Abstract
Introduction:
Social media, including Facebook outreach, is increasingly being used as a participant recruitment tool, and may be particularly useful in tobacco and smoking cessation studies. The Recruitment Innovation Center at Vanderbilt University Medical Center partnered with Project LUNA, a smoking cessation study, to conduct a pilot social media campaign aimed at increasing study recruitment.
Methods:
Two posts encouraging study participation were developed and promoted on Facebook to users with an interest in smoking-related topics, with a link to a study-specific webpage. Facebook and website analytics were collected, including impressions, clicks, click through rates, website traffic, and clicks to the study screening form. Study screening and enrollment data were also collected.
Results:
The Facebook campaign ran in June 2019 in the greater Houston area. In total, the Facebook posts logged 1,179,844 impressions, 6,490 clicks, and an overall click-through rate of 0.55%. There were no differences in response to the two different promotional posts. Approximately 3,812 unique individuals visited an intermediary study page, with 473 expressing interest in the study. Forty-three potential participants contacted the study team, resulting in study enrollment and randomization of 23 participants, with an estimated cost per enrolled participant of $441.
Conclusions:
The social media campaign was successful at increasing outreach and interest in the LUNA study. However, the price-per-participant enrolled was higher than in comparable tobacco cessation studies. These results and lessons learned may be beneficial to others considering social media as a recruitment method for their clinical research trial.
Keywords: social media, smoking cessation, clinical trial recruitment
INTRODUCTION
Social media is increasingly used as a participant recruitment tool for clinical research.1 However, data regarding its efficacy and cost-efficiency remains less developed. Emerging research suggests that social media may increase and speed trial enrollment while reducing per-participant cost.1,2 Others are reported to have experienced varying levels of success.3 Social media has been employed to recruit for studies on tobacco use or smoking cessation for at least 10 years, including surveys, observational studies, and trials.4–12 Facebook may be an especially attractive recruitment tool, as a large population of potential participants can be targeted and reached using keywords related to tobacco use. A few studies have demonstrated that Facebook can be highly cost effective,4,5 especially when conducting survey-based research. Others recruiting for web-based or in-person tobacco cessation interventions have reported higher costs.6–13 The Recruitment Innovation Center (RIC), funded by the US National Center for Advancing Translational Sciences, works with trial investigators to develop, test, and share innovations that enhance participant recruitment and retention, and in doing so, facilitates the success of clinical trials and ultimately the improvement of health outcomes. To add to the collective evidence base, the RIC collaborated with Project LUNA, a smoking cessation study, to explore the value of using a short social media campaign aimed at increasing study recruitment.
METHODS
Project LUNA
Project LUNA is a randomized controlled trial evaluating the efficacy of three smoking cessation treatment strategies in long-term smokers eligible for low-dose CT lung cancer screening. LUNA began recruitment at MD Anderson (Houston, Texas campus) on June 16, 2017 with a goal of 630 participants. Recruitment was originally driven by two strategies: 1) identifying eligible participants from a similar 5-year lung cancer screening study and 2) radio advertising in Houston. Initial enrollment averaged 7 to 13 participants per month.
Leveraging Facebook to aid LUNA recruitment
The LUNA study team partnered with the RIC, along with an advertising agency (Red Deluxe, Memphis, TN), to implement a short pilot exploration using Facebook to recruit study participants. This campaign included design and refinement of outreach posts (Figure 1) and testing to compare two different creative approaches. These two approaches, one focusing on smoking cessation and the other on early lung cancer detection, were used to determine which messaging would resonate most with potential participants. Timing and placement of both posts were determined by Facebook’s algorithms. The campaign targeted individuals aged 50 to 70 years, within 50 miles of MD Anderson, who were tagged by Facebook as having smoking-related interests (Smoking; Cigarette; Electronic Cigarette; Cigar; Tobacco).14,15 We also included country music as a targeted interest, inspired by LUNA’s previous success with advertisements on country music radio (note: radio ads did not run concurrently with the Facebook campaign). A simple Facebook page, “Smoking Cessation Research,” was created to push the outreach posts. Approximately $10,000 was budgeted for this campaign, which included Facebook fees and administrative costs associated with Red Deluxe.
