Abstract
Objective:
To investigate whether epileptiform discharge burden can identify those at risk for delayed cerebral ischemia (DCI) after subarachnoid hemorrhage (SAH).
Methods:
Retrospective analysis of 113 moderate to severe grade SAH patients who had continuous EEG (cEEG) recordings during their hospitalization. We calculated the burden of epileptiform discharges (ED), measured as number of ED per hour.
Results:
We find that many SAH patients have an increase in ED burden during the first 3–10 days following rupture, the major risk period for DCI. However, those who develop DCI have a significantly higher hourly burden from days 3.5–6 after SAH vs. those who do not. ED burden is higher in DCI patients when assessed in relation to the onset of DCI (area under the receiver operator curve 0.72). Finally, specific trends of ED burden over time, assessed by group-based trajectory analysis, also help stratify DCI risk.
Conclusions:
These results suggest that ED burden is a useful parameter for identifying those at higher risk of developing DCI after SAH. The higher burden rate associated with DCI supports the theory of metabolic supply-demand mismatch which contributes to this complication.
Significance:
ED burden is a novel biomarker for predicting those at high risk of DCI.
Keywords: EEG, SAH, delayed cerebral ischemia, epileptiform discharges, burden
1. Introduction
Delayed cerebral ischemia (DCI) is a leading complication following aneurysmal subarachnoid hemorrhage (SAH) (Dupont et al., 2010; Rowland et al., 2012; Vergouwen et al., 2011). Progress in preventing DCI that leads to improved outcomes has stagnated, due in part to challenges identifying those at high risk early after ictus. Transcranial doppler remains the standard of care for DCI detection (Naqvi et al., 2013; Suarez et al., 2002), but has limited sensitivity and specificity (Carrera et al., 2009; Kumar et al., 2016), and poor time resolution in that they are typically performed once or twice daily. There is also growing evidence that large vessel vasospasm is only one of several causes of DCI (Dreier et al., 2013, 2009; Rowland et al., 2012; Woitzik et al., 2012). Thus, other diagnostic modalities are needed to more broadly monitor for impending DCI.
Continuous EEG (cEEG) is increasingly used to monitor for early signs of DCI. Pathological low frequency spectral activity increases and higher frequency activity decreases hours to days prior (Claassen et al., 2005, 2004; Gollwitzer et al., 2015; Rots et al., 2016; Stuart et al., 2010; Vespa et al., 1997). Less is known about epileptiform abnormalities, which include sporadic epileptiform discharges (spikes and sharp waves), periodic discharges, and lateralized rhythmic delta activity (Sivaraju and Gilmore, 2016). We previously showed that late onset epileptiform discharges are associated with increased DCI risk (Kim et al., 2017; Rosenthal et al., 2018). We proposed that these abnormalities worsen metabolic supply-demand mismatch in an already injured brain leading to secondary ischemia. It is unknown whether specific features of these epileptiform abnormalities further inform DCI risk. Burden of seizures and frequency (per second) of periodic discharges have been linked to worsened neurologic outcomes (De Marchis et al., 2016; Vespa et al., 2016; Jens Witsch et al., 2017). However, the association between burden of epileptiform discharges and DCI has not yet been characterized. Assessing features of these abnormalities, such as sporadic and periodic epileptiform discharge (ED) burden, by manually marking discharges in multi-day recordings is untenable. By utilizing automated detection of these ED (Scheuer et al., 2017) we investigated whether ED burden over time differentiates those who do vs do not develop DCI.
2. Methods:
2.1. Study population:
We evaluated EEG reports and medical records from intensive care unit patients at a tertiary care center (Massachusetts General Hospital Neurosciences intensive care unit) who met study inclusion criteria between September 2011 and January 2015. Inclusion criteria were (previously published (Kim et al., 2017)): age ≥18 years; Hunt Hess 4–5 or Fisher grade 3 SAH; cEEG data was available lasting at least 24 hours; and cEEG monitoring was not discontinued more than 24 hours before any clinically diagnosed DCI events. We excluded one patient who developed status epilepticus (defined as ≥5 minutes of continuous clinical and/or electrographic seizure activity or recurrent seizure activity without recovery between seizures(Brophy et al., 2012)) because mechanisms of secondary brain injury in frank status epilepticus may be distinct. cEEG monitoring for ischemia detection was performed as part of routine clinical care which entailed recording within 48 hours of admission and continuing for 10 days, unless otherwise indicated. Retrospective collection and analysis of clinical data was performed under a protocol approved by the local institutional review board.
