Abstract
Background:
Negative stress significantly impacts major depressive disorder (MDD), given the shared brain circuitry between the stress response and mood. Thus, interventions that target this circuitry will have an important impact on MDD. The aim of this study was to evaluate the acute effects of a novel respiratory-gated auricular vagal afferent nerve stimulation (RAVANS) technique in the modulation of brain activity and connectivity in women with MDD in response to negative stressful stimuli.
Methods:
Twenty premenopausal women with recurrent MDD in an active episode were included in a cross-over experimental study that included two functional MRI visits within one week, randomized to receive exhalatory- (e-RAVANS) or inhalatory-gated (i-RAVANS) at each visit. Subjects were exposed to a visual stress challenge that preceded and followed RAVANS. A Factorial analysis was used to evaluate the effects of RAVANS on brain activity and connectivity and changes in depressive and anxiety symptomatology post-stress.
Results:
Compared with i-RAVANS, e-RAVANS was significantly associated with increased activation of subgenual anterior cingulate, orbitofrontal and ventromedial prefrontal cortices and increased connectivity between hypothalamus and dorsolateral prefrontal cortex, and from nucleus tractus solitarii to locus coeruleus and ventromedial prefrontal cortex. Changes in brain activity and connectivity after e-RAVANS were significantly associated with a reduction in depressive and anxiety symptoms.
Conclusions:
Our study suggests exhalatory-gated RAVANS effectively modulates brain circuitries regulating response to negative stress and is associated with significant acute reduction of depressive and anxiety symptomatology in women with recurrent MDD. Findings suggest a potential non-pharmacologic intervention for acute relief of depressive symptomatology in MDD.
Keywords: Major depression, vagus nerve, transcutaneous auricular vagus nerve stimulation, respiration, fMRI, stress response
INTRODUCTION
Major depressive disorder (MDD) has been associated with alterations of the stress response circuitry, including the hypothalamus (HYPO), amygdala (AMYG), hippocampus (HIPP), anterior cingulate cortex (ACC), ventromedial (vmPFC), dorsolateral (DLPFC) and orbital (OFC) prefrontal cortices (Mareckova et al., 2016; Mareckova et al., 2017). Many of these regions are morphologically and functionally sexually dimorphic and associated with vulnerability for sex differences in MDD and comorbid cardiovascular disease (CVD) risk, which has almost twice the rate in women than men (Goldstein et al., 2001; Goldstein et al., 2010; Goldstein et al., 2014). Neuroimaging studies, including ours (Garcia et al., 2020; Holsen et al., 2013; Mareckova et al., 2016; Mareckova et al., 2017) have suggested that alterations in this circuitry are implicated in mood dysregulation, increased activation of the hypothalamic-pituitary-adrenal (HPA) axis, and imbalance between the sympathetic and parasympathetic nervous system in depressed persons. These studies have demonstrated a differential and greater negative impact of depressed mood on brain activity and connectivity deficits in tandem with physiological dysregulation in women compared to men (Garcia et al., 2020). Thus, the development of novel interventions that regulate this system in a sex-dependent manner may have a significant impact on the improvement of clinical and physiological alterations of MDD.
Implantable Vagus Nerve Stimulation (VNS) is a neuromodulatory technique used for the management of treatment-resistant MDD (Aaronson et al., 2017; Daban et al., 2008; Nemeroff et al., 2006). However, VNS is invasive and associated with significant side effects and surgical morbidity (Daban et al., 2008), limiting broad applicability. Recently, a non-invasive variant of VNS, transcutaneous auricular vagus nerve stimulation (taVNS), which targets the auricular branch of the vagus nerve (ABVN) has been proposed (Ellrich, 2011; Ventureyra, 2000). Previous studies evaluating taVNS have shown promising antidepressant effects (Hein et al., 2013; Rong et al., 2016), however, responses to taVNS varied substantially (Wu et al., 2018). This may be due, in part, to individual variability in clinical presentation and history, but also to variability in neurobiology and specific brain targeting for taVNS in depressed individuals.
The mechanisms of action and neural pathways mediating taVNS effects are still unclear. However, neuroimaging and physiological studies have suggested that taVNS actions are mediated by modulation of the primary synapse for vagal afferents, located in the nucleus tractus solitarii (NTS) (Frangos et al., 2015; Garcia et al., 2017). From this brainstem nucleus, projections synapse with pontine monoaminergic nuclei, such as locus coeruleus (LC) and dorsal raphe nuclei (DRN), which have diffuse projections to higher-level stress response circuitry regions, including hypothalamus, amygdala, hippocampus, anterior cingulate cortex, and orbital, medial and dorsolateral prefrontal cortices (Badran et al., 2018; Frangos et al., 2015; Garcia et al., 2017; Kraus et al., 2007; Sclocco et al., 2019).
Interestingly, vagal afferent input and its regulatory actions on higher brain areas have been found to be affected by rhythmical oscillations in respiration (Garcia et al., 2017; Sclocco et al., 2019). The dorsal medullary vagal system operates in tune with respiration, such that NTS receives an inhibitory drive from the ventral respiratory group (VRG) medullary neurons during inhalation and a facilitatory input during exhalation (Baekey et al., 2010; Miyazaki et al., 1998; Miyazaki et al., 1999). Previous studies from our group have suggested that ABVN stimulation during exhalation, when medullary autonomic nuclei may be more receptive to facilitatory input, could enhance NTS modulation (Sclocco et al., 2019) as well as upstream neural circuitry involved in pain and mood regulation (Garcia et al., 2017).
In this study, we applied functional MRI (fMRI) to evaluate the effects of Respiratory-gated Auricular Vagal Afferent Nerve Stimulation (RAVANS), a novel form of respiratory-gated taVNS developed by our group (Garcia et al., 2017; Sclocco et al., 2019), in the modulation of the stress response circuitry of people with recurrent MDD. Given that our previous work demonstrated that depressed women exhibited greater alterations of this circuitry and associated physiological dysregulation than men (Garcia et al., 2020), we restricted this initial study to women. We hypothesized that compared to inhalatory-gated stimulation, exhalatory-gated RAVANS will more effectively modulate brain response to negative stressful stimuli with subsequent reduction of depressive and anxiety symptomatology in women with MDD.
MATERIAL AND METHODS
Subjects
Twenty premenopausal women (age: 30.3 ± 4.7 years) with recurrent MDD were included in the study. We chose to limit the reproductive stage of women to premenopausal first given the necessity to control for reproductive stage and menstrual phase due to the impact of gonadal hormones on regulation of this circuitry (Goldstein et al., 2010). A systematic psychiatric diagnostic interview was conducted during the screening visit including Structured Clinical Interview for DSM Diagnosis (SCID) (First et al., 2015) and the Hamilton Depression Rating Scale (HAM-D 17) (Hamilton, 1967). All subjects met criteria for recurrent MDD (≥ 2 episodes) and were in an active episode. 85% (n=17) of the participants were unmedicated at the time of inclusion, and three had received an SSRI or SNRI at a stable therapeutic dose for a minimum of 8 weeks. Exclusion criteria included contraindications for MRI, imminent suicide risk (i.e., current suicidal ideation or history of suicide attempt within last year), use of tricyclic antidepressants or other psychotropic medications except SSRI or SNRIs within four weeks prior to inclusion, diagnosis of bipolar affective disorder or substance abuse or dependence within past 12 months, presence of cardiac pacemakers or any implanted device, history of cardio,- cerebro-, or peripheral vascular disease, or clinically defined neurological disorder. The experimental procedure was approved by the Partners Human Research Committee, and participants were fully informed, gave written informed consent, and were paid for participation.
