Abstract
Advanced glycation end products (AGEs) accumulate with age in human lens capsules. AGEs in lens capsules potentiate the transforming growth factor beta-2-mediated mesenchymal transition of lens epithelial cells, which suggests that they play a role in posterior capsule opacification after cataract surgery. We measured AGEs by liquid chromatography-mass spectrometry in capsulorhexis specimens obtained during cataract surgery from nondiabetic and diabetic patients with and without established retinopathy. Our data showed that the levels of most AGEs (12 out of 13 measured) were unaltered in diabetic patients and diabetic patients with retinopathy compared to nondiabetic patients. There was one exception: glucosepane, which was significantly higher in diabetic patients, both with (6.85 pmol/μmol OH-proline) and without retinopathy (8.32 pmol/μmol OH-proline), than in nondiabetic patients (4.01 pmol/μmol OH-proline). Our study provides an explanation for the similar incidence of posterior capsule opacification between nondiabetic and diabetic cataract patients observed in several studies.
1. Introduction
The human lens capsule is a basement membrane secreted by a monolayer of lens epithelial cells. It surrounds the lens and allows selective molecules to pass through to the lens (Danysh and Duncan, 2009; Danysh et al., 2010). It thickens with age; in the adult lens, the average thickness is ~12 μm at the anterior pole and ~5 μm at the posterior pole (Barraquer et al., 2006). The lens capsule is a reservoir of growth factors that are needed for lens epithelial cells to proliferate and differentiate into fiber cells (Danysh and Duncan, 2009; Tholozan et al., 2007; VanSlyke et al., 2018).
Lens posterior capsule opacification (PCO) occurs in many patients after cataract surgery and implantation of an intraocular lens (IOL). The residual lens epithelial cells on the anterior capsule after cataract surgery proliferate, migrate and undergo changes to a myofibroblast phenotype through epithelial to mesenchymal transition (EMT) and secrete excessive extracellular matrix proteins, which causes PCO through fibrosis (Wormstone and Eldred, 2016; Wormstone et al., 2020). PCO often leads to diminished vision. The incidence of PCO increases with time after cataract surgery, ranging between 4.7–18.6% at 3 years and 7.1–22.6% at 5 years in patients with a single piece IOL implanted (Ursell et al., 2020). Nd:YAG capsulotomy can be performed to clear the fibrous tissue and restore vision. The biochemical mechanisms underlying PCO are not fully understood.
The lens capsule has been studied as a contributor to PCO. Transforming growth factor beta-2 (TGFβ2)-mediated signaling has been implicated (Boswell et al., 2017; Meacock et al., 2000). Integrins, especially αV, have been shown to be important for LECs to undergo EMT, possibly through activation of latent TGFβ2 in the capsule (Mamuya et al., 2014). Additionally, recent studies also implicate the immune response following cataract surgery as a possible cause (Jiang et al., 2018; Logan et al., 2017).
Advanced glycation end products (AGEs), which can potentiate TGFβ2-mediated signaling and accelerate EMT of LECs (Nam and Nagaraj, 2018; Raghavan et al., 2016), are formed as a result of a chemical reaction between carbonyl compounds and protein amino groups (mainly those of lysine and arginine residues). Since capsule proteins have negligible turnover, they accumulate AGEs with age. Many AGEs have been detected in human lens proteins (Nagaraj et al., 2012; Smuda et al., 2015). In addition, lens capsule proteins have been shown to accumulate AGEs with aging (Raghavan et al., 2016), and AGEs are elevated in capsules of cataractous as compared to noncataractous lenses (Raghavan et al., 2016). Our previous study also demonstrated that TGFβ2-mediated EMT of LECs is directly related to the levels of AGEs in the lens capsule (Raghavan et al., 2016). In addition, we demonstrated that capsule AGEs interact with a receptor for AGEs, RAGE, in LECs during enhancement of EMT (Raghavan and Nagaraj, 2016). Our recent study (Nam et al., 2021) shows that RAGE in LECs is required for TGFβ2-mediated EMT. Together, these data suggest that lens capsule AGEs play a role in PCO.
Hyperglycemia in diabetes promotes AGE formation in tissues (Vlassara and Uribarri, 2014). Many studies have shown that AGEs accumulate at a higher rate in tissue proteins in diabetes, and in skin collagen, AGEs are directly related to long-term glycemic control (Beisswenger et al., 1993; Lyons et al., 1991). Some studies, but not all (Ahmed et al., 2003), have shown higher levels of AGEs in diabetic lenses (Hashim and Zarina, 2011; Zarina et al., 2000), which could be a reason for accelerated age-related cataracts in diabetic patients. It is not known whether capsule proteins accumulate higher levels of AGEs in diabetic patients. Since capsule AGEs are likely to play a role in PCO, it was of significance to determine the effect of diabetes on capsule AGE levels.
Several studies have shown a similar or lower incidence of PCO in diabetic patients than in nondiabetic patients (Knorz et al., 1991; Praveen et al., 2014; Zaczek and Zetterstrom, 1999), but some studies have shown a higher incidence rate in diabetic patients (Hayashi et al., 2002; Ionides et al., 1994; Wu et al., 2018). Thus, the effect of diabetes on PCO is not completely resolved. In this study, we sought to determine whether capsule AGE levels are elevated in two separate groups, diabetic patients and diabetic patients with established retinopathy, relative to a patient group of nondiabetic patients undergoing cataract surgery.
2. Methods
All reagents used were of at least analytical grade. Liquid chromatography-mass spectrometry (LC-MS/MS) solvents were of mass spectrometry grade.
2.1. Sample collection and storage
Capsulorhexis specimens were obtained at the time of cataract surgery at the University of Colorado Health Sue-Anschutz Rodgers Eye Center, Aurora, CO. Upon collection, samples were stored at −80°C until processing. On the day of cataract surgery, blood samples were collected from consented patients for HbA1C measurement. The study was approved by the Institutional Review Board of the University of Colorado School of Medicine and was performed in compliance with the Helsinki Declaration (ClinicalTrials.gov Identifier: NCT02662010).