Figure 1:
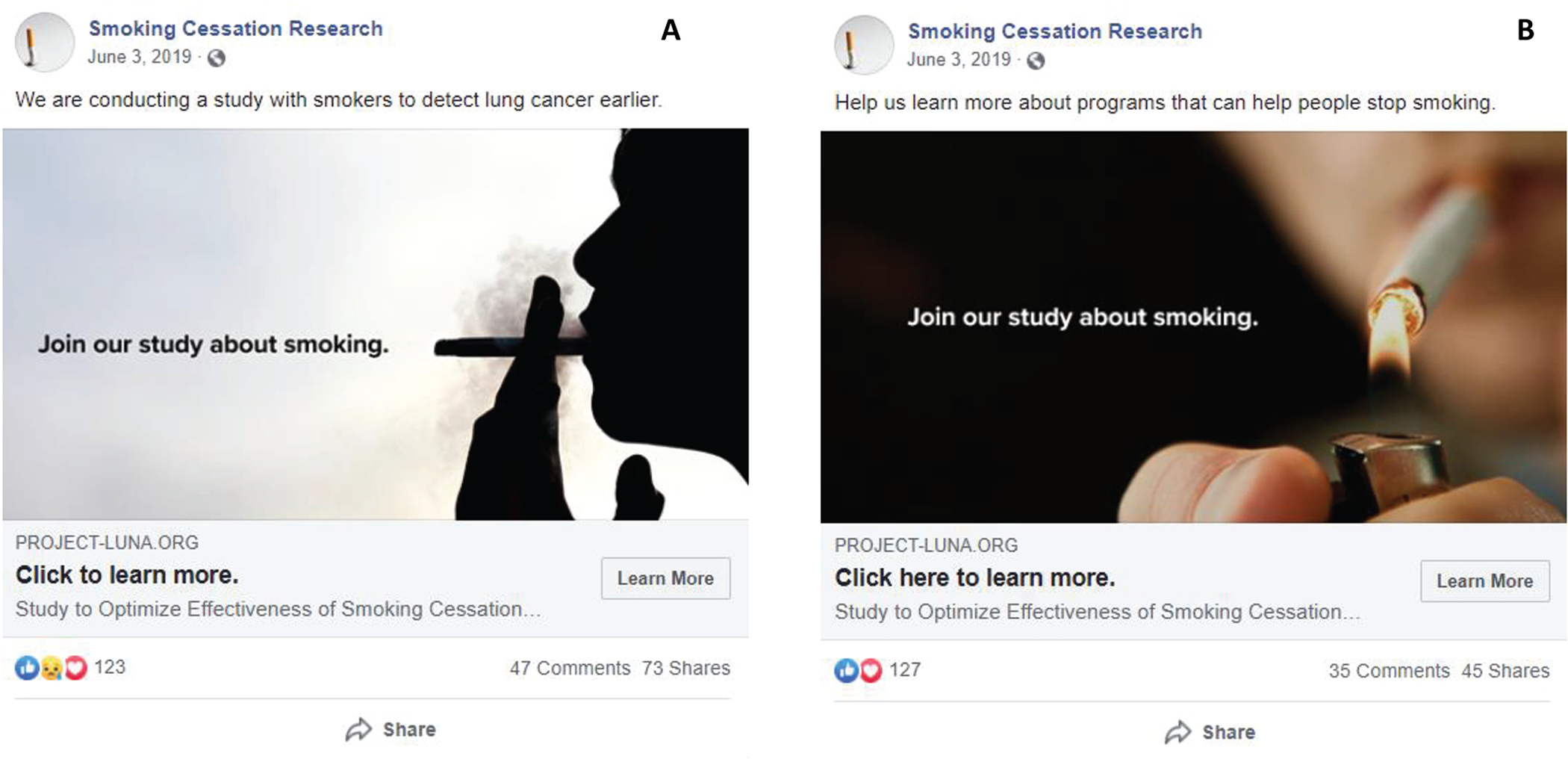
Facebook campaign creative materials, including A) lung cancer detection messaging approach and B) smoking cessation messaging approach
An intermediary website, which the Facebook posts linked to, allowed potential participants to learn more about the study (Supplemental Figure 1). This website was designed using Squarespace (New York, NY) and contained a study description, eligibility criteria, procedures and expectations, and contact information. The website also linked to the LUNA online study screening form, which included the research team’s telephone number as an alternative contact path. Potential participant contacts, screening, and recruitment outcomes were tracked by the LUNA team. Everyone who completed the LUNA screening form or called the coordinator was asked how they heard about the study.