Study design
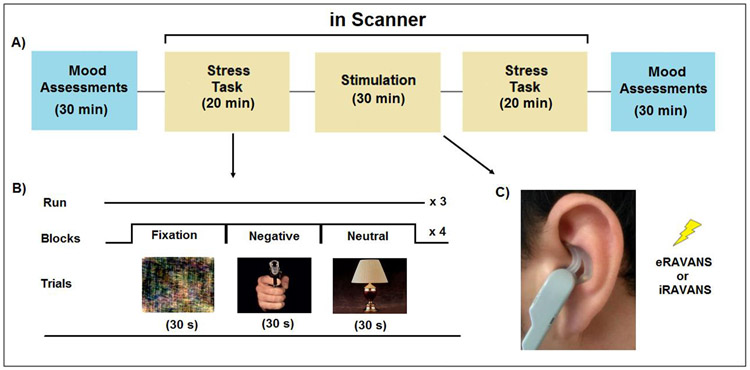
This study design was an experimental cross-over study of the effects of two types of taVNS on stress response circuitry and mood and anxiety symptoms in women undergoing a current episode of MDD. Subjects attended two scanning sessions within one-week interval during the follicular menstrual cycle phase. Participants were randomized to receive 30 minutes of exhalatory-gated (e-RAVANS) or inhalatory-gated taVNS (i-RAVANS) during the first or second scanning session (Figure 1). Before and after the stimulation period, subjects underwent three runs (360s each) of a mild visual stress challenge task in the scanner. Each run consisted of 12 blocks, four each of negative (high arousal/negative valence), neutral (low arousal/neutral valence) and fixation images (Fourier transform of neutral images) adapted from the International Affective Picture System (IAPS) (Lang, 2008), a task we developed and used for >16 years (Garcia et al., 2020; Goldstein et al., 2010; Holsen et al., 2013; Mareckova et al., 2016; Mareckova et al., 2017). IAPS images for e-RAVANS vs. i-RAVANS) were identical. During RAVANS administration, four scan runs totaling ~26 minutes (4 runs, duration for each run= 390 seconds) were collected (Figure 1).
Figure 1.
Experimental design. In a repeated-measures, cross-over design (A), participants attended two fMRI sessions in which they were exposed to a mild visual stress task (B) before and after exhalatory- (e-RAVANS) or inhalatory-gated (i-RAVANS) transcutaneous auricular vagus nerve stimulation (C). All subjects completed three runs (6 min each) of the mild visual stress task before and after RAVANS. Each run consisted of 12 blocks, 4 each of neutral (low arousal/neutral valence), negative (high arousal/negative valence) IAPS images and fixation stimuli (Fourier transforms of neutral images). Each block consisted of six different images, each presented for 5 seconds. During the stimulation period, 4 scan runs (6:30 min each) were collected.
Randomization and masking
A computer-generated randomization method was used to assign the order of e-RAVANS and i-RAVANS in recruited subjects. The randomization code was only given to the investigator operating the stimulation system on the first scanning session by an independent coordinator not involved with any other aspect of the study. Subjects as well as investigators involved in performing fMRI and behavioral evaluations and analyses remained blinded to the order of the intervention.
Behavioral Assessments
The Beck Depression Inventory-II (BDI-II (Beck et al., 1996)) and the State-Trait Anxiety Inventory (STAI (Spielberger et al., 1983), self-report questionnaires were administered outside the scanner at baseline (before the scan) and after the scan session (Figure 1) to evaluate acute changes in mood and anxiety symptoms. Subjective evaluation of image valence and arousal using the Self-assessment Manikin (SAM (Bradley and Lang, 1994)) was also collected. Behavioral data were analyzed using repeated measures ANOVA [mood and anxiety ratings by time (PRE-, POST-stimulation) and session (e-RAVANS, i-RAVANS)], controlled for baseline. A p<0.05 was designated for statistical significance.
Respiratory-gated auricular vagal afferent nerve stimulation
For taVNS, we used an MRI set-up of the respiratory-gated technique developed by our team (Garcia et al., 2017; Sclocco et al., 2019). Custom-built, ergonomically-shaped MR compatible electrodes (Bionik Medical Devices, Bucaramanga, Colombia) were placed in the left cymba concha of the auricle (Figure 1). Electrical stimulation to these electrodes was delivered by a current-constant stimulator (UROStim, Schwa Medico, Germany) and consisted of monophasic rectangular pulse trains with 300 μsec pulse width, duration of 0.8s and delivered at 30 Hz, during the exhalation (e-RAVANS) or inhalation (i-RAVANS) phase of respiration. The stimulation intensity was set by percept-matching across subjects, as described in our previous studies (Garcia et al., 2017; Sclocco et al., 2019).
Magnetic resonance imaging
All MRI scans were collected on a Siemens MAGNETOM Skyra 3T MRI scanner (Siemens Medical, Erlangen, Germany) with a 32-channel head coil. Subjects were instructed to relax and lay supine in the scanner with their eyes open while staying alert and awake. During the mild visual stress task fMRI, subjects were asked to press a button when each new image appeared to ensure attention. During RAVANS, they were asked to focus attention on any sensations experienced at the ear.
High-resolution (voxel size= 1 mm3) structural MRI scans were acquired using a T1-weighted MP-RAGE pulse sequence (TR= 2300 ms, TE= 2.95 ms, acceleration= GRAPPA factor 2, flip angle= 9°, FOV= 256 x 256 mm2, 176 axial slices). Whole-brain fMRI data were acquired with gradient-echo echo-planar imaging (EPI) using Simultaneous Multi-Slice (SMS) acquisition with multi-band factor 5 (TR= 1250ms, TE= 33 ms, flip angle= 65°, FOV= 200 x 200 mm2, 75 axial slices, voxel size= 2 mm3).
Peripheral physiological data were acquired during the study using a Powerlab system (ML880; ADInstruments Inc, Colorado Springs, CO) at a 1000 Hz sampling rate. We used a magnetic resonance-compatible pneumatic belt placed around the subject’s lower thorax to evaluate changes in respiratory volume. This system was constructed in-house and has been described in previous publications (Garcia et al., 2017; Sclocco et al., 2019). In addition, cardiac pulsatility data were acquired using a piezoelectric pulse transducer (AD instruments Inc., Colorado Springs, CO) attached to the right index finger.
Magnetic resonance imaging preprocessing
Imaging data were preprocessed using the Analysis of Functional Neuroimages (afni.nimh.nih.gov/afni) and the CONN toolbox. For each scan, pulse traces and respiration signals were resampled at 40 Hz and were used for physiological noise correction using AFNI’s RETROICOR function. Cardiac pulse annotation was performed using an automated method followed by manual confirmation, while respiratory volume per time was calculated using an automated algorithm and custom-made MATLAB scripts (The MathWorks Inc, Natick ,MA). Preprocessing of the fMRI data also included realignment, slice timing correction, non-linear volume-based spatial normalization (MNI152 brain template), spatial smoothing using a Gaussian filter (5 mm at FWHM), and artifact detection using artifact detection toolbox (ART) to identify outliers in the global mean image time series and movement. Outliers were included as covariates of no interest in the first-level, single-subject General Linear Model (GLM) analyses.
Magnetic resonance imaging data analysis
Comparisons of interest in the fMRI analysis (BOLD signal changes during exposure to negative vs neutral images) were tested in subjects that completed both stimulation sessions using linear contrasts and results from the individual subject level were submitted to a second-level factorial analysis with two factors: time of visual stress task (PRE- or POST-stimulation) and stimulation session (e-RAVANS or i-RAVANS). Significant interactions between these factors were then submitted to post-hoc analyses to determine directionality of the effect. Due to our a priori hypothesis of specific effects of RAVANS on stress response circuitry, we restricted the analysis to specific regions of interest (ROIs) (HYPO, AMYG, HIPP, ACC, subgenual ACC, mPFC, OFC) as described in previous publications from our group (Jacobs et al., 2015; Mareckova et al., 2016), using a small volume correction approach. We applied a voxel-wise height threshold of p<0.001 (uncorrected for multiple comparisons), and a cluster correction with FWE p-value<0.05. Mean beta weights within each significant cluster were extracted for each participant using the REX toolbox (Whitfield-Gabrieli, 2009) and were included in regression analyses in STATA (StataCorp, College Station TX) to investigate the link between variations in stress response circuitry activity and changes in depressive symptoms (BDI score difference: POST-PRE) after RAVANS administration. A p<0.05 was designated for statistical significance. Adjusted regression coefficients were calculated after including baseline BDI scores and use of antidepressant medications in the models.
