Abstract
The goal of this review is to provide a novel perspective on the nature and importance of the relationship between the circadian and pain systems. We provide: 1) An overview of the circadian and pain systems, 2) a review of direct and correlative evidence that demonstrates diurnal and circadian rhythms within the pain system; 3) a perspective highlighting the need to consider the role of a proposed feedback loop of circadian rhythm disruption and maladaptive pain; 4) a perspective on the nature of the relationship between circadian rhythms and pain. In summary, we propose that there is no single locus responsible for producing the circadian rhythms of the pain system. Instead, circadian rhythms of pain are a complex result of the distributed rhythms present throughout the pain system, especially those of the descending pain modulatory system, and the rhythms of the systems with which it interacts, including the opioid, endocrine, and immune systems.
Keywords: Circadian Rhythms, Pain, Pain System, Chronic Pain, Circadian Rhythms of Pain, Circadian Rhythms and Pain, Circadian Rhythm Disruption
1. Introduction
The vast majority of organisms on earth have evolved with internal manifestations of the external daily light-dark cycles. These circadian (circa = about; dies = day) rhythms are self-sustained, endogenous oscillations generated by circadian clocks that persist with a period of around 24-hours under constant conditions (Golombek and Rosenstein, 2010). Exposure to the daily external light-dark cycle synchronizes (entrains) these rhythms to the 24-hour cycle of the external world via signaling to a so-called ‘master clock’. Among mammals, this master clock is located in the suprachiasmatic nuclei (SCN) of the hypothalamus (Stephan and Zucker, 1972). Together, the SCN and the circadian clocks throughout the body comprise what is known as the circadian system.
Circadian clocks are self-sustaining and self-regulated through what is known as the transcription-translation feedback loop (TTFL). The TTFL comprises a family of core canonical ‘clock’ genes, including Clock, Bmal1, Per, and Cry, among several others. In brief, circadian rhythms are generated within the nucleus of cells by the autoregulatory TTFL of the core circadian genes. At the beginning of the circadian day, BMAL1 and CLOCK interact in the cytosol to form a heterodimer that then is translocated into the nucleus. This heterodimer binds to E-box promoter sequences of the Cry and Per genes to activate their transcription. The gene products of Per and Cry accumulate in the cytoplasm, dimerize, and then form a complex that is translocated back into the nucleus to repress their own transcription by interacting with CLOCK and BMAL1. Notably, a complete cycle of this feedback loop takes ~24 hours to occur. There are additional feedback loops interlocked with the core CLOCK-BMAL1/PER-CRY loop. Further intricate details of the TTFL are beyond the scope of this review, but they have been extensively characterized elsewhere (Partch et al., 2014). The daily oscillation in expression of these proteins is responsible for the generation of circadian rhythms at molecular, cellular, physiological, and behavioral levels.
In mammals, the TTFL of the circadian clock is present in nearly every cell (Yoo et al., 2004; Ruben et al., 2018; Nagoshi et al., 2004). Individual circadian clocks within the body can run with different period lengths, so without a coordinating signal, the circadian rhythms of various tissues and cells become misaligned. Alignment of internal rhythms generally occurs via signaling from the hypothalamic SCN.
The SCN acts as a conductor to align internal circadian rhythms with the external daily light-dark cycle by interpreting photic signaling from the retina and communicating that information with the rest of the brain and body. In mammals, photosensitive retinal cells, specifically, intrinsically photosensitive retinal ganglion cells containing the photopigment melanopsin, communicate time-of-day cues to the SCN via photic signaling information. Photic information is relayed along the retinohypothalamic tract to the SCN to inform time-of-day by modifying cellular activity and the expression of specific clock proteins within neurons and glia. For example, light pulses alter expression of Per1/Per2 within the rat SCN, but only during the night (Miyake et al., 2000). After receiving and interpreting retinal photic information, the SCN aligns internal rhythms with the external solar day via neuronal projections and humoral signaling (Pevet and Challet, 2011).
Many behavioral, physiological, and biochemical processes display circadian rhythms. Sleep-wake cycles, locomotor activity rhythms, body temperature fluctuations, as well as immune and endocrine function are examples of rhythmic processes regulated by the circadian system (Walker et al., 2020; Scheiermann et al., 2013; Gamble et al., 2014). Most importantly for the context of this review, pain is also regulated by the circadian system, although our current understanding of the interaction between the pain and circadian systems remains unspecified. Below, we have outlined our current understanding of the relationship between these two systems in order to inform potential hypotheses surrounding the nature of their function. We also highlight the importance of proper circadian rhythm health and the consequences of circadian rhythm disruption on pain.
2. The Pain System - An Overview
The pain system encodes and relays noxious sensory information from the periphery into the central nervous system to produce protective behavioral outcomes. Noxious information is relayed by peripheral nociceptors to the spinal cord, brainstem, midbrain, and forebrain where withdrawal or wound-protection behaviors are elicited to prevent or minimize injury (Baliki and Apkarian, 2015). Nociceptive information delivered to brain is processed into the sensory-discriminative and affective aspects of pain by a network of supraspinal structures that comprise what is commonly referred to as the pain matrix (Kulkarni et al., 2005; Garcia-Larrea and Peyron, 2013; Iannetti and Mouraux, 2010; Legrain et al., 2011; Auvray et al., 2010).
It is likely that none of the nuclei or structures considered to be part of the pain matrix exclusively process pain (Baliki and Apkarian, 2015). Instead, this matrix can be described as a ‘distributed nociceptive system’ because sensory-discriminative and affective components of pain can still be processed in the absence of one or more components of the system (Coghill, 2020). For the purpose of this review, we operationally classify supraspinal regions commonly implicated in the central processing of pain as part of the pain system while understanding that these regions do not exclusively process pain and may be equally involved in the processing of innocuous and noxious stimuli alike (Iannetti and Mouraux, 2010).
2.1. Functional Anatomy of the Pain System
Noxious stimuli are encoded and relayed central by peripheral pseudounipolar nociceptor neurons of two primary classes: A∂ fibers and C fibers. A∂ neurons are medium-sized myelinated fibers that rapidly transmit localized pain information, whereas C neurons are small unmyelinated fibers that transmit slow, low-resolution pain information. Upon exposure to a noxious stimulus, thermosensitive ion channels, mechanotransduction channels, or chemoreceptors on peripheral nociceptor terminals will transduce the noxious stimuli into depolarization events that activate local voltage-gated ion channels (Basbaum et al., 2009). For example, exposure to noxious heat above 43° C activates TRPV1 and other heat-sensitive ion channels, producing inward depolarizing currents at peripheral nociceptor terminals. Upon sufficient channel activation, action potentials will be generated and then propagated through the dorsal root ganglia (DRG) and into the dorsal horn of the spinal cord.
Nociceptor input into the dorsal horn of the spinal cord is processed and relayed rostrally to the brain. Action potentials from primary nociceptors produce excitatory post synaptic potentials on secondary nociceptive specific neurons and wide dynamic range neurons located within the gray matter of the dorsal horn via glutamate and co-transmitter release (such as substance P). These excitatory potentials are gated by feedforward inhibition produced by Aβ-fibers that excite inhibitory local glycinergic and GABAergic interneurons that synapse with secondary nociceptive specific and wide dynamic range neurons (Lu et al., 2013; Todd, 2010; Guo and Hu, 2014). Upon depolarization, nociceptive specific and wide dynamic range neurons relay nociceptive information across the anterior white commissure and into the brain along five main ascending tracts: the spinothalamic, the spinoreticular, the spinomesencephalic (or parabrachial tract), the cervicothalamic, and the spinohypothalamic tracts.
Supraspinal structures process nociceptive information in a distributed manner to produce the sensory-discriminative and affective components of pain. Sensory-discriminative components of pain (e.g., location, temporal quality, and intensity) are mainly produced by the primary somatosensory cortex (Bushnell et al., 1999; Rainville et al., 1997). Sensory discriminative information is relayed to the primary somatosensory cortex via projections from the ventral posterolateral and ventral posteromedial nuclei of the thalamus (Ab Aziz and Ahmad, 2006; Hsu et al., 2014). The secondary somatosensory cortex (Maihöfner et al., 2006; Timmermann et al., 2001), prefrontal cortex (PFC) (Ong et al., 2019), and insular cortex (Ostrowsky et al., 2002; Lu et al., 2016; Starr et al., 2009), also are suggested to play a role in sensory-discriminative pain processing.
Affective-motivational components of pain (e.g., subjective unpleasantness, motivation to escape painful stimulus) arise from the processing of nociceptive information within the PFC (including the anterior cingulate cortex (ACC)) (Ong et al., 2019; Xiao and Zhang, 2018; Porro et al., 2002; Metz et al., 2009), insula (Ostrowsky et al., 2002; Lu et al., 2016), amygdala (Neugebauer, 2015), and hypothalamus (Bernard, 2007). Nociceptive information is relayed to these regions from the intralaminar and ventromedial thalamic nuclei (Ab Aziz and Ahmad, 2006).
The ascending transmission of nociceptive information is regulated by the descending pain modulatory system. The descending pain modulatory system comprises primarily the periaqueductal gray (PAG), the locus coeruleus (LC), and the rostral ventromedial medulla (RVM) (Ossipov et al., 2014). Together, these three regions can both facilitate and inhibit the spinal transmission of nociceptive information. Descending regulation is achieved by direct descending projections from the LC and RVM to the dorsal horn of the spinal cord; these projections then either function to inhibit or facilitate nociceptive transmission. For example, pain “On” or “Off” neurons located in the RVM are thought to respectively facilitate or inhibit the transmission of nociceptive information within the spinal cord (Khasabov et al., 2015). Activity of the LC and RVM is regulated heavily by the PAG (Lau and Vaughan, 2014), which receives and processes pain signaling information from other supraspinal regions.
Other supraspinal regions in the pain system can also project directly to the PAG, LC, RVM, and dorsal horn of the spinal cord, forming an extended descending modulatory system. These regions include the PFC (Ong et al., 2019), nucleus submedius -> ventrolateral orbital cortex -> PAG circuit originating from the thalamus, (Tang et al., 2009), various regions of the hypothalamus (Bernard, 2007), and the amygdala (Pertovaara and Almeida, 2006). It is also notable that orexin/hypocretin neurons of the lateral hypothalamus appear to play a role in mediating analgesia via projections to the descending pain modulatory system, spinal cord, and other supraspinal components of the pain system (Colas et al., 2014; Ahmadi-Soleimani et al., 2020; Marcus and Elmquist, 2006).
The pain system adapts in response to painful stimuli. After acute pain stimulation, sites of original injury/stimulation can become hypersensitive to normal stimuli (allodynia) and noxious stimuli (hyperalgesia) in a process known as peripheral sensitization (Gangadharan and Kuner, 2013). Central plastic changes can also occur in the form of central sensitization; these changes enhance the reactivity and activity of the central pain system (Latremoliere and Woolf, 2009). Acute sensitization is thought to be useful for promoting wound-healing and wound-protection behavior. But sensitivity alterations to the pain system are not always beneficial, as they can contribute to the development of chronic pain states that may be useful for wound-protection and healing behaviors (de C Williams, 2016) yet are typically considered to be detrimental to the function and well-being of an individual (Mansour et al., 2014). Maladaptive pain states such as neuropathic or chronic pain (pain lasting for more than 6-months) can arise via sensitization when ‘useful’ pain information outlasts its acute protective function or when damage to the peripheral or central pain system occurs (Schaible, 2006).
3. Diurnal and Circadian Rhythms of the Pain System
In this section we present direct and correlative evidence of the interaction between the circadian and pain systems. We first briefly highlight behavioral studies of circadian rhythms of pain and then examine the involvement of specific nodes of the pain system in the circadian regulation of pain thresholds.
3.1. Circadian Rhythms of Pain
The pain system exhibits circadian rhythms in function (Segal et al., 2018; Palada et al., 2020). Clinical and experimental evidence suggest that pain responsiveness varies across the day in both sexes of diurnal and nocturnal species, including humans (Bruguerolle and Labrecque, 2007; Chassard and Bruguerolle, 2004). Indeed, daily variations in pain responsiveness have been observed in rodents housed in constant conditions, suggesting that these variations reflect true circadian rhythms (Oliverio et al., 1982; Pickard, 1987). In a meta-analysis of circadian rhythms of pain thresholds of healthy humans, pain thresholds were observed to be highest at the end of the active phase and during the night (Figure 1) (Hagenauer et al., 2017). However, pain threshold rhythms in humans vary dramatically in response to disease, with peaks and troughs of sensitivity varying inconsistently across different disease states (Kim et al., 2015).
Figure 1. The circadian regulation of pain.
