In the original article, there was a mistake in Figure 1, Location of sampling sites in the estuarine tidal flat wetlands of China. as published. There was a mistake in the scale label. The corrected Figure 1 appears below.
Figure 1.
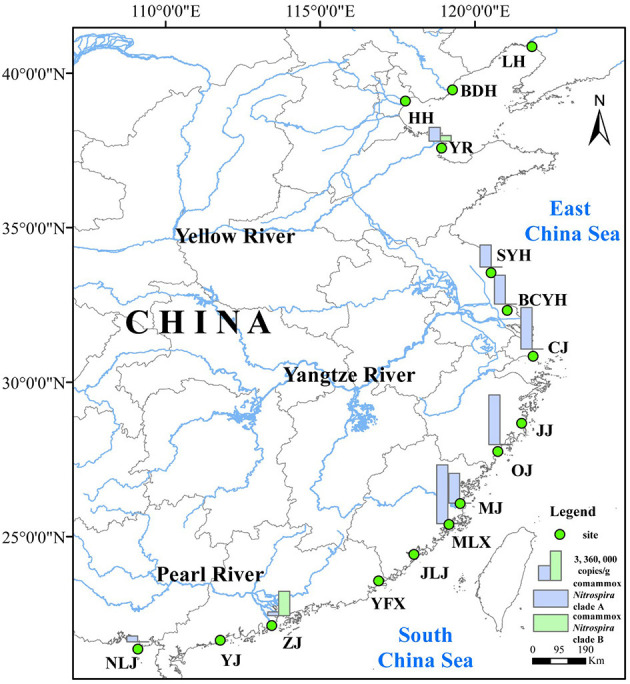
Location of sampling sites in the estuarine tidal flat wetlands of China.
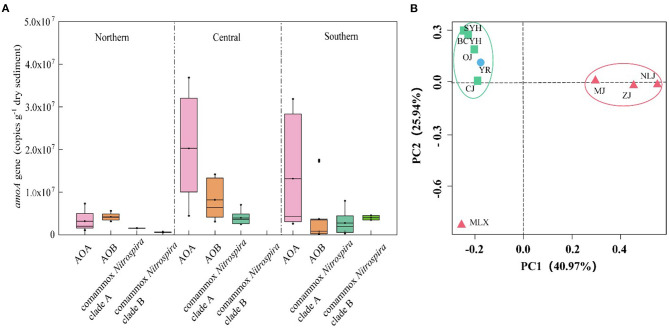
In the original article, there was a mistake in Figure 4, (A) Abundance of ammonia-oxidizers in the distinct areas based on qPCR results. as published. There was a mistake in the values on the Y-axis. The corrected Figure 4 appears below.
Figure 4.
(A) Abundance of ammonia-oxidizers in the distinct areas based on qPCR results. (B) UniFrac weighted PCoA analysis of comammox Nitrospira communities in the estuary tidal flat wetlands of China. Red triangle: Southern estuaries (MJ, ZJ, MLX, NLJ); Green square: Central estuaries (BCYH, SYH, CJ, OJ); Blue circle: Northern estuaries (YR).
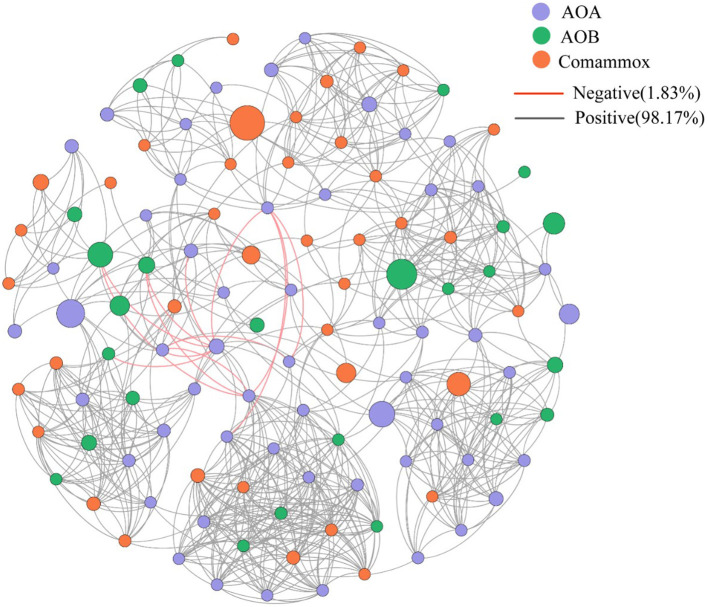
In the original article, there was a mistake in Figure 5, Network analysis of all ammonia oxidizers. Different colored circles represent different ammonia oxidants, orange lines represent negative interaction, black lines represent positive interaction. as published. The colors indicating negative and positive interactions were not clearly shown. The corrected Figure 5 appears below.