2.2. Sample processing
Capsule specimens were blinded and assigned a numeric identifier. They were subjected to enzymatic hydrolysis after being denuded of any residual lens fiber mass by rocking in 0.85% NaCl for 3 days at room temperature. Specimens were suspended in 150 μL PBS (PBS-only samples were used as blanks). Samples were incubated while mixing at 300 rpm at 37°C. Enzymes were added in the following order: at 0 and 24 h, 10 μL 0.7 mg/mL collagenase (Worthington, Lakewood, NJ, Cat# 5275); at 48 and 72 h, 10 μL 3 mg/ml protease Type XIV (Sigma-Aldrich, St. Louis, MO, Cat# P-5147); at 96 h, 4 μL leucine aminopeptidase suspension (Sigma-Aldrich, Cat# L5006); and at 120 h, 12 μL 0.5 mg/ml carboxypeptidase Y (Sigma-Aldrich, Cat# C3888). In all incubations, flushing with argon was conducted after the addition of each enzyme, and the entire digestion procedure was carried out in the presence of a few crystals of thymol (Sigma-Aldrich, Cat# T-0501). The digested material was passed through a 3-kDa molecular weight cutoff centrifugal filter (VWR International, Tualatin, OR). The filtrate was analyzed by liquid LC-MS/MS for acid-labile AGEs. Fifty microliters of the digest from each sample was acid hydrolyzed with 6 N HCl by incubating at 110°C for 24 h under argon in a sealed glass ampule. The acid-hydrolyzed samples were dried in a speed vac concentrator. To remove residual HCl, the dried pellet was suspended in 500 μL water and dried again. The final pellet was suspended in 100 μL water, sonicated and then centrifuged to sediment insoluble particles. The supernatant was analyzed with LC-MS/MS for acid stable AGEs.
2.3. LC-MS/MS
Samples were separated on a Waters Acquity UPLC system (Milford, MA) using a Waters Acquity UPLC HSS T3 column, 100 X 2.1 mm, 1.8 μm with appropriate VanGuard column at a temperature of 40°C, with a solvent flow of 0.6 ml/min. The solvents used were A: water and B: acetonitrile/water (80/20, v/v), each containing 0.12% heptafluorobutyric acid. The percentages of solvent A were: 0–2.2 min, 98%; 3.3 min, 92%; 7.6 min, 66%; 7.8 min, 0%; 9.5 min, 0% and 12.2 min, 98%. For mass spectrometric detection on a Sciex 4500 QTrap, scheduled multiple-reaction monitoring (sMRM) mode was used, utilizing collision-induced dissociation of the protonated molecules with compound-specific orifice potentials and fragment-specific collision energies. The ion source was run under the following conditions: temperature, 650°C; ion spray voltage, 2500 V; curtain gas, 35 ml/min; nebulizer gas, 65 mL/min; heating gas, 70 mL/min. The declustering potential, collision energy and cell exit potential for each of the monitored precursor-to-product ion transitions are shown in Table 1. After confirmation of the peak position, peaks were integrated and blank subtracted. Analytes were quantified using the standard addition method as previously described (Raghavan et al., 2016; Smuda et al., 2015) and corrected for enzyme hydrolysis efficiency. The enzyme efficiency percentage in each sample was calculated based on the CML and CEL contents in the enzyme digest/CML and CEL contents in acid hydrolysate*100. The data were normalized to 1 μmol hydroxy-proline (OH-Pro) in the samples.
Table 1.
Scheduled multiple reaction monitoring parameters
| precursorion | production 1 | production 2 | production 3 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AGE/amino acid internal standard | RT | m/z | DP | m/z | CE | CXP | m/z | CE | CXP | m/z | CE | CXP |
| OH-Pro | 0.84 | 132.0 | 38 | 86.0 | 19.1 | 6.9.0 | 68.0 | 26.1 | 5.1 | 57.9 | 36.7 | 9.1 |
| Glucosepane | 6.67* 6.84 |
429.3 | 20 | 384.5 | 38.0 | 19.0 | 269.2 | 55.0 | 20.0 | 339.2 | 55.0 | 20.0 |
| GOLD | 6.68 | 327.2 | 60 | 84.1 | 55.0 | 13.0 | 282.3 | 29.0 | 11.0 | 198.1 | 25.0 | 19.0 |
| MODIC | 7.10 | 357.3 | 25 | 312.2 | 31.0 | 14.0 | 267.3 | 45.0 | 15.0 | 197.4 | 45.0 | 14.0 |
| GALA | 2.36 | 205.2 | 40 | 142.1 | 19.0 | 10.0 | 84.1 | 25.0 | 13.0 | 159.1 | 15.0 | 13.0 |
| MOLD | 7.10 | 341.3 | 45 | 296.3 | 33.0 | 18.0 | 84.2 | 52.0 | 14.0 | 212.3 | 29.0 | 21.0 |
| MG-H3 | 6.00 | 229.2 | 45 | 114.1 | 22.5 | 9.0 | 70.1 | 45.0 | 14.0 | 116.1 | 21.0 | 9.0 |
| MG-H1 | 6.00 | 229.2 | 55 | 70.1 | 43.0 | 12.0 | 116.1 | 20.5 | 9.0 | 114.1 | 22.5 | 9.0 |
| CMA | 4.89 | 233.1 | 45 | 70.1 | 45.0 | 15.0 | 116.1 | 23.0 | 10.0 | 118.2 | 22.0 | 5.5 |
| Pyrraline | 6.81 | 255.2 | 38 | 175.2 | 17.0 | 14.0 | 237.2 | 12.0 | 11.0 | 148.3 | 25.0 | 10.0 |
| DT-Ha | 5.47 | 259.1 | 45 | 144.1 | 22.0 | 9.0 | 70.0 | 45.0 | 10.0 | 116.1 | 25.0 | 17.0 |
| CEA | 5.88 | 247.1 | 51 | 70.2 | 48.0 | 12.0 | 116.2 | 25.0 | 10.0 | 132.1 | 24.0 | 10.0 |
| CML | 2.36 | 205.1 | 40 | 130.2 | 17.0 | 11.0 | 84.1 | 25.0 | 13.0 | 56.1 | 50.0 | 10.0 |
| CEL | 4.24 | 219.1 | 54 | 84.1 | 33.0 | 11.0 | 130.1 | 18.0 | 12.0 | 56.1 | 59.0 | 8.0 |
RT: retention time (min), m/z: mass-to-charge ratio (atomic mass unit), DP: declustering potential (V), CE: collision energy (eV), CXP: cell exit potential (V)
The two isomer peaks with different retention times were integrated together and the sum was used.