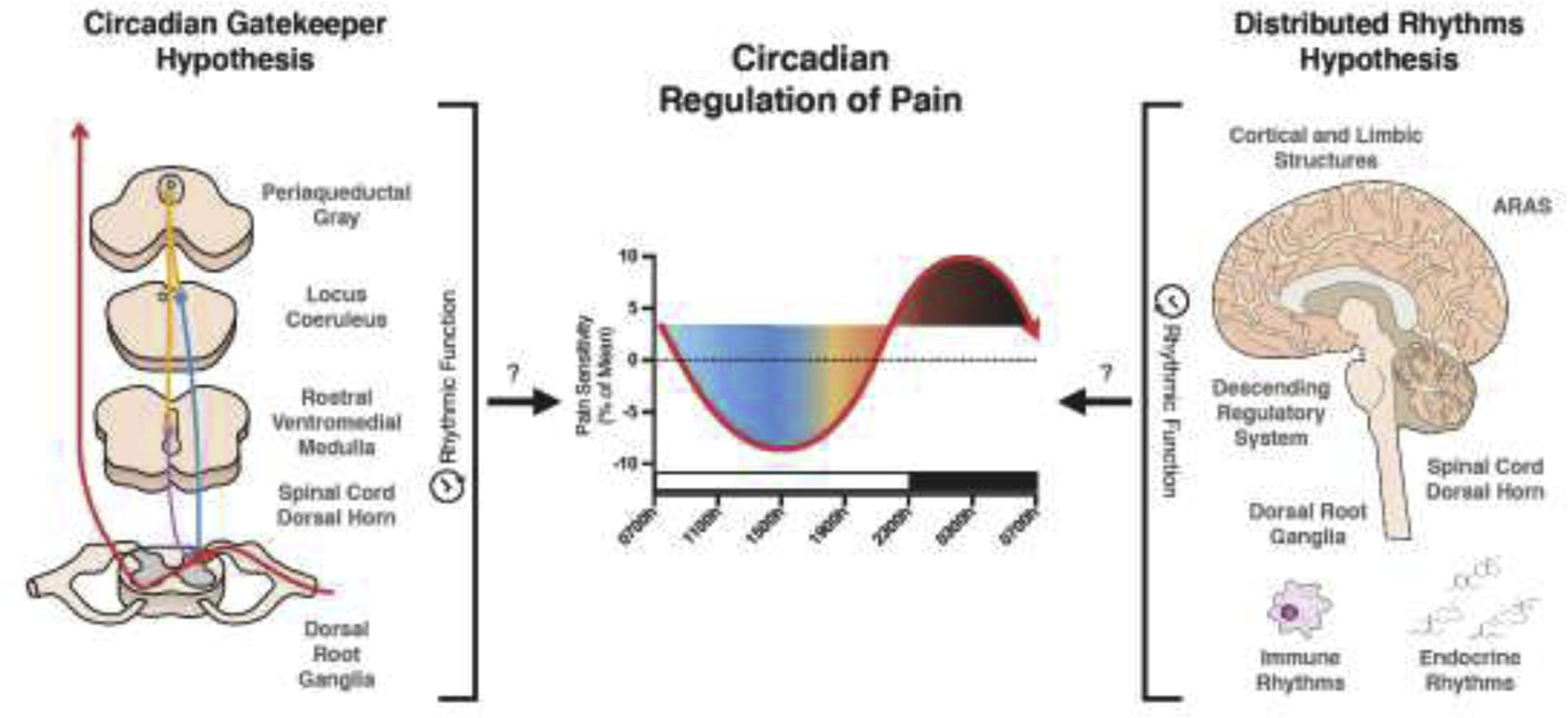
Two hypotheses are proposed to explain the potential origins of circadian pain rhythms. On the left, the “Circadian Gatekeeper” hypothesis is shown. This hypothesis proposes that the descending pain modulatory system, dorsal horn, and DRG regulate ascending transmission of nociceptive input in a time-specific manner to produce diurnal alterations in pain responsiveness. On the right, the “Distributed Rhythms” hypothesis is depicted. Similar to the description of the pain system as a “Distributed System” (Coghill, 2020), this hypothesis suggests that circadian rhythms present throughout the entire pain system and interacting systems function together to produce an integrated circadian rhythm of pain responsiveness. The center graph was adapted from Hagenhauer et al. (Hagenauer et al., 2017). The white and black bar above the x-axis represent typical wake/sleep periods, respectively.
If we assumed that pain threshold rhythms were phase-dependent (in common with locomotor activity rhythms), then we could reasonably predict that nocturnal species have rhythms that are antiphase to diurnal species. However, this is not always the case. Several rodent studies have reported highest pain responsiveness during the active phase (Oliverio et al., 1982; Martínez-Gómez et al., 1994; Frederickson et al., 1977), whereas others report the peak of sensitivity during the inactive phase (Kavaliers and Hirst, 1983). The phase of rhythms can even change based on whether rodents are entrained or free-running (Pickard, 1987). One study also reported antiphase thresholds in two strains of nocturnal mice (Castellano et al., 1985). Thus, further research is needed to understand the relationship between chronotype and pain threshold rhythms in order to optimally translate preclinical pain research, regardless of whether the research is focused on circadian biology.
3.2. Dorsal Root Ganglia
Daily rhythmic activity is observed within the primary nociceptors of the DRG. In mice, DRG express clock genes that drive the rhythmic expression of substance P across the day, likely via Tac1 transcriptional enhancer E-box sites (Zhang et al., 2012). Substance P is a pleiotropic neuropeptide that functions to transmit and modulate pain signaling within the pain system (Li et al., 2012). Synaptic release of glutamate and substance P by DRG in events of nociception results in depolarization of spinal neurons (Zieglgänsberger, 2019). This circadian pattern of substance P expression is correlated with circadian patterns of responsiveness to inflammatory pain induced by formalin injections (Zhang et al., 2012). DRG also exhibit a circadian oscillation in expression of α2δ-1, a voltage-gated calcium channel subunit (Kusunose et al., 2010). The expression of other nociceptive proteins in DRG is likely regulated by clock genes, considering the presence of the clock output gene Tef or the presence of prokineticin receptors (Lee et al., 2017; Negri et al., 2002). Additionally, diurnal variations in TRPV1 expression in the human esophagus have been observed (Yang et al., 2015). Though the findings of the previous study were not directly linked directly to the DRG, a study using male rats observed a diurnal profile of Trp channel expression in the DRG across the day (Kim et al., 2020), indicating that there may be daily variations of channel expression at nociceptor terminals.
3.3. Dorsal Horn of the Spinal Cord
The spinal cord displays circadian rhythms in nociceptive function. The circadian clock gene TTFL is present in neurons and astrocytes of the dorsal horn of the spinal cord (Morioka et al., 2012; Morioka et al., 2016); additional studies confirmed the expression of Rev-erbα and Per1 in the dorsal horn, although expression was only examined at single timepoints (Onishi et al., 2002; Yamamoto et al., 2001). The dorsal horn of the spinal cord displays a circadian pattern in enzymatic activity of the Na+ / K+ ATPase (Eblen-Zajjur et al., 2015), which is essential for neuronal function and plays an integral role in pain processing (LaCroix-Fralish et al., 2009). Although not yet reported, it is possible that the expression of Na+/K+ ATPase is directly or indirectly influenced by clock genes. Another study reported that the TTFL present in spinal astrocytes drives the rhythmic expression of cyclooxygenase-1 and glutamine synthase, both of which play roles in pain processing (Morioka et al., 2016).
Several studies to date have examined the relationship between pain threshold rhythms and circadian rhythms in the spinal cord. In a rat model of chronic constriction injury, a diurnal oscillation was detected in the NR2B-CREB-CRTC1 signaling pathway within the dorsal horn of the spinal cord. Diurnal rhythms of protein and mRNA expression were observed in each component of the pathway, including the NR2B NMDA glutamate receptor subunit and two of its response elements: CREB, and CRTC1 (Xia et al., 2016). This pathway regulates synaptic plasticity and the development of pain hypersensitivity (Xia et al., 2017). An adenovirus driven knockdown of CREB and CRTC1 expression increased mechanical withdrawal (von Frey filaments) thresholds and led to altered withdrawal threshold rhythms across the day (Xia et al., 2017). Next, rhythmic expression of substance P has been observed in the dorsal horn of the spinal cord (Zhang et al., 2012). Lastly, corticosterone-driven astrocytic ATP release in the spinal cord has been linked to diurnal rhythms of allodynia in a mouse model of nerve injury (Koyanagi et al., 2016). In this study, ablation of glucocorticoid secretion by adrenalectomy abolished diurnal astrocytic ATP release in the dorsal horn of the spinal cord and therefore diurnal allodynia rhythms (Koyanagi et al., 2016).
3.4. Periaqueductal Gray
The PAG exhibits circadian rhythms, but little is known about these rhythms in relation to circadian variations in pain modulation. The PAG receives direct input from ipRGCs (Kriegsfeld et al., 2004; Hattar et al., 2006), receives neuronal projections from the SCN (Zhang et al., 2009), and exhibits clock gene rhythms in vitro (Landgraf et al., 2016). One group reported daily differences in μ-opioid receptor mRNA expression in the PAG of mice with sham nerve ligation surgeries (Takada et al., 2013). In addition to its role in analgesia, the ventrolateral PAG acts in tandem with the ascending arousal system to gate rapid eye movement (REM) sleep. A population of ‘REM sleep-on’ and ‘REM sleep-off’ neurons are present throughout the vlPAG, serving as further evidence for diurnal variations in PAG activity (Sapin et al., 2009). Further, the PAG’s daily activity may be altered by fluctuations in levels of endogenous opioids (discussed below). Additional research is needed to understand how pain is modulated by daily molecular and physiological variations in the PAG.
3.5. Rostral Ventromedial Medulla
There is currently limited direct evidence on the role of the RVM in circadian rhythms of pain. The TTFL appears to be present in the caudal ventrolateral medulla (Monošíková et al., 2007), but has not yet been openly examined in the RVM or other regions of the medullary pain system. Additionally, diurnal variation in the activity of tryptophan-5-hydroxylase activity in the nucleus raphe magnus of the RVM has been reported, suggesting that there may be a temporal regulation of neuronal activity in the region (Hery et al., 1977). Importantly, the previously described pain On/Off neurons in the RVM appear to display circadian rhythmicity in activity that is intrinsically related to the sleep-wake cycle. In anesthetized animals, On-/Neutral cells fire spontaneously during waking and have little activity during sleep, whereas Off-cells fire sporadically during waking but have continuous activity during sleep (Leung and Mason, 1999). This observation is coincident with the hypothesis that circadian rhythms of pain are regulated at the level of the spinal cord via the differential activity of RVM On-/Off-neurons and their control over serotonergic projections to the dorsal horn (Foo and Mason, 2003).
3.6. Locus Coeruleus
As a core member of the ascending reticular activating system (which is responsible for regulating arousal and the sleep-wake cycle), there is abundant correlative evidence for the role of the LC in circadian pain processing. Although one study reported no evidence of the clock gene TTFL in the LC (Warnecke et al., 2005), another reported that Per1 is expressed in the LC and that expression levels vary across the day, suggesting that the LC does have its own clock gene loops (Mahoney et al., 2013). There is variation in LC tyrosine hydroxylase activity across the day (Natali et al., 1980). In rats, LC neurons are more active during the active period than the inactive period. But these rhythms are dependent on SCN signaling, as dorsal medial hypothalamic lesions abolished this circadian variation in LC neuronal activity (Aston-Jones et al., 2001). The LC plays a role in regulating the circadian rhythm of the sleep-wake cycle. Because of this, alteration of basal activity in the LC by chronic or even acute pain may modulate circadian rhythms (González and Aston-Jones, 2006). In relation to the varying activity of LC neurons across the day, NAα2 receptors located in the mPFC seem to have an analgesic affect when activated by NA released by the LC (Kaushal et al., 2016). However, NAα1 receptors, which have lower affinity for NA, seem to generate allodynia and hyperalgesia in chronic pain conditions (Kaushal et al., 2016). This may also be relevant for prolonged periods of disrupted circadian rhythms where LC activity is heightened.
3.7. Thalamus
Diurnal changes in human thalamic activity (Ku et al., 2018) and rodent corticothalamic connectivity have been observed (Cardoso-Cruz et al., 2011). Additionally, the paraventricular thalamus expresses clock gene rhythms (Feillet et al., 2008). Nonetheless, there is limited evidence for circadian rhythms in thalamic function in relation to pain processing.
