Structured Abstract
Background
A recent large clinical trial demonstrated an almost 50% decrease in postoperative infection in women who were laboring and/or had ruptured membranes for greater than four hours who received azithromycin in addition to standard preoperative antibiotic prophylaxis at the time of cesarean. Given these results, our institution made a policy change in May 2017 to add azithromycin to standard preoperative prophylaxis for all cesarean deliveries.
Objective
Evaluate the clinical effectiveness of adding azithromycin to cesarean preoperative antibiotic prophylaxis.
Study Design
We conducted a before-and-after cohort study of women delivered via cesarean at our institution. The pre-implementation group included women who delivered 3/1/16–2/28/17 (prior to institutional practice change adding azithromycin to standard preoperative prophylaxis), and the post-implementation group included women who delivered 9/1/17– 8/31/18 (allowing a 6-month period for uptake of the practice change). The primary outcome was a composite of postoperative infections (endometritis, wound infection, other maternal infections). Unadjusted and adjusted risk ratios (RR) and 95% confidence intervals were estimated using a modified Poisson regression model.
Results
In the pre-implementation (n=1171) and post-implementation (n=1168) groups, the incidence of the composite outcome was 4.7% and 5.3%, respectively (p=0.49). Both unadjusted (RR 1.13 [95% CI 0.78, 1.62]) and adjusted (a; aRR 1.06 [0.74, 1.52]) comparisons were not significantly different. Results also were statistically non-significant, but in the direction of lower rates of infection, in the after cohort for women in labor and/or with ruptured membranes for 4 or more hours (RR 0.88 [0.56, 1.39]; aRR 0.82 [0.52, 1.30]) and for women with clinical chorioamnionitis (RR 0.37 [0.08, 1.67]; data too sparse for adjusted analysis). In the subgroup of women who were not in labor, the after cohort had a statistically non-significant increased risk of the composite outcome in both unadjusted (RR 1.53 [0.86, 2.72]) and adjusted comparisons (aRR 1.48 [0.83, 2.65]).
Conclusions
In clinical practice, the addition of azithromycin to standard cesarean preoperative antibiotic prophylaxis may have an effect size smaller than seen in the large clinical trial prompting this practice change. Extrapolation of use to non-laboring women may be ineffective altogether.
Keywords: Cesarean, antibiotic, prophylaxis, azithromycin, infection, postoperative, endometritis, wound infection
Condensation
Expanded preoperative cesarean antibiotic prophylaxis may have a smaller effect size than expected based on previous trial
Introduction
The cesarean delivery rate in the United States rose from 5% in 1970 to 33% in 2009.1 The rate in 2009 represented a 59% increase since 1996.1,2 In 2018, the rate was 31.9%.3 Cesarean delivery is associated with a rate of surgical-site infection that is up to 10 times the rate associated with vaginal delivery.4,5 This situation offers an important opportunity for improvement in maternal health outcomes, as complications from cesarean delivery are key contributors to maternal morbidity and mortality in the United States.4–6
Clinical trials have shown that use of prophylactic antibiotics reduces the risk of post-cesarean infections by over 50%, with a significant reduction in endometritis, wound infection, urinary tract infection, and other serious maternal infection complications including bacteremia, sepsis, and death.7–9 Thus, antibiotic prophylaxis is recommended for all women undergoing cesarean delivery. Prior to a randomized controlled trial conducted by Tita et al. (described below), the recommendation had been for administration of a narrow-spectrum cephalosporin within 60 minutes before skin incision.10,11
In 2016, Tita et al. published results from the Cesarean Section Optimal Antibiotic Prophylaxis (C/SOAP) study, a multicenter placebo-controlled randomized trial evaluating the addition of a single 500-mg dose of azithromycin to the standard cefazolin for preoperative prophylaxis in women undergoing cesarean delivery after labor and/or with ruptured membranes for at least four hours.12 The primary outcome (a composite of postoperative infections) occurred in 49% fewer women receiving azithromycin in addition to cefazolin (6.1% vs 12.0%, p<0.001). Endometritis (3.8% vs 6.1%, p=0.02) and wound infection (2.4% vs 6.6%, p<0.001) were less common in the azithromycin group. There were no detected adverse effects of adding azithromycin, and neonatal outcomes were similar between the two groups.
