Calcium is an important cell signaling intermediate known to play a critical role in T-cell receptor (TCR)-mediated activation of both developing thymocytes and mature peripheral T cells. Upon antigen engagement, the TCR recruits PLCγ1 to the proximal signaling complex to be phosphorylated and activated by the membrane-bound kinase Itk. Activated PLCγ1 mediates the cleavage of the cell membrane lipid component PIP2 into the lipids diacylglycerol (DAG) and inositol 1,4,5-triphosphate (IP3). IP3 is diffusible within the cytosol and binds its receptors (IP3Rs) on the ER membrane to trigger the release of stored calcium from the ER lumen into the cytosol. Increased cytosolic calcium, in turn, triggers cluster formation by the calcium-binding transmembrane protein STIM1, which then pairs with calcium release-activated calcium channels (CRACs) on the plasma membrane. This induces the opening of CRACs on the plasma membrane to initiate calcium flux from outside of the cell. Increased cytosolic calcium flux induces the activation of calcium/calmodulin-dependent kinases and the phosphatase calcineurin. Calcineurin dephosphorylates and activates nuclear factor of activated T cells (NFAT), one of the most critical transcription factors in T cells, and triggers the gene expression programs responsible for cell survival, proliferation, and effector functions [1].
It has been well acknowledged that TCR signaling outcomes differ significantly between developing thymocytes and mature T cells. DP thymocytes (termed preselection DPs) are highly sensitive to low-affinity ligands, and during low-affinity ligand binding, a weaker signal is essential to promote cell survival. This phenomenon allows for positive selection of developing thymocytes by low-affinity cross-reactive self-peptides. However, TCR sensitivity is dramatically downregulated in post-selection mature T cells, which is considered helpful to prevent mature T cells from responding inappropriately to self-antigens [2]. However, the reason behind this difference remains elusive.
We have recently discovered a new TCR signaling regulatory mechanism in DP thymocytes mediated by the adaptor protein Tespa1. Tespa1 is highly expressed in thymic DP cells, but its function was previously unknown. We first found that Tespa1 deficiency in mice leads to a defect in positive selection in the thymus, which is closely related to a decline in calcium signaling [3]. In a subsequent mechanistic study, we revealed that Tespa1 could recruit ER calcium channel IP3Rs to the vicinity of TCRs during thymic T cell activation. This allowed IP3Rs to respond much more quickly to the IP3 produced by nearby PLCγ1, thus enabling thymocytes to respond to low-affinity ligands [4]. Our most recent work further showed that Tespa1-mediated IP3R recruitment exists in only immature DP T cells. As T cells mature, Tespa1 expression is remarkably downregulated. Consistently, mature WT and Tespa1 KO T cells show no difference in TCR-induced calcium flux. Thus, we propose that the stage-specific expression of Tespa1 in DP cells provides a unique mechanism to sensitize T cells to low-affinity self-antigens for positive selection [5]. Clustering of IP3R in the proximal region upon TCR stimulation has been observed [6, 7]. However, the physiological function and the mechanisms were not known before our study. Our findings thus provide an important mechanistic and physiological understanding of this phenomenon.
Our discovery of Tespa1 and its function also raises several new and interesting possibilities. Since Tespa1 plays a key role in TCR signaling, dysregulation of Tespa1 may lead to inappropriate T cell activation in response to self-antigen and trigger autoimmune diseases. Indeed, a recent study reported Tespa1 as one of the susceptibility genes reflecting abnormal TCR signaling in cooccurring primary Sjögren’s syndrome (pSS), systemic lupus erythematosus (SLE) and rheumatoid arthritis (RA) [6]. Although the mechanism by which Tespa1 contributes to these diseases remains elusive, these findings highlight an important role for the molecular control of TCR signaling in autoimmune diseases.