MD Anderson’s Office of Human Subjects Protections approved this campaign as an amendment to recruitment procedures for this study.
Data analysis
We summarize descriptive data from Facebook, including impressions (the number of times the awareness message was seen), clicks from the post to the website, click-through-rate (CTR) (clicks/impressions), and cost per thousand impressions (CPM; cost of one thousand user views). Analytics from the intermediary page included traffic (unique views, number of visits, and page views) and the number of clicks to the LUNA screening form.
RESULTS
The Facebook campaign ran from June 3 to June 30, 2019 (Table 1), with 1,179,844 impressions, and an overall spend of $10,135.49. The posts received 6,490 clicks, an overall CTR of 0.55%, and an overall CPM of $8.59. Comparison of the early lung cancer detection messaging versus the smoking cessation messaging outreach posts indicated that these strategies had roughly equivalent CTRs and cost. During the campaign, approximately 3,812 unique individuals visited the intermediary page, with 473 clicks on the “I am interested” button to reach the LUNA study screening form (Supplemental Table 1).
Table 1:
Facebook campaign summary
| Impressions* | Clicks | CTR | Total cost | CPM | |
|---|---|---|---|---|---|
| Cancer detection awareness message (Figure 1A) | 592,498 | 3294 | 0.56% | $5067.76 | $8.55 |
| Smoking cessation awareness message (Figure 1B) | 587,346 | 3195 | 0.54% | $5067.73 | $8.63 |
| Total | 1,179,844 | 6490 | 0.55% | $10,135.49 | $8.59 |
Key:
Each targeted Facebook user saw the LUNA post an average of 7 times; CTR click through rate; CPM cost per thousand impressions; cost and CPM represented in US dollars.
Study contacts and recruitment
The 473 clicks resulted in 32 individuals completing the online web screener and 10 contacting the study team through direct phone calls, for a total of 43 contacts from potential participants (1 unknown). All identified Facebook as their awareness source. Of those, 40 (93%) completed a requisite telephone screening survey. Twenty-three (57.5%) were eligible for the study. All 23 completed the baseline screening and enrollment visit, and all were randomized. In comparison, 11 participants were recruited via traditional strategies and randomized during the same period. The intermediary page resulted in a conversion rate of 4.86% (23 enrolled/473 clicks to LUNA screening form), more than double the 2.35% national average for landing page conversion.16 The estimated cost-per-enrolled participant associated with the Facebook campaign was $441 (Supplemental Table 2).
DISCUSSION
Overall, the LUNA social media campaign proved successful at outreach and recruitment of new study participants in the greater Houston, Texas area. In the months following the campaign, LUNA randomized 34 total participants, including 23 resulting from the social media campaign, compared to 11 from their usual recruitment sources. Thus, the social media campaign greatly accelerated the pace of recruitment, an important factor to consider when weighed against anticipated costs.
Our overall click-through rate of 0.55% fell within the range of other studies promoting smoking-related trials on Facebook, which reported rates from 0.18% to 2.79% (Supplemental Table 2). Our cost-per-click of $1.56, however, came in high, with other studies reporting from $0.34 to approximately $0.73 (AUD $0.95). At $441 per enrolled participant, the price-per-participant enrolled in LUNA was higher than comparable tobacco-cessation studies using social media to recruit for studies requiring in-person study visits ($42 to $103.66 per completed participant)9–11 or web-based cessation interventions ($8.80–$112.48),6–8,12,13 and far higher than survey research ($1.51–$4.28 per completed survey).4,5 While the LUNA social media campaign was successful at raising awareness and enrolling study participants, we recognize the high cost per enrolled participant may be prohibitive. This pilot was intended to explore the use of social media as a recruitment tool, along with gauging cost. Future research may inform design of Facebook strategies that maintain effectiveness while reducing cost, such as more precise targeting criteria and more engaging messaging.