Given these new data, in May 2017 the Section of Maternal-Fetal Medicine at the University of Oklahoma Health Sciences Center (OUHSC) published a clinical guideline recommending addition of azithromycin to antimicrobial prophylaxis for all women undergoing cesarean delivery at our institution. For patients with clinical chorioamnionitis undergoing cesarean, we recommended adding one perioperative dose of azithromycin to the intrapartum treatment regimen. The purpose of this before- and-after cohort study is to evaluate the clinical effectiveness (as opposed to clinical trial efficacy) of adding azithromycin to standard preoperative antibiotic prophylaxis at the time of all cesarean deliveries. We hypothesized that the addition of azithromycin would lead to a decrease in incidence of puerperal infection, both in the subgroup of women studied in the trial by Tita et al (i.e. those in labor and/or with rupture membranes for 4 hours or more), and in our extrapolation to women who were not included in that trial (i.e. women who were not in labor and who had either intact membranes or ruptured membranes for less than 4 hours, and women with chorioamnionitis).
Materials and Methods
We performed a before-and-after cohort study of women delivered via cesarean at OUHSC. Since our clinical guideline was published in May 2017, the pre-implementation group included women who delivered from March 1,2016 to February 28, 2017 and the post-implementation group included women who delivered September 1,2017 to August 31,2018, positioning a six-month washout period between groups to allow full implementation of the guideline. Women aged 18–49 who underwent cesarean at our institution were included in this analysis. Known fetal death and/or known fetal anomaly were exclusion criteria.
The usual practice at our institution for preoperative prophylaxis at the time of cesarean during the pre-implementation period was 2 grams (g) of cefazolin intravenously within 60 minutes prior to skin incision. During the post-implementation period, the new clinical guideline called for 2 g intravenous cefazolin and 500 mg intravenous azithromycin within 60 minutes prior to skin incision. If clinical circumstances precluded administration prior to incision, the antibiotics were to be administered as soon as possible after incision. In women with history of anaphylactic reaction to penicillin, 900 mg intravenous clindamycin and 1.5 mg/kg intravenous gentamicin (in the range of 80–160 mg) were advised. Re-dosing of antibiotics was advised if the procedure exceeded 4.5 hours in duration and/or estimated blood loss exceeded 1500 mL.
For women with a diagnosis of chorioamnionitis, intrapartum treatment consisted of 2 g intravenous ampicillin every 6 hours plus 1.5 mg/kg intravenous gentamicin every 8 hours (in the range of 80–160 mg); at the time of cesarean, 900mg intravenous clindamycin was given. During the post-implementation period, 500mg intravenous azithromycin was also given at the time of cesarean. The antibiotic regimen of ampicillin (dosing as above), gentamicin (dosing as above), and clindamycin (900mg intravenous every 8 hours) was continued for 48 hours after cesarean delivery.
The primary outcome was a composite of endometritis, wound infection, and other maternal infections (abdominopelvic abscess, maternal sepsis, pelvic septic thrombophlebitis, pyelonephritis, pneumonia, or meningitis) occurring within 6 weeks postpartum. The elements of this primary composite outcome were chosen to match the primary composite outcome of the original trial prompting our practice change.12 Endometritis was defined as a documented clinical diagnosis of endometritis with antibiotics administered for that indication. Wound infection was defined as a documented clinical diagnosis of wound infection with antibiotics administered for that indication; wound hematoma, seroma, or breakdown alone did not qualify as a wound infection. Maternal and neonatal data were abstracted via individual patient medical record review by maternal-fetal medicine specialists (SP), obstetrician-gynecologists (MD, SW), and ob/gyn residents (SG, CB, MK, MMW).
Outcomes were compared between the two overall groups (pre-implementation and post-implementation). Outcomes were also compared between the pre- and post-implementation groups within three pre-defined subgroups: 1) Women who underwent cesarean delivery during labor and/or with ruptured membranes for at least 4 hours, 2) women who underwent cesarean delivery without labor and with either intact membranes or ruptured membranes for < 4 hours, and 3) women with a clinical diagnosis of chorioamnionitis. Labor was defined as regular contractions with cervical dilation of 4 cm or more or with documented cervical change of at least 1 cm dilation or 50% effacement. Chorioamnionitis was defined as a diagnosis of chorioamnionitis documented by the medical team caring for the patient, and/or antibiotics given for this indication. Analyses of subgroups defined by labor and membrane rupture were examined with and without the inclusion of patients with chorioamnionitis.
Continuous variables were compared between groups using Wilcoxon rank sum tests. Categorical variables were compared using uncorrected chi-square tests and Fisher’s exact tests. Risk ratios and 95% confidence intervals were estimated using a modified Poisson regression model. The following variables were assessed for confounding: maternal age (< 35 vs 35 and older), race/ethnicity (white, non-white), parity (0 vs 1 or more), BMI at delivery (obese, non-obese), prior cesarean (yes or no), cervical ripening (yes or no), labor induction (no labor, induced, or spontaneous), payer status (commercial or government/self), gestational age (preterm vs term), gestational hypertension (yes/no), preeclampsia (yes/no), and diabetes (yes/no). A two-sided p value of 0.05 was considered statistically significant.