2.4. Statistical analyses
Levels for each of the analytes were compared across groups using linear regression, and linear contrasts were used to test pairwise comparisons. P-values for comparisons across the three groups were adjusted for multiple comparisons using the false discovery rate as described by Benjamini and Hochberg (Benjamini et al., 2001). These comparisons were also made after adjusting for age and sex. Sensitivity analyses using log-transformed values were performed. All analyses were performed using SAS version 9.4 (The SAS Institute, Cary, NC).
3. Results
The average ages of the 120 included patients among the three groups are shown in Table 2. The average age of diabetic patients with DR was 4 years lower than both diabetic and nondiabetic patients. The HbA1C levels were significantly higher in diabetic patients than in nondiabetic patients. We measured 13 AGEs in this study, among which 2 were derived from glycation initiated by glucose, 4 were derived from glyoxal, 6 were derived from methylglyoxal and one was derived from ascorbate (Fig. 1). The sMRM chromatograms for AGEs are shown in Supplementary Fig. 1. The levels of most AGEs were < 50 pmoles/μmol OH-Pro. Sensitivity analyses evaluating the comparison across groups after adjusting for age and gender or after log transformation yielded similar results; therefore, the unadjusted analyses using the original scale for each analyte are presented. Among the 13 AGEs measured, the levels of the majority of AGEs, 12 out of the 13, were similar in diabetic patients and diabetic patients with DR when compared to those in nondiabetic patients after correcting for multiple comparisons (Figs. 2 to 5). One exception was glucosepane, with a multiple testing corrected p-value < 0.01. The mean ± standard error levels were 8.32 ± 0.76 pmol/μmol OH-Pro in diabetic patients and 6.85 ± 0.89 pmol/μmol OH-Pro in diabetic patients + DR, which were significantly higher (p<0.01 and p=0.01, respectively) than those in nondiabetic patients (4.01 ± 0.71 pmol/μmol OH-Pro) (Fig. 2). However, there was no difference between diabetic patients and diabetic patients + DR (p=0.21). The other glucose-derived AGE, pyrraline, showed no differences between groups.
Table 2.
Patient demographics
| Nondiabetic (ND) (n = 48) | Diabetic w/o retinopathy (DB) (n = 42) | Diabetic w/retinopathy (DB+DR) (n = 30) | p-value* | |
|---|---|---|---|---|
| Female | 30 (63%) | 25 (60%) | 9 (30%) | 0.01 |
| Age (years), mean ± SD | 68 ± 6 | 68 ± 7 | 64 ± 8 | 0.02 |
| HbA1C, mean ± SD | 5.5 ± 0.3 | 7.6 ± 1.7 | 8.1 ± 1.7 | <0.01 |
SD = standard deviation.
p-values were calculated with either a chi-squared test or an analysis of variance, as appropriate.
Fig. 1. Structure of AGEs measured in this study.
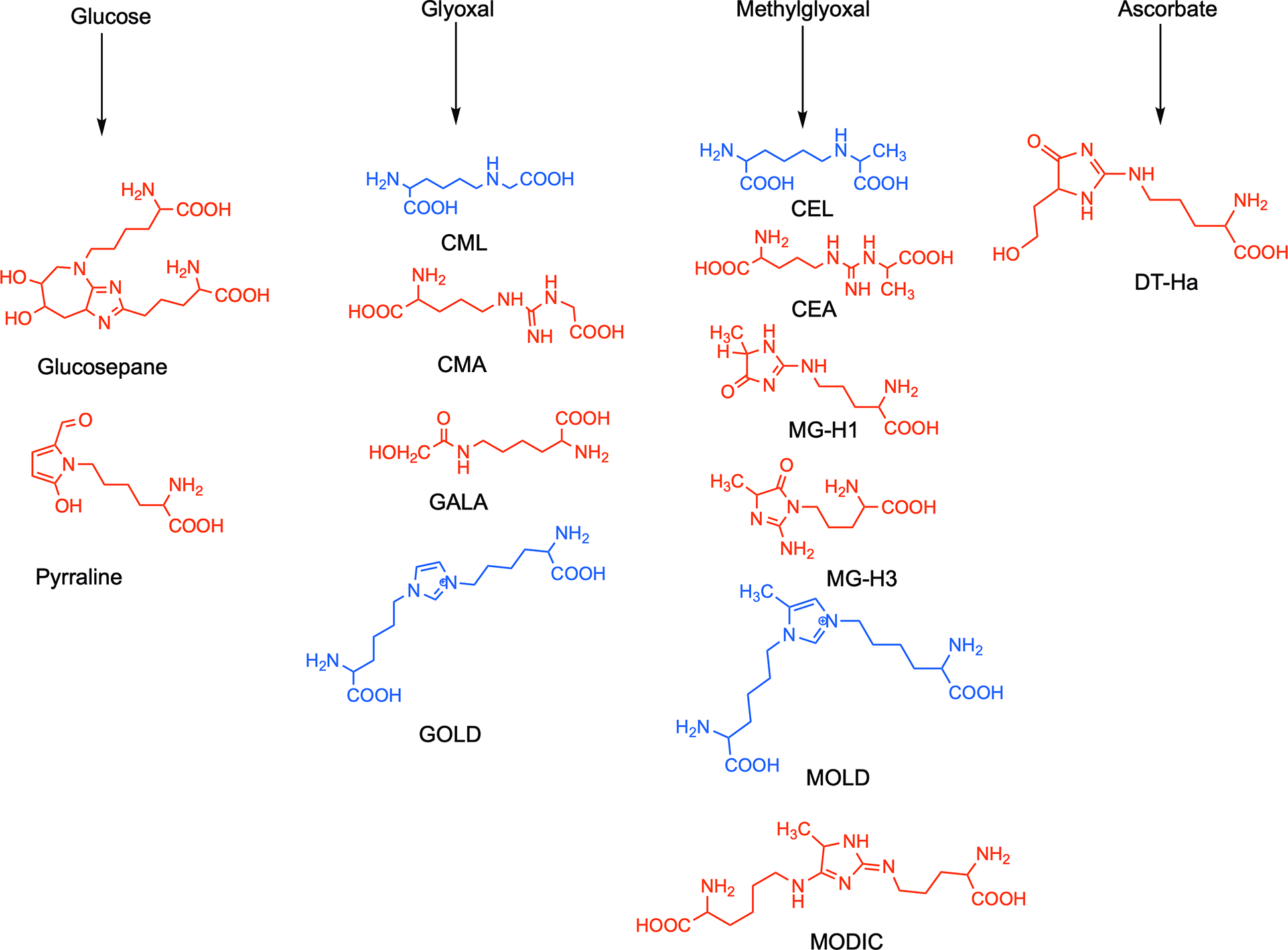
They are categorized into four classes based on their primary precursor carbonyl compounds. The structures shown in red are measured in enzyme-digested material, and those in blue are measured in acid-hydrolyzed material.