3.8. Hypothalamus
Few studies have examined the role of the hypothalamus in regulating diurnal variations in pain thresholds. However, there is correlative evidence of circadian rhythms in many nociceptive nuclei within the hypothalamus. Given that the SCN is located within the hypothalamus and projects to the hypothalamic preoptic area, paraventricular nucleus, dorsomedial hypothalamic nuclei, ventromedial hypothalamus (Kriegsfeld et al., 2004), it is not unexpected that the TTFL has been reported in each of these regions (Girotti et al., 2009; Kriegsfeld et al., 2003; Kalil et al., 2016; Orozco-Solis et al., 2016; Moriya et al., 2009). Beta-endorphin and met-enkephalin display rhythmic levels of protein expression in the hypothalamus of rat brains (Takahashi et al., 1986), and levels of endogenous opioids display diurnal fluctuations in the rat hypothalamus (Asai et al., 2007), suggesting that the hypothalamic processing of nociceptive input may vary across the day. The hypothalamus has also been implicated to play a role in cluster headaches (Holland and Goadsby, 2007; Burish et al., 2019), which exhibit diurnal variations in occurrence (Pringsheim, 2002). The diurnal variation in incidence of cluster headaches suggests a causative relationship to the rhythmic activity of the SCN and other hypothalamic regions, but this role has yet to be directly determined.
3.9. Lateral Hypothalamus (Orexin)
The orexin system has been implicated to play a role in pain regulation (Razavi and Hosseinzadeh, 2017), and its involvement in the circadian regulation of the sleep-wake cycle suggests a correlative relationship in the circadian regulation of pain. A diurnal variation of orexin-A is observed in the cerebrospinal fluid of humans, rats, and diurnal squirrel monkeys, although it is worth noting that the pattern of orexin-A levels in human CSF did not follow the predicted levels (Salomon et al., 2003; Fujiki et al., 2001; Zeitzer et al., 2003). Plasma orexin-A levels do not exhibit a circadian pattern of expression (Mäkelä et al., 2018). Importantly, orexin-A is observed to have anti-nociceptive activity (Razavi and Hosseinzadeh, 2017). Ablation of the SCN abolishes CSF orexin-A rhythms, suggesting that these rhythms are either directly or indirectly regulated by the SCN (Zhang et al., 2004).
Orexin neurons display circadian rhythms of firing activity, with peaks of activity observed during the active period as indicated by c-fos expression (Marston et al., 2008; Estabrooke et al., 2001). The rhythmic activity of the LC is directed in part by orexin neurons of the DMH (Gompf and Aston-Jones, 2008), consistent with the flip-flop switch model of arousal (Schwartz and Roth, 2008) and the demonstrated circadian rhythmicity of orexin-A levels in the pons and the lateral/medial hypothalamus (Taheri et al., 2000). It has also been proposed that projections from the DMH, mPOA, and SPVZ to orexin act as indirect projections from the SCN to help maintain entrainment (Deurveilher and Semba, 2005). Orexin neurons project to various components of the descending pain modulatory system and modulate their activity, providing further correlative evidence for the involvement of this region in pain rhythms (Ahmadi-Soleimani et al., 2020).
3.10. Cortical and Limbic Structures
Direct evidence for the involvement of cortical and limbic regions in the circadian regulation of pain is scarce. However, circadian variations are present in these regions, suggesting that they may play a role in the temporal variation of pain thresholds. Rhythmic clock gene expression is present in the human ACC, dorsolateral prefrontal cortex, nucleus accumbens, and amygdala (Li et al., 2013). The presence of clock gene expression has also been observed in rodent cortical and limbic regions, including the ACC, prelimbic and infralimbic cortices, ventral orbital cortex, insular cortex, amygdala, and nucleus accumbens (Woodruff et al., 2016; Chun et al., 2015; Christiansen et al., 2016; Lamont et al., 2005). The SCN do not directly innervate the prefrontal cortex; instead, entrainment of these regions is proposed to occur via a relay circuit from the SCN -> paraventricular thalamic nucleus -> PFC (Sylvester et al., 2002). One study reported a slight but significant variation in the expression of μ-opioid receptor expression across the day in the frontal cortex (Takada et al., 2013). There are also diurnal variations in cortical ACh release in rats, with higher release during the active phase (Mitsushima et al., 1996). Anterior insular lesions disrupt the sleep-wake cycle and disturb locomotor activity cycles, suggesting that the insula plays a role in the behavioral regulation of circadian rhythms (Chen et al., 2016a). Rhythmic expression of serotonin, its 5HIAA metabolite, and 3-methoxy-4-hydroxyphenylglycol, the main metabolites of norepinephrine are present in the amygdala (Moriya et al., 2015). Another group reported diurnal rhythms of serotonin and dopamine in the amygdala, though specific statistical analyses were not provided (Izumo et al., 2012). Lastly, there are diurnal variations in cortical synaptic activity and spine density (Hayashi et al., 2013a). Further research explicitly examining circadian rhythms in cortical and limbic pain processing seems warranted.
4. Interacting Systems
In this section, we discuss circadian rhythms of the endogenous opioid, immune, and endocrine systems to consider how their interaction with the pain system may influence circadian rhythms of pain.
4.1. Endogenous Opioid System
The endogenous opioid system functions to regulate pain, emotional, and stress responses (Ferdousi and Finn, 2018). Circadian variations in opioid levels and binding activity throughout the day suggest that the opioid system plays a role in the circadian regulation of pain, potentially via the modulation of the activity of the pain system. Leu-enkephalin and met-enkephalin display circadian fluctuations within various forebrain regions of the rat (Asai et al., 2007; Kurumaji et al., 1988). Melatonin can induce dose-dependent increases of met-enkephalin, but this effect is not entirely mediated by binding to melatonin receptors (Asai et al., 2007). Dynorphin, but not beta-endorphin, levels also fluctuate across the day in the rat hypothalamus and pituitary (Reid et al., 1982). Diurnal rhythms of plasma met-enkephalin and beta-endorphin have been reported in humans (Mozzanica et al., 1991; Mozzanica et al., 1992; Petraglia et al., 1983). Daily variation in whole brain met-enkephalin levels in rats was observed when tissue was collected immediately after prolonged exposure to a hot plate; higher levels of met-enkephalin during the dark phase were associated with increased withdrawal latencies (Wesche and Frederickson, 1981). Hypophysectomy did not abolish this daily variation but did blunt the levels of whole brain enkephalin (Wesche and Frederickson, 1981).
Pain responses induced by morphine and naloxone injections vary across the day, suggesting an endogenous fluctuation in the expression of opioid receptors or in the expression of components of their downstream signaling pathway (Frederickson et al., 1977; Kavaliers and Hirst, 1983). Recent work examining the analgesic effects of green light exposure during the light phase determined that green light induced analgesia is dependent on descending RVM signaling and opioid signaling in the spinal cord of male rats (Ibrahim et al., 2017; Martin et al., 2021). These data indicate a role of photic signaling and potentially circadian rhythm entrainment in the modulation of pain by the endogenous opioid system. Lastly, there is diurnal variation in opioid receptor expression in the rodent PAG and frontal cortex that was correlated with circadian variations in hot plate withdrawal thresholds (Takada et al., 2013).
Further research will be needed to identify whether the circadian rhythms of the endogenous opioid system modulate the pain system, or if the rhythms of the opioid system are an output of rhythms within the pain system. Taken together, the evidence above correlates with diurnal variations in reported opioid analgesic efficacy and requests for opioid analgesics in the clinic (Junker and Wirz, 2010). Additional insights into the interactions between these systems can improve patient treatment and chronotherapeutic efforts for pain management.
4.2. Endocrine System
Many hormones that interact with the pain system exhibit circadian rhythms, including cortisol, gonadal hormones, and melatonin. Cortisol modulates acute pain responsiveness and is thought to play a role in the development of chronic pain (Benson et al., 2019). Rhythms of cortisol concentrations are phase-dependent and fluctuate across the day; levels rise in the hours before waking and peak just after the onset of activity (Weitzman et al., 1971; Albers et al., 1985). One report suggests that cortisol rhythms are not necessary for diurnal variations in pain thresholds (Heybach and Vernikos-Danellis, 1978). But, as described above, one study reported that circadian corticosterone rhythms are necessary for the development of diurnal patterns of neuropathic allodynia in mice (Koyanagi et al., 2016). The rhythmic release of ATP from astrocytes in the dorsal horn of the spinal cord is dependent on corticosterone rhythms, as demonstrated by abolished ATP rhythms in adrenalectomized mice; it was suggested that the rhythmic release of ATP induces a rhythmic activation of microglia via P2Y12R signaling (Koyanagi et al., 2016). However, differences in microglial activation were not observed as determined by Iba-1 staining. Perhaps the cortisol-dependent allodynia rhythms observed are instead a result of diurnal differences in neuronal synaptic transmission driven by the diurnal activity of astrocytic ATP production. Regardless of mechanisms, this study contributes an important understanding to the role of glucocorticoids in daily changes in neuropathic pain thresholds.
Gonadal hormones can play both pro- and anti-nociceptive roles and likely contribute to sex differences in pain thresholds; as an oversimplification, androgens tend be anti-nociceptive whereas estrogens are pro-nociceptive (Craft et al., 2004; Aloisi and Bonifazi, 2006). Androgen levels have rhythmic expression (Bremner et al., 1983), and although ovarian estrogen and progesterone levels in females are altered throughout estrous cycles, evidence suggests that they do exhibit circadian rhythms in secretion during the luteal phase (Spies et al., 1974; Kriegsfeld et al., 2002).
Melatonin has a primarily analgesic effect, although the exact mechanism by which is produces analgesia is unknown (Wilhelmsen et al., 2011; Chen et al., 2016b). Melatonin concentrations fluctuate in a phase-independent manner; its concentrations peak and persist in the dark phase and are almost entirely absent in the light phase (Brown, 1994).
4.3. Immune System
The immune system is regulated by circadian rhythms and displays circadian variations in function. Briefly, the TTFL is present in immune and glial cells, driving rhythmic immune activity and the expression of various inflammatory mediators involved in both innate and adaptive immune function (Logan and Sarkar, 2012). For example, microglia may be involved in the circadian regulation of pain (Inoue and Tsuda, 2018). Via circadian expression of cathepsin S, microglia drive the previously mentioned diurnal variations in cortical synaptic activity and spine density (Hayashi et al., 2013a). P2Y12, the purinergic receptor that regulates microglia activation and neuropathic pain transmission within the spinal cord (Yu et al., 2019) is regulated by circadian rhythms (Hayashi et al., 2013b). Numerous other examples of the circadian regulation of immune and glial function and their influence on pain have been extensively reviewed (Segal et al., 2018). As the pain system is modulated by the activity of the immune system, there are considerable implications for the effects of rhythmic immune activity on pain rhythms.
5. Disrupted Circadian Rhythms and Pain
Within the past two centuries, humans have adopted lifestyles and environmental modifications that routinely disrupt our circadian rhythms. As a result of environmental or behavioral disturbances, our internal circadian rhythms can become shifted from the external world, blunted, or abolished. Examples of environmental or behavioral factors that disrupt circadian rhythms are numerous: between 15–25% of the world’s working population is involved in shift work (Drake and Wright, 2011), 70% of the population regularly experiences shifted sleep-wake cycles on weekend and work days - a phenomenon known as social “jet lag” (Roenneberg et al., 2012), and 80% percent of the global population is exposed to light pollution at night (Falchi et al., 2016). Other examples of circadian disruption include mistimed eating and jet lag resulting from transmeridian travel (Thaiss et al., 2014; Gibson et al., 2010; Challet, 2019; Zheng et al., 2020).
5.1. Circadian Rhythm Disruption and Pain
Multiple clinical and foundational science studies report that circadian rhythm disruption can directly alter pain thresholds. Disrupted circadian rhythms are linked to inflammation and altered endocrine function, both of which have direct implications on pain. In addition to directly affecting pain, many other consequences of disrupted circadian rhythms may be linked to altered pain, including increased risks for obesity (McHill and Wright, 2017), cancer (Davis and Mirick, 2006; Reiter et al., 2007; Haus and Smolensky, 2013), cardiovascular dysfunction (Chellappa et al., 2019), depression (Tsuneki et al., 2018) and altered immune (Logan and Sarkar, 2012) and endocrine function (Russart and Nelson, 2018).
The effects of circadian rhythm disruption on pain in humans have been examined in the context of night shift work and sleep disruption. Night shift work is associated with an increased risk for the incidence of lower back pain (Takahashi et al., 2015; Eriksen et al., 2004; Zhao et al., 2012). Night-shift work is also correlated with reduced pain thresholds. One cross-over study reported that night-shift workers have higher sensitivity to electrical and heat pain, but not cold or pressure pain (Matre et al., 2017). In another study, night shift workers had lower cold pain thresholds immediately after finishing a 12-hour shift in comparison to a normal sleep day before and after the shift (Pieh et al., 2018). Selective and total sleep deprivation also heightens pain sensitivity, although inconsistently in human studies (Onen et al., 2001). Total sleep deprivation reduces heat pain thresholds (Kundermann et al., 2004; Kundermann et al., 2008), as well as mechanical pain thresholds (Onen et al., 2001).