Our experience indicates that future campaigns should allow for intermittent adjustments in message targeting during the course of the campaign, as our a priori plan led to a high rate of exposure to the study posts, with individuals seeing the message an average of 7 times. This high exposure frequency may have surpassed the point of diminishing returns17 without meaningful impact on enrollment; Facebook now allows setting a cap on the number of times an individual may see a promotional post. Additionally, our campaign required individuals to go through a multi-step process to express interest in LUNA. While this was ideal for collecting data around website traffic, it may have been overly burdensome for potential study participants, causing substantial dropouts with each additional required “click.” It may be beneficial to send individuals directly to a study’s website to complete the online screening, rather than through an intermediary page.
Our study found no significant difference in response to our two creative approaches, which respectively focused on either cancer screening or smoking cessation. Both messages featured an indistinct face of a person smoking, which may have been too similar in approach to yield meaningful differences in response. In contrast, some studies have found that loss-frame messages (with respect to continuing to smoke) outperformed gain-frame messages (from stopping smoking) when targeting current smokers.13,18 Others have reported varying response rates to ads with differing styles, suggesting the value in comparing and pilot-testing different awareness messages, especially those targeting different demographic groups, to maximize recruitment success.4,13
Limitations
One limitation of this pilot experience was its short-term implementation, lasting for approximately one month; however, recruitment and enrollment constitute a longer process. It is possible that the social media campaign may be having ongoing and longer-term impacts on enrollment yields that were not captured in our data. The focus on a single social media platform (Facebook) may have also limited reach. Further, an individual’s decision to engage in screening behavior, smoking cessation, and study participation is influenced by multiple factors;10 it is unclear how these may affect openness to respond to social media recruitment messages. Our findings may be generalizable to other English-speaking countries with comparable cultures, as similar predictors of smoking cessation have been noted.19 However, smoking patterns and behaviors can vary markedly in other regions of the world.20 Thus, the use of social media for smoking cessation study recruitment may not produce similar results.
CONCLUSIONS:
A social media campaign to increase enrollment to a smoking cessation intervention should be viewed as one tool in the recruitment “tool chest”. While a targeted campaign can significantly boost enrollment within a short timeframe, this benefit needs to be balanced against a potentially substantial cost-per-participant. A period of pilot testing before implementing a full-scale campaign could be instructive. Campaign metrics, including cost-per-enrollee from social media, should be tracked to allow for ongoing refinement of strategies during implementation, and should be compared to metrics from other recruitment channels. In sum, trials may find social media to be a valuable complement to existing strategies for participant recruitment.
Supplementary Material
ACKNOWLEDGEMENTS:
We would like to thank Red Deluxe for their assistance in developing and implementing this social media campaign. We would also like to thank Meredith O’Brien for her contributions in developing and overseeing the LUNA study Facebook page. Lastly, we extend our appreciation to the individuals who expressed interest in the LUNA study and for those who enrolled as study participants.
FUNDING:
This project was supported by awards no. UL1 TR002243 from the National Center for Advancing Translational Sciences (NCATS), no. U24TR001579 from the NCATS and the National Library of Medicine, and no. R01CA207078 and no. CA016672 from the National Cancer Institute. It was also supported by generous philanthropic contributions to The University of Texas MD Anderson Moon Shots Program. Its contents are solely the responsibility of the authors and do not necessarily represent official views of the National Center for Advancing Translational Sciences, the National Library of Medicine, the National Cancer Institute, or the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DECLARATION OF INTERESTS:
The authors have no conflicts of interest to declare.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
REFERENCES
