Abstract
Background
Approximately 5% of patients with coronavirus disease (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 develop severe COVID-19. Severe COVID-19 requires respiratory management with mechanical ventilation and an extended period of treatment. Prolonged infectious virus shedding is a concern in severe COVID-19 cases, but few reports have examined the duration of infectious virus shedding. Therefore, we investigated the duration of infectious virus shedding in patients transferred to Hiroshima University Hospital with severe COVID-19 requiring mechanical ventilation.
Methods
Nasopharyngeal swab specimens were collected and analyzed using both viral culture and reverse transcriptase-quantitative polymerase chain reaction (RT-qPCR) tests between December 2020 and February 2021.
Results
Of the 23 patients tested, the proportions of those with positive test results at first specimen collection (the median number of days to first specimen collection after symptom onset was 10) on RT-qPCR and viral culture tests were 95·7% and 30·4%, respectively. All six patients with positive viral culture test results who were followed-up tested negative 24 days after symptom onset but remained positive on RT-qPCR. Viral loads based on PCR testing did not decrease over time, but those determined via culture tests decreased over time. The longest negative conversion time was observed in a dialysis patient on immunosuppressive drugs.
Conclusions
This study indicated that patients with severe COVID-19 remain culture positive for ≥ 10 days after symptom onset. Additionally, immunosuppressed patients with severe COVID-19 could consider isolation for ≥ 20 days.
Keywords: SARS-CoV-2, Severe coronavirus disease, Virus shedding
1. Introduction
Cases of coronavirus disease (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) continue to increase worldwide. Approximately 5% of patients with COVID-19 develop severe COVID-19 and require mechanical ventilation [1]. Numerous reports have shown that viral culture test results become negative 10 days after symptom onset [2,3]. It has also been reported that specimens collected more than 8 days after symptom onset were culture-negative, even when a high viral load was detected on quantitative polymerase chain reaction (PCR) testing [4,5]. In mild cases, from day 10 onwards, isolation is recommended for 24 h after the disappearance of symptoms such as fever. In contrast, severe cases are believed to exhibit prolonged infectious virus shedding, and isolation for about 20 days is recommended by the Centers for Disease Control and Prevention (CDC) and the European Centre for Disease Prevention and Control [6,7]. Nevertheless, an insufficient number of reports have examined the duration of infectious virus shedding in severe cases, and determining when to end isolation remains a major concern for medical institutions treating patients with severe COVID-19. A positive virus culture test result has been reported from a specimen collected 20 days after symptom onset [8]. A positive viral culture test result has even been reported on around day 70 after symptom onset in a patient with T-cell deficiency [9].
We assessed the duration of virus shedding in patients with severe COVID-19 by performing viral culture and reverse transcriptase-quantitative polymerase chain reaction (RT-qPCR) testing of patients with severe COVID-19 who required mechanical ventilation and were admitted to Hiroshima University Hospital for treatment.
2. Methods
2.1. Study participants and procedure
The study included 23 patients with severe COVID-19 who required mechanical ventilation and were admitted to Hiroshima University Hospital for treatment between December 2, 2020 and February 5, 2021. Hiroshima University Hospital accepts hospital transfers of patients with severe COVID-19 who require intubation management in Hiroshima Prefecture and provides treatment in its intensive care unit.
Nasopharyngeal swab specimens were collected with a swab and 1 mL of physiological saline. The first specimen was collected within one to two days after transfer to Hiroshima University Hospital. When the first viral culture test result was positive and circumstances allowed for follow-up, the duration of infectious virus shedding was investigated by collecting specimens every three to four days until negative viral culture conversion was observed. Patient information (date of symptom onset, medical history, medications, body mass index [BMI]) was extracted from the hospital's electronic medical records. Medical history of patients was only included in this study when there was evidence of a link between having a medical history and the occurrence of severe COVID-19 [10].
2.2. Cell and viral culture
SARS-CoV-2 was cultured in Vero cells expressing TMRPSS2 (VeroE6/TMRPSS2 cells, procured from JCRB cell bank, Japan). Cultures were observed for seven days and checked for cytopathic effects (CPEs). Infectivity titer was measured based on 50% tissue culture infectious dose (TCID50), while TCID50/mL was calculated using a method described in a previous report [11]. When no CPEs were observed within seven days, the culture was considered negative.