Basic research has examined the effects of dim light at night exposure, mistimed eating, simulated jet lag, and sleep deprivation on pain in rodents. Male Swiss Webster mice exposed to dim light at night (~5 lux of light) experienced mechanical allodynia and cold hyperalgesia (Bumgarner et al., 2020). Notably, cold hyperalgesia was observed in these mice after only four nights of exposure to dim light at night. These behavioral effects were correlated with upregulated expression of Il-6 and μ-opioid receptor expression in the RVM and PAG, respectively. Another study examined the role of mistimed eating on allodynia in a rodent model of neuropathy. It was observed that food consumption restricted to the inactive phase exacerbated mechanical allodynia in male mice with chronic constriction injury (Xu et al., 2018). Another study reported that jet lag can induce mechanical allodynia and heat hyperalgesia in female mice (Das et al., 2018). At the conclusion of the experiment, mice that experienced 14-weeks of weekly alternating light-dark cycles had lower mechanical thresholds and shorter hot plate withdrawal latencies than mice that received 6-weeks of shifts and had 8-weeks of typical light-dark cycles afterward. Appropriate control groups were missing in additional experiments of this study. Finally, multiple rodent studies have indicated that sleep deprivation heightens pain responsiveness (Lautenbacher et al., 2006). Together, these studies demonstrate a link between circadian rhythm disruption and altered function of the pain system.
5.2. Disrupted Rhythms of Pain
Multiple chronic and maladaptive pain conditions are associated with altered circadian rhythms in pain thresholds (Junker and Wirz, 2010). Altered pain rhythms manifest inconsistently across various diseases states. The peak of breakthrough pain episodes in cancer patients occurs in the late morning/early afternoon (Campagna et al., 2019; Saini et al., 2013). This is somewhat consistent with reported early morning peaks of pain in patients with fibromyalgia (Bellamy et al., 2004) and rheumatoid arthritis (Bellamy et al., 1991; Harkness et al., 1982), although one group reported no diurnal variation in rheumatoid arthritis pain (Dekkers et al., 2000). Morning peaks in reported pain for these diseases contrasts sharply with the reported night peaks of pain associated with diabetic neuropathy and postherpic neuralgia (Odrcich et al., 2006), evening peaks of pain in patients with varying forms of intractable pain (Folkard et al., 1976), and nocturnal painful spasms associated with multiple sclerosis patients who have pyramidal tract dysfunction (Solaro et al., 2000). Considering the heterogenous physiological consequences of these various disease states, the observed differences in acrophases of pain thresholds across the day could be explained by varying disruption of individual components of the pain system or other circadian regulatory systems.
Many chronic pain conditions are also associated with disrupted circadian rhythms, including endocrine and sleep-wake rhythms. Cervical spinal cord injury in humans disrupts daily serum melatonin and cortisol rhythms (Fatima et al., 2016). In more severe instances of chronic pain, such as fibromyalgia, sleep-wake rhythms are highly perturbed (Korszun, 2000). However, sleep-wake disturbances are also reported in chronic pain patients (McCracken and Iverson, 2002; Smith et al., 2000). Altered sleep rhythms and decreased sleep quality can in turn have numerous negative consequences on the function of the circadian system (Palada et al., 2020).
Lastly, disrupted circadian rhythms have been observed in several rodent models of neuropathy. For example, sciatic nerve ligation alters the rhythmic expression of the melatonin 1A and 1B receptor in male mice (Odo et al., 2014). Spinal cord injury in rats disrupted circadian rhythms of locomotor activity, glucocorticoid secretion, inflammatory gene expression, and clock gene expression (Gaudet et al., 2018).
5.3. Circadian Disruption and Maladaptive Pain: A Feedback Loop?
The evidence reviewed in sections 5.1 and 5.2 lay the groundwork for describing a feedback loop that arises between circadian rhythm disruption and chronic pain, particularly the chronic pain associated with disease conditions (Figure 2). Chronic pain can alter sleep-wake cycles and clock gene rhythms, among other rhythms, and circadian rhythm disruption can alter pain thresholds as well as the circadian rhythms of other interacting systems. Once initiated, this feedback loop likely results in states of chronically lowered pain thresholds and could contribute to prolonged periods of chronic pain. A clinically relevant example of this loop may be observed in fibromyalgia patients in whom sleep-wake cycles and other rhythmic processes are affected by intense chronic pain. This disruption of circadian rhythms will in turn likely reduce pain thresholds.
Figure 2. Disruption of circadian rhythms and maladaptive pain: A feedback loop.
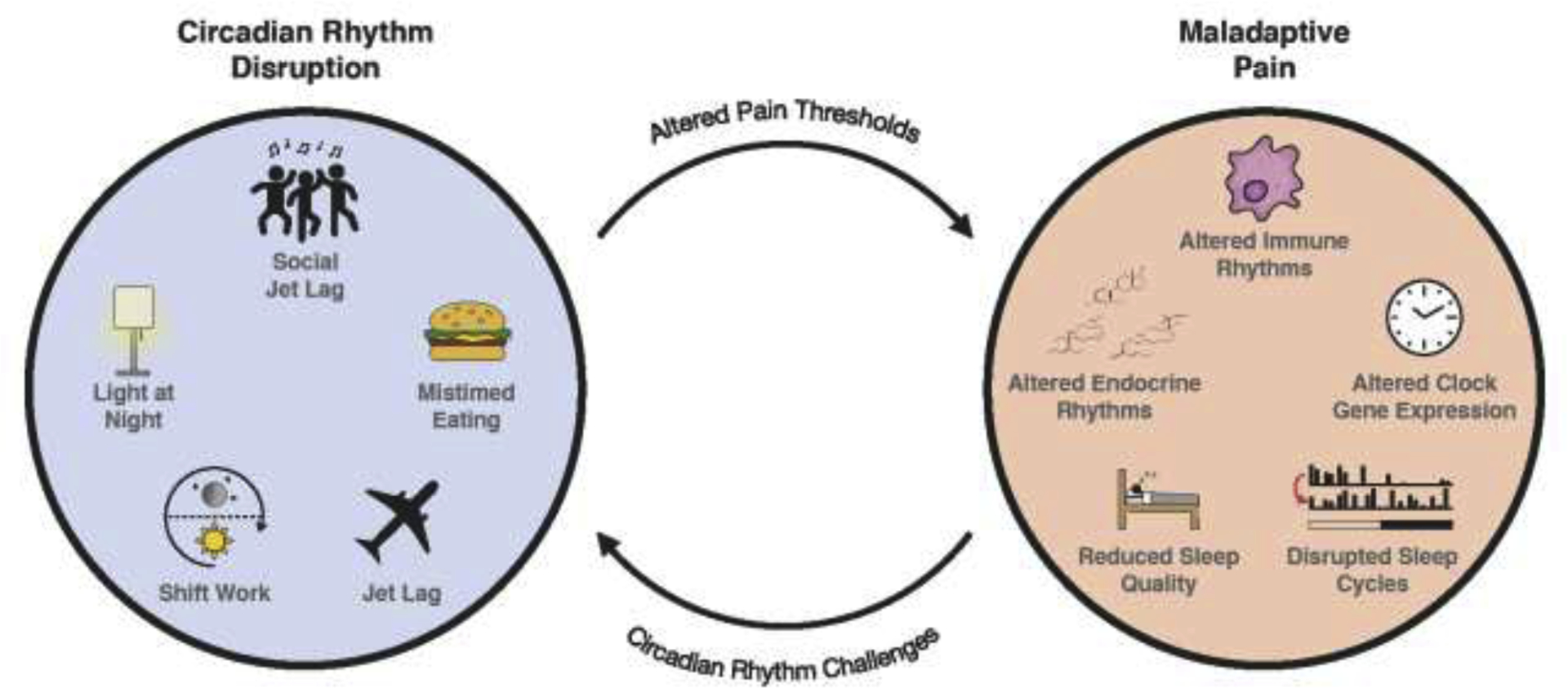
Maladaptive pain is negatively affected by common and seemingly innocuous forms of disrupted circadian rhythms, in addition to more severe forms of disruption. Many of the consequences of circadian rhythm disruption can negatively alter pain thresholds and affect maladaptive pain. The physiological consequences of maladaptive pain can induce various forms of disrupted circadian rhythms, thus generating a deleterious feedback loop. The consequences of this feedback loop should be considered in future clinical and preclinical research aiming to resolve and treat chronic and maladaptive pain.
It is unlikely that this loop drives pain thresholds to absolute minima or even close, particularly in healthy individuals. Though circadian rhythm disruption may temporarily or even chronically reduce pain thresholds, it is also unlikely that minor injuries would be sufficient to initiate the loop. Instead, this loop is likely more prominent in chronic pain caused by serious injury or maladaptive pain associated with disease states. As further research validates the relationship between the circadian and pain systems, it will be important to consider this feedback loop in the treatment and management of acute and chronic pain. Environmental manipulations that support circadian rhythms may help to ameliorate pain symptoms in chronic pain patients and others.
6. Limitations
Several limitations arise from this review. First, much of the evidence regarding the regulation of pain behavior by circadian rhythms is correlative rather than causative. This is particularly apparent when examining higher-order components of the pain system, including cortical and limbic structures. As such, conclusions regarding the involvement of numerous supraspinal structures in the circadian regulation of pain behavior are limited. Second, several of the non-human studies described in section 5.1 tested pain behavior during the light phase when nocturnal species are typically inactive, potentially hindering translational conclusions (Nelson et al., 2021). Lastly, there is a dramatic disparity in the sex of the animals in the reviewed literature. The vast majority of non-human studies only examined males (Supplemental Table 1). In the meta-analysis used to generate a model of human circadian rhythms in pain, only half of the 16 primary studies included females (Hagenauer et al., 2017). Because of known sex differences in circadian rhythms and pain behavior, this disparity leads to further potential limitations on the conclusions that can be drawn from the reviewed literature. Future human and non-human research should strive to examine circadian pain behaviors in both sexes.
7. Interpretations
The pain system exhibits circadian rhythms in function at all levels of hierarchical organization, but the origins of these rhythms are still not clear. Direct evidence has demonstrated that circadian regulation of pain occurs directly within DRG and the spinal cord, and abundant direct and correlative evidence have demonstrated that the supraspinal distributed pain system also processes pain differently across the day. Nonetheless, currently available evidence does not allow us to explicitly state that the circadian rhythmicity of pain thresholds exclusively arises from rhythms within the pain system alone. The variations in thresholds may instead be influenced or result from the integral relationships between the pain system and the circadian, endogenous opioid, immune, and endocrine systems as well as the ascending reticular activating system. Despite these uncertainties, the compiled evidence does outline several potential explanations of the origins of circadian rhythms of the pain system.
We propose that the circadian rhythms of the pain system may arise from a set of collective rhythms within the DRG, spinal cord, and descending pain modulatory system (Figure 1). Collectively, these structures may act as circadian ‘gatekeepers’ that diurnally modulate the transmission of nociceptive information into supraspinal structures responsible for the salient processing of pain. The sleep/wake dependent variation in activity of neurons within numerous components of the descending pain modulatory system supports this theory. For example, the modulation of serotonergic input to the spinal cord from the RVM is regulated by On/Off neurons whose activity fluctuates based on sleep/wake states (Foo and Mason, 2003). The ‘circadian gatekeeper’ system could also be influenced by other circadian rhythms, leaving room to explain inconsistently altered pain rhythms among various disease states.
Alternatively, circadian rhythms of the pain system may not be a result of rhythms only within the ‘gatekeeper’ system. Instead, we alternatively propose that a distributed network of circadian rhythms within the pain system and those with which it interacts. Just as there is no single region of the pain system responsible for processing pain, there may be no single region responsible for the circadian rhythmicity of pain thresholds. The rhythmicity of the pain system likely arises from rhythms within three groups of organization: the DRG and spinal cord, cortical and limbic structures, and the descending pain modulatory system. These groups each have their own rhythmicity and function, but their collective activity and interaction may produce the pain threshold rhythms observed in constant conditions. The rhythmic function of the disturbed system is likely also influenced by interactions with the ascending reticular activating system, circadian system, endocrine system, and immune system. Disease specific disruption of individual, but not all, of these modulatory systems may explain the difference in circadian pain threshold variations across the day observed in different disease states. The concept of distributed circadian rhythms within pain system will be difficult to elucidate, just as the examination of cerebral pain processing.
