in the methods, we used GSCAN data excluding 23andme participants because these were not released publicly), we detected a non-significant effect of DPW on PTSD-2.0 (see Table S6 for all estimates) in the absence of genetic instrument heterogeneity (Q = 3.28, df = 4, p = 0.512) and in the absence of horizontal pleiotropy (MR Egger intercept = −0.012, p = 0.271). We also examined this question using a larger subset of 163 DPW SNPS (p<5×10−5). We detected no effect of DPW on PTSD-2.0 (MR-RAPS β = 0.019, 95% CI [-0.061,0.099], p = 0.637); however, the estimates are inconsistent across methods (see Table S2). We detected nominal heterogeneity (Q = 196.57, df = 161, p = .029) and non-significant pleiotropy (MR Egger intercept = −0.005, p = 0.239).
Genetic Correlation
To reinforce that (1) the lack of association between PTSD and DPW and (2) the detected association between PTSD and AUD using a subset of genetic instruments reflect genome-wide information between the traits, we estimated the genetic correlation between each phenotype pair. PTSD and AUD were positively genetically correlated (rg = 0.28, 95% CI [0.123,0.437], p = 6.0×10−4). PTSD and DPW were not genetically correlated (rg = −0.07, 95% CI [-0.168,0.028], p = 0.177). These significant and non-significant correlations are consistent with the causal estimates between the two phenotypes.
Discussion
The primary goal of this study was to understand the mechanisms responsible for the known association between PTSD and alcohol phenotypes using MR methods applied to large-scale genome-wide association statistics among those of European ancestry. Although these datasets were generated from cohorts with different demographic characteristics and different ascertainment strategies (e.g., MVP is a biobank including mainly male participants while PGC and GSCAN are meta-analyses combining multiple samples with different characteristics), we observed a high genetic correlation among the traits investigated in these cohorts: MVP AUD vs. PGC AD rg = 0.98, 95% CI [0.724,1], (Zhou et al., 2020); MVP PTSD vs. PGC PTSD rg = 0.96, 95% CI [0.744,1] (Stein et al., 2021). This supports the notion that the genetic effects analyzed in the present study are generalizable across cohorts with different demographic characteristics and ascertainment strategies—at least among those of European ancestry.
We found evidence for a putative causal effect of PTSD on AUD, but not the reverse (i.e., AUD on PTSD) when using the MR-RAPS method, but not when only including marginally significant SNPs. As done in prior MR studies using aggregate molecular genetic instruments (Polimanti et al., 2019), we interpret the results of MR-RAPS, which capitalizes on the high polygenicity of PTSD, alcohol phenotypes, and related psychiatric conditions (Geschwind, 2015, Smoller, 2016), using SNPs with weaker associations with the exposure phenotype. Although the potential causal relations between PTSD and the alcohol phenotypes (i.e., DPW, AUD) had not been tested using this approach before, our results are consistent with the MR work on the MDD-AD relation, such that MDD causally influences risk for AD, but the reverse is not true (Polimanti et al., 2019). Our finding that PTSD impacts AUD lends support to the self-medication model, positing that individuals may misuse alcohol in an attempt to avoid aversive trauma symptoms (Stewart, 1996, Read et al., 2012). The lack of evidence supporting AUD impacting PTSD (i.e., risky behavior model, Read, 2014) may mean there is not a causal effect of AUD on PTSD. Alternatively, it may mean that support for the risky behavior model might be isolated to a particular sociodemographic group, such as adolescents. For example, support for the risky behavior model has been found in a sample of urban African-American youth (Bountress, In Press). As this study and others have found support for the risky behavior model in earlier developmental periods (e.g., adolescence, Thompson et al., 2017), it may also be that AUD precedes PTSD primarily in adolescence. The non-significant effect of AUD on PTSD should also be interpreted in light of the current stage of discovery of the genetic architecture for these phenotypes. For example, as sample sizes grow and increased number of variants are discovered that can be incorporated into future MR analyses the pattern of findings may change. In short, while the current results are most consistent with evidence for the self-medication model, the study is only a first step in this line of inquiry.