2.2. EEG recordings:
Continuous EEG data was recorded using the International 10–20 scalp electrode placement (19 electrodes).
2.3. EEG ED Identification:
EEG data was processed using Persyst 13 (Persyst Development Corporation, San Diego) spike detection software, which has been validated against interrater agreement amongst EEG technologists (Scheuer et al., 2017). ED burden was calculated as number of discharges (sporadic or periodic) detected per hour. We compared ED burden between those who did and did not develop DCI over time.
2.4. DCI classification:
DCI events were diagnosed according to an international consensus definition (Vergouwen et al., 2010) as either “(1) new focal neurologic deficits and/or decrease in the Glasgow Coma Scale of at least 2 points, persisting for a minimum of one hour, not explained by other causes (e.g. complications of a procedure, sedation, spike in intracranial pressure, rerupture, hydrocephalus, systemic or metabolic abnormalities) by means of clinical assessment, imaging or laboratory data, or (2) the presence of cerebral infarction on CT or MRI imaging of the brain, acquired at the discretion of the clinical team, that was not present on any neuroimaging done within the first 48 hours following early aneurysm occlusion, and not attributable to other causes such as surgical clipping or endovascular treatment.”
As previously described (Kim et al., 2017), we used the above DCI definition and assessed patients via: “(1) prospective daily structured research coordinator interview with the clinical team, (2) independent medical record review by three of the authors (ESR, MBW, SFZ) blinded to cEEG findings, (3) consensus adjudication by the same three authors in cases of uncertainty or disagreement. If a patient experienced a DCI event at any point during hospitalization, this was documented, even if after cessation of cEEG recording. Patients with DCI events occurring >24 hours after discontinuation of cEEG monitoring were excluded (see Study Population above).”
2.5. Data analysis:
For data analysis we used Matlab (MathWorks; Natick, MA) and STATA (Statcorp, College Station, TX). To investigate the relationship between ED and DCI, we separated cases into those who did and did not develop DCI and calculated the mean frequencies and standard error of each group. We winsorized the data for outliers above and below the 5% limits of the population (Frey, 2018). For cumulative ED burden, each patient’s ED burden was summed progressively over each hour. When a patient was disconnected from EEG, the final recorded cumulative ED value was carried forward to day 15, to not alter the trajectory of the population average cumulative curve. Mean and standard error were calculated for hourly and cumulative ED burden and compared by t-test. Area under the receiver operator curves (AUC) were also calculated hourly. These calculations were made based on data aligned from day of SAH and relative to the day of DCI. Spline smoothing was applied with a parameter of 0.001. Sensitivity and specificity for all ROC curves were calculated based on the optimal threshold (i.e. threshold value with the highest Youden’s J value, J = sensitivity + specificity −1). To generate comparisons relative to the day of DCI between DCI and non-DCI patients, we first identified each DCI patient and found their cEEG start time and DCI onset time. Then we searched all non-DCI patients to determine who had recordings during that same time frame. This process was repeated iteratively for each DCI patient, such that multiple controls were matched to each DCI patient and each non-DCI patient could be re-used to match multiple DCI patients. Finally, we used group-based trajectory modeling (Elmer et al., 2018; Nagin, 2014), to identify distinct polynomial trajectories of ED burden over time. We scaled hourly ED burden to 0 to 1 and fit our model using a beta distribution (Elmer et al., 2018). We assigned each patient to a single trajectory group based on the maximal posterior probability of group membership after all available data were modeled. We then determined if trajectory group membership was associated with DCI. 2-tailed Freeman Halton extension of Fisher’s exact test was used to test significance of group separation.
2.6. Data availability:
Data are available upon request.
3. Results:
We found 113 patients who met our inclusion criteria, 58 of whom developed DCI. Mean age of the total cohort is 56±14 years old. And 79 of the patients (70%) were female. Seven patients had seizures on EEG. The median time to DCI was 6.5±3.4 days.
3.1. Hourly ED burden after SAH
ED burden was significantly higher in patients with DCI compared to those without DCI. Temporal raster plots show ED burden of each patient from the day of SAH with darker shading corresponding to higher frequencies (Figure 1a). The ED detection algorithm detected at least one ED during recording in all patients. The median duration (±standard deviation) of recordings across all patients was 7 (±3.1) days with a median start time of 2 (±1.8) days post-SAH. The mean ED burden was significantly higher in DCI vs non-DCI patients from day 3.5 to day 6 (black bars; t-test, p=0.05) after SAH (Figure 1b). Evaluating AUC generated each hour after SAH, we found the maximum AUC within the first 6 days of SAH to range from 0.61–0.62 at 3.5–5.3 days after SAH. The optimum threshold sensitivity and specificity were 0.6 and 0.7, respectively.