When assessing confounding for the adjusted models, we also evaluated gestational age at delivery, gestational hypertension, preeclampsia, and diabetes as potential confounding variables. We did not adjust for these variables in the final models because subgroup sample sizes would not support additional covariates beyond the number of pre-specified variables listed above, and our assessment indicated that additional adjustment for these comorbidities had minimal impact on point estimates.
The University of Oklahoma delivers about 4000 patients per year and had a cesarean delivery rate of 32% during the study period. We estimated that there should be about 2560 women who underwent cesarean delivery during the study period (1280 women each in the pre- and post-implementation groups). We also estimated that there would be about 45% (1152) of these women with cesarean after labor and/or ruptured membranes for at least 4 hours. Our a priori power calculation indicated we would need 906 women total (453 in each cohort) to have 80% power to detect a 45% reduction (from 12% to 6.6%) in the primary outcome in the clinical effectiveness evaluation for this subgroup. Since the rate of the primary outcome was expected to be lower in the non-labor subgroup and since ≤ 5% of the population would be expected to have chorioamnionitis, analyses in these subgroups were considered exploratory. We did not perform a separate power calculation for the overall before and after groups because we had no other evidence for estimates of efficacy with application of this intervention to all women undergoing cesarean. This study was approved, with waiver of informed consent, by the Institutional Review Board at OUHSC.
Results
Of the 2592 patients who underwent cesarean delivery during the study period, 253 were excluded from our analyses due to maternal age, fetal anomaly or fetal death (see Figure). Baseline characteristics of the 2339 patients in the pre- and post-implementation groups are shown in Table 1. Compared to women in the post-implementation group, the pre-implementation group had a lower median gestational age at delivery and lower rates of hypertensive disorders of pregnancy and diabetes. All other baseline characteristics were similar between groups.
Figure.
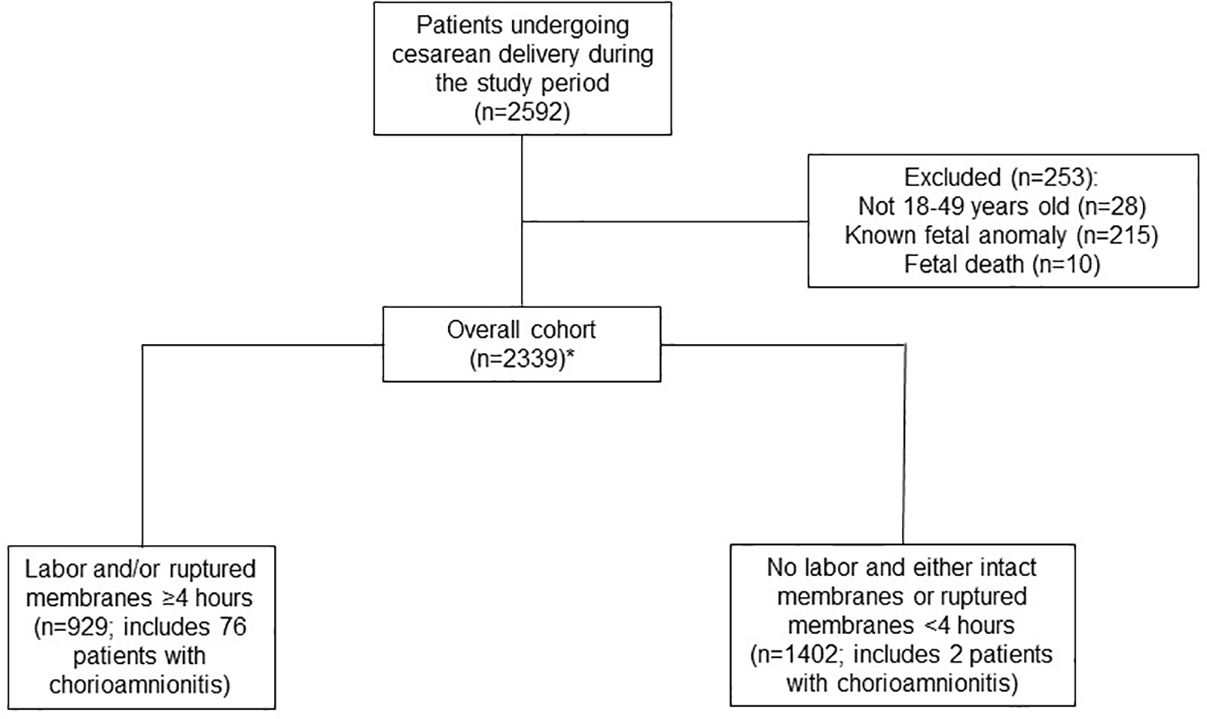
Flowchart showing exclusion criteria and patients excluded from final study sample.