2.3. RT-qPCR
RT-qPCR tests were performed using a method described in a previous report [11]. SARS-CoV-2 nucleic acids were extracted using a Viral Total Nucleic Acid Purification Kit (Promega Corporation, USA), while RT-qPCR was performed using a One Step PrimeScript™ III RT-qPCR mix (Takara Bio Inc., Japan). N genes were targeted using the forward primer (2.4 μM), 5′-AAA TTT TGG GGA CCA GGA AC-3′, reverse primer (3.2 μM), 5′-TGG CAG CTG TGT AGG TCA AC-3′, and probe (0.4 μM) 5′-FAM-ATG TCG CGC ATT GGC ATG GA-BHQ-3′. RT-qPCR was run according to the manufacturer's protocol. A known quantity of SARS-CoV-2 RNA was used as a positive control, and copies/mL were calculated from cycle threshold (Ct) values.
2.4. Ethics
This research was approved by the Ethical Committee for Epidemiology of Hiroshima University (approval number: E-2157). The requirement for written informed consent was waived because only the patients’ clinical specimens were used in this study, and the patients had the right to opt-out from the use of their surplus patient material and medical data for research.
2.5. Statistical analysis
Fisher's exact test and the Mann Whitney U test were used to compare patient characteristics. Statistical significance was set at p < 0.05. Statistical analysis was performed using JMP software version 15 (SAS Institute Inc., USA).
3. Results
3.1. Patient characteristics
Thirty-three nasopharyngeal swab specimens were collected from 23 patients. Characteristics of patients included in the study are shown in Table 1 . Patients were categorized into culture-positive (seven patients, 30·4%) and culture-negative (16 patients, 69·6%) groups, depending on initial viral culture test results. The median number of days prior to the first specimen collection was 10 days after symptom onset (culture-positive group: nine days, culture-negative group: 10.5 days; p = 0.21). The median patient age was 64.0 years (culture-positive group: 67.0 years, culture-negative group: 62.5 years; p = 0.66), and 82·6% of the patients were men (culture-positive group: 66.7%, culture-negative group: 87.5%; p = 0.35). The median BMI was 28.4 kg/m2 (culture-positive group: 26.4 kg/m2, culture-negative group: 30.8 kg/m2; p = 0.23), the most frequent comorbidity was hypertension (culture-positive group: 57·1%, culture-negative group: 50·0%; p = 0.56), and 30·4% of patients had diabetes mellitus (culture-positive group: 14·3%, culture-negative group: 37·5%; p = 0.28). Table 2 shows the viral culture results, PCR test results, and treatment according to the time since symptom onset. All specimens were collected before withdrawing mechanical ventilation. The median viral load of the first specimen was 4.0 × 10⁵copies/mL (culture-positive group: 2.7 × 10⁷copies/mL; culture-negative group: 1.1 × 10⁵copies/mL; P = 0.067). The median duration of corticosteroid therapy until the first specimen collection was 6 days (culture-positive group: 5 days; culture-negative group: 6 days; p = 0.14). Nine of the 23 patients (39.1%) received high-dose (methylprednisolone 1000 mg/day) pulse therapy (culture-positive group: 3/7 [42·9%]; culture-negative group: 6/16 [37·5%]; p > 0 0.99). Further, 9 of the 23 patients (39.1%) received antiviral drugs (culture-positive group: 4/7 [57·1%]; culture-negative group: 5/16 [31·3%]; p = 0.36). No significant difference between the two groups was found in any characteristics.
Table 1.
Patient characteristics (N = 23).