Fig. 2. The levels of glucose-derived AGEs.
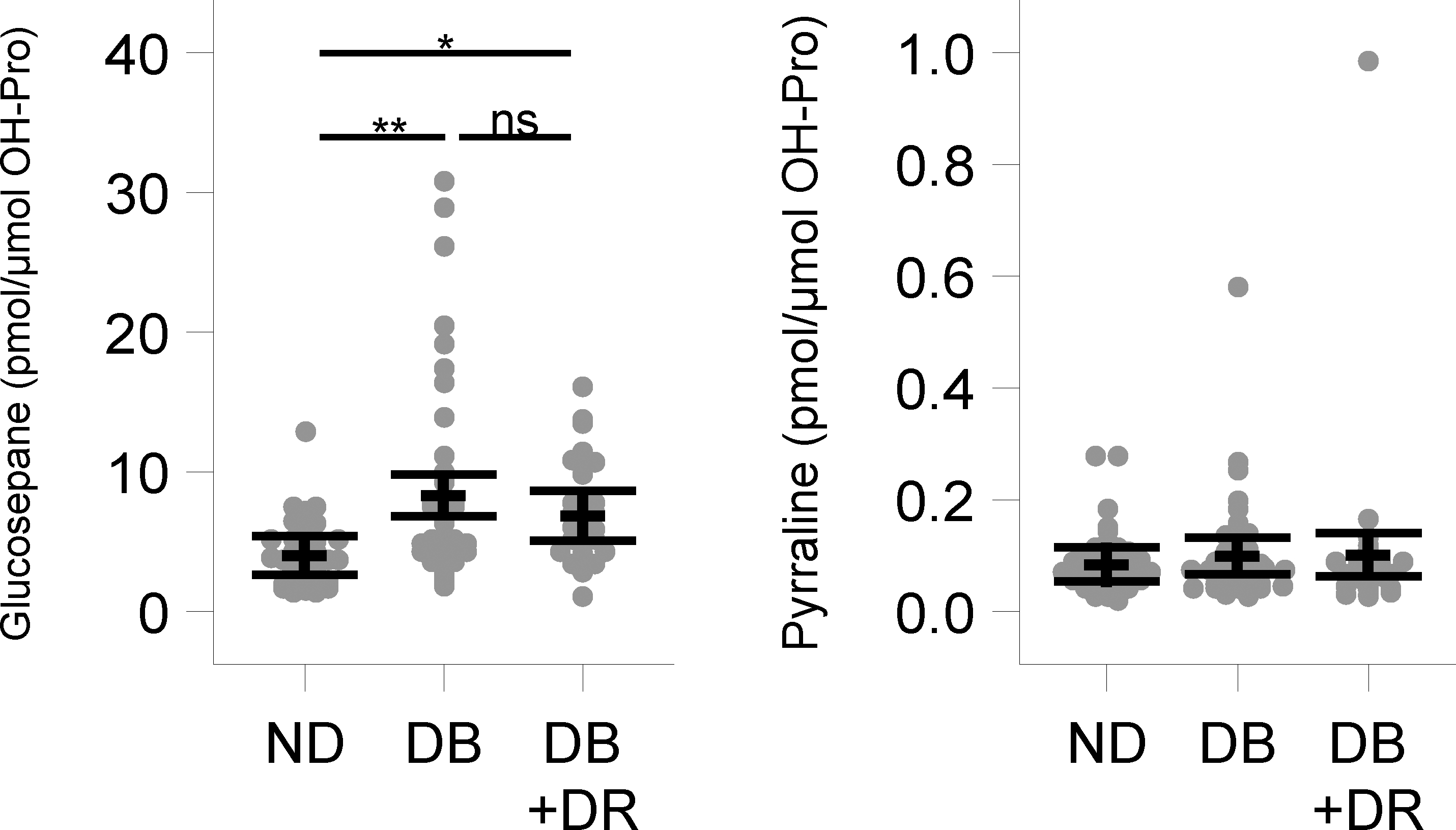
In nondiabetic (ND), diabetic (DB) and diabetic with retinopathy (DB + DR) capsulorhexis specimens. Points represent observed values, and the mean and 95% confidence intervals are displayed with dashed lines and whiskers, respectively. *p=0.01, **p<0.01, ns=nonsignificant
Fig. 5. The levels of an ascorbate-derived AGE.