The current study did not find evidence for causal effects between PTSD and DPW. However only a fraction of the expected genetic variants thought to influence each outcome were used in instrument construction. If future studies incorporating larger numbers of robustly associated molecular variants replicate the current results, this suggests that PTSD does not necessarily lead to more consumption, but increases the propensity of individuals to use alcohol in a way that leads to pathological consequences. This finding that PTSD is causally related to AUD but not DPW is consistent with the self-medication literature, finding that those who consume alcohol to avoid aversive symptoms tend to do so at hazardous, not low to moderate, levels (Mc Hugh and McBride, 2020, Crum et al., 2013). While of course consumption and AUD are correlated (Saha et al., 2007), this finding is consistent with work suggesting that even in cases of high levels of use, not everyone develops problems (i.e., there is variability in terms of “addiction resistance”; Kendler and Myers, 2015). It is also consistent with work finding that a related phenotype, MDD, was positively and significantly associated with alcohol problems but negatively and significantly associated with consumption (Sanchez-Roige et al., 2019). It is also consistent with work finding stronger associations between MDD and disordered alcohol use compared to MDD and quantity of alcohol consumed (Polimanti et al., 2019). Thus, it may be that clinical levels of PTSD symptoms drive individuals to misuse alcohol leading to more consequences, but does not simply result in an uptick in the amount of alcohol consumed.
In terms of the secondary research questions, all effects of PTSD on DPW/AUD and vice versa suggested non-significant horizontal pleiotropy, meaning that the association between PTSD and alcohol misuse may be not due to shared genetic effects among these phenotypes. Although there is some work suggesting shared latent genetic risk between PTSD and alcohol phenotypes (Xian et al., 2000, McLeod et al., 2001b), the literature on the potential shared risk factors is relatively small (e.g., Read et al., 2014). Current study findings are more consistent with the self-medication model and less consistent with the shared risk model; however, replication is needed as sample sizes become larger. Additionally, the general lack of genetic instrument heterogeneity suggests some consistency in the largely non-significant causal relations observed.
Despite this study’s strengths, including answering a substantive question using the largest GWAS results currently available, it is important to acknowledge its limitations. First, although we used summary statistics from large GWAS, only a small proportion of the expected heritability was used in the current study. As the power of GWAS studies grow and the number of discovered loci and proportion of heritability for each outcome also grow, so will our confidence in the conclusion of our results. Second, although we found a potentially causal effect of PTSD on AUD, it is possible that other constructs, such as trauma severity, could be driving this association. Third, these analyses were conducted using summary statistics on those of EA and were collapsed across sex, so the findings may not generalize to different ancestry groups, and findings may vary for men versus women. There are important sex differences in PTSD, such that females are more likely to meet criteria than males (Kessler et al., 1995) and alcohol use disorder, such that males are more likely to meet criteria than females (Grant et al., 2015). Thus, examining these questions separately by sex, in addition to among different ancestral groups, and among those at different developmental periods is important to test whether these study findings hold for other groups. However, to address these important questions in the current data is not possible to do power limitations.
Although this study has a number of limitations, it advances the field in a few ways. First, these findings extend research on latent genetic studies (i.e., twin studies/latent heritability) and molecular genetic correlations (i.e., PTSD-alcohol dependence genetic correlation studies) to describe directionality. Given the extensive extant literature finding associations between PTSD and alcohol phenotypes, these findings begin to clarify the nature of this association. Specifically, this pattern of results is more consistent with the self-medication hypothesis compared to the risky behavior or shared risk models—both in terms of finding that PTSD causally influences AUD (but not the reverse) and also that PTSD does not causally influence more normative alcohol consumption. These findings are useful in thinking about the causal links that may at least partially explain PTSD-AUD comorbidity, and they have important implications for prevention. Specifically, these findings provide a focus for future work, such as prioritizing “deep dives” into the mechanisms by which PTSD impacts AUD. For example, it may be useful within pre-clinical studies to attempt to understand the biologic pathways by which animals with PTSD come to misuse ethanol in the laboratory. It may also be useful within human clinical studies to examine the mechanisms by which trauma-related anxiety drives alcohol misuse (e.g., aberrations in fear learning and extinction mediating PTSD on AUD). Additionally, these findings are important to think about in the context of how to prioritize time with providers within the broader medical system (e.g., in primary care). These findings are important because they suggest that if medical providers come across patients with PTSD, but only have a few minutes with these patients, they ought to prioritize providing/referring for treatment for PTSD, as well as AUD prevention services (e.g., harm reduction), as PTSD appears to exert a potentially causal impact on AUD. In contrast, those who meet briefly with those with an AUD may not necessarily decide to prioritize skills or services that might help prevent onset of trauma/PTSD, as we did not find support for this link.