Figure 1:
Epileptiform discharge (ED) burden is higher in patients with delayed cerebral ischemia (DCI) compared to non-DCI patients. a) Swimmer plots of hourly ED burden (gray color scale) of individual patients (rows) with DCI (left) and without DCI (right) across time after subarachnoid hemorrhage (SAH). Gray color scale: white to black, range: 5–95% max ED burden, respectively. Purple shading corresponds to duration of EEG collection. b) Winsorized mean and standard error of DCI (red) and non-DCI (blue) patients for each hour, with an overlaying spline smoothed curve. Bars above the mean curves indicated statistical significance (t-test of p<0.05 for ≥2hours). c) AUC of ED burden prediction calculated for each hour after SAH (black), with an overlaying spline smoothed curve. Maximum AUC of 0.62 found at 3.5–5.3 days after SAH.
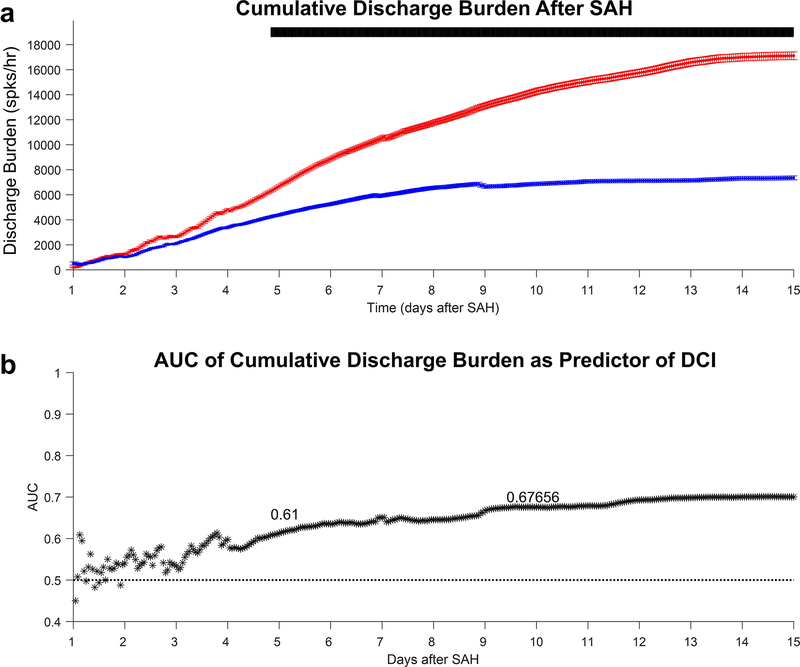
3.2. Cumulative ED burden after SAH
The AUCs calculated above were based on treating each hour independently but did not consider any prior ED burden information about a patient. A simplified way to investigate whether prior ED burden history further discriminates DCI from non-DCI groups, we calculated cumulative ED frequencies additively (Figure 2a). Within the first 5 days after subarachnoid hemorrhage, the peak AUC of cumulative ED burden was 0.61 and occurred at day 4.8, with an optimum threshold sensitivity of 0.66 and specificity of 0.60. Within the first 10 days, peak AUC was 0.68, occurring at day 9.6 (Figure 2b), with a sensitivity of 0.73 and specificity of 0.54.
Figure 2:
Cumulative epileptiform discharge (ED) burden after subarachnoid hemorrhage (SAH). a) Winsorized mean and standard error of delayed cerebral ischemia (DCI; red) and non-DCI (blue) patients for each cumulative hour. Bars above the mean curves indicate statistical significance (t-test of p<0.05 for ≥2hours). b) AUC of cumulative ED burden prediction of each cumulative hour after SAH (black). Maximum AUC within 5 days is 0.61 (day 4.8) and within 10 days of SAH is 0.68 (day 9.6).