Importantly, why are there circadian rhythms in pain thresholds across the day? As of now, this trait has only been observed in mammals. But, considering the highly conserved characteristics of nociceptive signaling, it is not unreasonable to speculate that these rhythms exist in other organisms; a discovery of rhythms in other organisms may point an evolutionarily/adaptive beneficial function. Admittedly, it may be wrong to assume that these rhythms are functionally relevant. To a skeptical eye, the overlap between the ascending reticular activating system and pain system could suggest that rhythms in pain thresholds might only be a behavioral byproduct that ultimately serves no adaptive benefit. Additional research will be needed to address these points. Conversely, variations in pain thresholds may allow for optimal behavioral function throughout the active hours by allowing us to ignore small scrapes and wounds that may only distract from survival until activity slows down before rest. Or, as suggested by Foo (Foo and Mason, 2003), circadian variations in the rostral projection of nociceptive information may be crucial for sustained sleep. Regardless of the evolutionary origins of these rhythms, their nature must be considered. Further characterization of circadian rhythms of the pain system will allow us to adapt pain management and treatment strategies that optimize patient outcome and well-being.
Supplementary Material
8. Acknowledgments.
The authors would like to thank artist Madelyn Brodie for her assistance in the preparation of our figures. Preparation of this review was supported by grant awards NINDS R01NS092388 (RJN), NCCIH 1R21AT011238 (RJN), and NIGMS under Award Number 5U54GM104942-03. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
9. References
- Ab Aziz CB, and Ahmad AH, 2006. The role of the thalamus in modulating pain. Malays. J. Med. Sci 13, 11. [PMC free article] [PubMed] [Google Scholar]
- Ahmadi-Soleimani SM, Mianbandi V, Azizi H, Zarmehri HA, Jandabi MG, Abbasi-Mazar A, Mohajer Y, and Darana SP, 2020. Coregulation of sleep-pain physiological interplay by orexin system: An unprecedented review. Behav. Brain Res 112650. [DOI] [PubMed] [Google Scholar]
- Albers HE, Yogev LEAH, Todd RB, and Goldman BD, 1985. Adrenal corticoids in hamsters: Role in circadian timing. J. Psychiatry Neurosci 248, R434–R438. [DOI] [PubMed] [Google Scholar]
- Aloisi AM, and Bonifazi M, 2006. Sex hormones, central nervous system and pain. Horm. Behav 50, 1–7. [DOI] [PubMed] [Google Scholar]
- Asai MAM, Mayagoitia LML, García DGD, Matamoros-Trejo GM-TG, Valdés-Tovar MV-TM, and Leff PLP, 2007. Rat brain opioid peptides-circadian rhythm is under control of melatonin. Neuropeptides 41, 389–397. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G, Chen S, Zhu Y, and Oshinsky ML, 2001. A neural circuit for circadian regulation of arousal. Nat. Neurosci 4, 732–738. [DOI] [PubMed] [Google Scholar]
- Auvray M, Myin E, and Spence C, 2010. The sensory-discriminative and affective-motivational aspects of pain. Neurosci. Biobehav. Rev 34, 214–223. [DOI] [PubMed] [Google Scholar]
- Baliki MN, and Apkarian AV, 2015. Nociception, pain, negative moods, and behavior selection. Neuron 87, 474–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basbaum AI, Bautista DM, Scherrer G, and Julius D, 2009. Cellular and molecular mechanisms of pain. Cell 139, 267–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellamy N, Sothern RB, Campbell J, and Buchanan WW, 1991. Circadian rhythm in pain, stiffness, and manual dexterity in rheumatoid arthritis: Relation between discomfort and disability. Ann. Rheum. Dis 50, 243–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellamy N, Sothern RB, and Campbell J, 2004. Aspects of diurnal rhythmicity in pain, stiffness, and fatigue in patients with fibromyalgia. J. Rheumatol 31, 379–389. [PubMed] [Google Scholar]
- Benson S, Siebert C, Koenen LR, Engler H, Kleine-Borgmann J, Bingel U, Icenhour A, and Elsenbruch S, 2019. Cortisol affects pain sensitivity and pain-related emotional learning in experimental visceral but not somatic pain: A randomized controlled study in healthy men and women. Pain 160, 1719–1728. [DOI] [PubMed] [Google Scholar]
- Bernard J-F, 2007. Hypothalamus and nociceptive pathways, in: Schmidt R, Willis W (Eds.), Encyclopedia of Pain, Berlin, Heidelberg, pp. 944–948. [Google Scholar]
- Bremner WJ, Vitiello MV, and Prinz PN, 1983. Loss of circadian rhythmicity in blood testosterone levels with aging in normal men. J. Psychiatry Neurosci 56, 1278–1281. [DOI] [PubMed] [Google Scholar]
- Brown GM, 1994. Light, melatonin and the sleep-wake cycle. J. Psychiatry Neurosci 19, 345. [PMC free article] [PubMed] [Google Scholar]
- Bruguerolle B, and Labrecque G, 2007. Rhythmic pattern in pain and their chronotherapy. Am. J. Epidemiol 59, 883–895. [DOI] [PubMed] [Google Scholar]
- Bumgarner JR, Walker WH, Liu JA, Walton JC, and Nelson RJ, 2020. Dim light at night exposure induces cold hyperalgesia and mechanical allodynia in male mice. Neuroscience 434, 111–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burish MJ, Chen Z, and Yoo S, 2019. Emerging relevance of circadian rhythms in headaches and neuropathic pain. Acta Physiologica 225, e13161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bushnell MC, Duncan GH, Hofbauer RK, Ha B, Chen JI, and Carrier B, 1999. Pain perception: Is there a role for primary somatosensory cortex. Proc. Natl. Acad. Sci. U S A 96, 7705–7709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campagna S, Sperlinga R, Milo A, Sannuto S, Acquafredda F, Saini A, Gonella S, Berruti A, Scagliotti GV, and Tampellini M, 2019. The circadian rhythm of breakthrough pain episodes in terminally-ill cancer patients. Cancers 11, 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardoso-Cruz H, Sameshima K, Lima D, and Galhardo V, 2011. Dynamics of circadian thalamocortical flow of information during a peripheral neuropathic pain condition. Front. Integr. Neurosci 5, 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellano C, Puglisi-Allegra S, Renzi P, and Oliverio A, 1985. Genetic differences in daily rhythms of pain sensitivity in mice. Pharmacol. Biochem. Behav 23, 91–92. [DOI] [PubMed] [Google Scholar]
- Challet E, 2019. The circadian regulation of food intake. J. Neurosci. Res 15, 393–405. [DOI] [PubMed] [Google Scholar]
- Chassard D, and Bruguerolle B, 2004. Chronobiology and anesthesia. Curr. Opin. Neurobiol 100, 413–427. [DOI] [PubMed] [Google Scholar]
- Chellappa SL, Vujovic N, Williams JS, and Scheer FAJL, 2019. Impact of circadian disruption on cardiovascular function and disease. Crit. Rev. Oncog 30, 767–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen MC, Chiang WY, Yugay T, Patxot M, Özçivit İB, Hu K, and Lu J, 2016a. Anterior insula regulates multiscale temporal organization of sleep and wake activity. J. Biol. Rhythms 31, 182–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W, Zhang X, and Huang W, 2016b. Pain control by melatonin: Physiological and pharmacological effects. Exp. Ther. Med 12, 1963–1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christiansen SL, Bouzinova EV, Fahrenkrug J, and Wiborg O, 2016. Altered expression pattern of clock genes in a rat model of depression. Int. J. Neuropsychopharmacol 19, pyw061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chun LE, Woodruff ER, Morton S, Hinds LR, and Spencer RL, 2015. Variations in phase and amplitude of rhythmic clock gene expression across prefrontal cortex, hippocampus, amygdala, and hypothalamic paraventricular and suprachiasmatic nuclei of male and female rats. J. Biol. Rhythms 30, 417–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coghill RC, 2020. The distributed nociceptive system: A framework for understanding pain. Trends Neurosci [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colas D, Manca A, and Mourrain P, 2014. Orexin a and orexin receptor 1 axonal traffic in dorsal roots at the cns/pns interface. Front. Neurosci 8, 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craft RM, Mogil JS, and Aloisi AM, 2004. Sex differences in pain and analgesia: The role of gonadal hormones. J. Psychiatry Neurosci 8, 397–411. [DOI] [PubMed] [Google Scholar]
- Das V, Kc R, Li X, Varma D, Qiu S, Kroin JS, Forsyth CB, Keshavarzian A, van Wijnen AJ, Park TJ, Stein GS, O-Sullivan I, Burris TP, and Im HJ, 2018. Pharmacological targeting of the mammalian clock reveals a novel analgesic for osteoarthritis-induced pain. Gene 655, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis S, and Mirick DK, 2006. Circadian disruption, shift work and the risk of cancer: A summary of the evidence and studies in Seattle. Physiol. Behav 17, 539–545. [DOI] [PubMed] [Google Scholar]
- de C Williams AC, 2016. What can evolutionary theory tell us about chronic pain. Pain 157, 788–790. [DOI] [PubMed] [Google Scholar]
- Dekkers JC, Geenen R, Godaert GLR, Doornen LJP, and Bijlsma JWJ, 2000. Diurnal courses of cortisol, pain, fatigue, negative mood, and stiffness in patients with recently diagnosed rheumatoid arthritis. Int. J. Behav. Med 7, 353–371. [Google Scholar]
- Deurveilher S, and Semba K, 2005. Indirect projections from the suprachiasmatic nucleus to major arousal-promoting cell groups in rat: Implications for the circadian control of behavioural state. Neuroscience 130, 165–183. [DOI] [PubMed] [Google Scholar]
- Drake CL, and Wright KP, 2011. Shift work, shift-work disorder, and jet lag. Eur. J. Neurosci 1, 784–798. [Google Scholar]
- Eblen-Zajjur A, Marín R, Vanegas H, Proverbio F, and Proverbio T, 2015. Diurnal changes in ouabain-sensitive Na+, K+-ATPase activity in the rat spinal dorsal horn. Neuroscience 9, 266–270. [Google Scholar]
- Eriksen W, Bruusgaard D, and Knardahl S, 2004. Work factors as predictors of intense or disabling low back pain; a prospective study of nurses’ aides. Pharmacol. Ther 61, 398–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estabrooke IV, McCarthy MT, Ko E, Chou TC, Chemelli RM, Yanagisawa M, Saper CB, and Scammell TE, 2001. Fos expression in orexin neurons varies with behavioral state. J. Neurosci 21, 1656–1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falchi F, Cinzano P, Duriscoe D, Kyba CCM, Elvidge CD, Baugh K, Portnov BA, Rybnikova NA, and Furgoni R, 2016. The new world atlas of artificial night sky brightness. Cancer Res 2, e1600377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fatima G, Sharma VP, and Verma NS, 2016. Circadian variations in melatonin and cortisol in patients with cervical spinal cord injury. Spinal Cord 54, 364–367. [DOI] [PubMed] [Google Scholar]
- Feillet CA, Mendoza J, Albrecht U, Pévet P, and Challet E, 2008. Forebrain oscillators ticking with different clock hands. Mol. Cell. Neurosci 37, 209–221. [DOI] [PubMed] [Google Scholar]
- Ferdousi M, and Finn DP, 2018. Stress-induced modulation of pain: Role of the endogenous opioid system, in: O’Mara S (Eds.), Progress in Brain Research, pp. 121–177. [DOI] [PubMed] [Google Scholar]
- Folkard S, Glynn CJ, and Lloyd JW, 1976. Diurnal variation and individual differences in the perception of intractable pain. Brain Res 20, 289–301. [DOI] [PubMed] [Google Scholar]
- Foo H, and Mason P, 2003. Brainstem modulation of pain during sleep and waking. Proc. Natl. Acad. Sci. U S A 7, 145–154. [DOI] [PubMed] [Google Scholar]
- Frederickson RC, Burgis V, and Edwards JD, 1977. Hyperalgesia induced by naloxone follows diurnal rhythm in responsivity to painful stimuli. Science 198, 756–758. [DOI] [PubMed] [Google Scholar]