Functional connectivity of the stress response circuitry
Functional connectivity was computed using seed-based correlation analysis with the CONN toolbox (Whitfield-Gabrieli and Nieto-Castanon, 2012). The seeds were located in NTS and DRN, key relay stations in the vagal afferent pathway (Garcia et al., 2017; Yuan and Silberstein, 2016), using previously described ROIs that were identified and significantly activated in response to RAVANS in our previous fMRI studies (Garcia et al., 2017; Sclocco et al., 2019). An additional HYPO seed (Halle et al., 2017) was used for this analysis, given the well-known importance of this structure in the mediation of vagal afferent effects in the regulation of neuroendocrine, autonomic and inflammatory responses to stress (Goldstein et al., 2019).
Functional connectivity was initially evaluated during RAVANS stimulation runs by extracting fMRI time series from the selected ROIs and using them in a weighted GLM and semi-partial correlation analyses. Resultant whole-brain parameter estimates and their variance from each individual were passed to group-level analyses to evaluate NTS and DRN connectivity differences between e-RAVANS and i-RAVANS sessions. For evaluation of functional connectivity during IAPS, the extracted fMRI time series from the selected ROIs and its interaction with the regressors for negative and neutral content were used to evaluate connectivity differences between the visual stress tasks during PRE- and POST-stimulation, for e-RAVANS and i-RAVANS sessions. All reported results were significant at an FDR-corrected p-value < 0.05. Average connectivity values (beta weights) in significant target clusters were extracted using REX.
General linear models (GLM) were then conducted in STATA (StataCorp, College Station TX) to investigate the link between variations in NTS, DRN and HYPO connectivity and changes in depressive symptoms in response to RAVANS administration. A p<0.05 was designated for statistical significance. Adjusted regression coefficients were calculated after including baseline BDI scores and use of antidepressant medications in the models. The study is registered with ClinicalTrials.gov, NCT04467164.
RESULTS
Subjects
Women in the study had a mean age at symptom onset of 18.2 ± 3.6 years. Mean current HAM-D score was 20.1 ± 3.4, and median menstrual cycle times for scans were day 4 (range=2-6) and day 11 (range= 9-13). Twenty subjects attended an exhalatory-gated fMRI scan and 18 inhalatory-gated.
Clinical Characteristics and Behavioral Assessments
BDI and STAI state scores at baseline were similar for e-RAVANS and i-RAVANS (Table 1). A repeated-measures ANOVA revealed a session by time significant interaction for BDI scores (F(1,36)= 4.79, p=0.03), with post-hoc analyses revealing a significant reduction in depressive symptoms after e-RAVANS compared to i-RAVANS (Table 1). In fact, 7 (35%) women after e-RAVANS administration had a reduction >30% in BDI scores from baseline compared to only 2 (11.1%) following i-RAVANS stimulation.
Table 1.
Mood and Anxiety Ratings in MDD women by RAVANS Session
| Rating scale | e-RAVANS | i-RAVANS | p value |
|---|---|---|---|
| BDI | |||
| Prescan | 28.1 ± 6.92 | 26.5 ± 7.77 | 0.52 |
| Postscan | 19.8 ± 9.05 | 22.9 ± 9.73 | 0.33 |
| Post-Pre difference ** | −8.21 ± 7.4 | −3.58 ± 4.83 | 0.03 |
| STAI (State anxiety score) | |||
| Prescan | 55.2 ± 6.29 | 54.1 ± 8.65 | 0.65 |
| Postscan | 47.8 ± 7.49 | 52.3 ± 7.97 | 0.09 |
| Post-Pre difference * | −7.36 ± 10.8 | −1.76 ± 5.79 | 0.06 |
| IAPS Stimuli Ratings | |||
| Negative arousal | 4.72 ± 1.79 | 5.28 ± 2.43 | 0.41 |
| Negative valence | 7.29 ± 1.38 | 7.58 ± 0.97 | 0.46 |
A repeated-measures ANOVA revealed a session x time significant interaction for BDI scores (F(1,36)= 4.79, p=0.03).
A repeated-measures ANOVA revealed a trend toward a session x time significant interaction for STAI state scores (F(1,36)= 3.62, p=0.06). Post-hoc analysis revealed that MDD subjects reported a significant decrease in STAI state scores after e-RAVANS stimulation compared to baseline values (p<0.01).
With respect to anxiety, women exposed to e-RAVANS showed a significant reduction in state anxiety (−7.36±10.8, p<0.01), with a trend toward a statistically significant session by time interaction, i.e. compared with i-RAVANS (F(1,36)= 3.62, p=0.06). No significant differences between sessions were found for IAPS stimuli ratings, despite change in depressive symptoms (Table 1). Evaluation of order effects did not reveal significant statistical differences.
Effects of RAVANS on Stress Response Circuitry Activity
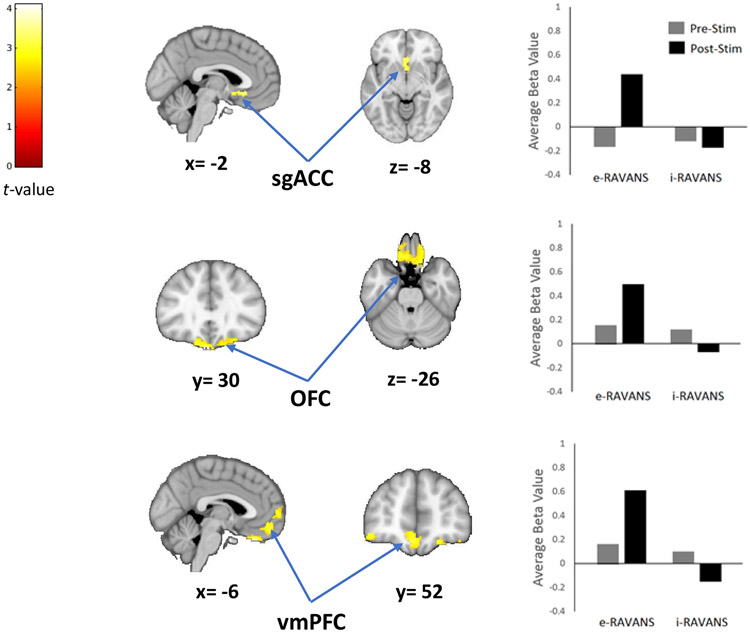
All subjects tolerated the auricular electrical stimulation procedure and there were no significant differences in average electrical current intensity (4.89 ± 1.28 mA vs 5.01 ± 1.08 mA, p=0.75). A factorial analysis comparing the brain response to the IAPS stress task before and after e-RAVANS and i-RAVANS sessions demonstrated a significant stimulation type by time interaction. Post-hoc analysis revealed e-RAVANS significantly increased activation of bilateral sgACC (100 voxels, T=2.9) during post-stimulation period compared to pre-stimulation and in contrast to i-RAVANS (Figure 2, Table 2A). The administration of e-RAVANS also resulted in significant post-stimulation effects on OFC (559 voxels, T=4.1) and vmPFC (722 voxels, T=4.1) activity in contrast to i-RAVANS (Figure 2, Table 2A).
Figure 2.
RAVANS effects on brain activity response to a mild-visual stress task. The results of a factorial analysis demonstrated a significant increased activation of subgenual anterior cingulate cortex (sgACC), orbitofrontal (OFC) and ventromedial (vmPFC) prefrontal cortices after e-RAVANS administration in contrast to i-RAVANS.
