Abstract
Objective:
The purpose of this study was to evaluate the prognostic value of quantitative myocardial blood flow and flow reserve (MFR), here reflecting the integrated effects of diffuse atherosclerosis and microvascular dysfunction, in patients with systemic inflammatory disorders.
Background:
Rheumatoid arthritis (RA), systemic lupus erythematosus (SLE), and psoriasis (PsO) are common inflammatory conditions with excess cardiovascular (CV) risk compared to the general population. Systemic inflammation perturbs endothelial function and has been linked to coronary vasomotor dysfunction. However, the prognostic significance of this vascular dysfunction is not known.
Methods:
This is a retrospective study of patients with RA, SLE, and PsO undergoing clinically indicated rest/stress myocardial perfusion positron emission tomography (PET). Patients with an abnormal myocardial perfusion study or left ventricular dysfunction were excluded. MFR was calculated as the ratio of myocardial blood flow (MBF, ml/min/g) at peak stress compared to that at rest.
Results:
Among the 198 patients (median age 65 years, 80% female), 20.7% had SLE, 31.8% PsO, and 47.5% RA. There was no difference in mean MFR between these conditions. Over a median follow-up of 7.8 years, there were 51 deaths and 63 MACE events. Patients in the lowest tertile (MFR<1.65) had higher all-cause mortality compared to the highest tertile, which remained significant after adjusting for age, sex, and the pretest clinical risk score (HR 2.4 95% CI 1.05–5.4 p=0.038). Similarly, compared to the highest MFR tertile, those in the lowest tertile had a lower MACE-free survival after adjusting for age, sex and the pretest clinical risk score (HR 3.6 (95% CI: 1.7–7.6, p=0.001).
Conclusions:
In patients with systemic inflammatory disorders, impaired coronary vasodilator reserve was associated with worse cardiovascular outcomes and all-cause mortality.
Keywords: Inflammation, microvascular dysfunction, nuclear imaging/PET
INTRODUCTION
Autoimmune systemic inflammatory diseases are associated with increased cardiovascular risk, including atherosclerosis and myocardial infarction (MI) (1–5). Although an increased prevalence of CV (cardiovascular) risk factors has been implicated in systemic inflammatory diseases, these pathophysiologic features provide only a partial explanation for the excess risk of MI, heart failure and death (6–9). Prior work has identified that the presence of impaired myocardial flow reserve (MFR) in patients without overt obstructive CAD identifies patients at increased risk of adverse cardiac events and improves the ability of models to discriminate patients with elevated CV risk, independent of traditional CV risk factors and stress test scores (10,11). This suggests that diffuse atherosclerosis and coronary microvascular dysfunction (CMD) play a more important role in the pathophysiologic abnormalities leading to increased risk than previously thought. There is ample laboratory evidence that inflammation plays a major role in all stages of atherothrombosis, including the early functional abnormalities in vascular endothelial and smooth muscle cell function (12). There is also increasing in vivo human evidence that increased systemic inflammation is associated with coronary vascular dysfunction (13).
An increased prevalence of coronary vasomotor dysfunction has been identified in patients with autoimmune systemic inflammatory conditions, including psoriasis, systemic lupus erythematosus (SLE) and rheumatoid arthritis (RA) (14–19). However, whether impaired coronary vasomotor dysfunction is also associated with adverse cardiac events or mortality in this patient population has not been studied. Our objective was to determine the prognostic value of impaired coronary vasodilator reserve in patients with common autoimmune systemic inflammatory diseases (psoriasis, RA, and SLE).
METHODS
Study Population
This retrospective study population included consecutive patients with a diagnosis of psoriasis, RA, or SLE prior to the date of cardiovascular assessment who underwent cardiac positive emission tomography (PET) for evaluation of CV symptoms (e.g. chest pain and/or dyspnea) between 2006 and 2019 at our institution. Patient demographics, clinical history, medications, and indications for testing were collected prospectively at the time of PET. The cohort consisted of patients enrolled in our cardiovascular PET database and with an ICD-9 and/or ICD-10 for psoriasis, RA, or SLE prior to the date of PET (Supplemental Figure 1). All electronic medical records were then independently reviewed by a physician to confirm a diagnosis of psoriasis, RA, or SLE. SLE diagnosis was confirmed based on the 1997 updated American College Rheumatology (ACR) criteria (20,21) and further validated by cross-reference with the Brigham and Women’s Hospital Rheumatology SLE Registry. Likewise, RA was further confirmed by cross-reference to a validated RA cohort at Brigham and Women’s Hospital Rheumatology (22). Patients with left ventricular ejection fraction (LVEF) < 50%, an abnormal myocardial perfusion study (summed stress score ≥ 3), prior coronary artery bypass surgery (CABG), or prior heart transplantation were excluded. The study was approved by the Partners Healthcare Institutional Review Board and conducted in accordance with institutional guidelines.
Quantification of Myocardial Blood Flow and Vasodilator Reserve
Myocardial blood flow and vasodilator reserve was assessed with quantitative PET imaging using a hybrid whole-body PET-computed tomography (CT) scanner (Discovery RX or STE LightSpeed 64, GE Healthcare, Milwaukee, Wisconsin) with 13N-ammonia or 82Rubidium as flow tracers. Myocardial perfusion images were obtained at rest and in response to vasodilator-stress, as previously described (23,24). Summed rest, stress, and difference scores were computed. Rest and stress LVEF were calculated from gated myocardial perfusion images with commercially available software (Corridor4DM, INVIA Medical Imaging Solutions, Ann Arbor, Michigan). Regional and global rest and stress myocardial blood flow (MBF in mL/min/g) was quantified using a validated tracer kinetic model as previously described (23,24). Regional and global MFR was calculated as the ratio of stress to rest myocardial blood flow. In our laboratory, the intra-class correlation coefficient for MFR among four readers was 0.94 (95% CI 0.88–0.98) (25), reflecting high reproducibility.
Outcomes
For the outcomes of all-cause mortality and first major adverse cardiovascular event (MACE), patients were followed from the time of PET imaging until death, first MACE event or censoring. Censoring was defined by the time at which medical records were adjudicated if the patient was still actively seen within the Partner’s Health Care System or by the last date of follow-up available. MACE was defined as a composite of cardiovascular death or hospitalization for nonfatal myocardial infarction, late coronary revascularization, cerebral vascular accident (CVA), or heart failure. All adverse CV events were ascertained >28 days after the time of cardiac PET scan. Of the 198 patients, 146 had record of clinical follow-up through 2019 in the electronic medical record. The remaining 52 patients had a last clinical record between 2007–2018 and were censored at the last date of follow-up within the EMR. Ascertainment of nonfatal myocardial infarction, CVA, or heart failure required a discharge note with a primary hospitalization diagnosis of myocardial infarction, CVA or heart failure. In addition, we only included events that met the 2018 Fourth Universal Definition of Myocardial Infarction or defined clinical criteria for the presence of symptoms, signs, and escalation of therapy for heart failure, were classified as such (26). Ascertainment of clinical endpoints, including cause of death, was determined by blinded adjudication of the electronic health medical records (EHR), Partners HealthCare Research Patient Data Registry, and the National Death Index (27).