Figure 5.
Network analysis of all ammonia oxidizers. Different colored circles represent different ammonia oxidants, orange lines represent negative interaction, black lines represent positive interaction.
In the original article, there was a mistake in Table 1, Distribution of ammonia-oxidizers in different ecosystems. as published. The values and units were not correctly indicated in the Table. The corrected Table 1 appears below.
Table 1.
Distribution of ammonia-oxidizers in different ecosystems.
| Country | Ecosystem | AOA | AOB | comammox Nitrospira clade A | comammox Nitrospira clade B | References |
|---|---|---|---|---|---|---|
| America | Recirculating aquaculture systems | 0.94 × 108-3.4 × 108 (copies/g) | 2.6 × 103-5.0 × 105 (copies/g) | 1.6 × 108-4.2 × 108 (copies/g) | – | Bartelme et al., 2017 |
| Denmark | Drinking water | 1.2 × 103−3.4 × 103 (copies/m3) | 1.6 × 107-10.0 × 107 (copies/m3) | 0.82 × 108-2.58 × 108 (copies/m3) | – | Tatari et al., 2017 |
| Austria | Waste water treatment plant | – | 1.3 × 103-2.1 × 103 (copies/ng DNA) | 3.4 × 102-6.8 × 102 (copies/ng DNA) | – | Pjevac et al., 2017 |
| China | Overlying water in river | 3.34 × 103-2.18 × 107 (copies/L) | 1.06 × 105-2.98 × 107 (copies/L) | 1.25 × 104 (copies/L) | – | Zhang et al., 2019 |
| China | Agriculture soil | – | – | 4.14 × 104-1.65 × 107 (copies/g) | 9.44 × 102-2.12 × 106 (copies/g) | Xu et al., 2020 |
| Italy | Rice paddy soil | 2.1 × 103-3.1 × 103 (copies/ng DNA) | – | 3.6 × 102-4.6 × 102 (copies/ng DNA) | 3.5 × 102-4.5 × 102 (copies/ng DNA) | Pjevac et al., 2017 |
| Italy | Forest soil | 1.4 × 102-2.6 × 102 (copies/ng DNA) | 1.7 × 103-3.5 × 103 (copies/ng DNA) | – | 2.9 × 102-4.9 × 102 (copies/ng DNA) | Pjevac et al., 2017 |
| China | River sediment | 1.84 × 102-3 × 102 (copies/ng DNA) | 9.3 × 101-3.4 × 103 (copies/ng DNA) | 1.8 × 102-2.8 × 102 (copies/ng DNA) | – | Zhao et al., 2019 |
| China | Intertidal sediment | 1.7 × 102-4.9 × 103 (copies/ng DNA) | 2.2 × 102-5.4 × 103 (copies/ng DNA) | 1.6 × 102-3.2 × 102 (copies/ng DNA) | – | Zhao et al., 2019 |
| China | Estuary tidal wetland sediment | 1.15 × 10 6 -1.66 × 10 7 (copies/g) or 5.71 × 10 1 -6.27 × 10 3 (copies/ng DNA) | 1.76 × 10 5 -1.73 × 10 7 (copies/g) or 1.05 × 10 1 -1.57 × 10 3 (copies/ng DNA) | 4.15 × 10 5 -6.67 × 10 6 (copies/g) or 2.74 × 10 1 -7.02 × 10 2 (copies/ng DNA) | 6.28 × 10 5 -4.01 × 10 6 (copies/g) or 1.1 × 10 2 -2.65 × 10 2 (copies/ng DNA) | This study |
In the original article, there was an error. There were some errors in the values of copy numbers.