Table 2.
RAVA NS effects on brain activity and connectivity
| A- RAVANS effects on brain activity in response to a mild visual stress task | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Contrast | ROI | Side | Voxels | Peak MNI coordinates | Peak T-value |
p-value (FWE-corrected) |
|||
| x | y | z | |||||||
| Exh>Inh | sgACC | L | 100 | −2 | 24 | −8 | 2.9 | 0.032 | |
| OFC | L | 559 | −14 | 30 | −26 | 4.1 | 0.022 | ||
| vmPFC | L | 722 | −6 | 52 | −14 | 4.1 | 0.027 | ||
| B- Brain connectivity during RAVANS administration | |||||||||
| Contrast | Seed ROI | Target ROI | Side | Voxels | Peak coordinates | Peak T-value |
p-value (FWE-corrected) |
||
| x | y | z | |||||||
| Inh>Exh | DRN | PCC | L | 418 | −12 | −54 | 44 | 4.93 | <0.001 |
| Exh>Inh | DRN | dlPFC | L | 198 | −40 | 44 | 28 | 4.67 | 0.035 |
| C- Effects of RAVANS on brain connectivity in response to a mild-visual stress task | |||||||||
| Contrast | Seed ROI | Target ROI | Side | Voxels | Peak coordinates | Peak T-value |
p-value (FWE-corrected) |
||
| x | y | z | |||||||
| Exh>Inh | NTS | LC | L | 393 | −10 | −32 | −26 | 3.2 | 0.007 |
| vmPFC | L | 227 | −10 | 54 | 0 | 3.9 | |||
| HYPO | dlPFC | R | 878 | 30 | 34 | 24 | 4.3 | <0.001 | |
sgACC, subgenual anterior cingulate cortex; OFC, orbitofrontal cortex; vmPFC, ventromedial prefrontal cortex; DRN, dorsal raphe nuclei; PCC, posterior cingulate cortex; dlPFC, dorsolateral prefrontal cortex; LC, locus coeruleus.
A GLM analysis revealed a statistically significant reduction in depressive symptomatology associated with BOLD signal change in sgACC (β=−5.01, t (16) =−3.23, p=0.007, Adj R2=0.38), OFC (β =−6.64, t (16) =−3.53, p=0.004, Adj R2=0.43) and vmPFC (β =−4.64, t (16) =−3.28, p=0.007, Adj R2=0.39) after e-RAVANS. Additional GLM analyses revealed a significant relationship between reduction in anxiety symptoms (STAI score) after e-RAVANS stimulation and BOLD signal changes in sgACC (β=−5.50, t (16) =−3.55, p=0.004, Adj R2=0.72), OFC (β =−6.74, t (16) =−3.46, p=0.005, Adj R2=0.71) and vmPFC (β =−4.83, t (16) =−3.50, p=0.004, Adj R2=0.71). No significant associations were found for the i-RAVANS session.
Effects of RAVANS on Stress Response Circuitry Connectivity
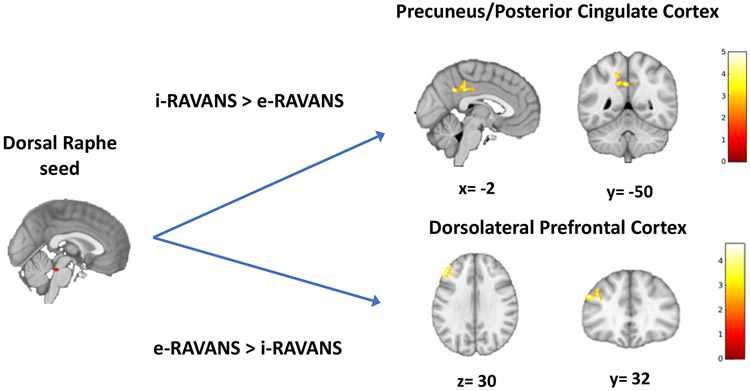
The voxel-wise evaluation of maps contrasting functional connectivity of brainstem nuclei (NTS, DRN) to the brain during e-RAVANS vs i-RAVANS revealed greater connectivity of DRN to left DLPFC during e-RAVANS (198 voxels, T=4.67, p=0.035), whereas increased DRN connectivity to precuneus and posterior cingulate cortex (418 voxels, T=4.93, p<0.001) was greater during i-RAVANS vs e-RAVANS (Figure 3, Table 2B).
Figure 3.
Functional connectivity from dorsal raphe to higher brain regions in response to RAVANS stimulation. Difference in maps contrasting functional dorsal raphe connectivity during inhalatory- (i-RAVANS) vs exhalatory-gated (e-RAVANS) stimulation noted greater connectivity to precuneus and posterior cingulate cortex during i-RAVANS, whereas an increased connectivity to left dorsolateral prefrontal cortex was observed during e-RAVANS.
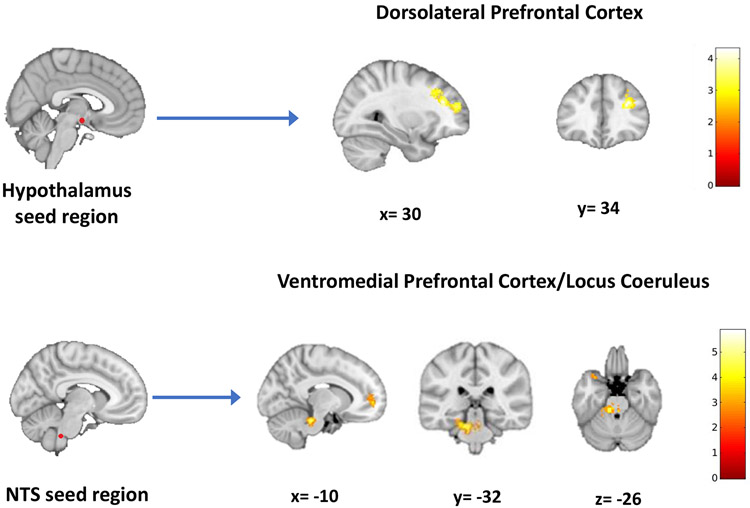
The effects of RAVANS on modulation of the stress response circuitry connectivity revealed that e-RAVANS significantly increased connectivity from HYPO to right DLPFC (878 voxels, T=4.3, p <0.001), and NTS to LC (393 voxels, T=3.2, p=0.007) and vmPFC (227 voxels, T=3.9) during post-stimulation compared with i-RAVANS (Figure 4, Table 2C) with no significant i-RAVANS > e-RAVANS findings. A GLM analysis revealed a statistically significant reduction in depressive symptomatology associated with increased NTS connectivity to LC (β=−9.02, t (16) =−4.36, p=0.001, Adj R2=0.55) and vmPFC (β =−8.48, t (16) =−3.50, p=0.004, Adj R2=0.43) after e-RAVANS. Additional GLM analyses revealed a significant association between reduction in anxiety symptoms after e-RAVANS and NTS connectivity to LC (β=−10.02, t (16) =−.72, p=0.003, Adj R2=0.73) and vmPFC (β =−8.19, t (16) =−2.97, p=0.012, Adj R2=0.67).
Figure 4.
RAVANS effects on brain connectivity during a mild-visual stress task. The results of a factorial analysis demonstrated a stimulation by time interaction with post-hoc testing revealing that e-RAVANS significantly increased connectivity during a mild visual stress task from the hypothalamus to dorsolateral prefrontal cortex and from nucleus tractus solitary to the locus coeruleus and ventromedial prefrontal cortex in contrast to i-RAVANS administration.