3.3. ED burden aligned to time of DCI
We also investigated whether ED burden differs in the days leading up to DCI. We aligned the data of all DCI patients to the date of DCI diagnosis. A temporal raster plot of each DCI patient aligned relative to the time of DCI (DCI = day 0) is shown in Figure 3A. Our control-matching process for comparison to DCI patients aligned to their DCI onset yielded 935 controls (see Methods). We found a higher ED burden in DCI patients prior to DCI onset (Figure 3B). AUC assessments at each hour prior to DCI yielded the highest AUC of 0.72 within 5 days prior to DCI based on the spline smoothed curve. This AUC was similar between −4.4 to −3.3 days prior to DCI onset, based on this curve (Figure 3C). Sensitivity and specificity were 0.74 and 0.70, respectively. AUC for ED burden beyond 5 days prior to DCI were high, but the number of patients with late DCI who recorded at these times points is low as reflected by the increased variance.
Figure 3:
Epileptiform discharge (ED) burden is higher in patients with delayed cerebral ischemia (DCI) prior to their DCI onset. a) Swimmer plots of hourly ED burden (gray color scale) of individual patients (rows) with DCI aligned to their onset of DCI (time 0). Gray color scale: white to black, range: 5–95% max ED burden, respectively. Purple shading corresponds to duration of EEG collection. b) winsorized mean and standard error of DCI (red) and non-DCI (blue) patients for each hour prior to the onset of their DCI, with an overlaying spline smoothed curve. Control patients were matched based on available EEG recordings during the same time frame as each individual DCI patient. Bars above the mean curves indicated statistical significance (t-test of p<0.05 for ≥2hours). c) AUC of rated burden prediction calculated for each hour prior to DCI onset, with an overlaying spline smoothed curve. Within 5 days of DCI, maximum AUC of 0.72 found at −3.3 days prior to DCI onset.
3.4. ED burden trajectories over time for DCI discrimination
Given the heterogeneity in ED burden patterns in individual patients, we sought to determine if there are groups of DCI patients who follow particular trajectories in discharge burden over time, and whether these trajectories are associated with DCI risk. Using group-based trajectory analysis, we found that a 3-group model best fit the data (Figure 4a). Based on these trajectories we calculated the probability of developing DCI based upon group membership (Figure 4b). In group 3, 79% of patients developed DCI, whereas in group 1, only 37% developed DCI and in group 2 52% had DCI. The differences in group membership related to DCI outcome was significant (p=0.011).
Figure 4:
Group based trajectory analysis differentiates delayed cerebral ischemia (DCI) from non-DCI patients based on rated burden patterns over time. a) Plot of group trajectories with mean epileptiform discharge (ED) burden and overlaying polynomial fit function (Days 2–10 post-SAH). Three unique group trajectories were identified based on their burden trajectories: group 1 (yellow), group 2 (gray), group 3 (green). b) Each group was assessed for their probability of DCI, with group 1 having a lower rate of DCI compared to group 3. 2-tailed Freeman-Halton extension of Fisher’s exact test p =0.01.
4. Discussion:
Our data demonstrate that automated, quantitative ED burden calculations can differentiate patients who do and do not develop DCI. Curve separation occurs around Day 3, corresponding to the beginning time period of DCI risk. Mean ED burden is significantly higher in DCI patients from day 3.5 to 6, with moderate discrimination. Interestingly, even non-DCI patients have increased ED burden during the peak DCI window of days 3–14. This may suggest a continuum of neuronal dysregulation that tips toward clinical deterioration beyond a certain threshold. As previously published (Kim et al., 2017), we propose that epileptiform abnormalities contribute to supply-demand mismatch and may contribute to the development of DCI. These data suggest that not only presence of EDs, but their burden confer increased DCI risk. In our previous model of DCI(Kim et al., 2017), we focused on daily presence of EA and determined the first occurrence of each subtype in DCI and non-DCI patients. This method allowed us to differentiate between groups at day 6. Our current model allows us to detect earlier, starting day 3.5. Our results are also complementary to our prior multi-state model(Rosenthal et al., 2018) and functional outcomes prediction model(Zafar et al., 2018), by assessing for continuous, automated feature analysis rather than dependence on manual reporting of EEG abnormalities. This continuous assessment allowed us to include patients with shorter EEG durations (24–72 hours) compared to prior Rosenthal et al. study (≥72 hours). Of note, the burden definition used here is separate than the ACNS definition for burden (Hirsch et al., 2013) used in our prior paper, because here the ED detection algorithm allowed for automated, hourly assessment of ED rates compared to a single daily assessment of burden.
