Objective:
We aimed to examine biomarkers for screening unhealthy alcohol use in the trauma setting.
Summary and Background Data:
Self-report tools are the practice standard for screening unhealthy alcohol use; however, their collection suffers from recall bias and incomplete collection by staff.
Methods:
We performed a multi-center prospective clinical study of 251 adult patients who arrived within 24 hours of injury with external validation in another 60 patients. The Alcohol Use Disorders Identification Test served as the reference standard. The following biomarkers were measured: (1) PEth; (2) ethyl glucuronide; (3) ethyl sulfate; (4) gamma-glutamyl-transpeptidase; (5) carbohydrate deficient transferrin; and (6) blood alcohol concentration (BAC). Candidate single biomarkers and multivariable models were compared by considering discrimination (AUROC). The optimal cutpoint for the final model was identified using a criterion for setting the minimum value for specificity at 80% and maximizing sensitivity. Decision curve analysis was applied to compare to existing screening with BAC.
Results:
PEth alone had an AUROC of 0.93 [95% confidence interval (CI): 0.92–0.93] in internal validation with an optimal cutpoint of 25 ng/mL. A 4– variable biomarker model and the addition of any single biomarker to PEth did not improve AUROC over PEth alone (P > 0.05). Decision curve analysis showed better performance of PEth over BAC across most predicted probability thresholds. In external validation, sensitivity and specificity were 76.0% (95% CI: 53.0%–92.0%) and 73.0% (95% CI: 56.0%–86.0%), respectively.
Conclusion and Relevance: PEth alone proved to be the single best biomarker for screening of unhealthy alcohol use and performed better than existing screening systems with BAC. PEth may overcome existing screening barriers.
Keywords: alcohol biomarker, screening, trauma, unhealthy alcohol use
Unhealthy alcohol use is the leading cause of premature mortality in the United States (US) 1 with death rates rising since 1997. 2 Alcohol-related health consequences contributed to an increasing number of Emergency Department visits in the US between 2006 and 2014, and acute care settings carry the highest prevalence of individuals with unhealthy alcohol use. 3 These data highlight the need for effective measures in screening to provide secondary prevention in high prevalence settings such as the Emergency Department and trauma center.
Currently, Level I and II trauma centers are required by the American College of Surgeons Committee on Trauma to provide brief interventions for patients who screen positive for unhealthy alcohol use, and programs such as screening and brief intervention (SBI) are recommended. 1,4 Between one-third and one-half of patients in trauma centers arrive with detectable blood alcohol concentrations (BAC). Providing SBI can reduce trauma recidivism by nearly 50 percent and is cost-effective in some studies but the evidence remains mixed and not consistent as others have shown no benefit. 5–7
Self-report methods such as the alcohol use disorders identification test (AUDIT) are the current practice standard for screening unhealthy alcohol use and are recommended by the United States Preventive Services Task Force. 8 However, questionnaires suffer from recall bias, social desirability, and barriers to communication, and limitations due to staffing of screeners for routine collection. BAC is another commonly employed screening approach, but it quickly becomes undetectable and carries a high false-negative rate. Indirect alcohol biomarkers such as gamma-glutamyl transpeptidase (GGT) and carbohydrate-deficient transferrin (CDT) are potential solutions but are confounded by sex, age, nonalcohol comorbidities, and acute organ dysfunction. 9
Direct alcohol biomarkers are promising measures for alcohol consumption. Ethyl glucuronide (EtG) and ethyl sulfate (EtS) are biomarkers found in body fluids and urine that can detect alcohol consumption that has been consumed several days prior. 10 Phospha-tidylethanol (PEth) is an ethanol metabolite formed in the red blood cell membrane and has a half-life up to 28 days after alcohol consumption. PEth has been validated to identify both acute and chronic alcohol consumption patterns in a variety of ambulatory care settings. 11–13 Many direct alcohol biomarkers have not been evaluated in trauma centers. We hypothesized PEth would provide the best screening metrics among direct and indirect alcohol biomarkers for unhealthy alcohol use in the trauma setting.
Methods
Study Setting and Criteria
The study was conducted at 2 Level I Trauma Centers in Illinois and California. Loyola University Medical Center (LUMC) served as the site for development and internal validation while the Zuckerberg San Francisco General Hospital & Trauma Center affiliated with the University of California San Francisco (UCSF) served as the external validation site. The Institutional Review Boards of LUMC and UCSF approved this study.
At LUMC, patients were screened and informed consent was obtained between August 2017 and April 2019. At UCSF, patients were screened and informed consent was obtained between April 2019 and December 2019. Currently, LUMC and UCSF use BAC for screening patients in their trauma center. Patients eligible for inclusion were adults (> 18 years) who arrived within 24 hours of injury. Patients were excluded if any of the following criteria were met: (1) death expected due to injuries within 48 hours of admission as assessed by the admitting physician; and (2) pregnancy state.