Statistical Analysis
We used Pearson’s chi-squared and one-way analysis of variance or Kruskal-Wallis to evaluate for differences in categorical and continuous baseline characteristics, respectively, across the different systemic inflammatory disease and MFR tertiles. The Kruskal-Wallis test, which can be interpreted as an assessment of whether at least one distribution in a set of distributions is more likely to produce larger values than the others or not, was used when the normality of the observed data was questionable. Categorical variables are reported as frequencies with percentage (%). Continuous variables are expressed as mean (± standard deviation) or median (interquartile range (IQR)). CRP was log transformed and Pearson’s correlation was used to compare the relationship between CRP and MFR. To study the baseline effect of MFR and stress MBF on all-cause mortality and on cardiovascular events, univariable Cox proportional hazards (PH) modeling was performed for overall survival and survival free from cardiovascular events using available covariates. Multivariable adjustment was performed using covariates with known clinical significance or that had significant univariable association with the outcome in question. To avoid overfitting, demographic and medical history variables (age, sex, symptoms, hypertension, diabetes, hyperlipidemia, smoking history, family history of premature CAD, body mass index, and oestrogen status) were incorporated into the validated Morise pretest clinical risk score for diagnosing significant CAD (with values 0–8, 9–15, and 16–24 indicating low, intermediate, and high pretest risk, respectively), as previously described (28,29). Kaplan-Meier curves were constructed by MFR tertiles or stress MBF tertiles to illustrate overall and cardiovascular event-free survival. Differences were tested with the log-rank test. Cox proportional hazards models, with multivariable adjustment using the previously identified covariates, were used to examine the association between events and MFR or stress MBF tertiles. Cox PH assumptions tests were assessed using graphical methods to verify that PH assumptions were met. All tests were 2-sided, and a value of p <0.05 was considered statistically significant. Statistical analysis was performed with the use of SAS University Edition 9.4 (2018).
RESULTS
Baseline characteristics of the study cohort
There were 198 patients in this cohort: 41 with SLE (20.7%), 63 with psoriasis (31.8%) and 94 with RA (47.5%) (Table 1). The mean age was 65.3 years (SD 11.8) and 80% were female. There was a high prevalence of coronary risk factors including hypertension (75.3%), dyslipidemia (56.6%), diabetes mellitus (DM, 30.3%), and obesity (39.9%). Comparison of demographics and coronary risk factor burden among the three systemic inflammatory diseases showed that patients with SLE were younger, more likely to be female, and less likely to have diabetes and dyslipidemia compared to patients with RA or psoriasis (Supplemental Table 1).
Table 1.
Baseline Demographics of the Study Cohort
| Total cohort (n=198) | |
|---|---|
| Rheumatoid Arthritis | 94 (47.5%) |
| Age at PET, mean (SD) (years) | 65.3 (11.8) |
| Female | 158 (80%) |
| Diabetes | 60 (30.3%) |
| Previously known CAD | 24 (12.1%) |
| Dyslipidemia | 112 (56.6%) |
| Hypertension | 149 (75.3%) |
| Family history of CAD | 56 (28.3%) |
| Obesity | 79 (39.9%) |
| Current tobacco use | 9 (4.6%) |
Impaired Coronary Vasodilator Reserve in Systemic Inflammatory Disease
At rest, MBF was regionally homogeneous and similar across the three groups of systemic inflammatory disease conditions. During maximal hyperemia, there was a similar magnitude of flow augmentation (Figure 1A) and resulting MFR (Figure 1B) in RA, SLE and psoriasis patients (Table 2).
Figure 1. Distribution of MFR and Stress MBF in Systemic Inflammatory Disease within Study Cohort.
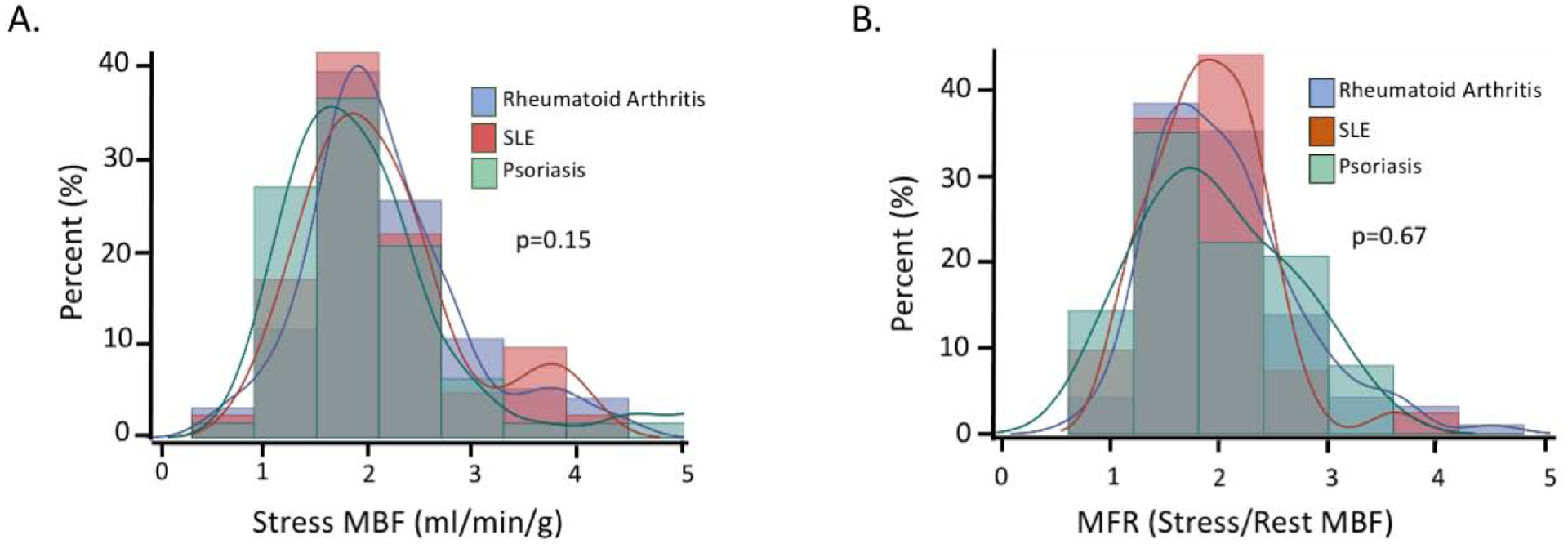
A. Overlay histogram demonstrates that the distribution of stress MBF was similar in SLE, RA, and psoriasis patients (p=0.15) B. Overlay histogram demonstrates that the distribution of MFR was similar in SLE, RA, and psoriasis patients (p=0.67).