- Fujiki N, Yoshida Y, Ripley B, Honda K, Mignot E, and Nishino S, 2001. Changes in csf hypocretin-1 (orexin a) levels in rats across 24 hours and in response to food deprivation. Neuroreport 12, 993–997. [DOI] [PubMed] [Google Scholar]
- Gamble KL, Berry R, Frank SJ, and Young ME, 2014. Circadian clock control of endocrine factors. Nat. Rev. Endocrinol 10, 466–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gangadharan V, and Kuner R, 2013. Pain hypersensitivity mechanisms at a glance. Dis. Model Mech 6, 889–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Larrea L, and Peyron R, 2013. Pain matrices and neuropathic pain matrices: A review. PAIN® 154, S29–S43. [DOI] [PubMed] [Google Scholar]
- Gaudet AD, Fonken LK, Ayala MT, Bateman EM, Schleicher WE, Smith EJ, D’Angelo HM, Maier SF, and Watkins LR, 2018. Spinal cord injury in rats disrupts the circadian system. Eneuro 5, ENEURO.0328–18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson EM, Wang C, Tjho S, Khattar N, and Kriegsfeld LJ, 2010. Experimental ‘jet lag’inhibits adult neurogenesis and produces long-term cognitive deficits in female hamsters. PLoS One 5, e15267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girotti M, Weinberg MS, and Spencer RL, 2009. Diurnal expression of functional and clock-related genes throughout the rat hpa axis: System-wide shifts in response to a restricted feeding schedule. Am. J. Physiol. Endocrinol. Metab 296, E888–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golombek DA, and Rosenstein RE, 2010. Physiology of circadian entrainment. Physiol. Rev 90, 1063–1102. [DOI] [PubMed] [Google Scholar]
- Gompf HS, and Aston-Jones G, 2008. Role of orexin input in the diurnal rhythm of locus coeruleus impulse activity. Brain Res 1224, 43–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González MMC, and Aston-Jones G, 2006. Licircadian regulation of arousal: Role of the noradrenergic locus coeruleus system and light exposure. Sleep 29, 1327–1336. [DOI] [PubMed] [Google Scholar]
- Guo D, and Hu J, 2014. Spinal presynaptic inhibition in pain control. Neuroscience 283, 95–106. [DOI] [PubMed] [Google Scholar]
- Hagenauer MH, Crodelle JA, Piltz SH, Toporikova N, Ferguson P, and Booth V, 2017. The modulation of pain by circadian and sleep-dependent processes: A review of the experimental evidence, in: Layton A, and Miller L (Eds.), Women in Mathematical Biology, pp. 1–21. [Google Scholar]
- Harkness JA, Richter MB, Panayi GS, Van de Pette K, Unger A, Pownall R, and Geddawi M, 1982. Circadian variation in disease activity in rheumatoid arthritis. Br. Med. J. (Clin. Res. Ed.) 284, 551–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattar S, Kumar M, Park A, Tong P, Tung J, Yau K, and Berson DM, 2006. Central projections of melanopsin- expressing retinal ganglion cells in the mouse. J. Comp. Neurol 497, 326–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haus EL, and Smolensky MH, 2013. Shift work and cancer risk: Potential mechanistic roles of circadian disruption, light at night, and sleep deprivation. Physiol. Behav 17, 273–284. [DOI] [PubMed] [Google Scholar]
- Hayashi Y, Koyanagi S, Kusunose N, Okada R, Wu Z, Tozaki-Saitoh H, Ukai K, Kohsaka S, Inoue K, Ohdo S, and Nakanishi H, 2013a. The intrinsic microglial molecular clock controls synaptic strength via the circadian expression of cathepsin s. Sci. Rep 3, 2744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi Y, Koyanagi S, Kusunose N, Takayama F, Okada R, Wu Z, and Nakanishi H, 2013b. Diurnal spatial rearrangement of microglial processes through the rhythmic expression of p2y12 receptors. J. Neurol. Disord 1, 10.4172. [Google Scholar]
- Hery F, Chouvet G, Kan JP, Pujol JF, and Glowinski J, 1977. Daily variations of various parameters of serotonin metabolism in the rat brain. Ii. Circadian variations in serum and cerebral tryptophan levels: Lack of correlation with 5-ht turnover. Brain Res 123, 137–145. [DOI] [PubMed] [Google Scholar]
- Heybach JP, and Vernikos-Danellis J, 1978. The effect of pituitary- adrenal function in the modulation of pain sensitivity in the rat. J. Psychiatry Neurosci 283, 331–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland P, and Goadsby PJ, 2007. The hypothalamic orexinergic system: Pain and primary headaches: Cme. Headache: J. of Head and Face Pain 47, 951–962. [DOI] [PubMed] [Google Scholar]
- Hsu DT, Kirouac GJ, Zubieta JK, and Bhatnagar S, 2014. Contributions of the paraventricular thalamic nucleus in the regulation of stress, motivation, and mood. Front. Behav. Neurosci 8, 73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iannetti GD, and Mouraux A, 2010. From the neuromatrix to the pain matrix (and back). Exp. Brain Res 205, 1–12. [DOI] [PubMed] [Google Scholar]
- Ibrahim MM, Patwardhan A, Gilbraith KB, Moutal A, Yang X, Chew LA, Largent-Milnesᵇ T, Malan TP, Vanderah TW, and Porreca F, 2017. Long-lasting antinociceptive effects of green light in acute and chronic pain in rats. Pain 158, 347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue K, and Tsuda M, 2018. Microglia in neuropathic pain: Cellular and molecular mechanisms and therapeutic potential. Nat. Rev. Neurosci 19, 138–152. [DOI] [PubMed] [Google Scholar]
- Izumo N, Ishibashi Y, Ohba M, Morikawa T, and Manabe T, 2012. Decreased voluntary activity and amygdala levels of serotonin and dopamine in ovariectomized rats. Behav. Brain Res 227, 1–6. [DOI] [PubMed] [Google Scholar]
- Junker U, and Wirz S, 2010. Chronobiology: Influence of circadian rhythms on the therapy of severe pain. Journal of oncology pharmacy practice 16, 81–87. [DOI] [PubMed] [Google Scholar]
- Kalil B, Ribeiro AB, Leite CM, Uchôa ET, Carolino RO, Cardoso TSR, Elias LLK, Rodrigues JA, Plant TM, and Poletini MO, 2016. The increase in signaling by kisspeptin neurons in the preoptic area and associated changes in clock gene expression that trigger the lh surge in female rats are dependent on the facilitatory action of a noradrenaline input. Endocrinology 157, 323–335. [DOI] [PubMed] [Google Scholar]
- Kaushal R, Taylor BK, Jamal AB, Zhang L, Ma F, Donahue R, and Westlund KN, 2016. Gaba-a receptor activity in the noradrenergic locus coeruleus drives trigeminal neuropathic pain in the rat; contribution of naα1 receptors in the medial prefrontal cortex. Neuroscience 334, 148–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavaliers M, and Hirst M, 1983. Daily rhythms of analgesia in mice: Effects of age and photoperiod. Brain Res 279, 387–393. [DOI] [PubMed] [Google Scholar]
- Khasabov SG, Malecha P, Noack J, Tabakov J, Okamoto K, Bereiter DA, and Simone DA, 2015. Activation of rostral ventromedial medulla neurons by noxious stimulation of cutaneous and deep craniofacial tissues. J. Neurophysiol 113, 14–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HK, Lee S-Y, Koike N, Kim E, Wirianto M, Burish MJ, Yagita K, Lee HK, Chen Z, and Chung JM, 2020. Circadian regulation of chemotherapy-induced peripheral neuropathic pain and the underlying transcriptomic landscape. Sci. Rep 10, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MJ, Chung JW, Kho H-S, and Park JW, 2015. The circadian rhythm variation of pain in the orofacial region. Journal of Oral Medicine and Pain 40, 89–95. [Google Scholar]
- Korszun A, 2000. Sleep and circadian rhythm disorders in fibromyalgia. Curr. Rheumatol. Rep 2, 124–130. [DOI] [PubMed] [Google Scholar]
- Koyanagi S, Kusunose N, Taniguchi M, Akamine T, Kanado Y, Ozono Y, Masuda T, Kohro Y, Matsunaga N, and Tsuda M, 2016. Glucocorticoid regulation of atp release from spinal astrocytes underlies diurnal exacerbation of neuropathic mechanical allodynia. Nature Comm 7, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriegsfeld LJ, LeSauter J, Hamada T, Pitts SM, and Silver R, 2002. Circadian rhythms in the endocrine system, in: Pfaff DW, Arnold AP, Fahrbach SE, Etgen AM, and RT R (Eds.), Hormones, Brain and Behavior, Cambridge, pp. 33–91. [Google Scholar]
- Kriegsfeld LJ, Korets R, and Silver R, 2003. Expression of the circadian clock gene period 1 in neuroendocrine cells: An investigation using mice with a per1::gfp transgene. Eur. J. Neurosci 17, 212–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriegsfeld LJ, Leak RK, Yackulic CB, LeSauter J, and Silver R, 2004. Organization of suprachiasmatic nucleus projections in syrian hamsters (mesocricetus auratus): An anterograde and retrograde analysis. J. Comp. Neurol 468, 361–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ku J, Lee YS, Chang HW, Earley CJ, Allen RP, and Cho YW, 2018. Diurnal variation of default mode network in patients with restless legs syndrome. Sleep Med 41, 1–8. [DOI] [PubMed] [Google Scholar]
- Kulkarni B, Bentley DE, Elliott R, Youell P, Watson A, Derbyshire SWG, Frackowiak RSJ, Friston KJ, and Jones AKP, 2005. Attention to pain localization and unpleasantness discriminates the functions of the medial and lateral pain systems. Eur. J. Neurosci 21, 3133–3142. [DOI] [PubMed] [Google Scholar]
- Kundermann B, Hemmeter-Spernal J, Huber MT, Krieg JC, and Lautenbacher S, 2008. Effects of total sleep deprivation in major depression: Overnight improvement of mood is accompanied by increased pain sensitivity and augmented pain complaints. Psychosom. Med 70, 92–101. [DOI] [PubMed] [Google Scholar]
- Kundermann B, Spernal J, Huber MT, Krieg JC, and Lautenbacher S, 2004. Sleep deprivation affects thermal pain thresholds but not somatosensory thresholds in healthy volunteers. Psychosom. Med 66, 932–937. [DOI] [PubMed] [Google Scholar]
- Kurumaji A, Takashima M, Ohi K, and Takahashi K, 1988. Circadian fluctuations in pain responsiveness and brain met-enkephalin-like immunoreactivity in the rat. Pharmacol. Biochem. Behav 29, 595–599. [DOI] [PubMed] [Google Scholar]
- Kusunose N, Koyanagi S, Hamamura K, Matsunaga N, Yoshida M, Uchida T, Tsuda M, Inoue K, and Ohdo S, 2010. Molecular basis for the dosing time-dependency of anti-allodynic effects of gabapentin in a mouse model of neuropathic pain. Mol. Pain 6, 1744–8069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaCroix-Fralish ML, Mo G, Smith SB, Sotocinal SG, Ritchie J, Austin J-S, Melmed K, Schorscher-Petcu A, Laferriere AC, and Lee TH, 2009. The β3 subunit of the na+, k+-atpase mediates variable nociceptive sensitivity in the formalin test. Neuroscience 144, 294–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamont EW, Robinson B, Stewart J, and Amir S, 2005. The central and basolateral nuclei of the amygdala exhibit opposite diurnal rhythms of expression of the clock protein period2. Proc. Natl. Acad. Sci. U S A 102, 4180–4184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landgraf D, Long JE, and Welsh DK, 2016. Depression- like behaviour in mice is associated with disrupted circadian rhythms in nucleus accumbens and periaqueductal grey. Eur. J. Neurosci 43, 1309–1320. [DOI] [PubMed] [Google Scholar]
- Latremoliere A, and Woolf CJ, 2009. Central sensitization: A generator of pain hypersensitivity by central neural plasticity. J. Pain 10, 895–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau BK, and Vaughan CW, 2014. Descending modulation of pain: The gaba disinhibition hypothesis of analgesia. Curr. Opin. Neurobiol 29, 159–164. [DOI] [PubMed] [Google Scholar]
- Lautenbacher S, Kundermann B, and Krieg JC, 2006. Sleep deprivation and pain perception. Pharmacol. Ther 10, 357–369. [DOI] [PubMed] [Google Scholar]
