Abstract
The prefrontal cortex (PFC) is a cortical structure involved in a variety of complex functions in the cognitive and affective domains. The intrinsic function of the PFC is defined by the interaction of local glutamatergic and GABAergic neurons and their modulation by long-range inputs. The ensuing interactions generate a ratio of excitation and inhibition (E-I) in each output neuron, a balance which is refined during the adolescent to adult transition. In this short review, we aim to describe how an increase in GABAergic transmission during adolescence modifies the E-I ratio in adults. We further discuss how this new setpoint may change the dynamics of PFC networks observed during the transition to adulthood.
Keywords: prefrontal cortex, adolescence, synaptic activity, excitatory-inhibitory balance
Introduction
The prefrontal cortex (PFC) undergoes a period of protracted development that spans adolescence until early adulthood [1, 2]. It is thought such a delayed maturation is responsible for the acquisition of adult cognitive abilities later in life particularly in the domains of decision-making, attention, learning, memory, and affect regulation. Due to this developmental feature, the time window during which the PFC is susceptible to environmental interference is longer than for other cortical regions. When compounded with the increase in experimentation and risk-taking also observed during adolescence [3], PFC disruption can have negative consequences in the acquisition of adult behavior. Here we discuss how PFC maturation during adolescence can be understood as a function of the excitation-inhibition (E-I) balance.
The E-I ratio and PFC development
The biological underpinnings behind prefrontal maturation are only beginning to be understood. At the cellular level, PFC activity is defined by the interaction of local GABAergic interneurons and pyramidal cells as well as glutamatergic projections from other limbic structures, and most notably, frontal-projecting dopamine cells in the ventral tegmental area (VTA). Thus, the resulting PFC output function is broadly the sum of inhibitory (GABAergic) and excitatory (glutamatergic) transmission impinging onto local pyramidal neurons, regulated by a layer of neuromodulators including but not limited to catecholamines, acetylcholine, and serotonin. The computation of these synaptic signals by pyramidal neurons can be condensed into a ratio of excitatory over inhibitory activity [4], hereby referred to as E-I ratio. Synaptic integration by pyramidal neurons in the PFC is especially important in deep layers, which make up the bulk of prefrontal output with projections to limbic structures such as the amygdala and the VTA, and subcortical circuits including the basal ganglia and the thalamus [5].
The importance of GABAergic and glutamatergic transmission in PFC function was established early on by application of receptor agonists and antagonist directly into the PFC. These early studies demonstrate that either increasing or decreasing inhibitory signaling in the PFC of adult animals have drastic consequences for PFC-dependent behaviors [6]. Similarly, inhibition of glutamatergic activity has been shown to alter prefrontal physiology and associated behaviors [7, 8]. Based on these studies, it can be inferred that any deviation of the established E/I ratio can have profound effects in the adult PFC. Indeed, proof of concept studies have shown that acutely increasing the excitability of pyramidal cells in the prelimbic cortex is enough to disrupt PFC-dependent behaviors in adult animals [9]. However, fewer studies have defined how, and most importantly, when, the balance between excitation and inhibition is achieved. The answer to that question will allow us to define how susceptible is the E-I balance to outside experience and how much of it is already predisposed.
Studies in animal models
Over the years, our laboratory and others have documented a developmental recruitment of inhibitory transmission in the PFC up to late adolescence (postnatal day - P- 50 in rats) without a concurrent increase in basal excitatory transmission [10–13]. In other words, the E-I ratio of layer V pyramidal neurons is actively readjusting during adolescence due to the gain of local GABAergic transmission (Figure 1). This fits with the model of protracted development of the GABAergic component in the PFC during adolescence [14]. By using models of non-contingent drug administration, the data show that any insult experienced up to late adolescence (P50) is enough to disrupt the normal increase of GABAergic function. The same results were not true for adult animals indicating that any insult sustained during adolescence preferentially disrupts the normal development of PFC GABAergic function and local E-I balance [10, 12, 13, 15] (Figure 1).