Supplementary Material
Acknowledgments
We would like to acknowledge funding from NIAAA (1K01AA028058-01, 1K01AA025692-01A1, F32MH122058, R21 DA047527). The PGC-SUD Working Group receives support from the National Institute on Drug Abuse and the National Institute of Mental Health via MH109532. Statistical analyses for the PGC were carried out on the Genetic Cluster Computer (http://www.geneticcluster.org) hosted by SURFsara and financially supported by the Netherlands Scientific Organization (NWO 480-05-003), along with a supplement from the Dutch Brain Foundation and the VU University Amsterdam. Financial support for the PTSD PGC was provided by the Stanley Center for Psychiatric Genetics at the Broad Institute, One Mind, Cohen Veterans Bioscience, and the National Institute of Mental Health (NIMH; R01MH106595) grants.
Footnotes
This sample size excludes 23andMe participants that were not included in the publicly available release.
References
- BENJET C, BROMET E, KARAM EG, KESSLER RC, MCLAUGHLIN KA, RUSCIO AM, SHAHLY V, STEIN DJ, PETUKHOVA M, HILL E, ALONSO J, ATWOLI L, BUNTING B, BRUFFAERTS R, CALDAS-DE-ALMEIDA JM, DE GIROLAMO G, FLORESCU S, GUREJE O, HUANG Y, LEPINE JP, KAWAKAMI N, KOVESS-MASFETY V, MEDINA-MORA ME, NAVARRO-MATEU F, PIAZZA M, POSADA-VILLA J, SCOTT KM, SHALEV A, SLADE T, TEN HAVE M, TORRES Y, VIANA MC, ZARKOV Z & KOENEN KC 2016. The epidemiology of traumatic event exposure worldwide: results from the World Mental Health Survey Consortium. Psychol Med, 46, 327–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BLANCO C, XU Y, BRADY K, PEREZ-FUENTES G, OKUDA M & WANG S 2013. Comorbidity of posttraumatic stress disorder with alcohol dependence among US adults: results from National Epidemiological Survey on Alcohol and Related Conditions. Drug Alcohol Depend, 132, 630–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOUNTRESS K, AGGEN SA, & KLIEWER W In Press. Is delinquency associated with subsequent victimization by community violence in adolescents? A test of the risky behavior model ina primarily African-American sample. Psychology of Violence. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRESLAU N 2009. Trauma and mental health in US inner-city populations. Gen Hosp Psychiatry, 31, 501–2. [DOI] [PubMed] [Google Scholar]
- BULIK-SULLIVAN BK, LOH PR, FINUCANE HK, RIPKE S, YANG J, PATTERSON N, DALY MJ, PRICE AL & NEALE BM 2015. LD Score regression distinguishes confounding from polygenicity in genome-wide association studies. Nat Genet, 47, 291–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BURGESS S, DAVIES NM & THOMPSON SG 2016. Bias due to participant overlap in two-sample Mendelian randomization. Genet Epidemiol, 40, 597–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CLARKE TK, ADAMS MJ, DAVIES G, HOWARD DM, HALL LS, PADMANABHAN S, MURRAY AD, SMITH BH, CAMPBELL A, HAYWARD C, PORTEOUS DJ, DEARY IJ & MCINTOSH AM 2017. Genome-wide association study of alcohol consumption and genetic overlap with other health-related traits in UK Biobank (N=112 117). Mol Psychiatry, 22, 1376–1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CRUM