| Patient No. | Age (years) | Sex | BMI (kg/m2) | Medical history |
|---|---|---|---|---|
| Culture-positive group (N = 7) | ||||
| P3 | 61 | Male | 20·3 | Rheumatoid arthritis (receiving prednisolone and abatacept), end-stage renal failure (undergoing dialysis), autoimmune leukopenia |
| P7 | 48 | Male | 28·4 | Fatty liver |
| P8 | 52 | Male | 32·4 | Diabetes mellitus (HbA1C: 11·2%) |
| P11 | 70 | Male | 26·4 | Hypertension |
| P12 | 67 | Male | 25·7 | Hypertension |
| P14 | 73 | Female | 24·5 | Hypertension |
| P22 | 68 | Female | 27·9 | Rheumatoid arthritis (receiving Janus kinase inhibitor and prednisolone), hypertension |
| Culture-negative group (N = 16) | ||||
| P1 | 53 | Male | 23·8 | Diabetes mellitus (HbA1C: 7·6%), interstitial pneumonitis |
| P2 | 42 | Male | 34·7 | Not significant |
| P4 | 50 | Male | 40·7 | Diabetes mellitus (HbA1C: 9·8%) |
| P5 | 66 | Male | 31·0 | Diabetes mellitus (HbA1C: 8·7%) |
| P6 | 72 | Male | 29·7 | Diabetes mellitus (HbA1C: 11·9%), hypertension |
| P9 | 68 | Male | 30·9 | Diabetes mellitus (HbA1C: 7·2%), hypertension |
| P10 | 65 | Male | 26·1 | Chronic hepatitis C |
| P13 | 57 | Male | 23·5 | Hypertension |
| P15 | 81 | Male | 24·6 | Hypertension |
| P16 | 77 | Female | 33·2 | Diabetes mellitus (HbA1C: 7·4%) |
| P17 | 68 | Male | 31·7 | Hypertension |
| P18 | 64 | Male | 22·3 | Emphysema, hypertension |
| P19 | 59 | Male | 23·7 | Hypertension |
| P20 | 54 | Male | 30·7 | Hypertension |
| P21 | 29 | Male | 36·4 | Crohn's disease |
| P23 | 61 | Female | 32·6 | Rheumatoid arthritis (receiving methotrexate) |
BMI, body mass index; HbA1C, glycated hemoglobin; P, patient.
Table 2.
Timing of SARS-CoV-2 testing and treatment according to days since symptom onset (N = 23).
| Days since symptom onset |
|||||
|---|---|---|---|---|---|
| Patient No. | First specimen collection (copies/mL) | Negative culture result (copies/mL) | Mechanical ventilation | Antiviral drugs | Corticosteroid therapy |
| Culture-positive group (N = 7) | |||||
| P3 | 12 (2.1 × 10⁹) | 24 (4.8 × 10⁶) | 11–44 | Favipiravir: 3–10 | 8–18 |
| P7 | 9 (2.7 × 10⁸) | 16 (3.0 × 10⁴) | 9–16 | Favipiravir: 3–8 | 4–11; High-dose pulse therapy: 9 |
| P8 | 10 (1.9 × 10⁵) | N.D. | 9–11 | None | 6–11 |
| P11 | 9 (4.1 × 10⁷) | 13 (4.5 × 10⁷) | 7–34 | None | 4–13 |
| P12 | 10 (5.3 × 10⁵) | 13 (1.8 × 10⁶) | 10–14 | None | 9–15 |
| P14 | 8 (9.7 × 10⁸) | 15 (8.0 × 10⁴) | 6–17 | Favipiravir: 4–5 | 4–17; High-dose pulse therapy: 4–6, 12–14 |
| P22 | 4 (2.7 × 10⁷) | 11 (4.7 × 10⁵) | 4–29 | Remdesivir: 3 | 3–10; High-dose pulse therapy: 3 |
| Culture-negative group (N = 16) | |||||
| P1 | 14 (6.4 × 10³) | 10–15 | Favipiravir: 1–6 | 3–16; High-dose pulse therapy: 5–7 |
|
| P2 | 11 (7.3 × 10⁵) | 8–13 | None | 5–13 | |
| P4 | 11 (7.0 × 10⁴) | 11–18 | None | 8–11 | |
| P5 | 9 (4.6 × 10⁴) | 9–13 | Favipiravir: 5–7; Remdesivir: 7–8 |
7–9; High-dose pulse therapy: 9 |
|
| P6 | 11 (4.9 × 10⁶) | 11–17 | Favipiravir: 7–9; Remdesivir: 9–10; |
7–10; High-dose pulse therapy: 10 |
|
| P9 | 9 (3.7 × 10⁷) | 9–12 | Favipiravir: 0–3; Remdesivir: 3–7 |
3–7 | |
| P10 | 8 (2.7 × 10⁵) | 9–25 | None | 2–8; High-dose pulse therapy: 5–7 |
|
| P13 | 12 (3.9 × 10⁶) | 9–24 | None | 7–15; High-dose pulse therapy: 7–8 |
|
| P15 | 12 (3.2 × 10³) | 3–15 | None | 3–12 | |
| P16 | 9 (6.5 × 10³) | 2–9 | None | 2–8 | |
| P17 | 10 (2.7 × 10⁶) | 5–28 | None | 9–15 | |
| P18 | 12 (1.6 × 10⁶) | 7–25 | None | 7–13 | |
| P19 | 6 (−) | 1–11 | None | 1–10 | |
| P20 | 10 (1.3 × 10³) | 4–20 | None | 4–10 | |
| P21 | 13 (4.8 × 10⁴) | 11–20 | None | 8–13; High-dose pulse therapy: 9–11 |
|
| P23 | 8 (1.1 × 10⁵) | 8–13 | Favipiravir: 3–6; Remdesivir: 6–7 |
7–13 | |
High-dose pulse therapy: methylprednisolone 1000 mg/day.