- 1.Brøgger-Mikkelsen M, Ali Z, Zibert JR, Andersen AD, Thomsen SF. Online Patient Recruitment in Clinical Trials: Systematic Review and Meta-Analysis. Journal of Medical Internet Research. 2020;22(11):e22179. doi: 10.2196/22179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Darmawan I, Bakker C, Brockman TA, Patten CA, Eder M. The Role of Social Media in Enhancing Clinical Trial Recruitment: Scoping Review. Journal of Medical Internet Research. 2020;22(10):e22810. doi: 10.2196/22810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Topolovec-Vranic J, Natarajan K. The Use of Social Media in Recruitment for Medical Research Studies: A Scoping Review. J Med Internet Res. 2016;18(11):e286. doi: 10.2196/jmir.5698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ramo DE, Prochaska JJ. Broad Reach and Targeted Recruitment Using Facebook for an Online Survey of Young Adult Substance Use. Journal of Medical Internet Research. 2012;14(1):e28. doi: 10.2196/jmir.1878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carter-Harris L, Bartlett Ellis R, Warrick A, Rawl S. Beyond Traditional Newspaper Advertisement: Leveraging Facebook-Targeted Advertisement to Recruit Long-Term Smokers for Research. J Med Internet Res. 2016;18(6). doi: 10.2196/jmir.5502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bunge EL, Taylor LA, Bond M, et al. Facebook for recruiting Spanish- and English-speaking smokers. Internet Interv. 2019;17. doi: 10.1016/j.invent.2019.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Watson NL, Mull KE, Heffner JL, McClure JB, Bricker JB. Participant Recruitment and Retention in Remote eHealth Intervention Trials: Methods and Lessons Learned From a Large Randomized Controlled Trial of Two Web-Based Smoking Interventions. J Med Internet Res. 2018;20(8). doi: 10.2196/10351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Akers L, Gordon JS. Using Facebook for Large-Scale Online Randomized Clinical Trial Recruitment: Effective Advertising Strategies. Journal of Medical Internet Research. 2018;20(11):e290. doi: 10.2196/jmir.9372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brodar KE, Hall MG, Butler EN, et al. Recruiting Diverse Smokers: Enrollment Yields and Cost. Int J Environ Res Public Health. 2016;13(12). doi: 10.3390/ijerph13121251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Frandsen M, Thow M, Ferguson SG. The Effectiveness Of Social Media (Facebook) Compared With More Traditional Advertising Methods for Recruiting Eligible Participants To Health Research Studies: A Randomized, Controlled Clinical Trial. JMIR Res Protoc. 2016;5(3). doi: 10.2196/resprot.5747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Frandsen M, Walters J, Ferguson SG. Exploring the Viability of Using Online Social Media Advertising as a Recruitment Method for Smoking Cessation Clinical Trials. Nicotine & Tobacco Research. 2014;16(2):247–251. doi: 10.1093/ntr/ntt157 [DOI] [PubMed] [Google Scholar]
- 12.Byaruhanga J, Tzelepis F, Paul C, Wiggers J, Byrnes E, Lecathelinais C. Cost Per Participant Recruited From Rural and Remote Areas Into a Smoking Cessation Trial Via Online or Traditional Strategies: Observational Study. J Med Internet Res. 2019;21(11). doi: 10.2196/14911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ramo DE, Rodriguez TMS, Chavez K, Sommer MJ, Prochaska JJ. Facebook recruitment of young adult smokers for a cessation trial: Methods, metrics, and lessons learned. Internet Interventions. 2014;1(2):58–64. doi: 10.1016/j.invent.2014.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Facebook: About Detailed Targeting. Facebook Business Help Center. AccessedMay 7, 2021. https://www.facebook.com/business/help/182371508761821
- 15.Facebook: Use Detailed Targeting. Facebook Business Help Center. AccessedMay 7, 2021. https://www.facebook.com/business/help/440167386536513
- 16.What’s a Good Conversion Rate? (It’s Higher Than You Think). AccessedMay 7, 2021. https://www.wordstream.com/blog/ws/2014/03/17/what-is-a-good-conversion-rate
- 17.A Data-Driven Exploration of Optimal Ad Frequency. Facebook IQ.AccessedMay 7, 2021. https://www.facebook.com/business/news/insights/a-data-driven-exploration-of-optimal-ad-frequency
- 18.Graham AL, Fang Y, Moreno JL, et al. Online Advertising to Reach and Recruit Latino Smokers to an Internet Cessation Program: Impact and Costs. Journal of Medical Internet Research. 2012;14(4):e116. doi: 10.2196/jmir.2162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hyland A, Borland R, Li Q, et al. Individual‐level predictors of cessation behaviours among participants in the International Tobacco Control (ITC) Four Country Survey. Tob Control. 2006;15(Suppl 3):iii83–iii94. doi: 10.1136/tc.2005.013516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Corrao MA, Guindon GE, Cokkinides V, Sharma N. Building the evidence base for global tobacco control. Bull World Health Organ. 2000;78(7):884–890. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