In nondiabetic (ND), diabetic (DB) and diabetic with retinopathy (DB + DR) capsulorhexis specimens. Points represent observed values, and the mean and 95% confidence intervals are displayed with dashed lines and whiskers, respectively. The multiple testing corrected p-value comparing all three groups was >0.5 for all analytes, so the pairwise comparisons are not presented.
Among the glyoxal-derived AGEs, the levels of CMA were the highest for all patient groups: 208.1 ± 20.9 pmol/μmol OH-Pro, 248.8 ± 22.4 pmol/μmol OH-Pro and 211.9 ± 26.4 pmol/μmol OH-Pro and not significantly different among the nondiabetic, diabetic and diabetic patients + DR, respectively (Fig. 3). The levels of other glyoxal-derived AGEs, GALA and CML did not exhibit any significant differences across groups. However, the observation of CMA levels that were several-fold higher than the levels of CML was unexpected. Further studies are required to determine the reasons underlying this observation. We note here that ~10–15% of CML measured could have formed as a result of acid hydrolysis of proteins, which could have caused an increase in the measured amounts in samples.
Fig. 3. The levels of glyoxal-derived AGEs.
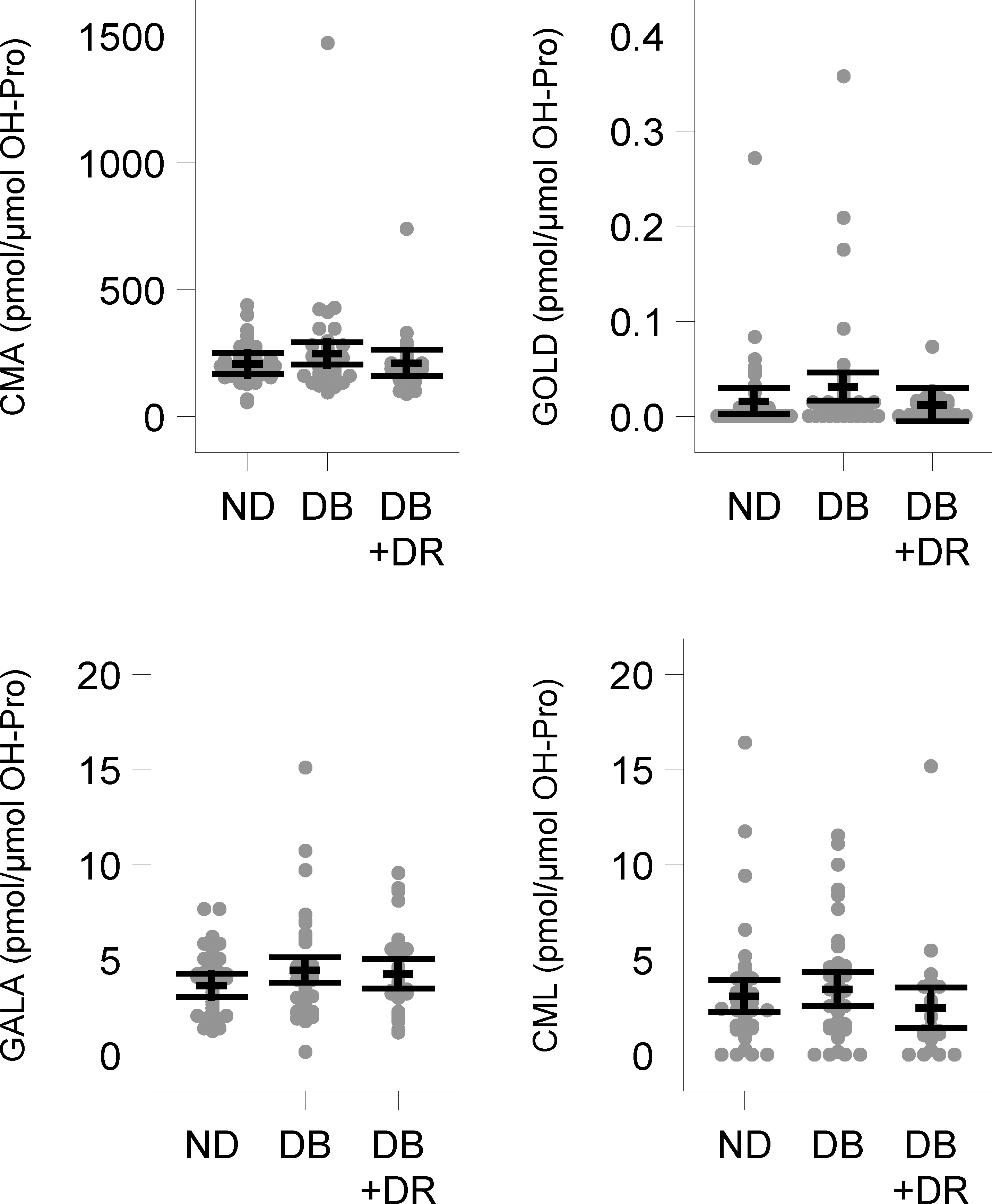
In nondiabetic (ND), diabetic (DB) and diabetic with retinopathy (DB + DR) capsulorhexis specimens. Points represent observed values, and the mean and 95% confidence intervals are displayed with dashed lines and whiskers, respectively. The multiple testing corrected p-value comparing all three groups was >0.5 for all analytes, so the pairwise comparisons are not presented.
Likewise, methylglyoxal-derived AGEs, MODIC, MG-H1, MG-H3, CEA and CEL showed no differences among the three groups (Fig. 4). Finally, the levels of DT-Ha, a 3-deoxythresone (ascorbate)-derived AGE (Rakete and Nagaraj, 2016), were not different across the three groups (Fig. 5).
Fig. 4. The levels of methylglyoxal-derived AGEs.