DISCUSSION
Our results showed that exhalatory-gated RAVANS (e-RAVANS) had a significant and positive modulatory effect on brain response to negative stressful stimuli in women with recurrent major depression undergoing an active depressive episode. Activation of sgACC, OFC and vmPFC under stress after stimulation was stronger for e-RAVANS compared to inhalatory-gated (i-RAVANS) stimulation. Further, regional brain activations and their connectivities were associated with a significant acute reduction in depressive and anxiety symptomatology, measured by changes in BDI and STAI post-stimulation, at a level suggesting clinical relevance (Button et al., 2015). Our study used a within-subject, crossover design which significantly reduced variability between the active and control interventions (i.e., each participant was her own control), leading to reducing the impact of potential confounders that may affect e-RAVANS vs. i-RAVANS differences. Importantly, this provided adequate statistical power to detect medium to large effect sizes (Cohen’s d= 0.73) for e-RAVANS vs. i-RAVANS on reduction of depressive and anxiety symptoms. The fact that e-RAVANS vs. i-RAVANS had an acute effect on mood/anxiety underscores its potential clinical utility for MDD. In fact, acute antidepressant effects are limited to essentially only two interventions (i.e. ketamine for MDD (Fava et al., 2018; Murrough et al., 2013; Zarate et al., 2006) and brexanolone for postpartum depression (Zheng et al., 2019).
Activations of anterior cingulate cortex and prefrontal cortical regions (i.e., sgACC, OFC and vmPFC) are involved in the inhibitory control of arousal and have been implicated in regulating emotional behavior, stress response, and mood disorders (Drevets et al., 2008), as demonstrated here. Additionally, we found increased connectivity between brainstem raphe nuclei and left prefrontal cortex (DLPFC), the former being a primary site of serotonin production and the latter involved with inhibitory control of arousal and mood regulation. Post e-RAVANS effects were also significantly associated with increased connectivity between hypothalamus and right DLPFC and brainstem NTS with locus coeruleus and vmPFC. These results support a neurophysiological model by which e-RAVANS may effectively inhibit arousal in circuitry involved in mood, anxiety and regulation of stress with potential antidepressant (implicated by connections with dorsal raphe nucleus) and anti-anxiety (implicated by connections with locus coeruleus) effects in MDD.
Interestingly, the sgACC is a key target for more invasive forms of neuromodulation, such as brain stimulation therapies in managing treatment-resistant depression (TRD) (Holtzheimer et al., 2017; Kennedy et al., 2011), based on significant findings underscoring the role of sgACC in MDD (Mayberg et al., 2005). Open label deep brain stimulation (DBS) of sgACC induced significant clinical improvement in two-thirds of patients with TRD (Kennedy et al., 2011), although findings were not replicated in a randomized sham-controlled study (Holtzheimer et al., 2017). Indirect modulation of sgACC with repetitive transcranial magnetic stimulation (rTMS) also consistently reduced depressive symptomatology in MDD (Philip et al., 2018). In previous studies with DBS or rTMS, clinical efficacy was directly associated with reduction of sgACC activity (Drevets et al., 2008; Philip et al., 2018). In contrast, our findings revealed a significant association between increased sgACC activation during a mild stress task and acute reduction of depressive symptomatology after e-RAVANS, again suggesting inhibitory effects of e-RAVANS on response to stress. These results are consistent with studies evaluating the mechanisms of action of acute pharmacological therapy for MDD, i.e., fast-acting antidepressant action of ketamine (Downey et al., 2016; Nugent et al., 2014).
Inhibitory control of arousal is also suggested by e-RAVANS impact on orbitofrontal cortex (OFC) response to negative stress during a depressive episode. Previously, we demonstrated reduced OFC-hypothalamus connectivity in women with increasing levels of dysphoric mood (Mareckova et al., 2016), significantly associated with stress physiology (i.e. lower cardiovagal activity (Garcia et al., 2020) and hypercortisolemia in response to stress (Mareckova et al., 2017)). Our results of enhanced OFC activation after e-RAVANS suggests significant effects on the regulation of visceromotor response to negative stressful stimuli (Koenig et al., 2018), which has clinical importance given the role of OFC in affective disorders and schizophrenia (Chen et al., 2018).
Further evidence for this is seen in the significant effects of e-RAVANS on ventromedial prefrontal cortex (vmPFC) activity and upregulation of brainstem NTS connectivity with vmPFC, suggesting an e-RAVANS effect on modulation of the medial visceromotor network involved in implicit regulation of emotional response (Etkin et al., 2015). This system plays an important role in evaluating the emotional significance of stimuli, without conscious monitoring, and making behavioral and physiological adjustments (Roy et al., 2012). Activation of vmPFC has been associated with successful suppression of emotional responses to negative stimuli, inhibition of fear and regulation of emotional conflict (Hansel and von Kanel, 2008). Further, vmPFC is involved in the ability to shift affective states and may be helpful in reducing persistent depressed mood in MDD (Holtzheimer and Mayberg, 2011).
The enhanced regulation of the visceromotor network post-stimulation and significant associations with reduction of depressive and anxiety symptomatology in our study suggests that modulation of this circuitry may activate acute antidepressant and anti-anxiety effects of e-RAVANS. The observed increased connectivity between key brainstem and hypothalamic nuclei and prefrontal cortex after e-RAVANS suggests an effect of cortical inhibitory control over HPA axis response to negative stressful stimuli in MDD, which may have important implications in the regulation of physiological alterations associated with MDD (Holsen et al., 2013; Mareckova et al., 2017).
Other studies have evaluated the effects of taVNS on brain activity and connectivity in MDD (Fang et al., 2016; Fang et al., 2017; Li et al., 2019; Liu et al., 2016; Tu et al., 2018; Wang et al., 2018) and found that one month of taVNS treatment was associated with reduction of functional connectivity between the default mode network (DMN) with anterior insula and parahippocampus and upregulation of DMN connectivity with precuneus and OFC, and right amygdala and left DLPFC, which were significantly associated with reduced depressive symptomatology (Fang et al., 2016; Liu et al., 2016). These fMRI studies tested response to taVNS during rest, whereas ours introduced a mild negative stress task to “challenge” the brain to regulate negative stress in MDD during an episode. Given the importance of understanding the dysregulation of brain circuitry in response to negative stress in MDD, (Goldstein et al., 2014; Mareckova et al., 2017), our findings may be indicative of taVNS effects on modulation of core features implicated in initiation and maintenance of depressed mood and anxiety in MDD.
Previous studies evaluated antidepressant effects of taVNS and demonstrated a significant effect on reducing depressive symptoms after two and four weeks of daily stimulation sessions, with none reporting acute antidepressant effects (Wu et al., 2018). Our study uniquely demonstrated acute (fast-acting) effects of e-RAVANS on reducing depressive symptomatology in women with recurrent MDD, a finding that is novel compared to previous MDD studies of taVNS. This acute effect was remarkable given the typical time to response using oral antidepressants, and the fact that our subjects had recurrent MDD and were primarily unmedicated. Although the BDI-II scale is traditionally used for assessment of mood changes over longer periods of time (i.e. two weeks), studies evaluating the effects of fast-acting therapies, such as ketamine, have demonstrated that acute reduction of BDI-II scores is associated with a clinical response in MDD and well-correlated with significant changes in other mood rating scales (Diazgranados et al., 2010; Thomas et al., 2018). Our study also identified a significant effect of e-RAVANS on the reduction of anxiety symptoms as measured by the STAI questionnaire. Future studies will need to be conducted to evaluate the generalizability of the effects of RAVANS for anxiety disorders per se, and for other populations, such as men with MDD, bipolar disorder, first episode cases of depression, adolescents or childhood with major depression.
One of the strengths of our study was the use of an active control (inhalatory-gated stimulation) allowing us to maintain blinding of treatment allocation for subjects. The use of the BDI, a patient reported outcome, also allowed us to reduce interviewer bias in evaluation of depressive symptomatology. In addition, staff involved in data analysis were blinded to treatment allocation. Further, our design included a relatively homogeneous sample of people with MDD with similar clinical characteristics (premenopausal women with recurrent MDD in an active episode, who were scanned during the follicular (early to beginning of mid) phase of their menstrual cycle) and were primarily unmedicated. We would argue this reduced the variability of the sample, allowing for increased internal validity and better precision in evaluating the effects of our intervention.