*Within the overall cohort, 8 patients had an unknown duration of membrane rupture. These were included in the analysis of the overall cohort, but were not included in the subgroup analyses.
Table 1.
Characteristics of patients pre- and post-addition of azithromycin to the previous standard of cefazolin for antimicrobial prophylaxis for cesarean delivery
| Pre (n= 1171) | Post (n= 1168) | p value | |
|---|---|---|---|
| Maternal Age at delivery (years) | 29 (25, 34) | 29 (25, 34) | 0.52a |
| Gestational Age (days) | 272 (254, 275) | 268 (253, 274) | 0.03a |
| Body Mass Index at time of delivery (kg/m2) | 33.9 (29.3, 39.8) | 33.7 (28.9, 38.9) | 0.19a |
| Race/ethnicity | 0.03b | ||
| White | 49.6% | 48.7% | |
| Black | 16.4% | 17.3% | |
| Hispanic | 21.8% | 18.0% | |
| Native American | 5.4% | 6.9% | |
| Asian | 2.7% | 2.5% | |
| Other | 4.1% | 6.6% | |
| Payer Status | 0.68b | ||
| Government/self | 70.4% | 69.6% | |
| Commercial | 29.6% | 30.4% | |
| Nulliparous* | 24.3% | 27.4% | 0.08b |
| Prior Cesarean | 57.4% | 55.5% | 0.35b |
| Chronic hypertension | 14.7% | 14.5% | 0.88b |
| Gestational hypertension | 10.4% | 15.9% | <0.0001b |
| Preeclampsia | 0.042b | ||
| Without severe features | 3.3% | 3.4% | |
| With severe features | 15.2% | 19.1% | |
| Diabetes Mellitus | <0.0001b | ||
| Gestational | 8.3% | 10.0% | |
| Pre-gestational | 4.4% | 8.7% | |
| Labor during admission | 35.8% | 38.1% | 0.23b |
| Labor induction | 18.7% | 20.5% | 0.69b |
| Cervical Ripening | 15.7% | 16.5% | 0.59b |
| Rupture of membranes ≥ 4 hours | 25.9% | 26.6% | 0.68b |
Abbreviations used: kg=kilogram, m=meter.
Body mass index at the time of delivery is reported.
Data are reported as median (25th, 75th percentile) or proportion of n and analyzed using Wilcoxon rank sum test or chi-square test, as appropriate.
Defined as women without a prior delivery at or beyond 20 weeks gestation
Wilcoxon rank sum test
Chi-square test
Among all women undergoing cesarean delivery during the study period, the incidence of the primary composite outcome was 4.7% in the pre-implementation group and 5.3% in the post-implementation group (p=0.49; Table 2). There were 929 women in the subgroup with labor and/or ruptured membranes for four hours or more (449 [48%] pre-implementation, and 480 [52%] post-implementation; includes 76 women with chorioamnionitis). In this subgroup, the primary composite outcome occurred in 8.0% of women in the pre-implementation period and in 7.1% of women in the post-implementation period (p=0.59; Table 2). In the subgroup without labor and who had either intact membranes or ruptured membranes for less than 4 hours, there were 1402 women (715 [51%] pre-implementation, and 687 [49%] post-implementation; includes 2 women with chorioamnionitis). The primary composite outcome occurred in 2.7% of women in the pre-implementation period and in 4.1% of women in the post-implementation period (p=0.14; Table 2). Finally, there were 78 women total who had chorioamnionitis (44 [56%] pre-implementation, and 34 [44%] post-implementation). Of the women with chorioamnionitis, the primary composite outcome occurred in 15.9% of women in the pre-implementation period and in 5.9% of women in the post-implementation period (p=0.28; Table 2).
Table 2.
Frequency of composite post-cesarean infection among patients in the pre- and post-azithromycin implementation periods for cesarean preoperative prophylaxis
| Pre-Azithromycin Implementation | Post-Azithromycin Implementation | ||||
|---|---|---|---|---|---|
|
| |||||
| Patient Population | Patients | Composite Infection n (%) | Patients | Composite Infection n (%) | p value |
| Overall | 1171 | 55 (4.7) | 1168 | 62 (5.3) | 0.49a |
| Subgroups* | |||||
| Labor and/or had ruptured membranes ≥4 hours** | 449 | 36 (8.0) | 480 | 34 (7.1) | 0.59a |
| Did not labor and had intact membranes or ruptured <4 hours** | 715 | 19 (2.7) | 687 | 28 (4.1) | 0.14a |
| Chorioamnionitis | 44 | 7 (15.9) | 34 | 2 (5.9) | 0.28b |
All subgroup analyses exclude the 8 patients in the overall cohort who had an unknown duration of membrane rupture.