By calculating cumulative ED burden, we utilize historical information about ED burden at each progressive time point. However, this approach did not significantly improve performance compared to independent performance calculations. This is likely due to the initial fluctuations in ED rates soon after SAH and the large spread in cumulative ED frequencies of individuals. Further work is needed to explore other time integrating methods which may improve DCI prediction.
We also investigated whether ED burden changes precede DCI. Our results show that discharge burden of DCI patients is elevated prior to DCI. Within the five-day window prior to DCI, the maximal AUC occurred 4 days prior to DCI. These results are consistent with prior studies suggesting that epileptiform abnormalities are notable 2–4 days prior to DCI onset (Rosenthal et al., 2018). One possible explanation for this observation is that brain physiology is altered long before any clinical or radiographic evidence of DCI. This time separation presents an opportunity to trial new therapies or earlier implementation of existing ones, within a time window of reversible tissue injury.
Additionally, we observe that our patient cohort could be classified into three patterns of ED burden over time. Using group-based trajectory analysis, we identified three distinct patterns among these varied trajectories. This type of modeling has not previously been applied in SAH patients, but has shown value in cardiac arrest prognostication (Elmer et al., 2016). We show that SAH patients can be described by three trajectory patterns over time and that belonging to either group 1 or 3 confers different DCI risk. An important direction for future work is to perform real-time evaluation of which trajectory group each patient belongs, dynamically updated based on accumulating data, for prospective assessment of how trajectory group belonging influences the probability of DCI, which is a more recently developed approach using GBTM (Elmer et al., 2019).
Overall, we found that most patients with SAH have increased ED burden 3–10 days after SAH, regardless of DCI status. This raises questions about why this timeframe is particularly injury prone. Perhaps patients with high ED burden reach a “tipping point” of decompensation, resulting in DCI. While SAH causes some direct neural injury, it also triggers a cascade of other processes, such as cortical spreading depression, inflammation, vasospasm and abnormal neural activity leading to metabolic supply-demand mismatch (Kim et al., 2017). There is evidence that seizures and other interictal patterns can increase metabolic demand (Vespa et al., 2016; J Witsch et al., 2017). This study cannot definitively determine whether high ED burden directly contributes to injury or is simply a marker of worsening metabolic supply-demand mismatch. However, it supports the hypothesis that prolonged abnormal neural activity over time could contribute to worsened injury after SAH, similar to the concept of the penumbra to ischemia threshold where prolonged mild reductions in cerebral blood lead to infarction.
Our study has limitations. First, ED detection algorithms are imperfect. While we used the most commonly available ED detection algorithm, developed using epilepsy monitoring unit data (Scheuer et al., 2017), there are limitations in its performance (Westover et al., 2017). Namely, false positive spike detections are a common issue for many algorithms (Ver Hoef et al., 2010; Scheuer et al., 2017). However, since we used the same algorithm across all patients and compared over time, we believe the type and rate of false detections are likely to be common across patients and the impact of these false positive detections is thereby somewhat mitigated. The relative comparisons both within and across patients is significant. However, it is possible that our assumption is incorrect and that the false detection rates for the algorithm used are somehow higher in the DCI patients than non-DCI patients. Thus, it is important for future studies could compare DCI prediction based on other ED detection algorithms (e.g. Jing et al. 2019). Second, while group-based trajectory modeling provides a method to assess subgroups of patients and their data over time, trajectory group membership is assigned probabilistically and updated as new data become available. We assigned group membership based on the final highest probability of group membership after all available data were modeled and did not explore its performance over time. Further work is needed to determine its predictive value in a real-time predictive setting. Finally, we focused on only one quantitative feature of EEG, which limits our overall performance in predicting DCI. While it is a common approach to assess the value of single features (Claassen et al., 2004; Kim et al., 2017; Vespa et al., 1997) in DCI prediction, future prediction algorithms should incorporate multiple EEG parameters, such as quantitative ED morphologic features which have been shown to be important for source localization(Wong, 1996, 1991; Wong and Wong, 1991), other epileptiform patterns (periodic discharges, lateralized rhythmic delta activity, etc.) and spectral features (Claassen et al., 2004; Gollwitzer et al., 2015; Rots et al., 2016; Vespa et al., 1997). We did not evaluate the effects of anti-seizure medications on the presence or burden of ED, which is an important future investigation. Further integration of other modalities, including transcranial doppler, radiology and clinical variables could a more powerful multimodal prediction algorithm for DCI. While our focus here was on DCI prediction, similar methods could be applied in future studies of other SAH outcomes, such as long-term functional status.