- Lee PR, Cohen JE, Iacobas DA, Iacobas S, and Fields RD, 2017. Gene networks activated by specific patterns of action potentials in dorsal root ganglia neurons. Sci. Rep 7, 43765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legrain V, Iannetti GD, Plaghki L, and Mouraux A, 2011. The pain matrix reloaded: A salience detection system for the body. Prog. Neurobiol 93, 111–124. [DOI] [PubMed] [Google Scholar]
- Leung CG, and Mason P, 1999. Physiological properties of raphe magnus neurons during sleep and waking. J. Neurophysiol 81, 584–595. [DOI] [PubMed] [Google Scholar]
- Li JZ, Bunney BG, Meng F, Hagenauer MH, Walsh DM, Vawter MP, Evans SJ, Choudary PV, Cartagena P, Barchas JD, Schatzberg AF, Jones EG, Myers RM, Watson SJ, Akil H, and Bunney WE, 2013. Circadian patterns of gene expression in the human brain and disruption in major depressive disorder. Proc. Natl. Acad. Sci. U S A 110, 9950–9955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W-W, Guo T-Z, Liang D. y., Sun Y, Kingery WS, and Clark JD, 2012. Substance p signaling controls mast cell activation, degranulation, and nociceptive sensitization in a rat fracture model of complex regional pain syndrome. Nature Comm 116, 882–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan RW, and Sarkar DK, 2012. Circadian nature of immune function. Mol. Cell. Endocrinol 349, 82–90. [DOI] [PubMed] [Google Scholar]
- Lu C, Yang T, Zhao H, Zhang M, Meng F, Fu H, Xie Y, and Xu H, 2016. Insular cortex is critical for the perception, modulation, and chronification of pain. Neurosci. Bull 32, 191–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y, Dong H, Gao Y, Gong Y, Ren Y, Gu N, Zhou S, Xia N, Sun YY, Ji RR, and Xiong L, 2013. A feed-forward spinal cord glycinergic neural circuit gates mechanical allodynia. J. Clin. Invest 123, 4050–4062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahoney CE, Brewer JM, and Bittman EL, 2013. Central control of circadian phase in arousal-promoting neurons. PLoS One 8, e67173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maihöfner C, Herzner B, and Otto Handwerker H, 2006. Secondary somatosensory cortex is important for the sensory-discriminative dimension of pain: A functional mri study. Eur. J. Neurosci 23, 1377–1383. [DOI] [PubMed] [Google Scholar]
- Mäkelä KA, Karhu T, Jurado Acosta A, Vakkuri O, Leppäluoto J, and Herzig KH, 2018. Plasma orexin-a levels do not undergo circadian rhythm in young healthy male subjects. Front. Endocrinol 9, 710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansour AR, Farmer MA, Baliki MN, and Apkarian AV, 2014. Chronic pain: The role of learning and brain plasticity. Restor. Neurol. Neurosci 32, 129–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus JN, and Elmquist JK, 2006. Orexin projections and localization of orexin receptors, in: (Eds.), The Orexin/Hypocretin System, Totowa, NJ, pp. 21–43. [Google Scholar]
- Marston OJ, Williams RH, Canal MM, Samuels RE, Upton N, and Piggins HD, 2008. Circadian and dark-pulse activation of orexin/hypocretin neurons. Mol. Brain 1, 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin LF, Moutal A, Cheng K, Washington SM, Calligaro H, Goel V, Kranz T, Largent-Milnes TM, Khanna R, and Patwardhan A, 2021. Green light antinociceptive and reversal of thermal and mechanical hypersensitivity effects rely on endogenous opioid system stimulation. J. Pain In Press, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez-Gómez M, Cruz Y, Salas M, Hudson R, and Pacheco P, 1994. Assessing pain threshold in the rat: Changes with estrus and time of day. Physiol. Behav 55, 651–657. [DOI] [PubMed] [Google Scholar]
- Matre D, Knardahl S, and Nilsen KB, 2017. Night-shift work is associated with increased pain perception. Scand. J. Work Env. Health 43, 260–268. [DOI] [PubMed] [Google Scholar]
- McCracken LM, and Iverson GL, 2002. Disrupted sleep patterns and daily functioning in patients with chronic pain. Pain Res. Manag 7, 75–79. [DOI] [PubMed] [Google Scholar]
- McHill AW, and Wright KP, 2017. Role of sleep and circadian disruption on energy expenditure and in metabolic predisposition to human obesity and metabolic disease. Pharmacol. Ther 18, 15–24. [DOI] [PubMed] [Google Scholar]
- Metz AE, Yau HJ, Centeno MV, Apkarian AV, and Martina M, 2009. Morphological and functional reorganization of rat medial prefrontal cortex in neuropathic pain. Proc. Natl. Acad. Sci. U S A 106, 2423–2438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsushima D, Mizuno T, and Kimura F, 1996. Age-related changes in diurnal acetylcholine release in the prefrontal cortex of male rats as measured by microdialysis. Neuroscience 72, 429–434. [DOI] [PubMed] [Google Scholar]
- Miyake S, Sumi Y, Yan L, Takekida S, Fukuyama T, Ishida Y, Yamaguchi S, Yagita K, and Okamura H, 2000. Phase-dependent responses of per1 and per2 genes to a light-stimulus in the suprachiasmatic nucleus of the rat. Neurosci. Lett 294, 41–44. [DOI] [PubMed] [Google Scholar]
- Monošíková J, Herichová I, Mravec B, Kiss A, and Zeman M, 2007. Effect of upregulated renin–angiotensin system on per2 and bmal1 gene expression in brain structures involved in blood pressure control in tgr(mren-2)27 rats. Brain Res 1180, 29–38. [DOI] [PubMed] [Google Scholar]
- Morioka N, Sugimoto T, Tokuhara M, Nakamura Y, Abe H, Hisaoka K, Dohi T, and Nakata Y, 2012. Spinal astrocytes contribute to the circadian oscillation of glutamine synthase, cyclooxygenase-1 and clock genes in the lumbar spinal cord of mice. Annu. Rev. Neurosci 60, 817–826. [DOI] [PubMed] [Google Scholar]
- Morioka N, Saeki M, Sugimoto T, Higuchi T, Zhang FF, Nakamura Y, Hisaoka-Nakashima K, and Nakata Y, 2016. Downregulation of the spinal dorsal horn clock gene per1 expression leads to mechanical hypersensitivity via c-jun n-terminal kinase and ccl2 production in mice. Neuroscience 72, 72–83. [DOI] [PubMed] [Google Scholar]
- Moriya S, Tahara Y, Sasaki H, Ishigooka J, and Shibata S, 2015. Phase-delay in the light-dark cycle impairs clock gene expression and levels of serotonin, norepinephrine, and their metabolites in the mouse hippocampus and amygdala. Sleep Med 16, 1352–1359. [DOI] [PubMed] [Google Scholar]
- Moriya T, Aida R, Kudo T, Akiyama M, Doi M, Hayasaka N, Nakahata N, Mistlberger R, Okamura H, and Shibata S, 2009. The dorsomedial hypothalamic nucleus is not necessary for food-anticipatory circadian rhythms of behavior, temperature or clock gene expression in mice. Eur. J. Neurosci 29, 1447–1460. [DOI] [PubMed] [Google Scholar]
- Mozzanica N, Finzi AF, Foppa S, Vignati G, and Villa ML, 1991. Association between circadian rhythms of endogenous hypothalamic opioid peptides and of natural killer cell activity. Int. J. Immunopharmacol 13, 317–321. [DOI] [PubMed] [Google Scholar]
- Mozzanica N, Villa ML, Foppa S, Vignati G, Cattaneo A, Diotti R, and Finzi AF, 1992. Plasma alpha-melanocyte-stimulating hormone, beta-endorphin, met-enkephalin, and natural killer cell activity in vitiligo. Pharmacol. Biochem. Behav 26, 693–700. [DOI] [PubMed] [Google Scholar]
- Nagoshi E, Saini C, Bauer C, Laroche T, Naef F, and Schibler U, 2004. Circadian gene expression in individual fibroblasts: Cell-autonomous and self-sustained oscillators pass time to daughter cells. Cell 119, 693–705. [DOI] [PubMed] [Google Scholar]
- Natali J-P, McRae-Degueurce A, Keane P, Debilly G, and Pujol J-F, 1980. Genetic studies of daily variations of first-step enzymes of monoamines metabolism in the brain of inbred strains of mice and hybrids. Ii. Daily variations of tyrosine hydroxylase activity in the locus coeruleus. Brain Res 191, 205–213. [DOI] [PubMed] [Google Scholar]
- Negri L, Lattanzi R, Giannini E, Metere A, Colucci M, Barra D, Kreil G, and Melchiorri P, 2002. Nociceptive sensitization by the secretory protein bv8. Br. J. Pharmacol 137, 1147–1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson RJ, Bumgarner JR, Walker WH, and DeVries AC, 2021. Time-of-day as a critical biological variable. Neurosci. Biobehav. Rev 127, 740–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neugebauer V, 2015. Amygdala pain mechanisms. Handb. Exp. Pharmacol 227, 261–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odo M, Koh K, Takada T, Yamashita A, Narita M, Kuzumaki N, Ikegami D, Sakai H, Iseki M, Inada E, and Narita M, 2014. Changes in circadian rhythm for mrna expression of melatonin 1a and 1b receptors in the hypothalamus under a neuropathic pain-like state. Synapse 68, 153–158. [DOI] [PubMed] [Google Scholar]
- Odrcich M, Bailey JM, Cahill CM, and Gilron I, 2006. Chronobiological characteristics of painful diabetic neuropathy and postherpetic neuralgia: Diurnal pain variation and effects of analgesic therapy. Pain 120, 207–212. [DOI] [PubMed] [Google Scholar]
- Oliverio A, Castellano C, and Puglisi-Allegra S, 1982. Opiate analgesia: Evidence for circadian rhythms in mice. Brain Res 249, 265–270. [DOI] [PubMed] [Google Scholar]
- Onen SH, Alloui A, Gross A, Eschallier A, and Dubray C, 2001. The effects of total sleep deprivation, selective sleep interruption and sleep recovery on pain tolerance thresholds in healthy subjects. J. Sleep Res 10, 35–42. [DOI] [PubMed] [Google Scholar]
- Ong WY, Stohler CS, and Herr DR, 2019. Role of the prefrontal cortex in pain processing. Mol. Neurobiol 56, 1137–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onishi H, Yamaguchi S, Yagita K, Ishida Y, Dong X, Kimura H, Jing Z, Ohara H, and Okamura H, 2002. Rev-erbalpha gene expression in the mouse brain with special emphasis on its circadian profiles in the suprachiasmatic nucleus. J. Neurosci. Res 68, 551–557. [DOI] [PubMed] [Google Scholar]
- Orozco-Solis R, Aguilar-Arnal L, Murakami M, Peruquetti R, Ramadori G, Coppari R, and Sassone-Corsi P, 2016. The circadian clock in the ventromedial hypothalamus controls cyclic energy expenditure. Cell Metab 23, 467–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ossipov MH, Morimura K, and Porreca F, 2014. Descending pain modulation and chronification of pain. Behav. Brain Res [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostrowsky K, Magnin M, Ryvlin P, Isnard J, Guenot M, and Mauguière F, 2002. Representation of pain and somatic sensation in the human insula: A study of responses to direct electrical cortical stimulation. Cereb. Cortex 12, 376–385. [DOI] [PubMed] [Google Scholar]
- Palada V, Gilron I, Canlon B, Svensson CI, and Kalso E, 2020. The circadian clock at the intercept of sleep and pain. Pain 161, 894–900. [DOI] [PubMed] [Google Scholar]
- Partch CL, Green CB, and Takahashi JS, 2014. Molecular architecture of the mammalian circadian clock. PLoS One 24, 90–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pertovaara A, and Almeida A, 2006. Descending inhibitory systems. Handb. Clin. Neurol 81, 179–192. [DOI] [PubMed] [Google Scholar]
- Petraglia F, Facchinetti F, Parrini D, Micieli G, De Luca S, and Genazzani AR, 1983. Simultaneous circadian variations of plasma acth, beta-lipotropin, beta-endorphin and cortisol. Horm. Res 17, 147–152. [DOI] [PubMed] [Google Scholar]
- Pevet P, and Challet E, 2011. Melatonin: Both master clock output and internal time-giver in the circadian clocks network. J Physiol Paris 105, 170–182. [DOI] [PubMed] [Google Scholar]
- Pickard GE, 1987. Circadian rhythm of nociception in the golden hamster. Brain Res 425, 395–400. [DOI] [PubMed] [Google Scholar]
- Pieh C, Jank R, Waiß C, Pfeifer C, Probst T, Lahmann C, and Oberndorfer S, 2018. Night-shift work increases cold pain perception. Sleep Med 45, 74–79. [DOI] [PubMed] [Google Scholar]