Subgroup includes patients with chorioamnionitis
Chi-square test
Fisher’s exact test
We also calculated risk ratios comparing the post-implementation period to the pre-implementation period (referent) for the overall group and for each subgroup (Table 3). For all women undergoing cesarean delivery in the study period, the risk of the composite infection outcome was similar in the post- and pre-implementation groups (unadjusted RR 1.13 (95% CI 0.78, 1.62). After adjusting for the pre-specified potential confounding variables, the risk ratio was near one (adjusted [a]RR 1.06 (95% CI 0.74, 1.52). For the subgroup with labor and/or ruptured membranes for four hours or more, an 18% reduction in risk of composite infection was seen in the post-implementation period, although this result did not achieve statistical significance (aRR 0.82 [95% CI: 0.52, 1.30]).
Table 3.
Unadjusted and adjusted risk ratios for composite post-cesarean infection outcomes in the post-implementation of azithromycin group compared to the pre-implementation group.
| Patient Population | Unadjusted RR (95% CI) | Adjusted RR (95% CI) |
|---|---|---|
| Overall | 1.13 (0.78, 1.62) | 1.06 (0.74, 1.52) |
| Subgroups | ||
| Labor and/or had ruptured membranes ≥4 hours* | 0.88 (0.56, 1.39) | 0.82 (0.52, 1.30) |
| Did not labor and had intact membranes or ruptured <4 hours* | 1.53 (0.86, 2.72) | 1.48 (0.83, 2.65) |
| Chorioamnionitis | 0.37 (0.08, 1.67) | ----** |
Data are reported as risk ratio (RR) (95% confidence interval).
Adjusted models control for maternal age at delivery, race, parity, obesity at delivery, prior cesarean, cervical ripening, labor induction, and payer status.
Subgroup includes patients with chorioamnionitis
Data too sparse to allow for adjusted analysis.
The above analyses included women with chorioamnionitis. When women with chorioamnionitis were excluded from the subgroup with labor and/or ruptured membranes for four hours or more, the numerical reduction in estimated risk of composite infection in the post-implementation group was attenuated, and also did not achieve statistical significance (aRR 0.96 [95% CI: 0.59, 1.57]).
For the subgroup without labor and who had either intact membranes or ruptured membranes for less than four hours, the RR for composite infection was numerically increased in the post-implementation group, but this difference was not statistically significant (unadjusted RR 1.53 [95% CI 0.86, 2.72]; aRR 1.48 [95% CI 0.83, 2.65]). When the two women with chorioamnionitis in this subgroup were excluded from the analysis, there was no impact on the RR point estimates.
For the subgroup with chorioamnionitis, the unadjusted comparison (RR 0.37 [95% CI: 0.08, 1.67]) did not reflect a significant difference between the pre- and post-implementation groups; data were too sparse to calculate an adjusted RR for this subgroup (Table 3).
Overall compliance with the policy change was high, with only 32 patients in the post-implementation group who did not have documentation of receiving peri-operative azithromycin. We re-examined our data with these patients excluded, and there was no meaningful impact on the RR point estimates. There were a total of seven patients who did not have documentation of receiving any peri-operative antibiotics (n=3 in the pre-implementation group and n=4 in the post-implementation group). Similarly, re-analysis of our data with these patients excluded did not substantially impact the RR point estimates.
Discussion
Principal Findings
After implementation of a policy to add azithromycin to standard preoperative prophylaxis for all cesarean deliveries at our institution, post-cesarean infection outcomes were numerically lower in the post-implementation group, but this difference did not reach statistical significance. When examining the subgroups of women 1) with labor and/or ruptured membranes for greater than four hours, 2) without labor and who had either intact membranes or ruptured membranes for less than four hours, and 3) with chorioamnionitis, post-cesarean infection outcomes were also not statistically different between the pre- and post-implementation periods.