5. Conclusions
Our data support the hypothesis that a higher burden of discharges identifies patients at increased risk for DCI. This study provides novel data to suggest that quantitative assessments of these abnormalities can quantify DCI risk. Despite being a single feature analysis, the ability to discriminate among groups was substantial. This provides the basis for future exploration of which epileptiform discharge features, in combination with other EEG features, best predict DCI.
Highlights.
Epileptiform discharges(ED) increase after subarachnoid hemorrhage during the high risk delayed cerebral ischemia(DCI) time-frame.
Quantitative ED burden is higher in those who develop DCI compared to those who do not.
ED burden can be used as a marker of DCI risk for automated prediction algorithm development.
Acknowledgements
During this research, Dr. Kim was supported by a NIH (R25N065743, K23NS112596), a American Heart Association Post-doctoral Award and a Bee Foundation Research Grant for SAH. During this research, Dr. Westover was supported by the Glenn Foundation for Medical Research and the American Federation for Aging Research through a Breakthroughs in Gerontology Grant; through the American Academy of Sleep Medicine through an AASM Foundation Strategic Research Award; from the Department of Defense through a subcontract from Moberg ICU Solutions, Inc, and by grants from the NIH (1R01NS102190, 1R01NS102574, 1R01NS107291, 1RF1AG064312). Dr. Elmer’s research time is supported by the NIH through grant K23NS097629. Dr. Zafar’s research time is supported by the NIH through grant K23NS114201. Eric S. Rosenthal was supported for this work by NIH/NINDS grant 1K23NS105950.
Footnotes
Disclosure/Conflict of Interest
None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Brophy GM, Bell R, Claassen J, Alldredge B, Bleck TP, Glauser T, et al. Guidelines for the evaluation and management of status epilepticus. Neurocrit Care 2012;17:3–23. 10.1007/s12028-012-9695-z. [DOI] [PubMed] [Google Scholar]
- Carrera E, Schmidt JM, Oddo M, Fernandez L, Claassen J, Seder D, et al. Transcranial doppler for predicting delayed cerebral ischemia after Subarachnoid hemorrhage. Neurosurgery 2009;65:316–23. 10.1227/01.NEU.0000349209.69973.88. [DOI] [PubMed] [Google Scholar]
- Claassen J, Hirsch LJ, Kreiter KT, Du EY, Connolly ES, Emerson RG, et al. Quantitative continuous EEG for detecting delayed cerebral ischemia in patients with poor-grade subarachnoid hemorrhage. Clin Neurophysiol 2004;115:2699–710. 10.1016/j.clinph.2004.06.017. [DOI] [PubMed] [Google Scholar]
- Claassen J, Mayer SA, Hirsch LJ. Continuous EEG monitoring in patients with subarachnoid hemorrhage. J Clin Neurophysiol 2005;22:92–8. https://doi.org/00004691-200504000-00002 [pii]. [DOI] [PubMed] [Google Scholar]
- De Marchis GM, Pugin D, Meyers E, Velasquez A, Suwatcharangkoon S, Park S, et al. Seizure burden in subarachnoid hemorrhage associated with functional and cognitive outcome. Neurology 2016;86:253–60. 10.1212/WNL.0000000000002281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreier J, Drenckhahn C, Woitzik J, Major S, Offenhauser N, Weber-Carstens S, et al. Spreading ischemia after aneurysmal subarachnoid hemorrhage. Acta Neurochir Suppl 2013;115:125–9. 10.1007/978-3-7091-1192-5_26. [DOI] [PubMed] [Google Scholar]
- Dreier JP, Major S, Manning A, Woitzik J, Drenckhahn C, Steinbrink J, et al. Cortical spreading ischaemia is a novel process involved in ischaemic damage in patients with aneurysmal subarachnoid haemorrhage. Brain 2009;132:1866–81. 10.1093/brain/awp102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupont SA, Wijdicks EFM, Lanzino G, Rabinstein A a. Aneurysmal subarachnoid hemorrhage: An overview for the practicing neurologist. Semin Neurol 2010;30:545–54. 10.1055/s-0030-1268862. [DOI] [PubMed] [Google Scholar]