Table 2:
Cardiac Stress Positron Emission Tomography/Computed Tomography Hemodynamics and Characteristics of the study cohort
| All N=198 | SLE N=41 | Psoriasis N=63 | RA N=94 | p-value | |
|---|---|---|---|---|---|
| Resting HR (bpm) | 69 (61–78) | 70 (63–76) | 68 (60–76) | 70 (61–80) | 0.38 |
| Peak HR (bpm) | 86 (78–96) | 83 (80–93) | 87 (78–93) | 87 (77–98) | 0.76 |
| Stress SBP (mm Hg) | 133 (118–155) | 133 (117–155) | 134 (117–154) | 133 (122–156) | 0.78 |
| Resting MAP (mm Hg) | 95.7 (88–107) | 91.7 (87–109) | 98 (87–107) | 95.8 (89–107) | 0.68 |
| Stress MAP (mm Hg) | 89 (80–100) | 90.3 (81–103) | 88.3 (80–100) | 89 (81–100) | 0.62 |
| Rest LVEF (%)* | 62 (55–68) | 57 (53–64) | 64 (55–72) | 62.5 (57–69) | 0.005 |
| Stress LVEF (%)^ | 67 (61–74) | 65 (56–74) | 68 (62–76) | 69 (62–72) | 0.13 |
| Stress MBF, (mL/min/g) | 1.96 (1.6–2.4) | 2.01 (1.6–2.4) | 1.83 (1.4–2.3) | 2.04 (1.7–2.5) | 0.15 |
| Rest MBF, (mL/min/g) | 1.01 (0.82–1.3) | 1.01 (0.88–1.4) | 0.99 (0.8–1.3) | 1.03 (0.82–1.3) | 0.66 |
| MFR | 1.87 (1.5–2.3) | 1.83 (1.6–2.2) | 1.80 (1.4–2.5) | 1.93 (1.5–2.3) | 0.67 |
SLE=systemic lupus erythematosus, RA=rheumatoid arthritis, MBF=myocardial blood flow, MFR=myocardial flow reserve, HR=heart rate, SBP=systolic blood pressure, MAP=mean arterial pressure. LVEF=left ventricular ejection fraction. All values shown are median with interquartile range (IQR, 25–75%).
Rest LVEF (%) n=193;
stress LVEF (%) n=190. P values refer to the comparison across the three groups of systemic inflammatory disease.
The entire cohort was then grouped into tertiles of increasing MFR as follows: low <1.65, intermediate ≥1.65 and ≤2.19, and high >2.19 (Table 3). Patients in the lowest MFR tertile were older and more likely to have a history of hypertension. There was no difference in the distribution of RA, SLE, and psoriasis patients across the MFR tertiles (Table 3). Disease-specific severity at the time of cardiac PET was not available for the cohort.
Table 3:
Characteristics of all autoimmune systemic inflammation disease patients (SLE, RA, and psoriasis) by myocardial flow reserve tertile.
| MFR < 1.65 (n=66) | 1.65 ≤ MFR ≤ 2.19 (n=66) | MFR > 2.19 (n=66) | p-value | |
|---|---|---|---|---|
| RA | 33 (50%) | 30 (45.5%) | 30 (47%) | |
| Age at PET, mean (SD) (years) | 67.2 (12.2) | 67.1 (11.7) | 60.6 (11.5) | 0.002 |
| Female | 55 (83.3%) | 54 (81.8%) | 49 (74.2%) | 0.38 |
| Obesity | 25 (37.9%) | 27 (40.9%) | 27 (40.9%) | 0.92 |
| Hypertension | 57 (86.4%) | 50 (75.8%) | 42 (63.6%) | 0.01 |
| Dyslipidemia | 44 (66.7%) | 38 (57.6%) | 30 (45.5%) | 0.05 |
| Diabetes | 26 (39.4%) | 20 (30.3%) | 14 (21.2%) | 0.08 |
| Previously known CAD | 9 (13.6%) | 6 (9.1%) | 9 (13.6%) | 0.65 |
| Any tobacco use | 3 (4.6%) | 2 (3%) | 4 (6%) | 0.71 |
| Family History of CAD | 21 (31.8%) | 25 (37.8%) | 10 (15.2%) | 0.01 |
SLE=systemic lupus erythematosus, RA=rheumatoid arthritis, CAD = coronary artery disease. MBF = myocardial blood flow. MFR = myocardial flow reserve. PET = positron emission tomography. SD = standard deviation.
However, we examined the relationship between C-reactive protein (CRP), a surrogate measure of systemic inflammation, and MFR in a subgroup of patients with CRP measurements within 30 days of the PET scan (n=33). Although the sample size was small, CRP was negatively correlated with MFR in this subgroup analysis (r=−0.39, p=0.02, Supplemental Figure 2), and this remained significant after adjusting for systemic inflammatory disease conditions.
Patient Outcomes
Over a median follow-up of 7.8 years (IQR 3.6–10.4) after PET, there were 51 deaths from any cause including 14 CV deaths. Compared with the highest tertile, the adjusted hazard ratio for all-cause death in the lowest tertile of MFR was 2.4 (95% CI: 1.05–5.4, p=0.038, Figure 2) whereas the corresponding adjusted HR for stress myocardial blood flow was 1.4 (CI: 0.7–2.9; p=0.31, Supplemental Figure 3A). In univariable analysis, MFR but not stress MBF was associated with all-cause death. Similar results were observed in separate multivariable models adjusted for age, sex, and pretest clinical score (Table 4).
Figure 2. Kaplan-Meier Estimate of Overall Survival.