Figure 1:
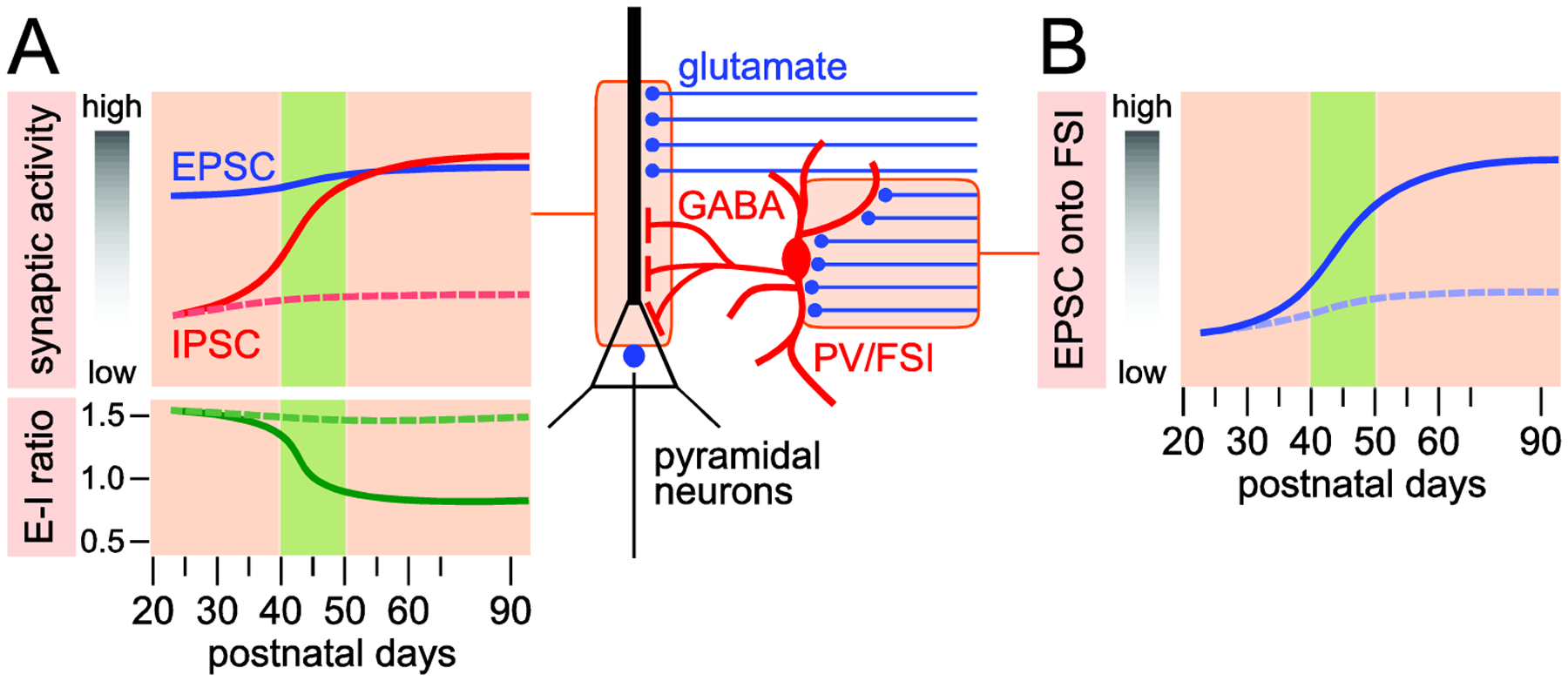
Adolescence is a critical period for synaptic activity in the PFC. A. The frequency of excitatory postsynaptic currents (EPSC) onto pyramidal output neurons remains relatively constant during the transition from adolescence to adulthood (blue line). Conversely, basal inhibitory postsynaptic currents (IPSC) increase sharply after postnatal day 40–45 (red line). Thus, the excitatory-inhibitory (E-I) ratio of synaptic activity became balanced after postnatal day 50 through adulthood. Dotted lines indicate the trajectory of IPSC and E-I ratio in the PFC upon disruption of NMDAR function [13] and parvalbumin (PV) expression [12] during adolescence. B. Characteristic developmental facilitation of EPSC frequency onto fast-spiking interneurons (FSI) in the PFC during adolescence. Dotted line illustrates trajectory of EPSC upon disruption of NMDAR function [13] and PV expression [12] in the PFC during adolescence.