RM, MOJTABAI R, LAZARECK S, BOLTON JM, ROBINSON J, SAREEN J, GREEN KM, STUART EA, LA FLAIR L, ALVANZO AAH & STORR CL 2013. A prospective assessment of reports of drinking to self-medicate mood symptoms with the incidence and persistence of alcohol dependence. JAMA psychiatry, 70, 718–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVEY SMITH G & HEMANI G 2014. Mendelian randomization: genetic anchors for causal inference in epidemiological studies. Hum Mol Genet, 23, R89–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIES NM, HOLMES MV & DAVEY SMITH G 2018. Reading Mendelian randomisation studies: a guide, glossary, and checklist for clinicians. Bmj, 362, k601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEBELL F, FEAR NT, HEAD M, BATT-RAWDEN S, GREENBERG N, WESSELY S & GOODWIN L 2014. A systematic review of the comorbidity between PTSD and alcohol misuse. Soc Psychiatry Psychiatr Epidemiol, 49, 1401–25. [DOI] [PubMed] [Google Scholar]
- EVANS DM & DAVEY SMITH G 2015. Mendelian Randomization: New Applications in the Coming Age of Hypothesis-Free Causality. Annu Rev Genomics Hum Genet, 16, 327–50. [DOI] [PubMed] [Google Scholar]
- GELERNTER J, KRANZLER HR, SHERVA R, ALMASY L, KOESTERER R, SMITH AH, ANTON R, PREUSS UW, RIDINGER M, RUJESCU D, WODARZ N, ZILL P, ZHAO H & FARRER LA 2014. Genome-wide association study of alcohol dependence:significant findings in African- and European-Americans including novel risk loci. Mol Psychiatry, 19, 41–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GELERNTER J, SUN N, POLIMANTI R, PIETRZAK R, LEVEY DF, BRYOIS J, LU Q, HU Y, LI B, RADHAKRISHNAN K, ASLAN M, CHEUNG KH, LI Y, RAJEEVAN N, SAYWARD F, HARRINGTON K, CHEN Q, CHO K, PYARAJAN S, SULLIVAN PF, QUADEN R, SHI Y, HUNTER-ZINCK H, GAZIANO JM, CONCATO J, ZHAO H & STEIN MB 2019. Genome-wide association study of post-traumatic stress disorder reexperiencing symptoms in >165,000 US veterans. Nat Neurosci, 22, 1394–1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GESCHWIND DH F. J 2015. Genetics and genomics of psychiatric disease. Science, 349, 1489–1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRANT BF, GOLDSTEIN RB, SAHA TD, CHOU SP, JUNG J, ZHANG H, PICKERING RP, RUAN WJ, SMITH SM & HUANG B 2015. Epidemiology of DSM-5 alcohol use disorder: results from the National Epidemiologic Survey on Alcohol and Related Conditions III. JAMA psychiatry, 72, 757–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HARTWIG FP, DAVIES NM, HEMANI G & DAVEY SMITH G 2016. Two-sample Mendelian randomization: avoiding the downsides of a powerful, widely applicable but potentially fallible technique. Int J Epidemiol, 45, 1717–1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HEMANI G, TILLING K & DAVEY SMITH G 2017. Orienting the causal relationship between imprecisely measured traits using GWAS summary data. PLoS Genet, 13, e1007081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JAKUPCAK M, TULL MT, MCDERMOTT MJ, KAYSEN D, HUNT S & SIMPSON T 2010. PTSD symptom clusters in relationship to alcohol misuse among Iraq and Afghanistan war veterans seeking post-deployment VA health care. Addict Behav, 35, 840–3. [DOI] [PubMed] [Google Scholar]