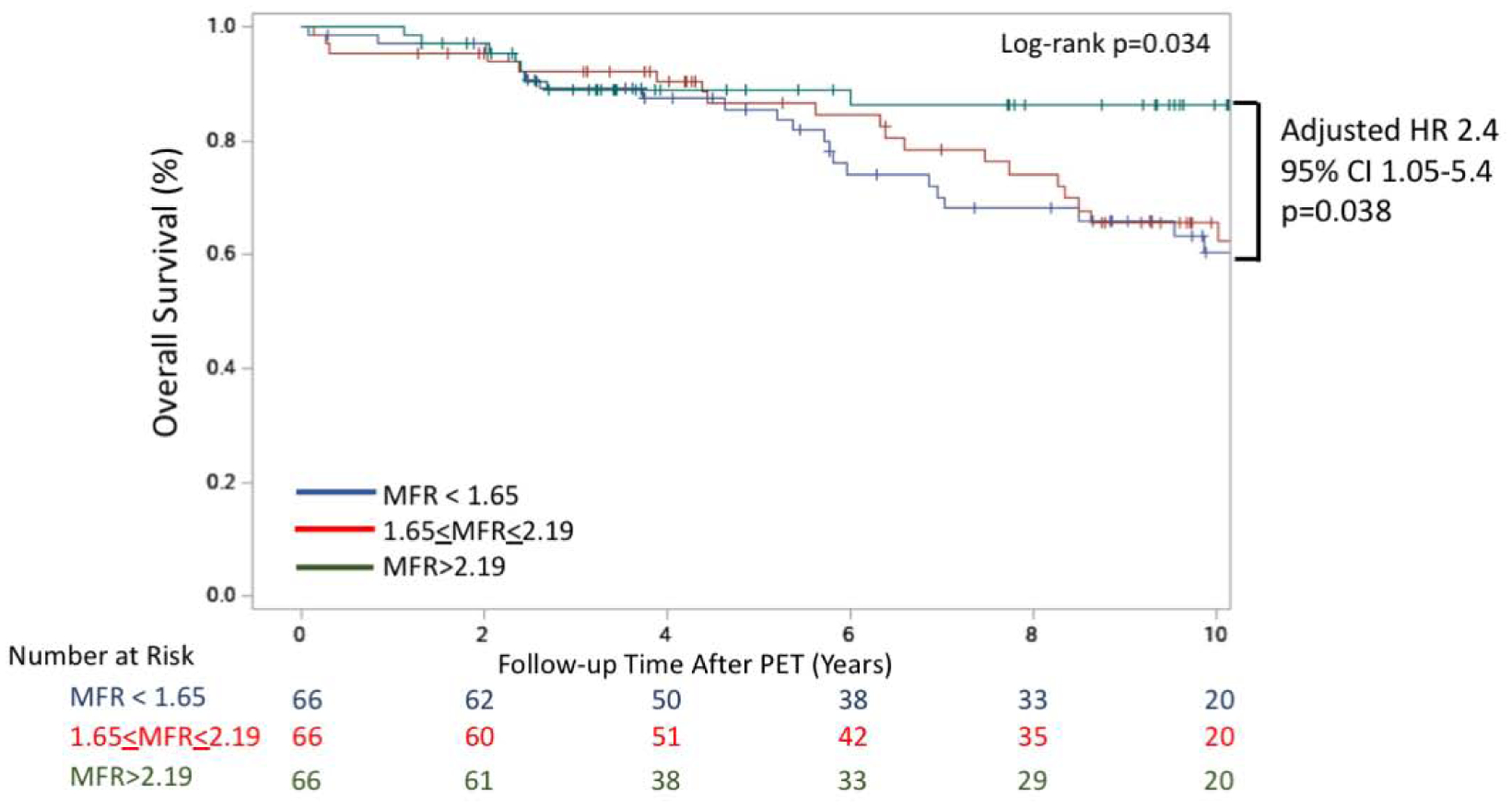
Overall survival for the cohort is presented stratified by MFR tertile. Multivariable analysis adjusted for age, sex and the Morise pre-test clinical risk score that incorporates cardiovascular risk factors (obesity, hypertension, hyperlipidemia, tobacco use, DM), age, sex and symptoms. Shown is lowest tertile compared to highest tertile [HR 2.4; 95% CI 1.05–5.4; p=0.038]. CI = confidence interval. HR = hazard ratio. MFR = myocardial flow reserve. PET = positron emission tomography.
Table 4.
Association between myocardial flow reserve and stress myocardial blood flow and clinical outcomes
| Outcomes | Univariable model hazard ratio (95% CI) | Multivariable model hazard ratio (95% CI) | ||||||
|---|---|---|---|---|---|---|---|---|
| MFR | p value | Stress MBF | p value | MFR | p value | Stress MBF | p value | |
| All-cause death | 1.92 (1.16–3.2) | 0.01 | 1.27 (0.9–1.77) | 0.16 | 1.72 (1.04–2.84) | 0.03 | 1.2 (0.86–17) | 0.3 |
| MACE | 2.3 (1.43–3.7) | <0.001 | 1.71 (1.2–2.4) | 0.002 | 2.16 (1.4–3.4) | 0.001 | 1.69 (1.2–2.4) | 0.002 |
Multivariable model was adjusted for age, sex and pre-test clinical score. MFR and stress myocardial blood flow (MBF) were modeled as continuous variables. Hazard ratios reflect a per unit decrease in MFR or stress MBF.
There were 63 MACE events (34 hospitalizations for heart failure, 11 hospitalizations for non-fatal myocardial infarction, seven hospitalizations for CVA, and 11 cardiovascular deaths). Compared with the highest tertile, the adjusted HR for MACE was 3.6 (95% CI: 1.7–7.6, p=0.001) and 2.5 (95% CI 1.1–5.4, p=0.02) for the lowest and middle tertile of MFR, respectively (Figure 3). Similar risk stratification in the highest tertile only was observed between tertiles of stress myocardial blood flow (Supplemental Figure 3B). In univariable analysis, MFR and stress MBF were significantly associated with MACE, and this association persisted in separate multivariable models that similarly included age, sex, and the pretest clinical risk score (Table 4).
Figure 3. Kaplan-Meier Estimate of CV Event-Free Survival.