Given that women are at two-fold risk for MDD, for which sex differences emerge in young adulthood, the inclusion of young premenopausal women alone in this initial study does not diminish the potential impact of our findings for the general population. Further work in men with recurrent MDD is needed and planned, given that our previous work on activation of this circuitry using the same fMRI stress challenge task as used here, demonstrated significant sex differences in activations (Goldstein et al., 2010).
In conclusion, our study suggests that exhalatory-gated RAVANS effectively modulates brain circuitries involved in mood, anxiety and stress response dysregulation in major depression. Evaluation of this intervention in longitudinal studies with larger sample sizes of women and men will be required to address the generalizability of findings and therapeutic efficacy and duration of the intervention. Although our study was not designed as a clinical trial, the statistically significant acute antidepressant effects found after e-RAVANS vs. i-RAVANS administration are highly promising and highlights respiration as an important physiological parameter that should be considered as critical for optimizing vagus nerve stimulation effects on major depressive disorder.
Highlights.
Exhalation-gated taVNS (e-RAVANS) upregulates brain response to negative stress
e-RAVANS has significant acute antidepressant and anxiolytic effects in MDD subjects
Respiration-gating may optimize taVNS therapeutic effects on major depression
Acknowledgements
We would like to thank Anne Remington, M.A for her contributions to the collection and management of data from this study. The authors would also like to thank Ben Pless for his insightful comments on the interpretation and presentation of the data and about translating these findings into a potential therapeutic.
Funding
This research was conducted with support from NIMH R21 MH103468 (JMG & VN, multi-PIs) and from Harvard Catalyst and the Harvard Clinical and Translational Science Center (NIH #1UL1 TRR001102). JMG, VN and RG’s time for analyses and writing was also supported, in part, by ORWH-NIMH U54MH118919 (JMG and Robert Handa, Multi-PIs) and Boston Biomedical Innovation Center (B-BIC), NHLBI U54HL119145; and VN and RG, in part, by NIH Office of The Director (OT2-OD023867). Finally, RG’s time for analyses and writing was, in part, supported by a NARSAD Young investigator Grant from the Brain & Behavior Research Foundation (Grant n.26236).
Role of the Funding Source
The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Interest
JMG and VN are on the scientific advisory board for Cala Health (a neuromodulation company that has licensed the RAVANS technology) and JMG has an equity interest. HA is a clinical consultant for Cala Health. However, the study reported in this manuscript was completed prior to the relationships with Cala Health. In addition, the investigators’ interests were reviewed and managed by the Massachusetts General Hospital and Mass General Brigham Healthcare in accordance with their institutional policies. Drs. Goldstein, Napadow and Garcia filed a patent on “System and methods for respiratory-gated nerve stimulation” (WO201901425). When originally funded, R21 MH103468 included Dr. Stanford as co-PI, a relationship that ended when she joined Alkermes prior to the initiation of the study. Aileen Gabriel and Jessica Stowell, BS and Drs. Cohen, Barbieri and Gitlin reported no financial interests or potential conflicts of interest.
REFERENCES
- Aaronson ST, Sears P, Ruvuna F, Bunker M, Conway CR, Dougherty DD, Reimherr FW, Schwartz TL, Zajecka JM, 2017. A 5-Year Observational Study of Patients With Treatment-Resistant Depression Treated With Vagus Nerve Stimulation or Treatment as Usual: Comparison of Response, Remission, and Suicidality. Am. J. Psychiatry 174(7), 640–648. 10.1176/appi.ajp.2017.16010034. [DOI] [PubMed] [Google Scholar]
- Badran BW, Dowdle LT, Mithoefer OJ, LaBate NT, Coatsworth J, Brown JC, DeVries WH, Austelle CW, McTeague LM, George MS, 2018. Neurophysiologic effects of transcutaneous auricular vagus nerve stimulation (taVNS) via electrical stimulation of the tragus: A concurrent taVNS/fMRI study and review. Brain Stimul. 11(3), 492–500. 10.1016/j.brs.2017.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baekey DM, Molkov YI, Paton JF, Rybak IA, Dick TE, 2010. Effect of baroreceptor stimulation on the respiratory pattern: insights into respiratory-sympathetic interactions. Respir. Physiol. Neurobiol. 174(1-2), 135–145. 10.1016/j.resp.2010.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Brown GK, 1996. Manual for the Beck Depression Inventory–Second Ed. Psychological Corporation, San Antonio, TX. [Google Scholar]
- Bradley MM, Lang PJ, 1994. Measuring emotion: the Self-Assessment Manikin and the Semantic Differential. J. Behav. Ther. Exp. Psychiatry 25(1), 49–59. 10.1016/0005-7916(94)90063-9. [DOI] [PubMed] [Google Scholar]
- Button KS, Kounali D, Thomas L, Wiles NJ, Peters TJ, Welton NJ, Ades AE, Lewis G, 2015. Minimal clinically important difference on the Beck Depression Inventory--II according to the patient's perspective. Psychol. Med 45(15), 3269–3279. 10.1017/S0033291715001270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Wang Y, Niu C, Zhong S, Hu H, Chen P, Zhang S, Chen G, Deng F, Lai S, Wang J, Huang L, Huang R, 2018. Common and distinct abnormal frontal-limbic system structural and functional patterns in patients with major depression and bipolar disorder. NeuroImage Clin. 20, 42–50. 10.1016/j.nicl.2018.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daban C, Martinez-Aran A, Cruz N, Vieta E, 2008. Safety and efficacy of Vagus Nerve Stimulation in treatment-resistant depression. A systematic review. J. Affect Disord 110(1-2), 1–15. 10.1016/j.jad.2008.02.012. [DOI] [PubMed] [Google Scholar]
- Diazgranados N, Ibrahim L, Brutsche NE, Newberg A, Kronstein P, Khalife S, Kammerer WA, Quezado Z, Luckenbaugh DA, Salvadore G, Machado-Vieira R, Manji HK, Zarate CA Jr., 2010. A randomized add-on trial of an N-methyl-D-aspartate antagonist in treatment-resistant bipolar depression. Arch. Gen. Psychiatry 67(8), 793–802. 10.1001/archgenpsychiatry.2010.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downey D, Dutta A, McKie S, Dawson GR, Dourish CT, Craig K, Smith MA, McCarthy DJ, Harmer CJ, Goodwin GM, Williams S, Deakin JF, 2016. Comparing the actions of lanicemine and ketamine in depression: key role of the anterior cingulate. Eur. Neuropsychopharmacol 26(6), 994–1003. 10.1016/j.euroneuro.2016.03.006. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Savitz J, Trimble M, 2008. The subgenual anterior cingulate cortex in mood disorders. CNS Spectr. 13(8), 663–681. 10.1017/s1092852900013754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellrich J, 2011. Transcutaneous Vagus Nerve Stimulation. Eur. Neurol. Rev 6(4), 254–256. 10.17925/ENR.2011.06.04.254. [DOI] [Google Scholar]
- Etkin A, Buchel C, Gross JJ, 2015. The neural bases of emotion regulation. Nat. Rev. Neurosci 16(11), 693–700. 10.1038/nrn4044. [DOI] [PubMed] [Google Scholar]