A correction has been made to the Abstract:
Complete ammonia oxidizers (comammox), able to individually oxidize ammonia to nitrate, are considered to play a significant role in the global nitrogen cycle. However, the distribution of comammox Nitrospira in estuarine tidal flat wetland and the environmental drivers affecting their abundance and diversity remain unknown. Here, we present a large-scale investigation on the geographical distribution of comammox Nitrospira along the estuarine tidal flat wetlands of China, where comammox Nitrospira were successfully detected in 9 of the 16 sampling sites. The abundance of comammox Nitrospira ranged from 4.15 × 105 to 6.67 × 106 copies/g, 2.21- to 5.44-folds lower than canonical ammonia oxidizers: ammonia-oxidizing bacteria (AOB) and ammonia-oxidizing archaea (AOA). Phylogenetic analysis based on the alpha subunit of the ammonia monooxygenase encoding gene (amoA) revealed that comammox Nitrospira Clade A, mainly originating from upstream river inputs, accounts for more than 80% of the detected comammox Nitrospira, whereas comammox Nitrospira clade B were rarely detected. Comammox Nitrospira abundance and dominant comammox Nitrospira OTUs varied within the estuarine samples, showing a geographical pattern. Salinity and pH were the most important environmental drivers affecting the distribution of comammox Nitrospira in estuarine tidal flat wetlands. The abundance of comammox Nitrospira was further negatively correlated with high ammonia and nitrite concentrations. Altogether, this study revealed the existence, abundance and distribution of comammox Nitrospira and the driving environmental factors in estuarine ecosystems, thus providing insights into the ecological niches of this recently discovered nitrifying consortium and their contributions to nitrification in global estuarine environments.
In the original article, there was an error. There were some errors in the values of copy numbers.
A correction has been made to RESULTS, Abundance of Comammox Nitrospira and Canonical Ammonia Oxidizers, Paragraph 1:
In the ammonia oxidizing community comammox Nitrospira was significantly less abundant than canonical ammonia-oxidizers (Supplementary Figure 3). While AOA and AOB were detected in all tested sediment samples, comammox Nitrospira were detected in only 9 of the 16 samples. Among those 9 samples, all contained Comammox Nitrospira clade A amoA, with abundances between 4.15 × 105 and 6.67 × 106 copies/g dry soil. Comammox Nitrospira clade B amoA was only detected in 2 samples, but dominated comammox Nitrospira abundance in these samples (6.28 × 105-4.01 × 106 copies/g dry soil). Comammox Nitrospira was widespread in most parts of the tested wetland areas, and their abundance showed spatial patterns, similar to those detected for the PNRs, with higher abundance in the central (9.41 × 106 ±1.28 × 106 copies/g dry soil) than southern (2.77 × 106 ± 2.53 × 106 copies/g dry soil) and northern (1.55 × 106 ± 6.3 × 105 copies/g dry soil) latitudes (Figure 4). The highest copy number of comammox Nitrospira amoA genes was detected at central latitude site MLX (6.66 × 106 copies/g dry soil), and the lowest one was recorded at the most southern site NLJ (6.47 × 105 copies/g dry soil). Again, no significant correlation with temperature (p > 0.05), but a significant positive correlation with Fe2C (r = 0.403, p < 0.01, n = 27) and a negative correlation with salinity (r = −0.321, p < 0.05, n = 27) were detected (Supplementary Figure 1), further indicating the strong effect of salinity and metal ions on ammonia oxidation.
In the original article, there was an error. There were some errors in the values of copy numbers.
A correction has been made to RESULTS, Abundance of Comammox Nitrospira and Canonical Ammonia Oxidizers, Paragraph 2:
Among the canonical ammonia oxidizers, which were detected in all samples, abundance ranged from 1.15 × 106 to 3.66 × 107 copies/g dry soil (AOA) and 1.76 × 105 to 1.73 × 107 copies/g dry soil (AOB) (Supplementary Figure 3). In 10 of the 16 estuarine tidal flat wetland samples AOA showed higher abundance than AOB (Supplementary Figure 4). The abundance of AOA was positively correlated with temperature (r = 0.44, p < 0.01, n = 48) with highest abundance in estuaries of central and southern latitudes. Contrary, AOB were mainly distributed across the central and northern latitudes, and dominated ammonia oxidizer abundances at the northern latitudes.