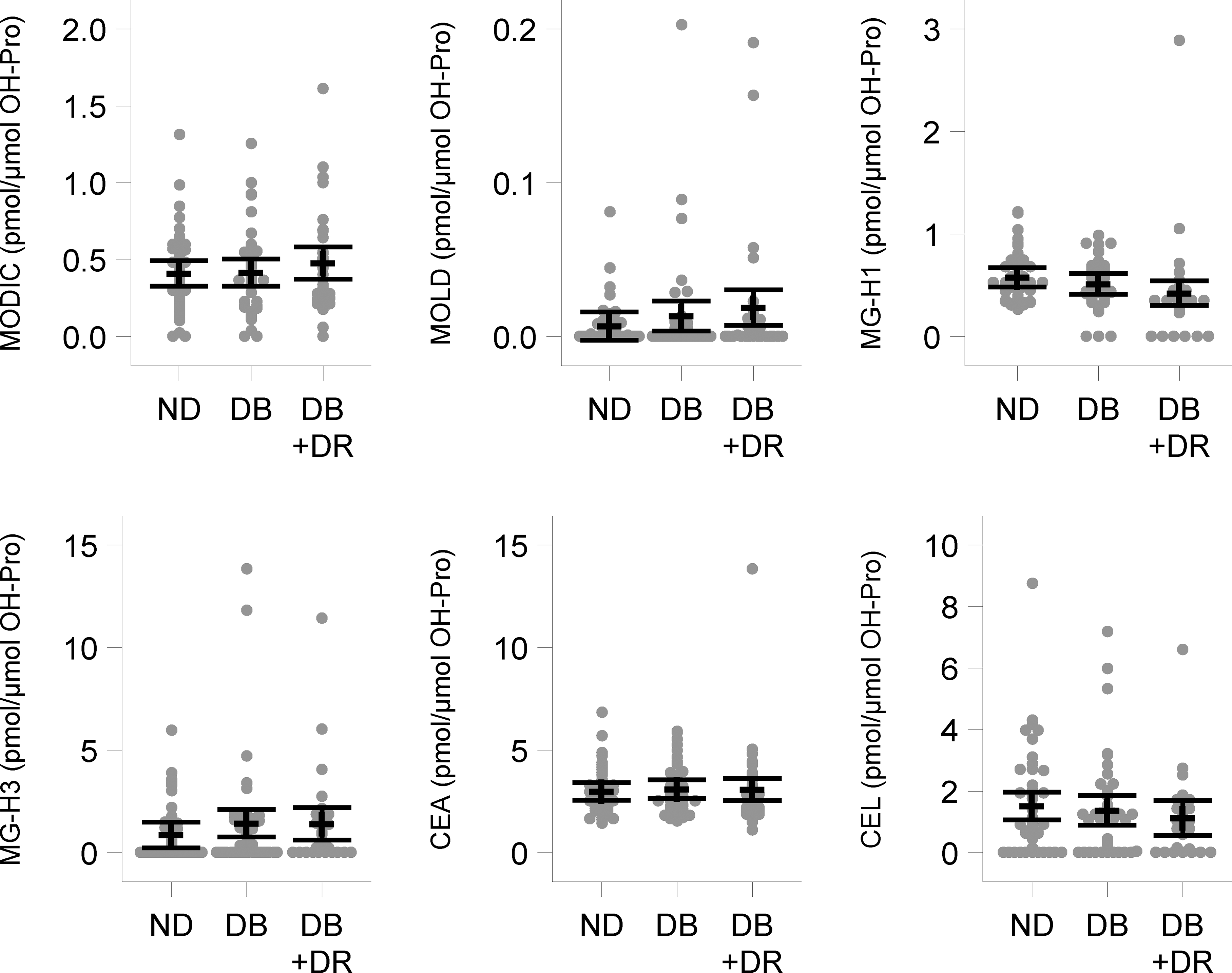
In nondiabetic (ND), diabetic (DB) and diabetic with retinopathy (DB + DR) capsulorhexis specimens. Points represent observed values, and the mean and 95% confidence intervals are displayed with dashed lines and whiskers, respectively. The multiple testing corrected p-value comparing all three groups was >0.5 for all analytes, so the pairwise comparisons are not presented.
4. Discussion
The objectives of this study were 1) to determine whether diabetic lens capsules contained higher levels of AGEs than nondiabetic lens capsules and 2) to determine whether AGE levels were higher in diabetic patients with established retinopathy than in diabetic patients without retinopathy. Based on the findings in other basement membranes, for example, in the tubular basement membrane of kidneys (Bendayan, 1998; Copeland et al., 1987) and Bruch’s membrane (Handa et al., 1999), we anticipated diabetic capsules, being basement membranes, to have higher levels of AGEs. However, 12 of the 13 AGEs measured were similar between diabetic patients and nondiabetic patients. This is interesting and, at the same time, unexpected. The lack of an increase in capsule AGEs in diabetes suggests the possibility that most glycation precursor levels are not elevated in the milieu of the lens capsule (aqueous humor) in diabetes. This possibility is supported by the observation that the AGE levels in aqueous humor in diabetic patients are similar to those in nondiabetic patients (Franke et al., 2003). Despite a significant elevation of AGEs in the aqueous humor of diabetic + DR patients compared to diabetic patients without DR (Endo et al., 2001), we found no difference in the majority of AGEs between the two groups. The absence of such an increase suggests that AGE-bearing proteins in aqueous humor are probably derived from plasma, not generated in situ in aqueous humor. Among all AGEs, only glucosepane was elevated in diabetic capsules relative to nondiabetic capsules. This AGE is derived solely from glucose (Biemel et al., 2002). Elevated glucose levels in the aqueous humor (Gomel et al., 2021) and/or in lens (Bron et al., 1993) could have led to this increase, but this needs to be investigated in a future study. An interesting observation in this study is ~60–80 times lower levels of CML than CMA. This was unexpected, as both AGEs are likely derived from glyoxal. Further work is needed to understand their mechanism of formation in lens capsules.