- Fang J, Rong P, Hong Y, Fan Y, Liu J, Wang H, Zhang G, Chen X, Shi S, Wang L, Liu R, Hwang J, Li Z, Tao J, Wang Y, Zhu B, Kong J, 2016. Transcutaneous Vagus Nerve Stimulation Modulates Default Mode Network in Major Depressive Disorder. Biol. Psychiatry 79(4), 266–273. 10.1016/j.biopsych.2015.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang J, Egorova N, Rong P, Liu J, Hong Y, Fan Y, Wang X, Wang H, Yu Y, Ma Y, Xu C, Li S, Zhao J, Luo M, Zhu B, Kong J, 2017. Early cortical biomarkers of longitudinal transcutaneous vagus nerve stimulation treatment success in depression. NeuroImage Clin. 14, 105–111. 10.1016/j.nicl.2016.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fava M, Freeman MP, Flynn M, Judge H, Hoeppner BB, Cusin C, Ionescu DF, Mathew SJ, Chang LC, Iosifescu DV, Murrough J, Debattista C, Schatzberg AF, Trivedi MH, Jha MK, Sanacora G, Wilkinson ST, Papakostas GI, 2018. Double-blind, placebo-controlled, dose-ranging trial of intravenous ketamine as adjunctive therapy in treatment-resistant depression (TRD). Mol. Psychiatry 25(7), 1592–1603. 10.1038/s41380-018-0256-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Williams JBW, Karg RS, Spitzer RL, 2015. Structured Clinical Interview for DSM-5- Research Version (SCID-F for DSM-5, Research Version; SCID-5-RV). American Psychiatric Association, Arlington, VA. [Google Scholar]
- Frangos E, Ellrich J, Komisaruk BR, 2015. Non-invasive Access to the Vagus Nerve Central Projections via Electrical Stimulation of the External Ear: fMRI Evidence in Humans. Brain Stimul. 8(3), 624–636. 10.1016/j.brs.2014.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia RG, Lin RL, Lee J, Kim J, Barbieri R, Sclocco R, Wasan AD, Edwards RR, Rosen BR, Hadjikhani N, Napadow V, 2017. Modulation of brainstem activity and connectivity by respiratory-gated auricular vagal afferent nerve stimulation in migraine patients. Pain 158(8), 1461–1472. 10.1097/j.pain.0000000000000930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia RG, Mareckova K, Holsen LM, Cohen JE, Whitfield-Gabrieli S, Napadow V, Barbieri R, Goldstein JM, 2020. Impact of sex and depressed mood on the central regulation of cardiac autonomic function. Neuropsychopharmacology 45(8), 1280–1288. 10.1038/s41386-020-0651-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein JM, Seidman LJ, Horton NJ, Makris N, Kennedy DN, Caviness VS, Faraone SV, Tsuang MT, 2001. Normal sexual dimorphism of the adult human brain assessed by in vivo magnetic resonance imaging. Cereb. Cortex 11(6), 490–497. 10.1093/cercor/11.6.490. [DOI] [PubMed] [Google Scholar]
- Goldstein JM, Jerram M, Abbs B, Whitfield-Gabrieli S, Makris N, 2010. Sex differences in stress response circuitry activation dependent on female hormonal cycle. J. Neurosci 30(2), 431–438. 10.1523/JNEUROSCI.3021-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein JM, Handa RJ, Tobet SA, 2014. Disruption of fetal hormonal programming (prenatal stress) implicates shared risk for sex differences in depression and cardiovascular disease. Front. Neuroendocrinol 35(1), 140–158. 10.1016/j.yfrne.2013.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein JM, Hale T, Foster SL, Tobet SA, Handa RJ, 2019. Sex differences in major depression and comorbidity of cardiometabolic disorders: impact of prenatal stress and immune exposures. Neuropsychopharmacology 44(1), 59–70. 10.1038/s41386-018-0146-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halle M, Talos I-F, Jakab M, Makris N, Meier D, Wald LL, Fischl B, Kikinis R, 2017. Multi-modality MRI-based Atlas of the Brain. Surgical Planning Laboratory, Department of Radiology, Brigham and Women's Hospital, Harvard Medical School, Boston, MA, USA. [Google Scholar]
- Hamilton M, 1967. Development of a rating scale for primary depressive illness. Br. J. Soc. Clin. Psychol 6(4), 278–296. 10.1111/j.2044-8260.1967.tb00530.x. [DOI] [PubMed] [Google Scholar]
- Hansel A, von Kanel R, 2008. The ventro-medial prefrontal cortex: a major link between the autonomic nervous system, regulation of emotion, and stress reactivity? BioPsychoSoc. Med. 2, 21. 10.1186/1751-0759-2-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hein E, Nowak M, Kiess O, Biermann T, Bayerlein K, Kornhuber J, Kraus T, 2013. Auricular transcutaneous electrical nerve stimulation in depressed patients: a randomized controlled pilot study. J. Neural. Transm. (Vienna) 120(5), 821–827. 10.1007/s00702-012-0908-6. [DOI] [PubMed] [Google Scholar]
- Holsen LM, Lancaster K, Klibanski A, Whitfield-Gabrieli S, Cherkerzian S, Buka S, Goldstein JM, 2013. HPA-axis hormone modulation of stress response circuitry activity in women with remitted major depression. Neuroscience 250, 733–742. 10.1016/j.neuroscience.2013.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtzheimer PE, Mayberg HS, 2011. Stuck in a rut: rethinking depression and its treatment. Trends Neurosci. 34(1), 1–9. 10.1016/j.tins.2010.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtzheimer PE, Husain MM, Lisanby SH, Taylor SF, Whitworth LA, McClintock S, Slavin KV, Berman J, McKhann GM, Patil PG, Rittberg BR, Abosch A, Pandurangi AK, Holloway KL, Lam RW, Honey CR, Neimat JS, Henderson JM, DeBattista C, Rothschild AJ, Pilitsis JG, Espinoza RT, Petrides G, Mogilner AY, Matthews K, Peichel D, Gross RE, Hamani C, Lozano AM, Mayberg HS, 2017. Subcallosal cingulate deep brain stimulation for treatment-resistant depression: a multisite, randomised, sham-controlled trial. Lancet Psychiatry 4(11), 839–849. 10.1016/S2215-0366(17)30371-1. [DOI] [PubMed] [Google Scholar]
- Jacobs EG, Holsen LM, Lancaster K, Makris N, Whitfield-Gabrieli S, Remington A, Weiss B, Buka S, Klibanski A, Goldstein JM, 2015. 17β-estradiol differentially regulates stress circuitry activity in healthy and depressed women. Neuropsychopharmacology 40(3), 566–576. 10.1038/npp.2014.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy SH, Giacobbe P, Rizvi SJ, Placenza FM, Nishikawa Y, Mayberg HS, Lozano AM, 2011. Deep brain stimulation for treatment-resistant depression: follow-up after 3 to 6 years. Am. J. Psychiatry 168(5), 502–510. 10.1176/appi.ajp.2010.10081187. [DOI] [PubMed] [Google Scholar]
- Koenig J, Westlund Schreiner M, Klimes-Dougan B, Ubani B, Mueller BA, Lim KO, Kaess M, Cullen KR, 2018. Increases in orbitofrontal cortex thickness following antidepressant treatment are associated with changes in resting state autonomic function in adolescents with major depression - Preliminary findings from a pilot study. Psychiatry Res. Neuroimaging 281, 35–42. https://doi.org/0.1016/j.pscychresns.2018.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraus T, Hösl K, Kiess O, Schanze A, Kornhuber J, Forster C, 2007. BOLD fMRI deactivation of limbic and temporal brain structures and mood enhancing effect by transcutaneous vagus nerve stimulation. J. Neural. Transm. (Vienna) 114(11), 1485–1493. 10.1007/s00702-007-0755-z. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN, 2008. International affective picture system (IAPS): affective ratings of pictures and instruction manual. Technical Report A-8, Gainesville, Fl: University of Florida. [Google Scholar]