The signal responsible for the increase in GABAergic transmission remains incompletely understood. Several lines of evidence point to glutamatergic afferents playing an important role. The PFC receives short and long-range glutamatergic projections from other cortical and subcortical structures, most notably, the hippocampus, the basolateral amygdala, and the thalamus [16, 17]. Many of these terminals contact the dendrites of pyramidal neurons with approximately 5% contacting GABAergic interneurons [18]. Although small, evidence suggests these glutamatergic inputs (e.g., vGlut1-positive) increase during adolescence, particularly in parvalbumin (PV)-positive/fast-spiking GABAergic interneurons [19, 20]. Presumably, these events drive the functional maturation of PV interneurons in the PFC and its contribution to sustaining local E-I balance [12].
In addition, several neuromodulators like dopamine, acetylcholine, and endocannabinoids have the ability to modify the strength of both glutamatergic and GABAergic synapses in the PFC, providing a nuanced layer of regulation that is also age-dependent (reviewed in [14]). Accordingly, disruption of these systems in the PFC during adolescence also decreases local GABAergic transmission [10, 21] and alters the E-I balance of prefrontal output. More recently, our lab has found that the age-dependent PFC modulation of behavior is likely due to the late-adolescent maturation of the ventral hippocampal recruitment of prefrontal GABAergic transmission [22].
The number of excitatory synapses largely outnumbers those of inhibitory synapses, even after adolescence when there is a loss of mostly excitatory, axospinous synapses and dendritic spines [23–25]. However, it is worth noting that the distribution of excitatory and inhibitory terminals in pyramidal neurons is not uniform: inhibitory synapses seem to be more concentrated at the soma and proximal dendrites, whereas the density of excitatory synapses is increased in the tufts or terminal segments of dendrites [26]. Thus, each segment seemingly has its own E-I ratio that can be modified to alter dendritic integration and output signal of each individual neuron [26]. This is an especially important consideration for the aforementioned neuromodulators and thalamic afferents, which typically innervate layer I in the PFC where dendritic tufts terminate.
The E-I balance in humans
The overproduction and subsequent loss of spines observed in rodents and non-human primates during adolescence is also recapitulated in the human PFC extending until early adulthood [27]. This late pruning of synapses and dendrites is likely to underlie the decrease in cortical thickness that occurs in frontal areas during development [2]. This is accompanied by an “stabilization” of remaining connections, which may mediate the changes in global E-I ratio observed during adolescence. Accordingly, several markers associated with glutamatergic and GABAergic synapses have been analyzed in human post-mortem tissue. Among those, complexins CX1 and CX2, presynaptic proteins expressed in inhibitory and excitatory synapses, respectively, change during postnatal development. CX1 increases until young adulthood whereas CX2 reaches a plateau during childhood, resulting in a reduced CX2/CX1 ratio in young adults [28]. Together with the above changes at the level of spines, it suggests that both excitatory and inhibitory inputs surviving the period of massive pruning strengthen their connections with post-synaptic targets. Conversely, the presynaptic transporters for glutamate and GABAA, vGlut and vGAT, respectively, do not appear to change substantially during adolescence [29], even when markers for several GABAergic interneurons are changing extensively [14, 30]. Thus, based on anatomical evidence and the expression of GABAergic and glutamatergic-associated proteins, there is a relative increase in the number of inhibitory synapses during the transition to adulthood. This may constitute the structural basis of the increased GABAergic function observed during adolescence.
Nonetheless, the link between individual neuronal E/I ratios and brain activity in humans remains obscure. Currently available methodologies used to map brain function in humans provide information over large areas in stark contrast to the high resolution, cell-type specific recordings of GABAergic and glutamatergic synapses from which E-I ratios are derived. Functional MRI has been valuable in mapping dynamic network activity changes during adolescence either in resting or task-dependent states [31], yet it fails to capture the functionality of excitatory and inhibitory synapses. Among human studies is the default-mode network, a set of brain structures with high correlated activity in the absence of goal-directed behaviors that becomes more functionally integrated in adults compared to children [32, 33], suggesting increased connectivity with long-range connections. On the other hand, goal-directed tasks in which the PFC is involved result in enhanced correlated activity with neighboring structures (e.g. anterior cingulate) [34]. In general, both resting-state and task-based studies suggest that during PFC maturation there is an increased connectivity with local and distal structures. Thus, it is conceivable that the developmental gain of GABAergic function in the PFC is a consequence of increased activity of long-range afferents, which in turn opens a window of communication with neighboring cortical areas through which information can be disseminated and relayed to the next processing hub.