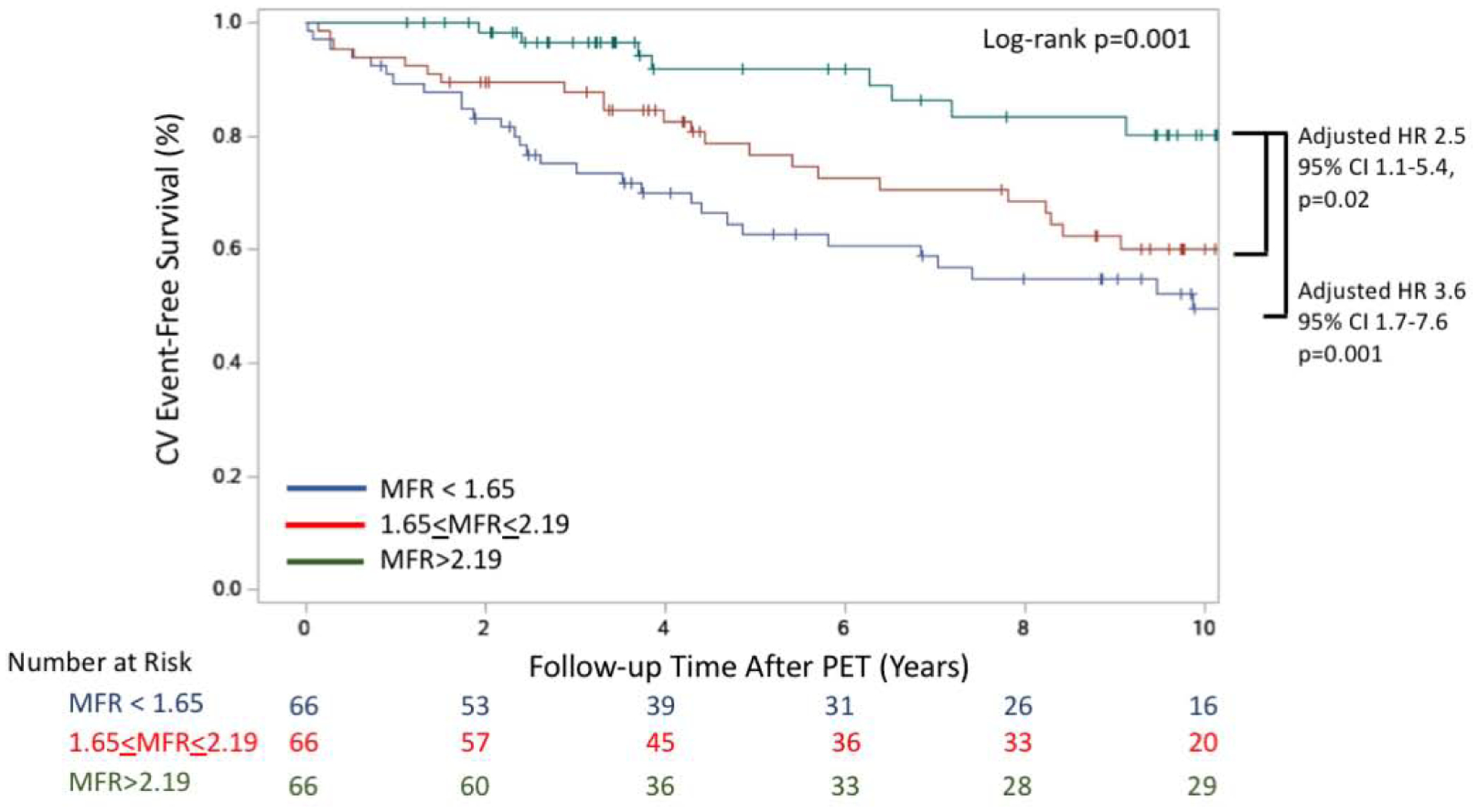
CV event-free survival for the cohort is presented stratified by MFR tertile. Multivariable analysis adjusted for presence of, age, sex and the Morise pretest clinical risk score that incorporates cardiovascular risk factors (obesity, hypertension, hyperlipidemia, tobacco use, DM), age, sex and symptoms. Shown is compared to highest tertile, middle tertile [HR 3.6; 95% CI 1.7–7.6; p=0.001], and lowest tertile [HR 2.5; 95% CI 1.1–5.4; p=0.02]. CI = confidence interval. CV = cardiovascular. HR = hazard ratio. MFR = myocardial flow reserve. PET = positron emission tomography.
To assess the relative strength of association between MFR and stress MBF with outcomes, we also run a multivariable model including both MFR and stress MBF that was also adjusted for age, sex, and pretest clinical score. In this integrated model, MFR but not stress MBF was associated with MACE (MFR HR: 1.76; 95% CI 1.03–3.01; p=0.037 per unit decrease in MFR; stress MBF HR: 1.34; 95% CI, 0.91–1.98; P=0.13 per unit decrease in stress MBF) and all-cause death (MFR HR: 1.73; 95% CI 0.98–3.1; P=0.059 and stress MBF HR: 1.0; 95% CI, 0.69–1.44; P=0.99 per unit decrease in stress MBF).
DISCUSSION
Autoimmune systemic inflammatory diseases are associated with coronary vasomotor dysfunction, reflecting a combination of diffuse atherosclerosis in conduit vessels and structural and functional abnormalities in the coronary microcirculation (13–19). Our data extend the previous observations of higher coronary vasodilator dysfunction burden in patients with systemic inflammatory disorders by demonstrating that such vascular dysfunction is associated with adverse outcomes, including death (Central Illustration). These results are novel and support the hypothesis of a link between systemic inflammation, coronary vascular dysfunction and adverse outcomes in this population.
Central Illustration. Systemic inflammatory disease, coronary vasodilator dysfunction and adverse outcomes.
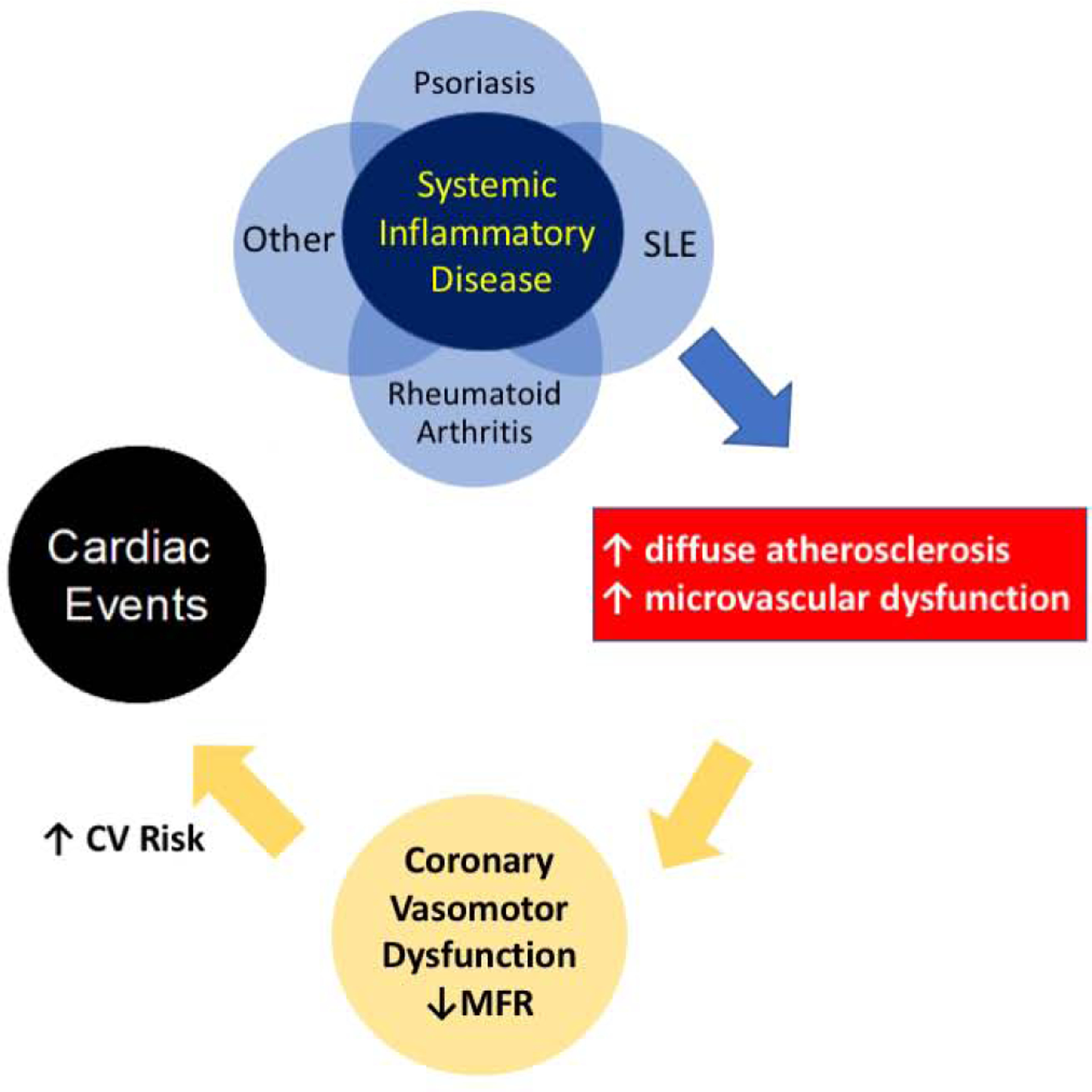
Systemic inflammatory disorders including psoriasis, SLE, rheumatoid arthritis and possible others increase the frequency of impaired coronary vasodilator function, resulting from an increased burden of diffuse atherosclerosis in conduit vessels and coronary microvascular dysfunction. In this study, we observed that the lowest myocardial flow reserve tertile (MFR < 1.65) had worse adjusted overall survival and worse adjusted cardiovascular event-free survival in patients with systemic inflammatory disease (SLE, RA, psoriasis).
The mechanisms through which measures of abnormal myocardial flow reserve are associated with adverse outcomes cannot be determined from this study. However, it is possible that high serum levels of pro-inflammatory cytokines including interleukin (IL)-17, IL-6, interferon-alpha and tumor necrosis factor, all of which are elevated in systemic inflammatory disorders (30–33) perturb endothelial function and vascular health resulting in accelerated epicardial atherosclerosis and coronary microvascular dysfunction. Indeed, the inverse association between levels of CRP and MFR observed in the sub-sample of patients in this cohort supports this hypothesis. We have recently shown that impaired coronary vasodilator reserve is associated with subclinical injury (34) and abnormalities in cardiac structure and function (29,35,36), and adverse outcomes (29,35,36) in patients without systemic inflammatory disorders. Whether there is one common pro-inflammatory pathway associated with coronary vascular dysfunction in patients with systemic inflammatory disorders is not known.