- Li XJ, Wang L, Wang HX, Zhang L, Zhang GL, Rong PJ, Fang JL, 2019. The effect of transcutaneous auricular vagus nerve stimulation on treatment-resistant depression monitored by resting-state fMRI and MRS: The first case report. Brain Stimul. 12(2), 377–379. 10.1016/j.brs.2018.11.013. [DOI] [PubMed] [Google Scholar]
- Liu J, Fang J, Wang Z, Rong P, Hong Y, Fan Y, Wang X, Park J, Jin Y, Liu C, Zhu B, Kong J, 2016. Transcutaneous vagus nerve stimulation modulates amygdala functional connectivity in patients with depression. J. Affect. Disord 205, 319–326. 10.1016/j.jad.2016.08.003. [DOI] [PubMed] [Google Scholar]
- Mareckova K, Holsen LM, Admon R, Makris N, Seidman L, Buka S, Whitfield-Gabrieli S, Goldstein JM, 2016. Brain activity and connectivity in response to negative affective stimuli: Impact of dysphoric mood and sex across diagnoses. Hum. Brain Mapp 37(11), 3733–3744. 10.1002/hbm.23271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mareckova K, Holsen L, Admon R, Whitfield-Gabrieli S, Seidman LJ, Buka SL, Klibanski A, Goldstein JM, 2017. Neural - hormonal responses to negative affective stimuli: Impact of dysphoric mood and sex. J. Affect Disord 222, 88–97. 10.1016/j.jad.2017.06.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayberg HS, Lozano AM, Voon V, McNeely HE, Seminowicz D, Hamani C, Schwalb JM, Kennedy SH, 2005. Deep brain stimulation for treatment-resistant depression. Neuron 45(5), 651–660. 10.1016/j.neuron.2005.02.014. [DOI] [PubMed] [Google Scholar]
- Miyazaki M, Arata A, Tanaka I, Ezure K, 1998. Activity of rat pump neurons is modulated with central respiratory rhythm. Neurosci. Lett 249(1), 61–64. 10.1016/s0304-3940(98)00402-9. [DOI] [PubMed] [Google Scholar]
- Miyazaki M, Tanaka I, Ezure K, 1999. Excitatory and inhibitory synaptic inputs shape the discharge pattern of pump neurons of the nucleus tractus solitarii in the rat. Exp. Brain Res 129(2), 191–200. 10.1007/s002210050889. [DOI] [PubMed] [Google Scholar]
- Murrough JW, Perez AM, Pillemer S, Stern J, Parides MK, aan het Rot M, Collins KA, Mathew SJ, Charney DS, Iosifescu DV, 2013. Rapid and longer-term antidepressant effects of repeated ketamine infusions in treatment-resistant major depression. Biol. Psychiatry 74(4), 250–256. 10.1016/j.biopsych.2012.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemeroff CB, Mayberg HS, Krahl SE, McNamara J, Frazer A, Henry TR, George MS, Charney DS, Brannan SK, 2006. VNS therapy in treatment-resistant depression: clinical evidence and putative neurobiological mechanisms. Neuropsychopharmacology 31(7), 1345–1355. 10.1038/sj.npp.1301082. [DOI] [PubMed] [Google Scholar]
- Nugent AC, Diazgranados N, Carlson PJ, Ibrahim L, Luckenbaugh DA, Brutsche N, Herscovitch P, Drevets WC, Zarate CA Jr., 2014. Neural correlates of rapid antidepressant response to ketamine in bipolar disorder. Bipolar Disord. 16(2), 119–128. 10.1111/bdi.12118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philip NS, Barredo J, Aiken E, Carpenter LL, 2018. Neuroimaging Mechanisms of Therapeutic Transcranial Magnetic Stimulation for Major Depressive Disorder. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 3(3), 211–222. 10.1016/j.bpsc.2017.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rong P, Liu J, Wang L, Liu R, Fang J, Zhao J, Zhao Y, Wang H, Vangel M, Sun S, Ben H, Park J, Li S, Meng H, Zhu B, Kong J, 2016. Effect of transcutaneous auricular vagus nerve stimulation on major depressive disorder: A nonrandomized controlled pilot study. J. Affect. Disord 195, 172–179. 10.1016/j.jad.2016.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy M, Shohamy D, Wager TD, 2012. Ventromedial prefrontal-subcortical systems and the generation of affective meaning. Trends Cogn. Sci 16(3), 147–156. 10.1016/j.tics.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sclocco R, Garcia RG, Kettner NW, Isenburg K, Fisher HP, Hubbard CS, Ay I, Polimeni JR, Goldstein J, Makris N, Toschi N, Barbieri R, Napadow V, 2019. The influence of respiration on brainstem and cardiovagal response to auricular vagus nerve stimulation: A multimodal ultrahigh-field (7T) fMRI study. Brain Stimul. 12(4), 911–921. 10.1016/j.brs.2019.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielberger C, Gorsuch R, Lushene R, Vagg P, Jacobs G, 1983. Manual for the state-trait anxiety inventory. Consulting Psychologists Press. Palo Alto, CA. [Google Scholar]
- Thomas RK, Baker G, Lind J, Dursun S, 2018. Rapid effectiveness of intravenous ketamine for ultraresistant depression in a clinical setting and evidence for baseline anhedonia and bipolarity as clinical predictors of effectiveness. J. Psychopharmacol 32(10), 1110–1117. 10.1177/0269881118793104. [DOI] [PubMed] [Google Scholar]
- Tu Y, Fang J, Cao J, Wang Z, Park J, Jorgenson K, Lang C, Liu J, Zhang G, Zhao Y, Zhu B, Rong P, Kong J, 2018. A distinct biomarker of continuous transcutaneous vagus nerve stimulation treatment in major depressive disorder. Brain Stimul. 11(3), 501–508. 10.1016/j.brs.2018.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ventureyra EC, 2000. Transcutaneous vagus nerve stimulation for partial onset seizure therapy. A new concept. Childs Nerv. Syst 16(2), 101–102. 10.1007/s003810050021. [DOI] [PubMed] [Google Scholar]
- Wang Z, Fang J, Liu J, Rong P, Jorgenson K, Park J, Lang C, Hong Y, Zhu B, Kong J, 2018. Frequency-dependent functional connectivity of the nucleus accumbens during continuous transcutaneous vagus nerve stimulation in major depressive disorder. J. Psychiatr. Res 102, 123–131. 10.1016/j.jpsychires.2017.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitfield-Gabrieli S, 2009. Region of Interest Extraction (REX) toolbox. Boston, MA. [Google Scholar]
- Whitfield-Gabrieli S, Nieto-Castanon A, 2012. Conn: a functional connectivity toolbox for correlated and anti correlated brain networks. Brain Connect. 2(3), 125–141. 10.1089/brain.2012.0073. [DOI] [PubMed] [Google Scholar]
- Wu C, Liu P, Fu H, Chen W, Cui S, Lu L, Tang C, 2018. Transcutaneous auricular vagus nerve stimulation in treating major depressive disorder: A systematic review and meta-analysis. Medicine 97(52), e13845. 10.1097/MD.0000000000013845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan H, Silberstein SD, 2016. Vagus Nerve and Vagus Nerve Stimulation, a Comprehensive Review: Part I. Headache 56(1), 71–78. 10.1111/head.12647. [DOI] [PubMed] [Google Scholar]
- Zarate CA Jr., Singh JB, Carlson PJ, Brutsche NE, Ameli R, Luckenbaugh DA, Charney DS, Manji HK, 2006. A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch. Gen. Psychiatry 63(8), 856–864. 10.1001/archpsyc.63.8.856. [DOI] [PubMed] [Google Scholar]
- Zheng W, Cai DB, Zheng W, Sim K, Ungvari GS, Peng XJ, Ning YP, Wang G, Xiang YT, 2019. Brexanolone for postpartum depression: A meta-analysis of randomized controlled studies. Psychiatry Res. 279, 83–89. 10.1016/j.psychres.2019.07.006. [DOI] [PubMed] [Google Scholar]