Outstanding Questions
Several observations in pre-clinical and clinical models point towards the E-I balance being disrupted in developmental disorders such as schizophrenia and autism. It is possible that multiple neurotransmitter systems contribute to fine-tuning the E-I balance in the PFC during adolescence. The synaptic contribution of each afferent/input to PFC output computation remains to be studied.
Similarly, while it is known that disruption of prefrontal E-I balance affects PFC-dependent behaviors, the mechanisms by which individual neuronal E-I ratios mediate cortical information processing are unknown. A balanced E-I ratio may increase cross-cortical firing that subserves recurrent activity to increase the receptivity of the PFC and associated cortical areas to modulation by a common afferent [4].
What is the relevant correlate of the E/I ratio in humans? The correlates of neuronal E-I ratio in humans remain incompletely defined. Better methods to analyze the correspondence of local field potentials or EEG to individual pyramidal neuron E-I ratios need to be implemented [35]. This needs to be further integrated into knowledge of how networks are changing to subserve the increase in inter-regional connectivity observed during the transition to adulthood [33].
Conclusions
The E-I ratio of PFC output neurons is refined during adolescence by an increase in GABAergic function. As such, adolescence constitutes a period of exquisite sensitivity to environmental disruption that can have long-lasting consequences in PFC output function. This susceptibility stems from an apparent arrest in the development of local prefrontal GABAergic network that reaches its maturation late in adolescence. Multiple layers of neuromodulators aid in the maturation of PFC GABAergic function and interruption of these processes can exert near-identical disruptions in the E-I balance. Understanding the underlying mechanisms contributing to the maturation of prefrontal excitatory and inhibitory synapses and its impact on local network function is necessary to reveal how developmental disorders where the PFC is involved emerge during adolescence.
Highlights.
The prefrontal excitatory-inhibitory (E-I) balance is actively changing during adolescence
A gain of GABA function during late adolescence dictates the prefrontal E-I balance
Reduced GABA function up to adolescence can permanently alter prefrontal E-I ratio
Declaration of Interest
This work was supported by the National Institute of Mental Health grants MH086507, MH105488, and MH123147 to KYT. Funding source had no role in the study design, data analysis, or writing of this report. Authors have no additional disclosures.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
None
References
- [1].Sowell ER, Peterson BS, Thompson PM, Welcome SE, Henkenius AL, Toga AW, Mapping cortical change across the human life span, Nat Neurosci 6(3) (2003) 309–15. [DOI] [PubMed] [Google Scholar]
- [2].Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, Nugent TF 3rd, Herman DH, Clasen LS, Toga AW, Rapoport JL, Thompson PM, Dynamic mapping of human cortical development during childhood through early adulthood, Proc Natl Acad Sci U S A 101(21) (2004) 8174–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Spear LP, The adolescent brain and age-related behavioral manifestations, Neurosci Biobehav Rev 24(4) (2000) 417–63. [DOI] [PubMed] [Google Scholar]
- [4].Lew SE, Tseng KY, Dopamine modulation of GABAergic function enables network stability and input selectivity for sustaining working memory in a computational model of the prefrontal cortex, Neuropsychopharmacology 39(13) (2014) 3067–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Thomson AM, Lamy C, Functional maps of neocortical local circuitry, Front Neurosci 1(1) (2007) 19–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Tse MT, Piantadosi PT, Floresco SB, Prefrontal cortical gamma-aminobutyric acid transmission and cognitive function: drawing links to schizophrenia from preclinical research, Biol Psychiatry 77(11) (2015) 929–39. [DOI] [PubMed] [Google Scholar]