- Elmer J, Gianakas JJ, Rittenberger JC, Baldwin ME, Faro J, Plummer C, et al. Group-Based Trajectory Modeling of Suppression Ratio After Cardiac Arrest. Neurocrit Care 2016;25:415–23. 10.1007/s12028-016-0263-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmer J, Jones BL, Nagin DS. Using the Beta distribution in group-based trajectory models. BMC Med Res Methodol 2018;18:10–4. 10.1186/s12874-018-0620-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmer J, Jones BL, Zadorozhny VI, Puyana JC, Flickinger KL, Callaway CW, et al. A novel methodological framework for multimodality, trajectory model-based prognostication. Resuscitation 2019;137:197–204. 10.1016/j.resuscitation.2019.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey BB. Winsorizing. SAGE Encycl. Educ. Res. Meas. Eval, SAGE Publications, Inc.; 2018. 10.4135/9781506326139.n747. [DOI] [Google Scholar]
- Gollwitzer S, Groemer T, Rampp S, Hagge M, Olmes D, Huttner HBB, et al. Early prediction of delayed cerebral ischemia in subarachnoid hemorrhage based on quantitative EEG: A prospective study in adults. Clin Neurophysiol 2015;126:1514–23. 10.1016/j.clinph.2014.10.215. [DOI] [PubMed] [Google Scholar]
- Hirsch LJ, LaRoche SM, Gaspard N, Gerard E, Svoronos A, Herman ST, et al. American Clinical Neurophysiology Society’s Standardized Critical Care EEG Terminology: 2012 version. J Clin Neurophysiol 2013;30:1–27. 10.1097/WNP.0b013e3182784729. [DOI] [PubMed] [Google Scholar]
- Ver Hoef L, Elgavish R, Knowlton RC. Effect of detection parameters on automated electroencephalography spike detection sensitivity and false-positive rate. J Clin Neurophysiol 2010;27:12–6. 10.1097/WNP.0b013e3181cb4294. [DOI] [PubMed] [Google Scholar]
- Jing J, Sun H, Kim JA, Herlopian A, Karakis I, Ng M, et al. Development of Expert-Level Automated Detection of Epileptiform Discharges during Electroencephalogram Interpretation. JAMA Neurol 2019;02114:1–6. 10.1001/jamaneurol.2019.3485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JA, Rosenthal ES, Biswal S, Zafar S, Shenoy AV., O’Connor KL, et al. Epileptiform abnormalities predict delayed cerebral ischemia in subarachnoid hemorrhage. Clin Neurophysiol 2017;128:1091–9. 10.1016/j.clinph.2017.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar G, Shahripour RB, Harrigan MR. Vasospasm on transcranial Doppler is predictive of delayed cerebral ischemia in aneurysmal subarachnoid hemorrhage: A systematic review and meta-analysis. J Neurosurg 2016;124:1257–64. 10.3171/2015.4.JNS15428. [DOI] [PubMed] [Google Scholar]
- Nagin DS. Group-Based Trajectory Modeling : An Overview 2014;15213:205–10. 10.1159/000360229. [DOI] [PubMed] [Google Scholar]
- Naqvi J, Yap KH, Ahmad G, Ghosh J. Transcranial Doppler ultrasound: A review of the physical principles and major applications in critical care. Int J Vasc Med 2013;2013:629378. 10.1155/2013/629378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal ES, Biswal S, Zafar SF, O’Connor KL, Bechek S, Shenoy A V., et al. Continuous Electroencephalography Predicts Delayed Cerebral Ischemia after Subarachnoid Hemorrhage: A Prospective Study of Diagnostic Accuracy. Ann Neurol 2018. 10.1002/ana.25232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rots ML, van Putten MJAM, Hoedemaekers CWE, Horn J. Continuous EEG Monitoring for Early Detection of Delayed Cerebral Ischemia in Subarachnoid Hemorrhage: A Pilot Study. Neurocrit Care 2016;24:207–16. 10.1007/s12028-015-0205-y. [DOI] [PubMed] [Google Scholar]
- Rowland MJ, Hadjipavlou G, Kelly M, Westbrook J, Pattinson KTS. Delayed cerebral ischaemia after subarachnoid haemorrhage: looking beyond vasospasm. Br J Anaesth 2012;109:315–29. 10.1093/bja/aes264. [DOI] [PubMed] [Google Scholar]
- Scheuer ML, Bagic A, Wilson SB. Spike detection: Inter-reader agreement and a statistical Turing test on a large data set. Clin Neurophysiol 2017;128:243–50. 10.1016/j.clinph.2016.11.005. [DOI] [PubMed] [Google Scholar]