The fact that the AGE levels are largely similar between diabetic and nondiabetic patients supports our hypothesis that capsule AGEs promote the TGFβ2-mediated EMT of lens epithelial cells during PCO and that the incidence of PCO should be similar between the two groups. This is in fact the case in several studies, but as mentioned above, the PCO incidence was higher in diabetic patients in some studies. In this context, it should be noted that the occurrence of PCO also depends on the nature of the implanted IOL; round-edged IOLs and acrylic hydrophilic IOLs have higher rates of PCO than square-edged IOLs and acrylic hydrophobic IOLs (Duman et al., 2015; Hazra et al., 2012). Thus, the discrepancy between studies could be attributable to differences in the implanted IOLs or due to other potential confounding factors, such as patient age.
In conclusion, our study revealed that AGE levels are similar between diabetic and nondiabetic human lens capsules. This provides a biochemical basis for the lack of difference in the PCO incidence in the two groups observed in several studies.
Supplementary Material
Fig. S1. Representative LC-MS/MS sMRM chromatograms for AGEs
Highlights.
Advanced glycation endproducts (AGEs) in human lens capsules were measured by LC-MS/MS
The levels of majority of AGEs were similar between diabetic and nondiabetic lens capsules
Glucosepane levels were significantly higher in diabetic than in nondiabetic lens capsules
Acknowledgements
This work was supported by the National Institutes of Health Grants EY028836 and EY023286 and an unrestricted grant from Research to Prevent Blindness to the Department of Ophthalmology, University of Colorado. We would like to thank Levi Bonnell, Jennifer Cathcart, Tara Churney and Ruth T. Eshete for their help with obtaining patient consent and specimen collection.
Footnotes
Commercial relationship
None.
Declaration of competing interest
The authors declare that they have no conflicts of interest
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Data availability statement
All data generated or analyzed during this study are included in this article.
References
- Ahmed N, Thornalley PJ, Dawczynski J, Franke S, Strobel J, Stein G, Haik GM, 2003. Methylglyoxal-derived hydroimidazolone advanced glycation end-products of human lens proteins. Invest Ophthalmol Vis Sci 44, 5287–5292. [DOI] [PubMed] [Google Scholar]
- Barraquer RI, Michael R, Abreu R, Lamarca J, Tresserra F, 2006. Human lens capsule thickness as a function of age and location along the sagittal lens perimeter. Invest Ophthalmol Vis Sci 47, 2053–2060. [DOI] [PubMed] [Google Scholar]
- Beisswenger PJ, Moore LL, Curphey TJ, 1993. Relationship between glycemic control and collagen-linked advanced glycosylation end products in type I diabetes. Diabetes Care 16, 689–694. [DOI] [PubMed] [Google Scholar]
- Bendayan M, 1998. Immunocytochemical detection of advanced glycated end products in rat renal tissue as a function of age and diabetes. Kidney Int 54, 438–447. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Drai D, Elmer G, Kafkafi N, Golani I, 2001. Controlling the false discovery rate in behavior genetics research. Behav Brain Res 125, 279–284. [DOI] [PubMed] [Google Scholar]
- Biemel KM, Friedl DA, Lederer MO, 2002. Identification and quantification of major maillard cross-links in human serum albumin and lens protein. Evidence for glucosepane as the dominant compound. J Biol Chem 277, 24907–24915. [DOI] [PubMed] [Google Scholar]
- Boswell BA, Korol A, West-Mays JA, Musil LS, 2017. Dual function of TGFbeta in lens epithelial cell fate: implications for secondary cataract. Mol Biol Cell 28, 907–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bron AJ, Sparrow J, Brown NA, Harding JJ, Blakytny R, 1993. The lens in diabetes. Eye (Lond) 7 ( Pt 2), 260–275. [DOI] [PubMed] [Google Scholar]
- Copeland KR, Yatscoff RW, Thliveris JA, Mehta A, Penner B, 1987. Non-enzymatic glycation and altered renal structure and function in the diabetic rat. Kidney Int 32, 664–670. [DOI] [PubMed] [Google Scholar]
- Danysh BP, Duncan MK, 2009. The lens capsule. Exp Eye Res 88, 151–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danysh BP, Patel TP, Czymmek KJ, Edwards DA, Wang L, Pande J, Duncan MK, 2010. Characterizing molecular diffusion in the lens capsule. Matrix Biol 29, 228–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duman R, Karel F, Ozyol P, Ates C, 2015. Effect of four different intraocular lenses on posterior capsule opacification. Int J Ophthalmol 8, 118–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endo M, Yanagisawa K, Tsuchida K, Okamoto T, Matsushita T, Higuchi M, Matsuda A, Takeuchi M, Makita Z, Koike T, 2001. Increased levels of vascular endothelial growth factor and advanced glycation end products in aqueous humor of patients with diabetic retinopathy. Horm Metab Res 33, 317–322. [DOI] [PubMed] [Google Scholar]
- Franke S, Stein F, Dawczynski J, Blum M, Kubetschka U, Stein G, Strobel J, 2003. Advanced glycation end-products in anterior chamber aqueous of cataractous patients. J Cataract Refract Surg 29, 329–335. [DOI] [PubMed] [Google Scholar]
- Gomel N, Barequet IS, Lipsky L, Bourla N, Einan-Lifshitz A, 2021. The effect of the glycemic control on the aqueous humor glucose levels in diabetic patients undergoing elective cataract surgery. Eur J Ophthalmol 31, 415–421. [DOI] [PubMed] [Google Scholar]
- Handa JT, Verzijl N, Matsunaga H, Aotaki-Keen A, Lutty GA, te Koppele JM, Miyata T, Hjelmeland LM, 1999. Increase in the advanced glycation end product pentosidine in Bruch’s membrane with age. Invest Ophthalmol Vis Sci 40, 775–779. [PubMed] [Google Scholar]
- Hashim Z, Zarina S, 2011. Advanced glycation end products in diabetic and non-diabetic human subjects suffering from cataract. Age (Dordr) 33, 377–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi K, Hayashi H, Nakao F, Hayashi F, 2002. Posterior capsule opacification after cataract surgery in patients with diabetes mellitus. Am J Ophthalmol 134, 10–16. [DOI] [PubMed] [Google Scholar]