- [7].Baldwin AE, Holahan MR, Sadeghian K, Kelley AE, N-methyl-D-aspartate receptor-dependent plasticity within a distributed corticostriatal network mediates appetitive instrumental learning, Behav Neurosci 114(1) (2000) 84–98. [DOI] [PubMed] [Google Scholar]
- [8].Floresco SB, Prefrontal dopamine and behavioral flexibility: shifting from an “inverted-U” toward a family of functions, Front Neurosci 7 (2013) 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Yizhar O, Fenno LE, Prigge M, Schneider F, Davidson TJ, O’Shea DJ, Sohal VS, Goshen I, Finkelstein J, Paz JT, Stehfest K, Fudim R, Ramakrishnan C, Huguenard JR, Hegemann P, Deisseroth K, Neocortical excitation/inhibition balance in information processing and social dysfunction, Nature 477(7363) (2011) 171–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Cass DK, Flores-Barrera E, Thomases DR, Vital WF, Caballero A, Tseng KY, CB1 cannabinoid receptor stimulation during adolescence impairs the maturation of GABA function in the adult rat prefrontal cortex, Mol Psychiatry 19(5) (2014) 536–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Gonzalez-Burgos G, Miyamae T, Pafundo DE, Yoshino H, Rotaru DC, Hoftman G, Datta D, Zhang Y, Hammond M, Sampson AR, Fish KN, Ermentrout GB, Lewis DA, Functional Maturation of GABA Synapses During Postnatal Development of the Monkey Dorsolateral Prefrontal Cortex, Cereb Cortex 25(11) (2015) 4076–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Caballero A, Flores-Barrera E, Thomases DR, Tseng KY, Downregulation of parvalbumin expression in the prefrontal cortex during adolescence causes enduring prefrontal disinhibition in adulthood, Neuropsychopharmacology 45(9) (2020) 1527–1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Flores-Barrera E, Thomases DR, Tseng KY, MK-801 Exposure during Adolescence Elicits Enduring Disruption of Prefrontal E-I Balance and Its Control of Fear Extinction Behavior, J Neurosci 40(25) (2020) 4881–4887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Caballero A, Granberg R, Tseng KY, Mechanisms contributing to prefrontal cortex maturation during adolescence, Neurosci Biobehav Rev 70 (2016) 4–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Thomases DR, Cass DK, Tseng KY, Periadolescent exposure to the NMDA receptor antagonist MK-801 impairs the functional maturation of local GABAergic circuits in the adult prefrontal cortex, J Neurosci 33(1) (2013) 26–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Oh SW, Harris JA, Ng L, Winslow B, Cain N, Mihalas S, Wang Q, Lau C, Kuan L, Henry AM, Mortrud MT, Ouellette B, Nguyen TN, Sorensen SA, Slaughterbeck CR, Wakeman W, Li Y, Feng D, Ho A, Nicholas E, Hirokawa KE, Bohn P, Joines KM, Peng H, Hawrylycz MJ, Phillips JW, Hohmann JG, Wohnoutka P, Gerfen CR, Koch C, Bernard A, Dang C, Jones AR, Zeng H, A mesoscale connectome of the mouse brain, Nature 508(7495) (2014) 207–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].DeNardo LA, Berns DS, DeLoach K, Luo L, Connectivity of mouse somatosensory and prefrontal cortex examined with trans-synaptic tracing, Nat Neurosci 18(11) (2015) 1687–1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Sun Q, Li X, Ren M, Zhao M, Zhong Q, Ren Y, Luo P, Ni H, Zhang X, Zhang C, Yuan J, Li A, Luo M, Gong H, Luo Q, A whole-brain map of long-range inputs to GABAergic interneurons in the mouse medial prefrontal cortex, Nat Neurosci 22(8) (2019) 1357–1370. [DOI] [PubMed] [Google Scholar]
