To the Editor
Prion diseases are fatal and incurable neurodegenerative diseases caused by a pathogenic form of prion protein (PrPSc) derived from a cellular form of prion protein (PrPC). Recent studies have reported that aquaporin 4 (AQP4) expression is dramatically upregulated in the brains of individuals with several different prion diseases. Since AQP4 is a key protein of the glymphatic system, which is the perivascular waste clearing system of the brain, and since altered expression of AQP4 has been observed in prion diseases, the glymphatic system may be associated with prion diseases. Thus, investigation of the association between the glymphatic system and prion diseases is important. We identified the altered expression pattern of glymphatic system-related molecules in prion-infected mice at 7 months post-injection and sporadic Creutzfeldt-Jakob disease (CJD) patients by western blotting and immunohistochemistry (IHC). In addition, we introduced glymphatic system-activated drugs, including dexmedetomidine and clonidine, which are known to increase glymphatic flow and remove brain waste. Then, we evaluated the protective effect of glymphatic system-activated drugs in prion-infected mice by western blotting and survival analysis. We identified altered band patterns of cleaved agrin and upregulation of neurotrypsin expression in prion-infected mice and sporadic CJD patients. We found dramatic clearance of PrPSc and amelioration of astrocytosis in the dexmedetomidine- and clonidine-treated prion-infected mice at 5 months post-injection. In addition, we observed a significantly delayed incubation period in the dexmedetomidine- and clonidine-treated prion-infected mice.
Prion diseases are fatal, progressive and irreversible brain proteinopathies characterized by astrocytosis and the accumulation of PrPSc originating from PrPC [1]. Recent studies have reported that AQP4 expression is dramatically elevated in the brains of individuals with several prion diseases [2–4]. Since AQP4 is a key protein of the glymphatic system in the brain and altered expression of AQP4 has been observed in prion diseases [5, 6], the glymphatic system is assumed to be associated with prion diseases. Thus, investigation of the association between the glymphatic system and prion diseases is important.
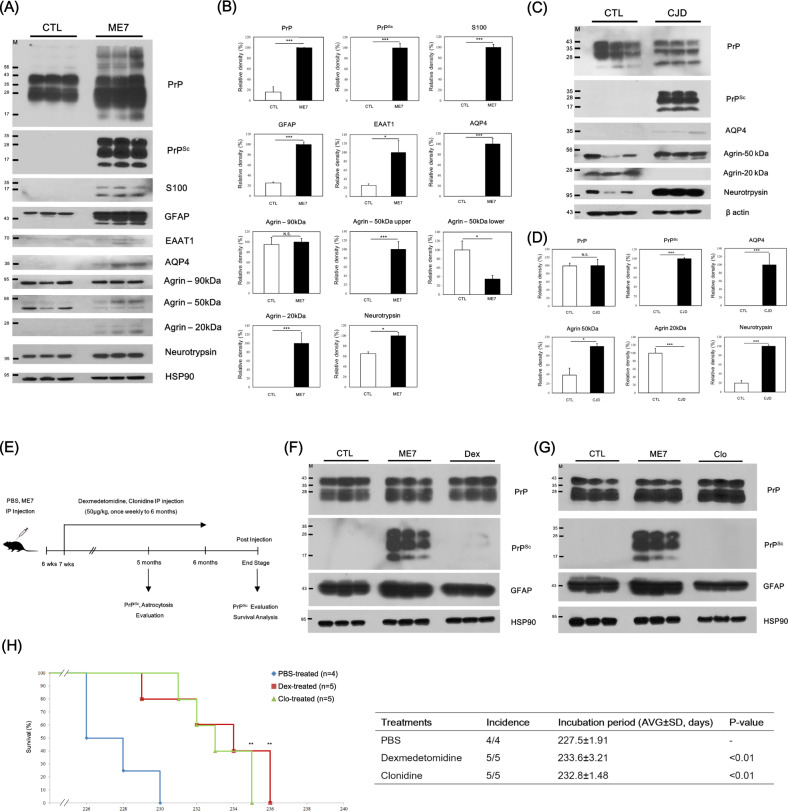
To identify novel glymphatic system-related biomarkers in prion-infected mice, we investigated the expression pattern of glymphatic system-related proteins in prion-infected mice and matched controls at 7 months post-injection by western blotting. In brief, total prion protein (PrP) and PrPSc levels were upregulated and accumulated in prion-infected mice compared to control mice. In addition, astrocyte markers, including S100, glial fibrillary acidic protein (GFAP) and excitatory amino acid transporter 1 (EAAT1), were significantly elevated in the prion-infected mice. In agreement with previous studies, AQP4 in this study was elevated in the prion-infected mice compared to the control mice. Notably, glymphatic system-related proteins, including agrin and neurotrypsin, showed different expression patterns in the prion-infected mice compared to the matched controls (Fig. 1A, B).