- Hazra S, Palui H, Vemuganti GK, 2012. Comparison of design of intraocular lens versus the material for PCO prevention. Int J Ophthalmol 5, 59–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ionides A, Dowler JG, Hykin PG, Rosen PH, Hamilton AM, 1994. Posterior capsule opacification following diabetic extracapsular cataract extraction. Eye (Lond) 8 ( Pt 5), 535–537. [DOI] [PubMed] [Google Scholar]
- Jiang J, Shihan MH, Wang Y, Duncan MK, 2018. Lens Epithelial Cells Initiate an Inflammatory Response Following Cataract Surgery. Invest Ophthalmol Vis Sci 59, 4986–4997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knorz MC, Soltau JB, Seiberth V, Lorger C, 1991. Incidence of posterior capsule opacification after extracapsular cataract extraction in diabetic patients. Metab Pediatr Syst Ophthalmol (1985) 14, 57–58. [PubMed] [Google Scholar]
- Logan CM, Bowen CJ, Menko AS, 2017. Induction of Immune Surveillance of the Dysmorphogenic Lens. Sci Rep 7, 16235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons TJ, Bailie KE, Dyer DG, Dunn JA, Baynes JW, 1991. Decrease in skin collagen glycation with improved glycemic control in patients with insulin-dependent diabetes mellitus. J Clin Invest 87, 1910–1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamuya FA, Wang Y, Roop VH, Scheiblin DA, Zajac JC, Duncan MK, 2014. The roles of alphaV integrins in lens EMT and posterior capsular opacification. J Cell Mol Med 18, 656–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meacock WR, Spalton DJ, Stanford MR, 2000. Role of cytokines in the pathogenesis of posterior capsule opacification. Br J Ophthalmol 84, 332–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagaraj RH, Linetsky M, Stitt AW, 2012. The pathogenic role of Maillard reaction in the aging eye. Amino Acids 42, 1205–1220. [DOI] [PubMed] [Google Scholar]
- Nam MH, Nagaraj RH, 2018. Matrix-bound AGEs enhance TGFbeta2-mediated mesenchymal transition of lens epithelial cells via the noncanonical pathway: implications for secondary cataract formation. Biochem J 475, 1427–1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nam MH, Pantcheva MB, Rankenberg J, Nagaraj RH, 2021. Transforming growth factor-β2-mediated mesenchymal transition in lens epithelial cells is repressed in the absence of RAGE. Biochem J Accepted for publication. [DOI] [PubMed] [Google Scholar]
- Praveen MR, Vasavada AR, Shah GD, Shah AR, Khamar BM, Dave KH, 2014. A prospective evaluation of posterior capsule opacification in eyes with diabetes mellitus: a case-control study. Eye (Lond) 28, 720–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghavan CT, Nagaraj RH, 2016. AGE-RAGE interaction in the TGFbeta2-mediated epithelial to mesenchymal transition of human lens epithelial cells. Glycoconj J 33, 631–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghavan CT, Smuda M, Smith AJ, Howell S, Smith DG, Singh A, Gupta P, Glomb MA, Wormstone IM, Nagaraj RH, 2016. AGEs in human lens capsule promote the TGFbeta2-mediated EMT of lens epithelial cells: implications for age-associated fibrosis. Aging Cell 15, 465–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakete S, Nagaraj RH, 2016. Identification of Kynoxazine, a Novel Fluorescent Product of the Reaction between 3-Hydroxykynurenine and Erythrulose in the Human Lens, and Its Role in Protein Modification. J Biol Chem 291, 9596–9609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smuda M, Henning C, Raghavan CT, Johar K, Vasavada AR, Nagaraj RH, Glomb MA, 2015. Comprehensive analysis of maillard protein modifications in human lenses: effect of age and cataract. Biochemistry 54, 2500–2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tholozan FM, Gribbon C, Li Z, Goldberg MW, Prescott AR, McKie N, Quinlan RA, 2007. FGF-2 release from the lens capsule by MMP-2 maintains lens epithelial cell viability. Mol Biol Cell 18, 4222–4231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ursell PG, Dhariwal M, O’Boyle D, Khan J, Venerus A, 2020. 5 year incidence of YAG capsulotomy and PCO after cataract surgery with single-piece monofocal intraocular lenses: a real-world evidence study of 20,763 eyes. Eye (Lond) 34, 960–968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanSlyke JK, Boswell BA, Musil LS, 2018. Fibronectin regulates growth factor signaling and cell differentiation in primary lens cells. J Cell Sci 131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlassara H, Uribarri J, 2014. Advanced glycation end products (AGE) and diabetes: cause, effect, or both? Curr Diab Rep 14, 453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wormstone IM, Eldred JA, 2016. Experimental models for posterior capsule opacification research. Exp Eye Res 142, 2–12. [DOI] [PubMed] [Google Scholar]
- Wormstone IM, Wormstone YM, Smith AJO, Eldred JA, 2020. Posterior capsule opacification: What’s in the bag? Prog Retin Eye Res, 100905. [DOI] [PubMed] [Google Scholar]
- Wu S, Tong N, Pan L, Jiang X, Li Y, Guo M, Li H, 2018. Retrospective Analyses of Potential Risk Factors for Posterior Capsule Opacification after Cataract Surgery. J Ophthalmol 2018, 9089285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaczek A, Zetterstrom C, 1999. Posterior capsule opacification after phacoemulsification in patients with diabetes mellitus. J Cataract Refract Surg 25, 233–237. [DOI] [PubMed] [Google Scholar]
- Zarina S, Zhao HR, Abraham EC, 2000. Advanced glycation end products in human senile and diabetic cataractous lenses. Mol Cell Biochem 210, 29–34. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Representative LC-MS/MS sMRM chromatograms for AGEs
Data Availability Statement
All data generated or analyzed during this study are included in this article.