The timing of the first specimen collection, the first specimen collection with a negative culture result, mechanical ventilation, antiviral drug administration, and corticosteroid therapy are shown according to the number of days since symptom onset.
Copies/mL were detected using polymerase chain reaction testing.
3.2. Test results by specimen
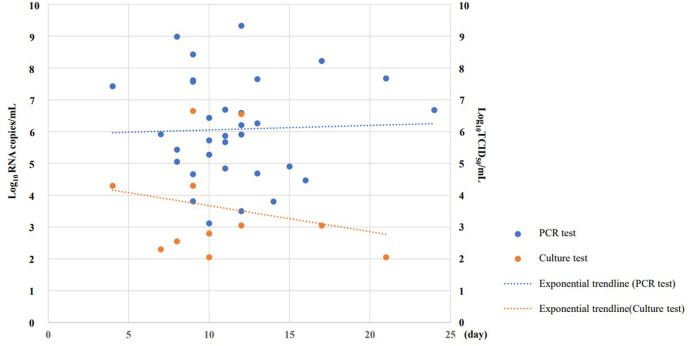
At first specimen collection, 95·7% of the 23 patients tested positive via RT-qPCR results, and 30·4% of the patients tested positive via viral culture. Of the 33 specimens tested, the proportion of those with positive viral culture test results was 33·3%. The longest time to produce a positive viral culture test result was 21 days after symptom onset, and the infectivity titer ranged from 1·1 × 102 to 3·6 × 106 TCID50/mL. Of the 33 specimens considered, the proportion of those with a positive RT-qPCR test result was 97·0% and detected viral loads ranged from 1·3 × 103 to 2·1 × 109 copies/mL (Fig. 1 ). RT-qPCR test results showed no change in viral load over time, but viral culture test results showed a decrease in infectivity titer over time.
Fig. 1.
SARS-CoV-2 viral load results and exponential trendline based on quantitative polymerase chain reaction testing and culture (N = 33).
PCR, polymerase chain reaction.
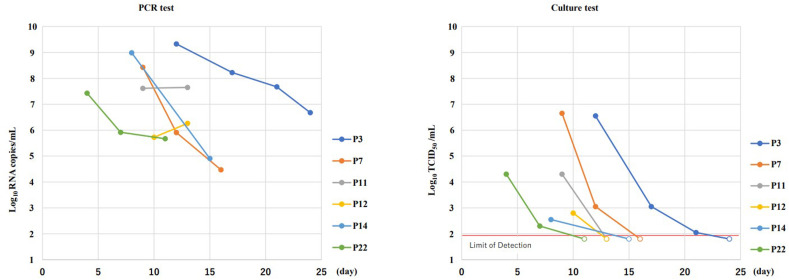
Of the seven patients with an initial positive viral culture test result, specimens were collected and viral culture and RT-qPCR testing were performed in six patients until negative viral culture conversion occurred (Fig. 2 ). Viral culture testing revealed that the infectivity titer decreased over time and negative viral culture conversion occurred in all six patients (median negative conversion time: 14 days). PCR testing revealed viral loads of at least 104 copies/mL, even in specimens with a negative viral culture test result. One of the seven patients, whose negative viral culture conversion time was 24 days, had been receiving prednisolone and abatacept for rheumatoid arthritis, undergoing dialysis, and had underlying autoimmune leukopenia.
Fig. 2.
Time-course of SARS-CoV-2 polymerase chain reaction and culture results in patients with one or more culture-positive results
PCR, polymerase chain reaction.