Fig. 1.
Strong association of the glymphatic system with prion diseases. A The expression patterns of glymphatic system-related proteins in the whole brains of wild-type and ME7 scrapie-infected mice at 7 months post-injection were analyzed by western blotting. B The expression levels of glymphatic system-related proteins were normalized to the HSP90 level. CTL: PBS-inoculated mice, n = 3; ME7: ME7 scrapie-inoculated mice, n = 3. *P < 0.05; **P < 0.01; ***P < 0.001; N.S. not significant. C The expression patterns of glymphatic system-related proteins in the frontal cortex of sporadic CJD patients and matched healthy controls were analyzed by western blotting. D The expression levels of glymphatic system-related proteins were normalized to the β actin level. CTL: age-matched healthy control, n = 3; CJD: CJD patients, n = 3. *P < 0.05; ***P < 0.001; N.S. not significant. E Experimental overview of the evaluation of the effects of glymphatic system-activated molecules in prion-infected mice. F Evaluation of the protective effects of dexmedetomidine (Dex) on PrPSc accumulation and astrocytosis in the prion-infected mice at 5 months post-injection by western blotting. G Evaluation of the protective effect of clonidine (Clo) on PrPSc accumulation and astrocytosis in the prion-infected mice at 5 months post-injection by western blotting. H The incubation periods in the PBS-treated ME7 scrapie-inoculated mice (n = 4), dexmedetomidine-treated ME7 scrapie-inoculated mice (n = 5) and clonidine-treated ME7 scrapie-inoculated mice (n = 5). **P < 0.01. The graph shows the percentage survival versus days after intraperitoneal (IP) inoculation
We also investigated the expression pattern of glymphatic system-related proteins in the cerebral cortex of the prion-infected mice and the matched controls at 7 months post-injection by IHC. Similar to the western blotting results, the astrocyte marker GFAP was elevated in the prion-infected mice. Notably, glymphatic system-related markers, including AQP4, agrin and neurotrypsin, were also elevated in the prion-infected mice compared to the matched controls (Supplementary Fig. 1).
We also investigated the expression pattern of glymphatic system-related proteins in the frontal cortex of sporadic CJD patients and matched controls by western blotting. In brief, the expression level of PrP was similar between the sporadic CJD patients and the matched controls. However, PrPSc accumulation was observed in the sporadic CJD patients but not in the matched controls. Notably, glymphatic system-related proteins, including AQP4, agrin and neurotrypsin, showed different expression patterns in the sporadic CJD patients compared to the matched controls (Fig. 1C, D).
To evaluate the protective effects of glymphatic system-activated drugs [7, 8], including dexmedetomidine and clonidine, against prion disease, we inoculated the ME7 scrapie strain into 6-week-old mice; treated them with PBS, dexmedetomidine and clonidine weekly; and sacrificed them at 5 months post-injection. Notably, PrPSc was dramatically eliminated in the dexmedetomidine- and clonidine-treated prion-infected mice compared to the PBS-treated prion-infected mice at 5 months post-injection. In addition, the astrocyte marker GFAP was significantly decreased in the dexmedetomidine- and clonidine-treated prion-infected mice (Fig. 1E–G, Supplementary Fig. 2).
To evaluate the protective effects of glymphatic system-activated drugs, including dexmedetomidine and clonidine, we performed a survival analysis. In brief, the incubation period of the PBS-treated prion-infected mice was 227.5 ± 1.91 days. Notably, the incubation periods of the dexmedetomidine (233.6 ± 3.21 days) and clonidine (232.8 ± 1.48 days)-treated prion-infected mice were significantly delayed compared to those of the PBS-treated prion-infected mice (Fig. 1H). PrPSc accumulation in the dexmedetomidine-treated prion-infected mice was similar to that in the PBS-treated prion-infected mice. However, the clonidine-treated prion-infected mice showed dramatic clearance of accumulated PrPSc (Supplementary Fig. 3A, B).
Although the drugs tested in this study did not completely cure the prion disease, we identified the therapeutic potential of glymphatic system-activated drugs in fatal neurodegenerative diseases and prion diseases. Thus, we suggest that the development of drugs to activate the glymphatic system is inevitable for the treatment of several neurodegenerative diseases in the future.
Supplementary information
Acknowledgements
This work was supported by a National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIT) (2021R1A2C1013213). This research was supported by the Basic Science Research Program through the National Research Foundation (NRF) of Korea funded by the Ministry of Education (2017R1A6A1A03015876). This work was supported by an NRF (National Research Foundation of Korea) Grant funded by the Korean Government (NRF-2019-Fostering Core Leaders of the Future Basic Science Program/Global Ph.D. Fellowship Program). SYW and YCK were supported by the BK21 Plus Program in the Department of Bioactive Material Sciences.
Author contributions
YCK, SYW and BHJ conceived and designed the experiment. YCK and SYW performed the experiments. YCK, SYW and BHJ analyzed the data. YCK, SYW and BHJ wrote the paper. All authors read and approved the final manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
These authors contributed equally: Yong-Chan Kim, Sae-Young Won.
Supplementary information
The online version contains supplementary material available at 10.1038/s41423-021-00747-z.
References
- 1.Prusiner SB. The prion diseases. Brain Pathol. 1998;8:499–513. doi: 10.1111/j.1750-3639.1998.tb00171.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rodriguez A, Perez-Gracia E, Espinosa JC, Pumarola M, Torres JM, Ferrer I. Increased expression of water channel aquaporin 1 and aquaporin 4 in Creutzfeldt-Jakob disease and in bovine spongiform encephalopathy-infected bovine-PrP transgenic mice. Acta Neuropathol. 2006;112:573–85. doi: 10.1007/s00401-006-0117-1. [DOI] [PubMed] [Google Scholar]
- 3.Costa C, Tortosa R, Rodriguez A, Ferrer I, Torres JM, Bassols A, et al. Aquaporin 1 and aquaporin 4 overexpression in bovine spongiform encephalopathy in a transgenic murine model and in cattle field cases. Brain Res. 2007;1175:96–106. doi: 10.1016/j.brainres.2007.06.088. [DOI] [PubMed] [Google Scholar]
- 4.Iwasaki Y, Mimuro M, Yoshida M, Hashizume Y, Ito M, Kitamoto T, et al. Enhanced Aquaporin-4 immunoreactivity in sporadic Creutzfeldt-Jakob disease. Neuropathology. 2007;27:314–23. doi: 10.1111/j.1440-1789.2007.00781.x. [DOI] [PubMed] [Google Scholar]
- 5.Hablitz LM, Pla V, Giannetto M, Vinitsky HS, Staeger FF, Metcalfe T, et al. Circadian control of brain glymphatic and lymphatic fluid flow. Nat. Commun. 2020;11:4411. doi: 10.1038/s41467-020-18115-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wolburg H, Noell S, Wolburg-Buchholz K, Mack A, Fallier-Becker P. Agrin, aquaporin-4, and astrocyte polarity as an important feature of the blood-brain barrier. Neuroscientist. 2009;15:180–93. doi: 10.1177/1073858408329509. [DOI] [PubMed] [Google Scholar]
- 7.Lilius TO, Blomqvist K, Hauglund NL, Liu G, Staeger FF, Baerentzen S, et al. Dexmedetomidine enhances glymphatic brain delivery of intrathecally administered drugs. J. Control Rel. 2019;304:29–38. doi: 10.1016/j.jconrel.2019.05.005. [DOI] [PubMed] [Google Scholar]
- 8.Feng ZX, Dong H, Qu WM, Zhang W. Oral delivered dexmedetomidine promotes and consolidates non-rapid eye movement sleep via sleep-wake regulation systems in mice. Front Pharm. 2018;9:1196. doi: 10.3389/fphar.2018.01196. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.