In contrast, the activation of peripheral T cells is known to be restrained by multiple mechanisms to prevent excessive immune activation. These mechanisms include inhibition by regulatory T cells and inhibitory receptors (known as checkpoint receptors in tumor biology). Overcoming the inhibitory signal in T cells has been proven to be very effective in enhancing antitumor immunity (a mechanism known as checkpoint blockade). Thus, an interesting question is whether we can achieve better antitumor T cell immunity by overexpressing Tespa1 in mature T cells. Indeed, overexpression of Tespa1 in Jurkat T cells leads to enhanced calcium flux, suggesting that T cell activation can be controlled by modulating Tespa1 expression levels [4]. In addition, a recent study of lung adenocarcinoma revealed that Tespa1 expression correlates significantly with antitumor T cell activation and the survival-related prognosis of lung adenocarcinoma, suggesting a possible role of Tespa1 in regulating the antitumor T cell response [7]. Subsequent studies will be needed to further explore this possibility.
Finally, do similar calcium flux tuning mechanisms also exist in T cells or other cells? When Tespa1 expression is downregulated in mature T cells, another IP3R-binding protein, SLAT (SWAP-70-like adaptor of T cells), has stable expression in peripheral T cells. SLAT is selectively expressed in T cells and has been shown to play a key role in initiating Ca2+ signaling [8]. T cells that are genetically deficient for SLAT display a severe defect in TCR-induced activation and effector functions. It was later shown that SLAT directly binds to ER-localized inositol triphosphate receptor (IP3R) following TCR activation and facilitates its Ca2+ ion channel function. This process is required for productive T cell activation and effector functions [9]. However, whether SLAT can induce similar IP3R translocation has not yet been examined and remains an open question. Moreover, SLAT seems to have a broader function in T-cells since it is also essential for initiating cytoskeletal changes. SLAT deficiency leads to more profound defects in both thymocyte development and peripheral T cell function [10]. Second, calcium signaling is also involved in BCR activation in B cells. BANK1, a B cell-specific scaffold protein, binds PLCγ2 and mediates the formation of the BANK1-Lyn-IP3R complex [11]. Overexpression of BANK1 elevates calcium flux in B cells, and its polymorphisms are associated with B cell-mediated autoimmune diseases like systemic lupus erythematosus. In addition, it has been shown that some of the IP3Rs in HeLa cells can cluster together and mobilize to the plasma membrane. IP3Rs tethered close to ER-plasma membrane junctions are optimally placed to be activated by endogenous IP3 and regulate Ca2+ entry [12]. Thus, facilitating calcium signaling by mobilizing IP3Rs may serve as a more general mechanism, which also exists in nonimmune cells. Of course, more evidence is needed to prove this idea (Fig. 1).
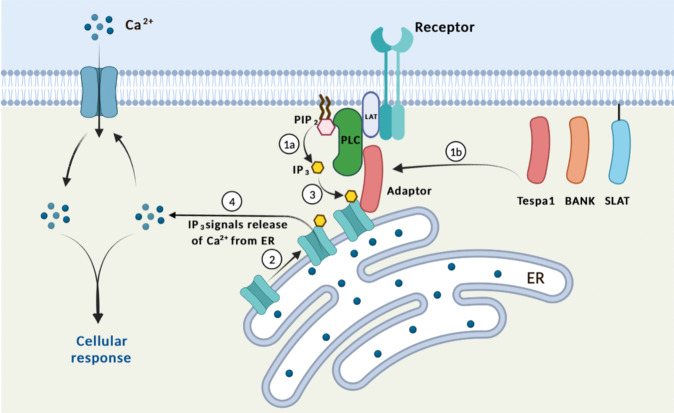
Fig. 1. Mobilizing ER IP3Rs as a mechanism to facilitate calcium signaling: Several IP3R binding proteins, including Tespa1, BANK1, and SLAT, have been found in T and B cells.
Upon TCR or BCR activation, PLC is recruited to the receptor complex and activated, which catalyzes the generation of IP3 from PIP2 (Step 1a). At the same time, IP3R binding adaptors are also recruited to the receptor complex by binding to PLC (Step 1b). These adaptors are capable of binding to IP3R and triggering the relocation of IP3R, the ER calcium channel, to near PLC, placing them very close to where their ligand, IP3, is produced (Step 2). This allows IP3 to bind to IP3R in a very quick and efficient way (Step 3), triggering downstream cellular responses (Step 4).