Dysregulated immune responses, often involving excessive off-target inflammatory activity, are seen in numerous conditions, and include common co-morbidities such as atherosclerosis and obesity (12,37,38). Given that each systemic inflammatory disease in this study has unique biologic targets and different immunopathobiology, we hypothesize that there are probably multiple immune dysregulatory mechanisms that can perturb vascular health and this is likely context specific. The link between specific immune dysregulation pathways and coronary vascular dysfunction is currently under investigation (NCTs Lipids, Inflammation, and CV Risk in RA, LiiRA [NCT02714881] and Effects of Tildrakizumab on CFR in Moderate-severe psoriasis, MiNIMA [NCT04271540]). The association between coronary vasodilator dysfunction and outcomes in patients with SLE, RA and psoriasis suggest that MFR could be used to identify patients with these diseases who are at risk for adverse cardiovascular events (39,40). Excess cardiovascular risk in these populations is increasingly becoming recognized. Indeed, the 2019 ACC/AHA 10-year cardiovascular risk factor guidelines have released updated guidance that systemic inflammatory diseases are considered a “risk enhancer.” These new recommendations can be used to stratify patients who are at intermediate risk in whom primary prevention statin therapy is being considered in clinical practice (41).
Study Limitations
Our study has several limitations. These include the lack of validated disease-specific measures of disease activity for each disease at the time of cardiac PET and the anti-inflammatory treatment history. Although psoriasis, RA and SLE are diseases characterized by systemic inflammation, each disease is distinct with unique characteristics and immune dysregulation. The small sample size for each disease limits the ability to interpret hard outcomes, including all-cause mortality and MACE, within each disease. However, there were similar values of MFR across the diseases suggesting that impaired coronary vasodilator reserve is a common finding across each disease state. In addition, it is possible that there was the potential for a small number of missed MACE events that may have occurred outside our health system. Furthermore, the patients in this cohort were commonly clinically referred for symptoms of dyspnea or chest pain, thus likely representing a higher risk population. Whether our results also apply to the younger patients (≤40) with psoriasis, RA, and SLE without symptoms warrants additional studies. However, even after excluding overt obstructive CAD and LV dysfunction, we found that patients with systemic inflammatory diseases and impaired MFR had higher all-cause mortality and MACE. In addition, we did not include a comparator arm of patients without systemic inflammatory disease in this study as this study was not designed to compare the prevalence of impaired MFR, as this has been previously shown in all three diseases (14,15,17–19). However, this study was designed to understand how MFR might be used as a biomarker for further risk stratify this population. Coronary angiography was infrequent in our patient cohort without regional perfusion defects on PET scanning. However, the blunted stress myocardial blood flow and MFR is likely a reflection of both coronary microvascular dysfunction and diffuse non-obstructive atherosclerosis. Future studies including coronary CT angiography would be required to decipher the relative role of diffuse non-obstructive atherosclerosis and microvascular dysfunction.
CONCLUSIONS
In conclusion, these findings demonstrate that all these three distinct autoimmune systemic inflammatory disorders may be associated with impaired coronary vasodilator reserve in a subset of patients. When present, this vascular dysfunction was associated with an increased risk of adverse cardiovascular outcomes and all-cause mortality.
COMPETENCY IN PATIENT CARE AND PROCEDURAL SKILLS:
There is an increasing recognition of the role of inflammation in cardiovascular disease. Patients with systemic inflammatory disease conditions are known to be at elevated cardiovascular risk; yet current risk-stratification strategies underestimate this risk. Advanced cardiovascular imaging techniques can enable early diagnosis, risk stratification and patient management in patients with suspected or known CVD. Quantification of myocardial blood flow and flow reserve with PET may serve as a marker of risk and improve risk prediction in patients with systemic inflammatory disease.