- Porro CA, Baraldi P, Pagnoni G, Serafini M, Facchin P, Maieron M, and Nichelli P, 2002. Does anticipation of pain affect cortical nociceptive systems. J. Neurosci 22, 3206–3214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pringsheim T, 2002. Cluster headache: Evidence for a disorder of circadian rhythm and hypothalamic function. Can. J. Neurol. Sci 29, 33–40. [DOI] [PubMed] [Google Scholar]
- Rainville P, Duncan GH, Price DD, Carrier B, and Bushnell MC, 1997. Pain affect encoded in human anterior cingulate but not somatosensory cortex. Science 277, 968–971. [DOI] [PubMed] [Google Scholar]
- Razavi BM, and Hosseinzadeh H, 2017. A review of the role of orexin system in pain modulation. Biomed. Pharmacother 90, 187–193. [DOI] [PubMed] [Google Scholar]
- Reid LD, Konecka AM, Przewłocki R, Millan MH, Millan MJ, and Herz A, 1982. Endogenous opioids, circadian rhythms, nutrient deprivation, eating and drinking. Life Sci 31, 1829–1832. [DOI] [PubMed] [Google Scholar]
- Reiter RJ, Tan DX, Korkmaz A, Erren TC, Piekarski C, Tamura H, and Manchester LC, 2007. Light at night, chronodisruption, melatonin suppression, and cancer risk: A review. Crit. Rev. Oncog 13, 303–328. [DOI] [PubMed] [Google Scholar]
- Roenneberg T, Allebrandt KV, Merrow M, and Vetter C, 2012. Social jetlag and obesity. Curr. Biol 22, 939–943. [DOI] [PubMed] [Google Scholar]
- Ruben MD, Wu G, Smith DF, Schmidt RE, Francey LJ, Lee YY, Anafi RC, and Hogenesch JB, 2018. A database of tissue-specific rhythmically expressed human genes has potential applications in circadian medicine. Sci. Transl. Med 10, eaat8806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russart KLG, and Nelson RJ, 2018. Light at night as an environmental endocrine disruptor. Physiol. Behav 190, 82–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saini A, Tucci M, Tampellini M, Maina D, Bouraouia K, Giuliano PL, Termine A, Castellano M, Campagna S, Laciura P, and Berruti A, 2013. Circadian variation of breakthrough pain in cancer patients. Eur. J. Pain 17, 264–270. [DOI] [PubMed] [Google Scholar]
- Salomon RM, Ripley B, Kennedy JS, Johnson B, Schmidt D, Zeitzer JM, Nishino S, and Mignot E, 2003. Diurnal variation of cerebrospinal fluid hypocretin-1 (orexin-a) levels in control and depressed subjects. Biol. Psychiatry 54, 96–104. [DOI] [PubMed] [Google Scholar]
- Sapin E, Lapray D, Bérod A, Goutagny R, Léger L, Ravassard P, Clément O, Hanriot L, Fort P, and Luppi PH, 2009. Localization of the brainstem gabaergic neurons controlling paradoxical (rem) sleep. PLoS One 4, e4272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaible H-G, 2006. Peripheral and central mechanisms of pain generation, in: Stein C (Eds.), Analgesia, Berlin, Heidelberg, pp. 3–28. [DOI] [PubMed] [Google Scholar]
- Scheiermann C, Kunisaki Y, and Frenette PS, 2013. Circadian control of the immune system. Nat. Rev. Immunol 13, 190–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz JR, and Roth T, 2008. Neurophysiology of sleep and wakefulness: Basic science and clinical implications. Curr. Neuropharmacol 6, 367–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal JP, Tresidder KA, Bhatt C, Gilron I, and Ghasemlou N, 2018. Circadian control of pain and neuroinflammation. J. Neurosci. Res 96, 1002–1020. [DOI] [PubMed] [Google Scholar]
- Smith MT, Perlis ML, Smith MS, Giles DE, and Carmody TP, 2000. Sleep quality and presleep arousal in chronic pain. J. Behav. Med 23, 1–13. [DOI] [PubMed] [Google Scholar]
- Solaro C, Uccelli MM, Guglieri P, Uccelli A, and Mancardi GL, 2000. Gabapentin is effective in treating nocturnal painful spasms in multiple sclerosis. Mult. Scler 6, 192–193. [DOI] [PubMed] [Google Scholar]
- Spies HG, Mahoney CJ, Norman RL, Clifton DK, and Resko JA, 1974. Evidence for a diurnal rhythm in ovarian steroid secretion in the rhesus monkey. J. Psychiatry Neurosci 39, 347–351. [DOI] [PubMed] [Google Scholar]
- Starr CJ, Sawaki L, Wittenberg GF, Burdette JH, Oshiro Y, Quevedo AS, and Coghill RC, 2009. Roles of the insular cortex in the modulation of pain: Insights from brain lesions. J. Neurosci 29, 2684–2694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephan FK, and Zucker I, 1972. Circadian rhythms in drinking behavior and locomotor activity of rats are eliminated by hypothalamic lesions. Proc. Natl. Acad. Sci. U S A 69, 1583–1586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sylvester CM, Krout KE, and Loewy AD, 2002. Suprachiasmatic nucleus projection to the medial prefrontal cortex: A viral transneuronal tracing study. Neuroscience 114, 1071–1080. [DOI] [PubMed] [Google Scholar]
- Taheri S, Sunter D, Dakin C, Moyes S, Seal L, Gardiner J, Rossi M, Ghatei M, and Bloom S, 2000. Diurnal variation in orexin a immunoreactivity and prepro-orexin mrna in the rat central nervous system. Neurosci. Lett 279, 109–112. [DOI] [PubMed] [Google Scholar]
- Takada T, Yamashita A, Date A, Yanase M, Suhara Y, Hamada A, Sakai H, Ikegami D, Iseki M, and Inada E, 2013. Changes in the circadian rhythm of mrna expression for μ- opioid receptors in the periaqueductal gray under a neuropathic pain- like state. Proc. Natl. Acad. Sci. U S A 67, 216–223. [DOI] [PubMed] [Google Scholar]
- Takahashi H, Motomatsu T, Nawata H, Kato K, Ibayashi H, and Nobunaga M, 1986. Influences of feeding and drinking on circadian rhythms of opioid peptides in plasma, hypothalamus and pituitary gland in rats. Physiol. Behav 37, 609–614. [DOI] [PubMed] [Google Scholar]
- Takahashi M, Matsudaira K, and Shimazu A, 2015. Disabling low back pain associated with night shift duration: Sleep problems as a potentiator. Pharmacol. Ther 58, 1300–1310. [DOI] [PubMed] [Google Scholar]
- Tang JS, Qu CL, and Huo FQ, 2009. The thalamic nucleus submedius and ventrolateral orbital cortex are involved in nociceptive modulation: A novel pain modulation pathway. Prog. Neurobiol 89, 383–389. [DOI] [PubMed] [Google Scholar]
- Thaiss CA, Zeevi D, Levy M, Zilberman-Schapira G, Suez J, Tengeler AC, Abramson L, Katz MN, Korem T, and Zmora N, 2014. Transkingdom control of microbiota diurnal oscillations promotes metabolic homeostasis. Cell 159, 514–529. [DOI] [PubMed] [Google Scholar]
- Timmermann L, Ploner M, Haucke K, Schmitz F, Baltissen R, and Schnitzler A, 2001. Differential coding of pain intensity in the human primary and secondary somatosensory cortex. J. Neurophysiol 86, 1499–1503. [DOI] [PubMed] [Google Scholar]
- Todd AJ, 2010. Neuronal circuitry for pain processing in the dorsal horn. Nat. Rev. Neurosci 11, 823–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuneki H, Wada T, and Sasaoka T, 2018. Chronopathophysiological implications of orexin in sleep disturbances and lifestyle-related disorders. Pharmacol. Ther 186, 25–44. [DOI] [PubMed] [Google Scholar]
- Walker WH, Walton JC, DeVries AC, and Nelson RJ, 2020. Circadian rhythm disruption and mental health. Transl. Psychiatry 10, 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warnecke M, Oster H, Revelli JP, Alvarez-Bolado G, and Eichele G, 2005. Abnormal development of the locus coeruleus in ear2(nr2f6)-deficient mice impairs the functionality of the forebrain clock and affects nociception. Genes Dev 19, 614–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weitzman ED, Fukushima D, Nogeire C, Roffwarg H, Gallagher TF, and Hellman L, 1971. Twenty-four hour pattern of the episodic secretion of cortisol in normal subjects. J. Psychiatry Neurosci 33, 14–22. [DOI] [PubMed] [Google Scholar]
- Wesche DL, and Frederickson RC, 1981. The role of the pituitary in the diurnal variation in tolerance to painful stimuli and brain enkephalin levels. Life Sci 29, 2199–2205. [DOI] [PubMed] [Google Scholar]
- Wilhelmsen M, Amirian I, Reiter RJ, Rosenberg J, and Gögenur I, 2011. Analgesic effects of melatonin: A review of current evidence from experimental and clinical studies. J. Pineal Res 51, 270–277. [DOI] [PubMed] [Google Scholar]
- Woodruff ER, Chun LE, Hinds LR, and Spencer RL, 2016. Diurnal corticosterone presence and phase modulate clock gene expression in the male rat prefrontal cortex. Endocrinology 157, 1522–1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia T, Chu S, Cui Y, Xu F, Liu Y, Song J, Qian Y, Shao X, Li X, Gu X, and Ma Z, 2017. The role of nr2b-creb-mir212/132-crtc1-creb signal network in pain regulation in vitro and in vivo. Anesth. Analg 124, 2045–2053. [DOI] [PubMed] [Google Scholar]
- Xia T, Cui Y, Qian Y, Chu S, Song J, Gu X, and Ma Z, 2016. Regulation of the nr2b-creb-crtc1 signaling pathway contributes to circadian pain in murine model of chronic constriction injury. Neuroscience 122, 542–552. [DOI] [PubMed] [Google Scholar]
- Xiao X, and Zhang YQ, 2018. A new perspective on the anterior cingulate cortex and affective pain. Neurosci. Biobehav. Rev 90, 200–211. [DOI] [PubMed] [Google Scholar]
- Xu F, Zhao X, Liu H, Shao X, Chu S, Gong X, Ma Z, and Gu X, 2018. Misaligned feeding may aggravate pain by disruption of sleep-awake rhythm. Mol. Pain 127, 255–262. [DOI] [PubMed] [Google Scholar]
- Yamamoto S, Shigeyoshi Y, Ishida Y, Fukuyama T, Yamaguchi S, Yagita K, Moriya T, Shibata S, Takashima N, and Okamura H, 2001. Expression of the per1 gene in the hamster: Brain atlas and circadian characteristics in the suprachiasmatic nucleus. J. Comp. Neurol 430, 518–532. [PubMed] [Google Scholar]
- Yang SC, Chen CL, Yi CH, Liu TT, and Shieh KR, 2015. Changes in gene expression patterns of circadian-clock, transient receptor potential vanilloid-1 and nerve growth factor in inflamed human esophagus. Sci. Rep 5, 13602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo SH, Yamazaki S, Lowrey PL, Shimomura K, Ko CH, Buhr ED, Siepka SM, Hong HK, Oh WJ, Yoo OJ, Menaker M, and Takahashi JS, 2004. Period2::luciferase real-time reporting of circadian dynamics reveals persistent circadian oscillations in mouse peripheral tissues. Proc. Natl. Acad. Sci. U S A 101, 5339–5346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu T, Zhang X, Shi H, Tian J, Sun L, Hu X, Cui W, and Du D, 2019. P2y12 regulates microglia activation and excitatory synaptic transmission in spinal lamina ii neurons during neuropathic pain in rodents. Sci. Rep 10, 165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeitzer JM, Buckmaster CL, Parker KJ, Hauck CM, Lyons DM, and Mignot E, 2003. Circadian and homeostatic regulation of hypocretin in a primate model: Implications for the consolidation of wakefulness. J. Neurosci 23, 3555–3560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Truong KK, and Zhou Q-Y, 2009. Efferent projections of prokineticin 2 expressing neurons in the mouse suprachiasmatic nucleus. PLoS One 4, e7151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Li H, Teng H, Zhang T, Luo Y, Zhao M, Li YQ, and Sun ZS, 2012. Regulation of peripheral clock to oscillation of substance p contributes to circadian inflammatory pain. Anesthesiol 117, 149–160. [DOI] [PubMed] [Google Scholar]
- Zhang S, Zeitzer JM, Yoshida Y, Wisor JP, Nishino S, Edgar DM, and Mignot E, 2004. Lesions of the suprachiasmatic nucleus eliminate the daily rhythm of hypocretin-1 release. Sleep 27, 619–627. [DOI] [PubMed] [Google Scholar]
- Zhao I, Bogossian F, and Turner C, 2012. The effects of shift work and interaction between shift work and overweight/obesity on low back pain in nurses: Results from a longitudinal study. Pharmacol. Ther 54, 820–825. [DOI] [PubMed] [Google Scholar]
- Zheng D, Ratiner K, and Elinav E, 2020. Circadian influences of diet on the microbiome and immunity. Trends. Immunol 41, 512–530. [DOI] [PubMed] [Google Scholar]
- Zieglgänsberger W, 2019. Substance p and pain chronicity. Cell Tissue Res 375, 227–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