- KAPRIO J, KOSKENVUO M, LANGINVAINIO H, ROMANOV K, SARNA S & ROSE RJ 1987. Genetic influences on use and abuse of alcohol: a study of 5638 adult Finnish twin brothers. Alcohol Clin Exp Res, 11, 349–56. [DOI] [PubMed] [Google Scholar]
- KENDLER KS & MYERS J 2015. Addiction resistance: Definition, validation and association with mastery. Drug Alcohol Depend, 154, 236–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KESSLER RC, SONNEGA A, BROMET E, HUGHES M & NELSON CB 1995. Posttraumatic stress disorder in the National Comorbidity Survey. Archives of General Psychiatry, 52, 1048–1060. [DOI] [PubMed] [Google Scholar]
- KNOPIK VS, HEATH AC, MADDEN PA, BUCHOLZ KK, SLUTSKE WS, NELSON EC, STATHAM D, WHITFIELD JB & MARTIN NG 2004. Genetic effects on alcohol dependence risk: re-evaluating the importance of psychiatric and other heritable risk factors. Psychol Med, 34, 1519–30. [DOI] [PubMed] [Google Scholar]
- KRANZLER HR, ZHOU H, KEMBER RL, VICKERS SMITH R, JUSTICE AC, DAMRAUER S, TSAO PS, KLARIN D, BARAS A, REID J, OVERTON J, RADER DJ, CHENG Z, TATE JP, BECKER WC, CONCATO J, XU K, POLIMANTI R, ZHAO H & GELERNTER J 2019. Genome-wide association study of alcohol consumption and use disorder in 274,424 individuals from multiple populations. Nat Commun, 10, 1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LABRECQUE J & SWANSON SA 2018. Understanding the Assumptions Underlying Instrumental Variable Analyses: a Brief Review of Falsification Strategies and Related Tools. Curr Epidemiol Rep, 5, 214–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LAWLOR DA 2016. Commentary: Two-sample Mendelian randomization: opportunities and challenges. Int J Epidemiol, 45, 908–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIU M, JIANG Y, WEDOW R, LI Y, BRAZEL DM, CHEN F, DATTA G, DAVILA-VELDERRAIN J, MCGUIRE D, TIAN C, ZHAN X, CHOQUET H, DOCHERTY AR, FAUL JD, FOERSTER JR, FRITSCHE LG, GABRIELSEN ME, GORDON SD, HAESSLER J, HOTTENGA JJ, HUANG H, JANG SK, JANSEN PR, LING Y, MÄGI R, MATOBA N, MCMAHON G, MULAS A, ORRÙ V, PALVIAINEN T, PANDIT A, REGINSSON GW, SKOGHOLT AH, SMITH JA, TAYLOR AE, TURMAN C, WILLEMSEN G, YOUNG H, YOUNG KA, ZAJAC GJM, ZHAO W, ZHOU W, BJORNSDOTTIR G, BOARDMAN JD, BOEHNKE M, BOOMSMA DI, CHEN C, CUCCA F, DAVIES GE, EATON CB, EHRINGER MA, ESKO T, FIORILLO E, GILLESPIE NA, GUDBJARTSSON DF, HALLER T, HARRIS KM, HEATH AC, HEWITT JK, HICKIE IB, HOKANSON JE, HOPFER CJ, HUNTER DJ, IACONO WG, JOHNSON EO, KAMATANI Y, KARDIA SLR, KELLER MC, KELLIS M, KOOPERBERG C, KRAFT P, KRAUTER KS, LAAKSO M, LIND PA, LOUKOLA A, LUTZ SM, MADDEN PAF, MARTIN NG, MCGUE M, MCQUEEN MB, MEDLAND SE, METSPALU A, MOHLKE KL, NIELSEN JB, OKADA Y, PETERS U, POLDERMAN TJC, POSTHUMA D, REINER AP, RICE JP, RIMM E, ROSE RJ, RUNARSDOTTIR V, STALLINGS MC, STANČÁKOVÁ A, STEFANSSON H, THAI KK, TINDLE HA, TYRFINGSSON T, WALL TL, et al. 2019. Association studies of up to 1.2 million individuals yield new insights into the genetic etiology of tobacco and alcohol use. Nat Genet, 51, 237–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MC HUGH R & MCBRIDE O 2020. Self-medicating low mood with alcohol use: Examining the role of frequency of alcohol use, quantity consumed and context of drinking. Addict Behav, 111, 106557. [DOI] [PubMed] [Google Scholar]