- [19].Chung DW, Wills ZP, Fish KN, Lewis DA, Developmental pruning of excitatory synaptic inputs to parvalbumin interneurons in monkey prefrontal cortex, Proc Natl Acad Sci U S A 114(4) (2017) E629–E637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Caballero A, Flores-Barrera E, Cass DK, Tseng KY, Differential regulation of parvalbumin and calretinin interneurons in the prefrontal cortex during adolescence, Brain Struct Funct 219(1) (2014) 395–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Cass DK, Thomases DR, Caballero A, Tseng KY, Developmental disruption of gamma-aminobutyric acid function in the medial prefrontal cortex by noncontingent cocaine exposure during early adolescence, Biol Psychiatry 74(7) (2013) 490–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Miguelez Fernandez AMM, Molla HM, Thomases DR, Tseng KY, Prefrontal alpha7nAChR signaling differentially modulates afferent drive and trace fear conditioning behavior in adolescent and adult rats, J Neurosci in (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Bourgeois JP, Goldman-Rakic PS, Rakic P, Synaptogenesis in the prefrontal cortex of rhesus monkeys, Cereb Cortex 4(1) (1994) 78–96. [DOI] [PubMed] [Google Scholar]
- [24].Koss WA, Belden CE, Hristov AD, Juraska JM, Dendritic remodeling in the adolescent medial prefrontal cortex and the basolateral amygdala of male and female rats, Synapse 68(2) (2014) 61–72. [DOI] [PubMed] [Google Scholar]
- [25].Drzewiecki CM, Willing J, Juraska JM, Synaptic number changes in the medial prefrontal cortex across adolescence in male and female rats: A role for pubertal onset, Synapse 70(9) (2016) 361–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Iascone DM, Li Y, Sumbul U, Doron M, Chen H, Andreu V, Goudy F, Blockus H, Abbott LF, Segev I, Peng H, Polleux F, Whole-Neuron Synaptic Mapping Reveals Spatially Precise Excitatory/Inhibitory Balance Limiting Dendritic and Somatic Spiking, Neuron 106(4) (2020) 566–578 e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Petanjek Z, Judas M, Simic G, Rasin MR, Uylings HB, Rakic P, Kostovic I, Extraordinary neoteny of synaptic spines in the human prefrontal cortex, Proc Natl Acad Sci U S A 108(32) (2011) 13281–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Salimi K, Glantz LA, Hamer RM, German TT, Gilmore JH, Jarskog LF, Regulation of complexin 1 and complexin 2 in the developing human prefrontal cortex, Synapse 62(4) (2008) 273–82. [DOI] [PubMed] [Google Scholar]
- [29].Fung SJ, Webster MJ, Weickert CS, Expression of VGluT1 and VGAT mRNAs in human dorsolateral prefrontal cortex during development and in schizophrenia, Brain Res 1388 (2011) 22–31. [DOI] [PubMed] [Google Scholar]
- [30].Fung SJ, Webster MJ, Sivagnanasundaram S, Duncan C, Elashoff M, Weickert CS, Expression of Interneuron Markers in the Dorsolateral Prefrontal Cortex of the Developing Human and in Schizophrenia, Am J Psychiat 167(12) (2010) 1479–1488. [DOI] [PubMed] [Google Scholar]
- [31].Stevens MC, The contributions of resting state and task-based functional connectivity studies to our understanding of adolescent brain network maturation, Neurosci Biobehav Rev 70 (2016) 13–32. [DOI] [PubMed] [Google Scholar]
- [32].Fair DA, Dosenbach NU, Church JA, Cohen AL, Brahmbhatt S, Miezin FM, Barch DM, Raichle ME, Petersen SE, Schlaggar BL, Development of distinct control networks through segregation and integration, Proc Natl Acad Sci U S A 104(33) (2007) 13507–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Fair DA, Cohen AL, Dosenbach NU, Church JA, Miezin FM, Barch DM, Raichle ME, Petersen SE, Schlaggar BL, The maturing architecture of the brain’s default network, Proc Natl Acad Sci U S A 105(10) (2008) 4028–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Oemisch M, Westendorff S, Everling S, Womelsdorf T, Interareal Spike-Train Correlations of Anterior Cingulate and Dorsal Prefrontal Cortex during Attention Shifts, J Neurosci 35(38) (2015) 13076–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Gao R, Peterson EJ, Voytek B, Inferring synaptic excitation/inhibition balance from field potentials, Neuroimage 158 (2017) 70–78. [DOI] [PubMed] [Google Scholar]