Translational outlook:
The integration of multiparametric imaging approaches, including cardiac PET, into routine clinical practice, has become powerful tools for both clinical care and research. MFR by PET provides a robust and reproducible measure of the burden of diffuse atherosclerosis and microvascular dysfunction on coronary vasomotor function. There is a need for future studies to investigate the link between specific immune dysregulation pathways and coronary vascular dysfunction and whether inhibition of specific pathways can improve coronary vascular health.
Supplementary Material
Grants or other financial supporters of the study:
NHLBI T32 HL094301 (BW, SD, JB) NIAMS K24 AR066109 (KC), NIH K23 HL135438 (VT) NHLBI R01HL150342 (SD)
Conflicts of Interest and Financial Disclosure: The authors have reported that they have no conflict of interest relationships relevant to the contents of this paper to disclose. The author financial disclosures include: Dr. Di Carli reports grants from Gilead Sciences and Spectrum Dynamics, and personal consulting fees from Janssen and Bayer, outside the submitted work. Dr. Dorbala reports grants from Pfizer and GE healthcare and personal consulting fees from GE Health Care and Pfizer, outside the submitted work. Dr. Blankstein reports grants from Amgen incorporation and Astellas and personal consulting fees from Amgen Inc, outside of the submitted work. Dr. Merola reports grant and or/personal consulting fees for Merck, Abbvie, Dermavant, Eli Lilly, Novartis, Janssen, UCB, Celgene, Sanofi, Regeneron, Arena, Sun Pharma, Biogen, Pfizer, EMD Sorono, Avotres and Leo Pharma. Dr. Massarotti reports grants from BMS and personal consulting fees from UCB, and Exagen. She additionally serves on DSMB on EMD Serono. Dr. Costenbader reports research support and colloborations with Merck, Asta Zeneca, Janssen and Glaxo Smith Kline and consulting fees from Merck
Abbreviations:
- ACR
American College Rheumatology
- CMD
coronary microvasculature dysfunction
- CVA
cerebral vascular accident
- MACE
major adverse cardiovascular event
- MBF
myocardial blood flow
- MFR
myocardial flow reserve
- PET
positron emission tomography
- PsO
psoriasis
- RA
rheumatoid arthritis
- SLE
systemic lupus erythematosus
- CRP
C-reactive protein
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Ethics: The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate.
References
- 1.Liao KP. Cardiovascular disease in patients with rheumatoid arthritis. Trends Cardiovasc Med 2017;27(2):136–40. Doi: 10.1016/j.tcm.2016.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Teague H, Mehta NN The Link Between Inflammatory Disorders and Coronary Heart Disease: a Look at Recent Studies and Novel Drugs in Development. Curr Atheroscler Rep 2016;18(1):3. Doi: 10.1007/s11883-015-0557-y. [DOI] [PubMed] [Google Scholar]
- 3.Liu Y, Kaplan MJ Cardiovascular disease in systemic lupus erythematosus: an update. Curr Opin Rheumatol 2018;30(5):441–8. Doi: 10.1097/BOR.0000000000000528. [DOI] [PubMed] [Google Scholar]
- 4.Mehta NN, Azfar RS, Shin DB, Neimann AL, Troxel AB, Gelfand JM Patients with severe psoriasis are at increased risk of cardiovascular mortality: cohort study using the General Practice Research Database. Eur Heart J 2010;31(8):1000–6. Doi: 10.1093/eurheartj/ehp567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Skaggs BJ, Hahn BH, McMahon M Accelerated atherosclerosis in patients with SLE--mechanisms and management. Nat Rev Rheumatol 2012;8(4):214–23. Doi: 10.1038/nrrheum.2012.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Armstrong EJ, Harskamp CT, Armstrong AW Psoriasis and major adverse cardiovascular events: a systematic review and meta-analysis of observational studies. J Am Heart Assoc 2013;2(2):e000062. Doi: 10.1161/JAHA.113.000062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Azfar RS, Seminara NM, Shin DB, Troxel AB, Margolis DJ, Gelfand JM Increased Risk of Diabetes and Likelihood of Receiving Diabetes Treatment in Patients with Psoriasis. Arch Dermatol 2012;148(9):995–1000. Doi: 10.1001/archdermatol.2012.1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Petri M, Perez-Gutthann S, Spence D, Hochberg MC Risk factors for coronary artery disease in patients with systemic lupus erythematosus. Am J Med 1992;93(5):513–9. Doi: 10.1016/0002-9343(92)90578-y. [DOI] [PubMed] [Google Scholar]
- 9.del Rincón I, Polak JF, O’Leary DH, et al. Systemic inflammation and cardiovascular risk factors predict rapid progression of atherosclerosis in rheumatoid arthritis. Ann Rheum Dis 2015;74(6):1118–23. Doi: 10.1136/annrheumdis-2013-205058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gupta A, Taqueti VR, van de Hoef TP, et al. Integrated Non-invasive Physiological Assessment of Coronary Circulatory Function and Impact on Cardiovascular Mortality in Patients with Stable Coronary Artery Disease. Circulation 2017;136(24):2325–36. Doi: 10.1161/CIRCULATIONAHA.117.029992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Osborne MT, Bajaj NS, Taqueti VR, et al. Coronary Microvascular Dysfunction Identifies Patients at High Risk of Adverse Events Across Cardiometabolic Diseases. J Am Coll Cardiol 2017;70(22):2835–7. Doi: 10.1016/j.jacc.2017.09.1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Libby P, Ridker PM, Hansson GK, Leducq Transatlantic Network on Atherothrombosis Inflammation in atherosclerosis: from pathophysiology to practice. J Am Coll Cardiol 2009;54(23):2129–38. Doi: 10.1016/j.jacc.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Recio-Mayoral A, Mason JC, Kaski JC, Rubens MB, Harari OA, Camici PG Chronic inflammation and coronary microvascular dysfunction in patients without risk factors for coronary artery disease. Eur Heart J 2009;30(15):1837–43. Doi: 10.1093/eurheartj/ehp205. [DOI] [PubMed] [Google Scholar]