- MCLEOD DS, KOENEN KC, MEYER JM, LYONS MJ, EISEN S, TRUE W & GOLDBERG J 2001a. Genetic and environmental influences on the relationship among combat exposure, posttraumatic stress disorder symptoms, and alcohol use. J Trauma Stress, 14, 259–75. [DOI] [PubMed] [Google Scholar]
- MCLEOD S, KOENEN KC, MEYER J, LYONS MJ, EISEN S, TRUE W & GOLDBERG D 2001b. Genetic and environmental influences on the relationship among combat exposure, posttraumatic stress disorder symptoms, and alcohol use. Journal of Traumatic Stress, 14, 259–275. [DOI] [PubMed] [Google Scholar]
- NIEVERGELT CM, MAIHOFER AX, KLENGEL T, ATKINSON EG, CHEN CY, CHOI KW, COLEMAN JRI, DALVIE S, DUNCAN LE, GELERNTER J, LEVEY DF, LOGUE MW, POLIMANTI R, PROVOST AC, RATANATHARATHORN A, STEIN MB, TORRES K, AIELLO AE, ALMLI LM, AMSTADTER AB, ANDERSEN SB, ANDREASSEN OA, ARBISI PA, ASHLEY-KOCH AE, AUSTIN SB, AVDIBEGOVIC E, BABIC D, BAEKVAD-HANSEN M, BAKER DG, BECKHAM JC, BIERUT LJ, BISSON JI, BOKS MP, BOLGER EA, BORGLUM AD, BRADLEY B, BRASHEAR M, BREEN G, BRYANT RA, BUSTAMANTE AC, BYBJERG-GRAUHOLM J, CALABRESE JR, CALDAS-DE-ALMEIDA JM, DALE AM, DALY MJ, DASKALAKIS NP, DECKERT J, DELAHANTY DL, DENNIS MF, DISNER SG, DOMSCHKE K, DZUBUR-KULENOVIC A, ERBES CR, EVANS A, FARRER LA, FEENY NC, FLORY JD, FORBES D, FRANZ CE, GALEA S, GARRETT ME, GELAYE B, GEUZE E, GILLESPIE C, UKA AG, GORDON SD, GUFFANTI G, HAMMAMIEH R, HARNAL S, HAUSER MA, HEATH AC, HEMMINGS SMJ, HOUGAARD DM, JAKOVLJEVIC M, JETT M, JOHNSON EO, JONES I, JOVANOVIC T, QIN XJ, JUNGLEN AG, KARSTOFT KI, KAUFMAN ML, KESSLER RC, KHAN A, KIMBREL NA, KING AP, KOEN N, KRANZLER HR, KREMEN WS, LAWFORD BR, LEBOIS LAM, LEWIS CE, LINNSTAEDT SD, LORI A, LUGONJA B, LUYKX JJ, LYONS MJ, MAPLES-KELLER J, MARMAR C, MARTIN AR, et al. 2019. International meta-analysis of PTSD genome-wide association studies identifies sex- and ancestry-specific genetic risk loci. Nat Commun, 10, 4558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- POLIMANTI R, PETERSON RE, ONG JS, MACGREGOR S, EDWARDS AC, CLARKE TK, FRANK J, GERRING Z, GILLESPIE NA, LIND PA, MAES HH, MARTIN NG, MBAREK H, MEDLAND SE, STREIT F, AGRAWAL A, EDENBERG HJ, KENDLER KS, LEWIS CM, SULLIVAN PF, WRAY NR, GELERNTER J & DERKS EM 2019. Evidence of causal effect of major depression on alcohol dependence: findings from the psychiatric genomics consortium. Psychol Med, 49, 1218–1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAVERA S, CARRASCO N, GELERNTER J & POLIMANTI R 2018. Phenomic Impact of Genetically-Determined Euthyroid Function and Molecular Differences between Thyroid Disorders. J Clin Med, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- READ JP, COLDER CR, MERRILL JE, OUIMETTE P, WHITE J & SWARTOUT A 2012. Trauma and posttraumatic stress symptoms predict alcohol and other drug consequence trajectories in the first year of college. J Consult Clin Psychol, 80, 426–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- READ JP, MERRILL JE, GRIFFIN MJ, BACHRACH RL & KHAN SN 2014. Posttraumatic stress symptoms and alcohol problems: self-medication or trait vulnerability? Am J Addict, 23, 108–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- READ JP, MERRILL JE, GRIFFIN MJ, BACHRACH RL, & KHAN SN 2014. Posttraumatic stress symptoms and alcohol problems: self-medication or trait vulnerability? The American Journal on Addictions, 23, 108–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROJAS SM, BUJARSKI S, BABSON KA, DUTTON CE & FELDNER MT 2014. Understanding PTSD comorbidity and suicidal behavior: associations among histories of alcohol dependence, major depressive disorder, and suicidal ideation and attempts. Journal of Anxiety Disorders, 28 318–325. [DOI] [PubMed] [Google Scholar]
- SAHA TD, STINSON FS & GRANT BF 2007. The role of alcohol consumption in future classifications of alcohol use disorders. Drug Alcohol Depend, 89, 82–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SANCHEZ-ROIGE S, PALMER AA, FONTANILLAS P, ELSON SL, ADAMS MJ, HOWARD DM, EDENBERG HJ, DAVIES G, CRIST RC, DEARY IJ, MCINTOSH AM & CLARKE TK 2019. Genome-Wide Association Study Meta-Analysis of the Alcohol Use Disorders Identification Test (AUDIT) in Two Population-Based Cohorts. Am J Psychiatry, 176, 107–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SARTOR CE, GRANT JD, LYNSKEY MT, MCCUTCHEON VV, WALDRON M, STATHAM DJ, BUCHOLZ KK, MADDEN PA, HEATH AC, MARTIN NG & NELSON EC 2012. Common heritable contributions to low-risk trauma, high-risk trauma, posttraumatic stress disorder, and major depression. Arch Gen Psychiatry, 69, 293–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHEERIN CM, BOUNTRESS KE, MEYERS JL, SAENZ DE VITERI SS, SHEN H, MAIHOFER AX, DUNCAN LE & AMSTADTER AB 2020. Shared molecular genetic risk of alcohol dependence and posttraumatic stress disorder (PTSD). Psychol Addict Behav. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SIMPSON TL, STAPPENBECK CA, VARRA AA, MOORE SA & KAYSEN D 2012. Symptoms of posttraumatic stress predict craving among alcohol treatment seekers: results of a daily monitoring study. Psychol Addict Behav, 26, 724–33. [DOI] [PubMed] [Google Scholar]
- SMOLLER JW 2016. The Genetics of Stress-Related Disorders: PTSD, Depression, and Anxiety Disorders. Neuropsychopharmacology, 41, 297–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STEIN MB, JANG KL, TAYLOR S, VERNON PA & LIVESLEY WJ 2002. Genetic and environmental influences on trauma exposure and posttraumatic stress disorder symptoms: a twin study. Am J Psychiatry, 159, 1675–81. [DOI] [PubMed] [Google Scholar]
- STEIN MB, LEVEY DF, CHENG Z, WENDT FR, HARRINGTON K, PATHAK GA, CHO K, QUADEN R, RADHAKRISHNAN K & GIRGENTI MJ 2021. Genome-wide association analyses of post-traumatic stress disorder and its symptom subdomains in the Million Veteran Program. Nature Genetics, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STEWART SH 1996. Alcohol abuse in individuals exposed to trauma: A critical review. Psychological Bulletin, 120, 83–112. [DOI] [PubMed] [Google Scholar]