Results
Tita et. al demonstrated in their large, multicenter, randomized controlled trial that the addition of azithromycin to the previous standard preoperative antibiotic prophylaxis significantly reduced the frequency of infection after cesarean delivery in women who were laboring and/or had ruptured membranes for 4 hours or more.12 In our study, this subgroup of laboring women had a composite infection rate that was numerically lower in the after-implementation cohort, and the risk of the composite infection outcome occurring was 12% lower in the unadjusted analysis and 18% lower in the fully-adjusted analysis for the after-implementation group. However, these differences did not reach statistical significance, and the effect size was substantially lower than the reduction of 49% reported in the C/SOAP trial.12
Clinically, our institution made the decision to extrapolate the results of the C/SOAP trial to all women by adding azithromycin to the preoperative antibiotic prophylaxis regimen for every cesarean delivery. We made this decision for two reasons: first, other studies that included non-laboring patients have demonstrated a benefit of adding azithromycin to preoperative cesarean prophylaxis.13–15 Second, extrapolating this regimen to all women would simplify preoperative antibiotic prophylaxis and minimize the risk for accidental omission of azithromycin. When examining outcomes for the subsets of women who would not have met enrollment criteria for the C/SOAP trial, we found that those who had a pre-labor cesarean did not have a statistically significant difference in rates of infection after implementation of the new preoperative prophylaxis regimen. In fact, the point estimates in both unadjusted and adjusted comparisons were higher in the azithromycin group. Given these data, we conclude that the addition of azithromycin in this group of non-laboring patients may be ineffective. In women with chorioamnionitis, we observed an imprecise (as expected with the small sample size of this subset) estimate in the direction of reduced postoperative infections in the post-implementation group, suggesting that extrapolating the expanded antibiotic prophylaxis regimen to women with chorioamnionitis undergoing cesarean may be beneficial.
Importantly, in examining the subgroup who labored, when we removed women with chorioamnionitis from the analyses we found that the association of azithromycin administration with numerically lower risk for postoperative infection was further attenuated. This suggests that the major benefit of expanded preoperative antibiotic prophylaxis may lie primarily in treatment of overt or subclinical chorioamnionitis that was present at the time of cesarean, and that the mechanism of action is prevention of realizing clinically-evident infection in laboring women with subclinical chorioamnionitis.
In our study the rates of diabetes, gestational hypertension, and preeclampsia were significantly higher in the after-implementation cohort. These differences mirror the trends of an increasingly co-morbid pregnant population in the US as described by others.16,17 It is well-known that the rates of pregnancy complications, especially infection, are higher in patients with medical comorbidities. 18–21 It is possible that the baseline risk for infection was higher in the after-implementation cohort due to the higher rate of comorbidities. However, in our analyses, adjusting for the presence of these comorbidities did not change the results.
There are multiple potential reasons for the differences in our results compared to the findings from the C/SOAP study. Perhaps most notably, our baseline composite infection outcome rate in the subgroup with labor and/or ruptured membranes for 4 hours or more (8.0%) was lower than expected and lower than reported in the original trial by Tita et al (12.0%). This difference in prevalence paired with our smaller effect size explains why the reduction in the composite outcome did not reach statistical significance in our cohort. Specifically, given the limitations as described above, the fact that a statistically significant difference in the composite outcome was not observed may represent a type II error.
It is worth noting that there are other examples in which results from retrospective or prospective observational studies differed from those found in a randomized controlled clinical trial.22–24 The potential reasons for these differences vary, ranging from narrow trial inclusion criteria to medication noncompliance in the regular clinical setting. Although the inherent biases and limitations of retrospective data must be considered, real-world observational data continues to be an important source of information to complement findings from clinical trials.25 Therefore, we think that such clinical effectiveness cohort studies are important.
Clinical Implications
Although our outcomes did not reach statistical significance, the numerically lower rate of the composite infection outcome in women in labor or with ruptured membranes for more than four hours in the post-implementation group suggests that the addition of azithromycin to preoperative cesarean prophylaxis may be beneficial. However, in non-laboring women the non-significant trend toward a higher rate of infection in the post-implementation group suggests that the addition of azithromycin may not be beneficial in this subgroup of women.
Given these results, we have decided that our extrapolation of azithromycin administration to non-laboring cesarean deliveries does not appear to be helpful, and have discontinued this practice. We are continuing to add azithromycin for cesarean delivery in women who are laboring and/or have ruptured membranes, and in women with chorioamnionitis.
Research Implications
We encourage additional cohort studies to investigate the clinical effectiveness of adding azithromycin to preoperative cesarean antibiotic prophylaxis. Judicious use of antibiotics has always been crucial in order to limit medication exposure and the development of resistant organisms. In addition, recent evidence suggests that maternal antibiotic use in pregnancy can affect the neonatal microbiome.26 Further research in this area is needed to ensure that the benefits of antibiotic use outweigh risks.