Competing interests
The authors declare no competing interests.
References
- 1.Lyu J, Wang L, Lu L. Thymocyte selection: from signaling to epigenetic regulation. Adv Immunol. 2019;144:1–22. doi: 10.1016/bs.ai.2019.08.005. [DOI] [PubMed] [Google Scholar]
- 2.Davey GM, Schober SL, Endrizzi BT, Dutcher AK, Jameson SC, Hogquist KA. Preselection thymocytes are more sensitive to T cell receptor stimulation than mature T cells. J Exp Med. 1998;188:1867–74. doi: 10.1084/jem.188.10.1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang D, Zheng M, Lei L, Ji J, Yao Y, Qiu Y, et al. Tespa1 is involved in late thymocyte development through the regulation of TCR-mediated signaling. Nat Immunol. 2012;13:560–8. doi: 10.1038/ni.2301. [DOI] [PubMed] [Google Scholar]
- 4.Liang J, Lyu J, Zhao M, Li D, Zheng M, Fang Y, et al. Tespa1 regulates T cell receptor-induced calcium signals by recruiting inositol 1,4,5-trisphosphate receptors. Nat Commun. 2017;8:15732. doi: 10.1038/ncomms15732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lyu J, Wang P, Xu T, Shen Y, Cui Z, Zheng M, et al. Thymic-specific regulation of TCR signaling by Tespa1. Cell Mol Immunol. 2019;16:897–907. doi: 10.1038/s41423-019-0259-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang Y, Chen S, Chen J, Xie X, Gao S, Zhang C, et al. Germline genetic patterns underlying familial rheumatoid arthritis, systemic lupus erythematosus and primary Sjogren’s syndrome highlight T cell-initiated autoimmunity. Ann Rheum Dis. 2020;79:268–75. doi: 10.1136/annrheumdis-2019-215533. [DOI] [PubMed] [Google Scholar]
- 7.Zhao R, Ding D, Yu W, Zhu C, Ding Y. The lung adenocarcinoma microenvironment mining and its prognostic merit. Technol Cancer Res Treat. 2020;19:1533033820977547. doi: 10.1177/1533033820977547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bécart S, Balancio AJ, Charvet C, Feau S, Sedwick CE, Altman A. Tyrosine-phosphorylation-dependent translocation of the SLAT protein to the immunological synapse is required for NFAT transcription factor activation. Immunity. 2008;29:704–19. doi: 10.1016/j.immuni.2008.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fos, C, Becart, S, Balancio, AJC, Boehning, D & Altman, A Association of the EF-hand and PH domains of the guanine nucleotide exchange factor SLAT with IP3 receptor 1 promotes Ca2+ signaling in T cells. Sci Signal. 2014;7:ra93. [DOI] [PMC free article] [PubMed]
- 10.Becart S, Altman A. SWAP-70-like adapter of T cells: a novel Lck-regulated guanine nucleotide exchange factor coordinating actin cytoskeleton reorganization and Ca2+ signaling in T cells. Immunol. Rev. 2009;232:319–33. doi: 10.1111/j.1600-065X.2009.00839.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yokoyama K, Su Ih IH, Tezuka T, Yasuda T, Mikoshiba K, Tarakhovsky A, et al. BANK regulates BCR-induced calcium mobilization by promoting tyrosine phosphorylation of IP(3) receptor. EMBO J. 2002;21:83–92. doi: 10.1093/emboj/21.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thillaiappan, NB, Chavda, AP, Tovey, SC, Prole, DL, Taylor, CW Ca2+ signals initiate at immobile IP3 receptors adjacent to ER-plasma membrane junctions. Nat Commun. 2017;8:1505. [DOI] [PMC free article] [PubMed]