- THOMPSON R, LEWIS T, NEILSON EC, ENGLISH DJ, LITROWNIK AJ, MARGOLIS B, PROCTOR L & DUBOWITZ H 2017. Child Maltreatment and Risky Sexual Behavior. Child Maltreat, 22, 69–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VLAHOV D, GALEA S, RESNICK H, AHERN J, BOSCARINO JA, BUCUVALES M, GOLD J & KILPATRICK D 2002. Increased use of cigarettes, alcohol, and marijuana amond Manhattan, New York residents after the September 11th terrorist attacks. American Journal of Epidemiology, 155, 988–996. [DOI] [PubMed] [Google Scholar]
- WALTERS RK, POLIMANTI R, JOHNSON EC, MCCLINTICK JN, ADAMS MJ, ADKINS AE, ALIEV F, BACANU S-A, BATZLER A, BERTELSEN S, BIERNACKA JM, BIGDELI TB, CHEN L-S, CLARKE T-K, CHOU Y-L, DEGENHARDT F, DOCHERTY AR, EDWARDS AC, FONTANILLAS P, FOO JC, FOX L, FRANK J, GIEGLING I, GORDON S, HACK LM, HARTMANN AM, HARTZ SM, HEILMANN-HEIMBACH S, HERMS S, HODGKINSON C, HOFFMANN P, JAN HOTTENGA J, KENNEDY MA, ALANNE-KINNUNEN M, KONTE B, LAHTI J, LAHTI-PULKKINEN M, LAI D, LIGTHART L, LOUKOLA A, MAHER BS, MBAREK H, MCINTOSH AM, MCQUEEN MB, MEYERS JL, MILANESCHI Y, PALVIAINEN T, PEARSON JF, PETERSON RE, RIPATTI S, RYU E, SACCONE NL, SALVATORE JE, SANCHEZ-ROIGE S, SCHWANDT M, SHERVA R, STREIT F, STROHMAIER J, THOMAS N, WANG J-C, WEBB BT, WEDOW R, WETHERILL L, WILLS AG, AGEE M, ALIPANAHI B, AUTON A, BELL RK, BRYC K, ELSON SL, FONTANILLAS P, FURLOTTE NA, HINDS DA, HUBER KE, KLEINMAN A, LITTERMAN NK, MCCREIGHT JC, MCINTYRE MH, MOUNTAIN JL, NOBLIN ES, NORTHOVER CAM, PITTS SJ, SATHIRAPONGSASUTI JF, SAZONOVA OV, SHELTON JF, SHRINGARPURE S, TIAN C, TUNG JY, VACIC V, WILSON CH, BOARDMAN JD, CHEN D, CHOI D-S, COPELAND WE, CULVERHOUSE RC, DAHMEN N, DEGENHARDT L, DOMINGUE BW, ELSON SL, FRYE MA, et al. 2018. Transancestral GWAS of alcohol dependence reveals common genetic underpinnings with psychiatric disorders. Nature Neuroscience, 21, 1656–1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WINDLE M 1994. Substance use, risky behaviors, and victimization among a US national adolescent sample. Addiction, 89, 175–82. [DOI] [PubMed] [Google Scholar]
- WIUM-ANDERSEN MK, ØRSTED DD, TOLSTRUP JS & NORDESTGAARD BG 2015. Increased alcohol consumption as a cause of alcoholism, without similar evidence for depression: a Mendelian randomization study. Int J Epidemiol, 44, 526–39. [DOI] [PubMed] [Google Scholar]
- XIAN H, CHANTARUJIKAPONG SI, SCHERRER JF, EISEN SA, LYONS MJ, GOLDBERG J, TSUANG M & TRUE WR 2000. Genetic and environmental influences on posttraumatic stress disorder, alcohol and drug dependence in twin pairs. Drug Alcohol Depend, 61, 95–102. [DOI] [PubMed] [Google Scholar]
- ZHOU H, SEALOCK JM, SANCHEZ-ROIGE S, CLARKE TK, LEVEY DF, CHENG Z, LI B, POLIMANTI R, KEMBER RL, SMITH RV, THYGESEN JH, MORGAN MY, ATKINSON SR, THURSZ MR, NYEGAARD M, MATTHEISEN M, BØRGLUM AD, JOHNSON EC, JUSTICE AC, PALMER AA, MCQUILLIN A, DAVIS LK, EDENBERG HJ, AGRAWAL A, KRANZLER HR & GELERNTER J 2020. Genome-wide meta-analysis of problematic alcohol use in 435,563 individuals yields insights into biology and relationships with other traits. Nat Neurosci, 23, 809–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