In the original article, there was an error. There were some errors in the values of copy numbers.
A correction has been made to DISCUSSION, Distribution of Comammox Nitrospira in Estuarine Tidal Flat Wetlands of China, Paragraph 1:
Comammox Nitrospira were detected from 9 of the 16 sampling sites. The abundance of comammox Nitrospira ranged from 4.15 × 105 to 6.66 × 106 copies/g, 2.21- to 5.44-folds lower than canonical ammonia oxidizers: AOA and AOB, which were both detected at every sampling location. The three types of microorganisms use ammonia as an energy substance, and hence are in direct nutrient competition. However, they are able to coexist in most environments. In the estuarine tidal flat wetlands nitrifying microbial network (AOA, AOB, and comammox Nitrospira) (Figure 5), the correlation between all species is mainly positive (98.17%) and their abundance is equally correlated with the detected PNRs. The average ratio of comammox Nitrospira to AOA and AOB is 0.18 and 0.46. From the proportion of abundance, the contribution of comammox Nitrospira to the PNRs and hence nitrification may be smaller than that of AOA and AOB. The abundance of AOA was higher than that of AOB in 10 of the 16 sediment samples, with the AOA/AOB ratio ranging from 0.22 up to 205. No significant decreases of PNRs could be observed in intertidal sediment after AOB were inhibited by ampicillin, implying that AOA might play the most important role for the nitrification potential in this specific ecosystem (Zheng et al., 2014).
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- Bartelme R. P., McLellan S. L., Newton R. J. (2017). Freshwater recirculating aquaculture system operations drive biofilter bacterial community shifts around a stable nitrifying consortium of ammonia-oxidizing archaea and comammox Nitrospira. Front. Microbiol. 8:101. 10.3389/fmicb.2017.00101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pjevac P., Schauberger C., Poghosyan L., Herbold C. W., Van Kessel M. A., Daebeler A., et al. (2017). AmoA-Targeted polymerase chain reaction primers for the specific detection and quantification of comammox Nitrospira in the environment. Front. Microbiol. 8:1508. 10.3389/fmicb.2017.01508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatari K., Musovic S., Gulay A., Dechesne A., Albrechtsen H., Smets B. F. (2017). Density and distribution of nitrifying guilds in rapid sand filters for drinking water production: dominance of Nitrospira spp. Water Res. 127, 239–248. 10.1016/j.watres.2017.10.023 [DOI] [PubMed] [Google Scholar]
- Xu S., Wang B., Li Y., Jiang D., Zhou Y., Ding A., et al. (2020). Ubiquity, diversity, and activity of comammox Nitrospira in agricultural soils. Sci. Total. Environ. 706:135684. 10.1016/j.scitotenv.2019.135684 [DOI] [PubMed] [Google Scholar]
- Zhang S., Xia X., Li S., Zhang L., Wang G., Li M., et al. (2019). Ammonia oxidizers in high-elevation rivers of the Qinghai-tibet plateau display distinctive distribution patterns. Appl. Environ. Microbiol. 85:e01701–19. 10.1128/AEM.01701-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Z., Huang G. H., He S., Zhou N., Wang M., Dang C., et al. (2019). Abundance and community composition of comammox bacteria in different ecosystems by a universal primer set. Sci. Total. Environ. 691, 146–155. 10.1016/j.scitotenv.2019.07.131 [DOI] [PubMed] [Google Scholar]
- Zheng Y., Hou L., Newell S. E., Liu M., Zhou J., Zhao H., et al. (2014). Community dynamics and activity of ammonia-Oxidizing prokaryotes in intertidal sediments of the Yangtze estuary. Appl. Environ. Microbiol. 80, 408–419. 10.1128/AEM.03035-13 [DOI] [PMC free article] [PubMed] [Google Scholar]