Strengths and Limitations
Our study has several strengths. Data extraction was performed via individual patient chart review by trained physicians, and we adjusted for demographic differences between cohorts. Our analysis has limitations inherent to a retrospective study. In particular, given that well over half of post-cesarean infection complications occur after the delivery hospitalization,27–29 we may have had incomplete ascertainment of infection outcomes if patients presented to outside hospitals for care. However, we would not expect that the proportion of women who followed up at outside hospitals would be different between the pre- and post-implementation groups. In addition, this analysis was performed with data from a single center, which may limit generalizability.
Conclusions
Our data suggest that in clinical practice, the addition of azithromycin to standard cesarean preoperative antibiotic prophylaxis may have an effect size smaller than that seen in the large clinical trial prompting this practice change. Extrapolation of this practice change to non-laboring women undergoing cesarean may be ineffective altogether. However, women with chorioamnionitis may benefit from the addition of azithromycin to cesarean preoperative prophylaxis. We think that additional similar analyses from other centers would be of interest.
AJOG at a Glance.
A. Why was the study conducted?
A recent trial demonstrated reduction in postoperative infections with addition of azithromycin to standard antibiotic prophylaxis at cesarean
We aimed to evaluate the clinical effectiveness, as opposed to clinical trial efficacy, of this regimen and its extrapolation to non-laboring patients
B. What are the key findings?
Addition of azithromycin was not associated with a significant change in postoperative infection when applied to all women undergoing cesarean
In women who were in labor and/or with ruptured membranes for ≥ four hours, azithromycin was associated with a numeric, but non-statistically significant, decrease in infection
C. What does this study add to what is already known?
Addition of azithromycin to standard cesarean prophylaxis may have a smaller effect size than that seen in the trial prompting this practice change
Extrapolation to patients not in labor and with intact membranes or ruptured < four hours may be inappropriate
Acknowledgments
Financial support: Support was provided by the National Institute of General Medical Sciences (U54GM104938). The funding source had no role in: study design; the collection, analysis, and interpretation of the data; the writing of the report; or the decision to submit the article for publication.
Footnotes
The authors report no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Martin JA, Hamilton BE, Ventura SJ, Osterman MJ, and Mathews TJ. Births: final data for 2011. Natl Vital Stat Rep. 2013June;62(1):1–69, 72. [PubMed] [Google Scholar]
- 2.MacDorman MF, Menacker F, and Declercq E. Cesarean birth in the United States: epidemiology, trends, and outcomes. Clin Perinatol. 2008June;35(2):293–307. [DOI] [PubMed] [Google Scholar]
- 3.Martin JA, Hamilton BE, Osterman MJK, and Driscoll AK. Births: Final data for 2018. National Vital Statistics Reports. November2019;68(13). [PubMed] [Google Scholar]
- 4.Gibbs RS. Clinical risk factors for puerperal infection. Obstet Gynecol. 1980May;55(5 Suppl):178S–184S. [DOI] [PubMed] [Google Scholar]
- 5.Duff P (2014). Maternal and fetal infection. In Creasy Robert K., Resnik Robert, lams Jay D., Lockwood Charles J., Moore Thomas R., and Greene Michael F. (Eds.). Creasy and Resnik’s Maternal-Fetal Medicine: Principles and Practice (7th ed., p. 802–51). Elsevier: Philadelphia. [Google Scholar]
- 6.DeFrances CJ and Hall MJ. 2005 National Hospital Discharge Survey. Adv Data. July 2007;12(385): p. 1–19. [PubMed] [Google Scholar]
- 7.Smaill FM and Grivell RM. Antibiotic prophylaxis versus no prophylaxis for preventing infection after cesarean section. Cochrane Database Syst Rev. 2014. October;(10):CD007482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chelmow D, Ruehli MS, and Huang E. Prophylactic use of antibiotics for nonlaboring patients undergoing cesarean delivery with intact membranes: a meta-analysis. Am J Obstet Gynecol. 2001March;184(4):656–61. [DOI] [PubMed] [Google Scholar]
- 9.Tita A, Rouse DJ, Blackwell S, Saade GR, Spong CY, and Andrews WW. Evolving concepts in antibiotic prophylaxis for cesarean delivery: a systematic review. Obstet Gynecol. 2009March;113(3):675–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bratzler DW, Houck PM; Surgical Infection Prevention Guideline Writers Workgroup. Antimicrobial prophylaxis for surgery: an advisory statement from the National Surgical Infection Prevention Project. Am J Surg. 2005April;189(4)395–404. [DOI] [PubMed] [Google Scholar]