- 14.Amigues I, Russo C, Giles JT, et al. Myocardial Microvascular Dysfunction in Rheumatoid ArthritisQuantitation by 13N-Ammonia Positron Emission Tomography/Computed Tomography. Circ Cardiovasc Imaging 2019;12(1):e007495. Doi: 10.1161/CIRCIMAGING.117.007495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liao KP, Huang J, He Z, et al. Coronary microvascular dysfunction in rheumatoid arthritis compared to diabetes mellitus and association with all-cause mortality. Arthritis Care Res (Hoboken) 2019. Doi: 10.1002/acr.24108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zanatta E, Colombo C, D’Amico G, d’Humières T, Dal Lin C, Tona F Inflammation and Coronary Microvascular Dysfunction in Autoimmune Rheumatic Diseases. Int J Mol Sci 2019;20(22). Doi: 10.3390/ijms20225563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ishimori ML, Martin R, Berman DS, et al. Myocardial Ischemia in the Absence of Obstructive Coronary Artery Disease in Systemic Lupus Erythematosus. JACC: Cardiovascular Imaging 2011;4(1):27–33. Doi: 10.1016/j.jcmg.2010.09.019. [DOI] [PubMed] [Google Scholar]
- 18.Weber B, Perez-Chada LM, Divakaran S, et al. Coronary microvascular dysfunction in patients with psoriasis. J Nucl Cardiol 2020. Doi: 10.1007/s12350-020-02166-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Osto E, Piaserico S, Maddalozzo A, et al. Impaired coronary flow reserve in young patients affected by severe psoriasis. Atherosclerosis 2012;221(1):113–7. Doi: 10.1016/j.atherosclerosis.2011.12.015. [DOI] [PubMed] [Google Scholar]
- 20.Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum 1997;40(9):1725. Doi: 10.1002/art.1780400928. [DOI] [PubMed] [Google Scholar]
- 21.Tan EM, Cohen AS, Fries JF, et al. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum 1982;25(11):1271–7. Doi: 10.1002/art.1780251101. [DOI] [PubMed] [Google Scholar]
- 22.Liao KP, Cai T, Gainer V, et al. Electronic medical records for discovery research in rheumatoid arthritis. Arthritis Care Res (Hoboken) 2010;62(8):1120–7. Doi: 10.1002/acr.20184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.El Fakhri G, Kardan A, Sitek A, et al. Reproducibility and accuracy of quantitative myocardial blood flow assessment with (82)Rb PET: comparison with (13)N-ammonia PET. J Nucl Med 2009;50(7):1062–71. Doi: 10.2967/jnumed.104.007831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lortie M, Beanlands RSB, Yoshinaga K, Klein R, Dasilva JN, DeKemp RA Quantification of myocardial blood flow with 82Rb dynamic PET imaging. Eur J Nucl Med Mol Imaging 2007;34(11):1765–74. Doi: 10.1007/s00259-007-0478-2. [DOI] [PubMed] [Google Scholar]
- 25.Murthy VL, Naya M, Foster CR, et al. Improved cardiac risk assessment with noninvasive measures of coronary flow reserve. Circulation 2011;124(20):2215–24. Doi: 10.1161/CIRCULATIONAHA.111.050427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thygesen K, Alpert JS, Jaffe AS, et al. Fourth Universal Definition of Myocardial Infarction (2018). J Am Coll Cardiol 2018:25285. Doi: 10.1016/j.jacc.2018.08.1038. [DOI] [PubMed] [Google Scholar]
- 27.Hicks Karen A, Tcheng James E, Bozkurt Biykem, et al. 2014 ACC/AHA Key Data Elements and Definitions for Cardiovascular Endpoint Events in Clinical Trials. Circulation 2015;132(4):302–61. Doi: 10.1161/CIR.0000000000000156. [DOI] [PubMed] [Google Scholar]
- 28.Morise AP, Haddad WJ, Beckner D Development and validation of a clinical score to estimate the probability of coronary artery disease in men and women presenting with suspected coronary disease. Am J Med 1997;102(4):350–6. Doi: 10.1016/s0002-9343(97)00086-7. [DOI] [PubMed] [Google Scholar]
- 29.Taqueti VR, Solomon SD, Shah AM, et al. Coronary microvascular dysfunction and future risk of heart failure with preserved ejection fraction. Eur Heart J 2018;39(10):840–9. Doi: 10.1093/eurheartj/ehx721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Feldmann M, Brennan FM, Maini R Cytokines in Autoimmune Disorders. International Reviews of Immunology 1998;17(1–4):217–28. Doi: 10.3109/08830189809084493. [DOI] [PubMed] [Google Scholar]
- 31.Dean GS, Tyrrell-Price J, Crawley E, Isenberg DA Cytokines and systemic lupus erythematosus. Annals of the Rheumatic Diseases 2000;59(4):243–51. Doi: 10.1136/ard.59.4.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brembilla NC, Senra L, Boehncke W-H The IL-17 Family of Cytokines in Psoriasis: IL-17A and Beyond. Front Immunol 2018;9. Doi: 10.3389/fimmu.2018.01682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sidiropoulos PI, Siakka P, Pagonidis K, et al. Sustained improvement of vascular endothelial function during anti-TNFalpha treatment in rheumatoid arthritis patients. Scand J Rheumatol 2009;38(1):6–10. Doi: 10.1080/03009740802363768. [DOI] [PubMed] [Google Scholar]
- 34.Taqueti VR, Everett BM, Murthy VL, et al. Interaction of impaired coronary flow reserve and cardiomyocyte injury on adverse cardiovascular outcomes in patients without overt coronary artery disease. Circulation 2015;131(6):528–35. Doi: 10.1161/CIRCULATIONAHA.114.009716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bajaj NS, Singh A, Zhou W, et al. Coronary Microvascular Dysfunction, Left Ventricular Remodeling, and Clinical Outcomes in Patients With Chronic Kidney Impairment. Circulation 2020;141(1):21–33. Doi: 10.1161/CIRCULATIONAHA.119.043916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhou W, Brown JM, Bajaj NS, et al. Hypertensive coronary microvascular dysfunction: a subclinical marker of end organ damage and heart failure. Eur Heart J n.d. Doi: 10.1093/eurheartj/ehaa191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Reilly SM., Saltiel AR. Adapting to obesity with adipose tissue inflammation. Nature Reviews Endocrinology 2017;13(11):633–43. Doi: 10.1038/nrendo.2017.90. [DOI] [PubMed] [Google Scholar]
- 38.Libby P Inflammation in atherosclerosis. Arterioscler Thromb Vasc Biol 2012;32(9):2045–51. Doi: 10.1161/ATVBAHA.108.179705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ridker PM, Everett BM, Thuren T, et al. Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease. N Engl J Med 2017;377(12):1119–31. Doi: 10.1056/NEJMoa1707914. [DOI] [PubMed] [Google Scholar]
- 40.Tardif J-C, Kouz S, Waters DD, et al. Efficacy and Safety of Low-Dose Colchicine after Myocardial Infarction. New England Journal of Medicine 2019;381(26):2497–505. Doi: 10.1056/NEJMoa1912388. [DOI] [PubMed] [Google Scholar]
- 41.Arnett Donna K, Blumenthal Roger S, Albert Michelle A, et al. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2019;140(11):e596–646. Doi: 10.1161/CIR.0000000000000678. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