- Sivaraju A, Gilmore EJ. Understanding and Managing the Ictal-Interictal Continuum in Neurocritical Care. Curr Treat Options Neurol 2016;18:8. 10.1007/s11940-015-0391-0. [DOI] [PubMed] [Google Scholar]
- Stuart RM, Waziri A, Weintraub D, Schmidt MJ, Fernandez L, Helbok R, et al. Intracortical EEG for the detection of vasospasm in patients with poor-grade subarachnoid hemorrhage. Neurocrit Care 2010;13:355–8. 10.1007/s12028-010-9414-6. [DOI] [PubMed] [Google Scholar]
- Suarez JI, Qureshi AI, Yahia AB, Parekh PD, Tamargo RJ, Williams MA, et al. Symptomatic vasospasm diagnosis after subarachnoid hemorrhage: evaluation of transcranial Doppler ultrasound and cerebral angiography as related to compromised vascular distribution. Crit Care Med 2002;30:1348–55. 10.1097/00003246-200206000-00035. [DOI] [PubMed] [Google Scholar]
- Vergouwen MDI, Etminan N, Ilodigwe D, Macdonald RL. Lower incidence of cerebral infarction correlates with improved functional outcome after aneurysmal subarachnoid hemorrhage. J Cereb Blood Flow Metab 2011;31:1545–53. 10.1038/jcbfm.2011.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vergouwen MDI, Vermeulen M, van Gijn J, Rinkel GJE, Wijdicks EF, Muizelaar JP, et al. Definition of delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage as an outcome event in clinical trials and observational studies: proposal of a multidisciplinary research group. Stroke 2010;41:2391–5. 10.1161/STROKEAHA.110.589275. [DOI] [PubMed] [Google Scholar]
- Vespa P, Tubi M, Claassen J, Blanco M, McArthur D, Velazquez AG, et al. Metabolic Crisis occurs with Seizures and Periodic Discharges after Brain Trauma. Ann Neurol 2016;79:579–90. 10.1002/ana.24606. [DOI] [PubMed] [Google Scholar]
- Vespa PM, Nuwer MR, Juhász C, Alexander M, Nenov V, Martin N, et al. Early detection of vasospasm after acute subarachnoid hemorrhage using continuous EEG ICU monitoring. Electroencephalogr Clin Neurophysiol 1997;103:607–15. [DOI] [PubMed] [Google Scholar]
- Westover MB, Halford JJ, Bianchi MT. What it should mean for an algorithm to pass a statistical Turing test for detection of epileptiform discharges. Clin Neurophysiol 2017;128:1406–7. 10.1016/j.clinph.2017.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jens Witsch, Frey HP, Schmidt JM, Velazquez A, Falo CM, Reznik M, et al. Electroencephalographic periodic discharges and frequency-dependent brain tissue hypoxia in acute brain injury. JAMA Neurol 2017. 10.1001/jamaneurol.2016.5325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witsch J, Frey HP, Schmidt JM, Velazquez A, Falo CM, Reznik M, et al. Electroencephalographic Periodic Discharges and Frequency-Dependent Brain Tissue Hypoxia in Acute Brain Injury. JAMA Neurol 2017;74:301–9. 10.1001/jamaneurol.2016.5325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woitzik J, Dreier JP, Hecht N, Fiss I, Sandow N, Major S, et al. Delayed cerebral ischemia and spreading depolarization in absence of angiographic vasospasm after subarachnoid hemorrhage. J Cereb Blood Flow Metab 2012;32:203–12. 10.1038/jcbfm.2011.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong PKH. Quantitative analysis of epileptic discharges. Brain Topogr, vol. 8, Springer; 1996, p. 209–14. 10.1007/BF01184771. [DOI] [PubMed] [Google Scholar]
- Wong PKH. Introduction to Brain Topography. Springer; US; 1991. 10.1007/978-1-4615-3716-8. [DOI] [Google Scholar]
- Wong PKH, Wong PKH. Source Modelling and Analysis. Introd. to Brain Topogr, Springer; US; 1991, p. 81–111. 10.1007/978-1-4615-3716-8_2. [DOI] [Google Scholar]
- Zafar SF, Postma EN, Biswal S, Boyle EJ, Bechek S, O’connor K, et al. Effect of epileptiform abnormality burden on neurologic outcome and antiepileptic drug management after subarachnoid hemorrhage. Clin Neurophysiol 2018;129:2219–27. 10.1016/j.clinph.2018.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available upon request.