- 11.Use of Prophylactic Antibiotics in Labor and Delivery. ACOG Practice Bulletin No. 199. American College of Obstetricians and Gynecologists. Obstet Gynecol. 2018;132(3):e103–e119. [DOI] [PubMed] [Google Scholar]
- 12.Tita AT, Szychowski JM, Boggess K, Saade G, Longo S, Clark E, et al. Adjunctive Azithromycin Prophylaxis for Cesarean Delivery. N Engl J Med. 2016September;375(13):1231–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Andrews WW, Hauth JC, Cliver SP, Savage K, and Goldenberg R. Randomized clinical trial of extended spectrum antibiotic prophylaxis with coverage for Ureaplasma urealyticum to reduce post-cesarean delivery endometritis. Obstet Gynecol. 2003June; 101 (6):1183–9. [DOI] [PubMed] [Google Scholar]
- 14.Tita AT, Hauth JC, Grimes A, Owen J, Stamm AM, and Andrews WW. Decreasing incidence of postcesarean endometritis with extended-spectrum antibiotic prophylaxis. Obstet Gynecol. 2008January;111(1):51–56. [DOI] [PubMed] [Google Scholar]
- 15.Tita AT, Owen J, Stamm AM, Grimes A, Hauth JC, and Andrews WW. Impact of extended-spectrum antibiotic prophylaxis on incidence of postcesarean surgical wound infection. Am J Obstet Gynecol. 2008September;199(3):303.e1–3. [DOI] [PubMed] [Google Scholar]
- 16.Correa A, Bardenheier B, Elixhauser A, Geiss LS, and Gregg E. Trends in prevalence of diabetes among delivery hospitalizations, United States, 1993–2009. Matern Child Health J. 2015March;19(3):635–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hutcheon JA, Lisonkova S, and Joseph KS. Epidemiology of pre-eclampsia and the other hypertensive disorders of pregnancy. Best Pract Res Clin Obstet Gynaecol. 2011August;25(4):391–403. [DOI] [PubMed] [Google Scholar]
- 18.Ketcheson F, Woolcott C, Allen V, and Langley JM. Risk factors for surgical site infection following cesarean delivery: a retrospective cohort study. CMAJ Open. 2017July;5(3):E546–E556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.La Rosa M, Jauk V, Saade GR, Boggess K, Longo S, Clark EAS, et al. Incidence and Risk Factors for Hospital Readmission or Unexpected Visits in Women Undergoing Unscheduled Cesarean Delivery. Am J Perinatol. 2019September;36(11):1115–1119. [DOI] [PubMed] [Google Scholar]
- 20.Schneid-Kofman N, Sheiner E, Levy A, and Holcberg G. Risk factors for wound infection following cesarean deliveries. Int J Gynaecol Obstet. 2005July;90(1):10–5. [DOI] [PubMed] [Google Scholar]
- 21.Wloch C, Wilson J, Lamagni T, Harrington P, Charlett A, and Sheridan E. Risk factors for surgical site infection following caesarean section in England: results from a multicentre cohort study. BJOG. 2012October;119(11):1324–33. [DOI] [PubMed] [Google Scholar]
- 22.Carls GS, Tuttle E, Tan R-D, Huynh J, et al. Understanding the gap between efficacy in randomized controlled trials and effectiveness in real-world use of GLP-1 RA and DPP-4 therapies in patients with type 2 diabetes. Diabetes Care. 2017November;40(11 ):1469–1478. [DOI] [PubMed] [Google Scholar]
- 23.Edelman SV and Polonsky WH. Type 2 diabetes in the real world: the elusive nature of glycemic control. Diabetes Care. 2017. November;40(11):1425–1432. [DOI] [PubMed] [Google Scholar]
- 24.Rothwell PM. External validity of randomised controlled trials: “to whom do the results of this trial apply?”. Lancet. 2005January1–7;365(9453):82–93. [DOI] [PubMed] [Google Scholar]
- 25.Blonde L, Khunti K, Harris BS, Meizinger C, and Skolnik NS. Interpretation and impact of real-world clinical data for the practicing clinician. Adv Ther. 2018November;35(11):1763–1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang M, et al. Association of prenatal antibiotics with measures of infant adiposity and the gut microbiome. Ann Clin Microbiol Antimicrob. 2019;18:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Figueroa D, Chapman Jauk V, Szychowski JM, Garner R, et al. Surgical staples compared with subcuticular suture for skin closure after cesarean delivery: a randomized controlled trial. Obstet Gynecol. 2013January;121(1):33–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cardoso Del Monte MC and Mendes Pinto Neto A. Postdischarge surveillance following cesarean section: the incidence of surgical site infection and associated factors. Am J Infect Control. 2010. August;38(6):467–72. [DOI] [PubMed] [Google Scholar]
- 29.Noy D and Creedy D. Postdischarge surveillance of surgical site infections: a multi-method approach to data collection. Am J Infect Control. 2002. November;30(7):417–24. [DOI] [PubMed] [Google Scholar]