4. Discussion
In patients with severe COVID-19 requiring mechanical ventilation, a large difference in the percentage of positive results between viral culture and PCR testing was revealed. Viral culture testing revealed that viral load decreased over time, while decreases were not observed via PCR testing. Previous reports have also shown that PCR test results remain positive for an extended period, up to 40 days or more in some cases [12,13]. The CDC does not recommend ending isolation based on PCR testing [6]. Although the PCR viral load tended to be higher in culture-positive specimens than in culture-negative specimens, we found many culture-negative specimens with viral loads of ≥10⁵ copies/mL. In the culture-positive group, the viral load did not decrease significantly before becoming culture-negative. The number of infectious viruses decreases with time, but inactivated viruses may remain in the body or nasal cavity and can be detected by PCR. Therefore, there may be a difference in the viral load obtained using PCR testing and culture. The results of this study also showed that it is difficult to evaluate whether a patient is infectious based on PCR testing, even if the viral load is known.
Improvement of symptoms is also a criterion for lifting isolation in mild cases. It is difficult to confirm the improvement of symptoms while patients are on mechanical ventilation. In severe cases, long-term mechanical ventilation is often required. In this study, all culture-negative specimens were those taken before withdrawing mechanical ventilation.
In terms of infectivity titers, we confirmed that both the viral load and percentage of positive results decreased over time. One patient required 24 days for negative viral culture conversion to occur. In this patient, factors associated with immunosuppression, including treatment with prednisolone and abatacept, and dialysis for renal failure likely influenced the results. The likelihood that these factors caused severe COVID-19 has already been identified. Previous studies revealed that a high proportion of patients receiving prednisolone for rheumatism developed severe COVID-19 [14], and the mortality rate of COVID-19 is reportedly higher in patients who are on dialysis than in their counterparts [15]. Nevertheless, the possibility that these factors may lead to viral culture test positivity for prolonged periods has not been fully examined. Though this affected only one patient in this study, the finding suggests that these factors may prolong the negative viral culture conversion time.
For all the other patients in this study, there was no difference in age, BMI, or medical history between the culture-positive and culture-negative groups, and negative viral culture conversion occurred between days 6 and 16 after symptom onset. Furthermore, in this study, approximately one third (30·4%) of all patients with severe COVID-19 were believed to have been shedding the infectious virus 10 days after symptom onset.
This study has a few limitations. This was a single-center study of a group with a small sample size, and only seven patients had positive viral culture test results. Patients were transferred to our hospital when they required mechanical ventilation and the timing of the first specimen collection was different for each patient. A few specimens were collected within seven days after symptom onset, and we could not investigate the percentage of positive viral culture test results obtained prior to initiating therapy. In addition, we did not collect specimens every day; hence, the time to culture negativity might have been overestimated. SARS-CoV-2 infectivity was assessed with a viral culture test that used Vero cells expressing TMPRSS2. Vero cells are well suited for SARS-CoV-2 culture [16], and Vero cells expressing TMPRSS2 are even more susceptible to SARS-CoV-2 infection [17]. However, additional knowledge is required to investigate what factors inhibit viral culture and the detection limits of viral culture.
This study showed that 30% of the critically-ill patients remained culture positive for ≥ 10 days after symptom onset. Further, one immunosuppressed patient required 24 days of testing before negative viral culture conversion occurred, suggesting that a longer period of isolation is required for patients with impaired cellular immunity. Further research investigating patients with COVID-19 who exhibit prolonged infectious virus shedding is required.
Author contribution
This study was designed and supervised by NS, TS, and HO. T. Nomura, HK, KO, and NS collected samples and clinical data, which were analyzed by T. Nomura, MK, and TN. All authors contributed to interpreting the data. The final manuscript was reviewed by all authors.
Funding
The authors received no funding for this study.
Declaration of competing interest
We declare no competing interests.
Acknowledgments
None.
Footnotes
All authors meet the ICMJE authorship criteria.
References
- 1.Centers for Disease Control and Prevention Interim clinical guidance for management of patients with confirmed coronavirus disease (COVID-19) 2021. https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-guidance-management-patients.html Available at: Accessed 21 February.
- 2.Millon M., Lagier J.C., Gautret P., Colson P., Fournier P.E., Amrane S., et al. Early treatment of COVID-19 patients with hydroxychloroquine and azithromycin: a retrospective analysis of 1061 cases in Marseille, France. Trav Med Infect Dis. 2020;35:101738. doi: 10.1016/j.tmaid.2020.101738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bullard J., Dust K., Funk D., Strong J.E., Alexander D., Garnett L., et al. Predicting infectious SARS-CoV-2 from diagnostic samples. Clin Infect Dis. 2020;71:2663–2666. doi: 10.1093/cid/ciaa638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wolfel R., Corman V.M., Guggemos W., Seilmaier M., Zange S., Müller M., et al. Virological assessment of hospitalized patients with COVID-2019. Nature. 2020;581:465–469. doi: 10.1038/s41586-020-2196-x. [DOI] [PubMed] [Google Scholar]
- 5.La Scola B., Le Bideau M., Andreani J., Thuan Hoang V., Grimaldier C., Colson P., et al. Viral RNA load as determined by cell culture as a management tool for discharge of SARS-CoV-2 patients from infectious disease wards. Eur J Clin Microbiol Infect Dis. 2020;39:1059–1061. doi: 10.1007/s10096-020-03913-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention Duration of isolation and precautions for adults with COVID-19. 2021. https://www.cdc.gov/coronavirus/2019-ncov/hcp/duration-isolation.html Available at: Accessed 21 February.
- 7.European Centre for Disease Prevention and Control Guidance for discharge and ending of isolation of people with COVID-19. 2021. https://www.ecdc.europa.eu/en/publications-data/guidance-discharge-and-ending-isolation-people-covid-19 Available at. Accessed 21 February.
- 8.van Kampen JJA, van de Vijver D.A.M.C., Fraaij P.L.A., Haagmans B.L., Lamers M.M., Okba N., et al. Duration and key determinants of infectious virus shedding in hospitalized patients with coronavirus disease-2019 (COVID-19) Nat Commun. 2021;12:267. doi: 10.1038/s41467-020-20568-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Avanzato V.A., Matson M.J., Seifert S.N., Pryce R., Williamson B.N., Anzick S.L., et al. Case Study: prolonged infectious SARS-CoV-2 shedding from an asymptomatic immunocompromised individual with cancer. Cell. 2020;183:1901–1912. doi: 10.1016/j.cell.2020.10.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Centers for Disease Control and Prevention People with certain medical conditions. 2021. https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/people-with-medical-conditions.html?CDC_AA_refVal=https%3A%2F%2Fwww.cdc.gov%2Fcoronavirus%2F2019-ncov%2Fneed-extra-precautions%2Fgroups-at-higher-risk.html Available at: Accessed 21 February.
- 11.Kitagawa H., Nomura T., Nazmul T., Omori K., Shigemoto N., Sakaguchi T., et al. Effectiveness of 222-nm ultraviolet light on disinfecting SARS-CoV-2 surface contamination. Am J Infect Contr. 2020;49:299–301. doi: 10.1016/j.ajic.2020.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.An J., Liao X., Xiao T., Qian S., Yuan J., Ye H., et al. Clinical characteristics of the recovered COVID-19 patients with re-detectable positive RNA test. MedRxiv. 2020;8:1084. doi: 10.21037/atm-20-5602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhou B., She J., Wang Y., Ma X. The duration of viral shedding of discharged patients with severe COVID-19. Clin Infect Dis. 2020;71:2240–2242. doi: 10.1093/cid/ciaa451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gianfrancesco M., Hyrich K.L., Al-Adely S., Carmona L., Danila M.I., Gossec L., et al. Characteristics associated with hospitalisation for COVID-19 in people with rheumatic disease: data from the COVID-19 Global Rheumatology Alliance physician-reported registry. Ann Rheum Dis. 2020;79:859–866. doi: 10.1136/annrheumdis-2020-217871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hsu C.M., Weiner D.E., Aweh G., Miskulin D.C., Manley H.J., Stewart C., et al. COVID-19 infection among US dialysis patients: risk factors and outcomes from a national dialysis provider. Am J Kidney Dis [Preprint] 2021 doi: 10.1053/j.ajkd.2021.01.003. [cited 2021 March 29]. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ogando N.S., Dalebout T.J., Zevenhoven-Dobbe J.C., Limpens R.W.A.L., van der Meer Y., Caly L., et al. SARS-coronavirus-2 replication in Vero E6 cells: replication kinetics, rapid adaptation and cytopathology. J Gen Virol. 2020;101:925–940. doi: 10.1099/jgv.0.001453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Matsuyama S., Nao N., Shirato K., Kawase M., Saito S., Takayama I., et al. Enhanced isolation of SARS-CoV-2 by TMPRSS2- expressing cells. Proc Natl Acad Sci U S A. 2020;117:7001–7003. doi: 10.1073/pnas.2002589117. [DOI] [PMC free article] [PubMed] [Google Scholar]