Sample Size Calculation
At LUMC, study recruitment was guided by an expected 30% prevalence of unhealthy alcohol use based on prior epidemiology 3,5,7 and an AUROC of 0.80 for the best biomarker(s). Accounting for dropout and missing data, recruitment of 250 patients was planned to achieve a 2-sided 95% confidence interval (CI) of (0.70, 0.90). At UCSF, a convenience sample was collected until study completion on December 31, 2019.
Reference Standard for Screening Unhealthy Alcohol Use
The AUDIT is currently recommended in the trauma setting for screening unhealthy alcohol use. 14 It is a 10–item questionnaire that scores risky alcohol use on a scale from 0 to 40 15 with sex-specific cut-points providing better validation in injured patients. 16 We defined unhealthy alcohol use by applying the sex-specific cutpoints of ≥5 and ≥8 for females and males to capture the lower limit of risk groups for unhealthy alcohol use. Some patients were not able to self-report so proxy reporting was used and has previously been validated to perform similarly to self-report. 17
Alcohol Biomarker Assays
Blood and urine samples were obtained within 24hours of presentation and the following biomarkers were measured: (1) dried whole blood spot PEth16:0/18:1; (2) urine EtG; (3) urine EtS; (4) serum GGT; (5) serum CDT; and (6) BAC. PEth samples from dried blood spot collections were analyzed at United States Drug Testing Laboratories (USDTL) (Des Plaines, IL) using liquid chromatography-mass spec-trometry as previously described. 18 This method assays a single isomer of PEth (palmitoyl/oleoyl), a phospholipid containing 16:0 and 18:1 fatty acids and is the most prevalent PEth homolog in human blood. The limit of detection is 2 ng/mL, the limit of quantitation is 8 ng/ml and the assay is linear up to 800 ng/mL. The nurse provider collected the venous whole blood sample into an EDTA tube for refrigerated storage. The blood samples were retrieved by research staff on the same day and 40 microliters (uL) was pipetted onto the PEth dry blood spot cards into 5 spots for a total of 200 uL. The collection kits provided by the testing laboratory, USDTL, included a blood spot drying box for storage and transport. All samples were sent to USDTL via paper envelopes and in accordance with lab protocol with a stated integrity of 1 year at room temperature for the blood spot cards.
BAC was measured using the hospital’s clinical laboratory headspace gas chromatography method with flame ionization detection. GGT, CDT, and urine samples were sent to an outside laboratory (Quest Diagnostics, Chantilly, VA) within 72 hours of sample collection. Blood was immediately centrifuged, and serum was separated and stored at 4°Ce until assayed for GGT and CDT. GGT activity in the sample is directly proportional to the change in absorbance at 410/480 nm due to the formation of 5–amino–2–nitro-benzoate. 19 Serum analysis to report levels of %CDT used rate-nephelometric determination after anion exchange separation. 20
Urine samples of EtG and EtS were measured using high performance liquid chromatography-mass spectrometry. Levels at <500 ng/mL for EtG and <100 ng/mL for EtS were considered negative. The analytical measurement range was validated up to 10,000 ng/mL and linear to 200,000ng/mL. The concentration of urine may artificially increase EtG and EtS so normalization was performed using measures of urine creatinine, and values standardized to a concentration of 100mg/dL. Normalized EtG and EtS were calculated as 100/Urine Creatinine × Urine EtG/EtS.
Analysis Plan
Statistical tests to compare patient characteristics across sites were conducted using Chi-square tests for proportions and Wil-coxon-Mann Whitney tests for quantitative variables (Table 1). P-values less than 0.05 were considered statistically significant.
Table 1.
Patient Characteristics at Development/Internal Validation Site and External Validation Site
| Development/Internal Validation Site (n = 251) | External Validation Site (n = 60) | P-value | |
|---|---|---|---|
| Demographics | |||
| Age in years, median (IQR) | 53.0 (33.5-65.0) | 38.0 (29.0-54.0) | <0.01 |
| Male sex, n (%) | 187 (74.5) | 50 (83.3) | 0.20 |
| Hispanic ethnicity, n (%) | 52 (20.7) | 21 (37.5) | 0.01 |
| White race, n (%) | 155 (61.8) | 33 (55.0) | 0.42 |
| Comorbidities/conditions | |||
| Unhealthy alcohol Use, n (%) | 80 (31.9) | 23 (38.3) | 0.42 |
| Drug Misuse, n (%) | 37 (14.7) | 17 (28.3) | 0.02 |
| Cirrhosis, n (%) | 3 (1.2) | 1 (1.7) | 0.99 |
| Psychosis, n (%) | 16 (6.4) | 15 (30.6) | <0.01 |
| Trauma characteristics | |||
| Admission Systolic BP, median (IQR) | 128 (117–142) | 122 (96–145) | 0.03 |
| PRBC transfused (ml), median (IQR) | 0 (0-0) | 0 (0–1487.5) | <0.01 |
| Mechanism (Blunt), n (%) | 212 (84.5) | 33 (55.0) | <0.01 |
| Glasgow Coma Scale <13, n (%) | 27 (10.8) | 10 (17.2) | 0.26 |
| ISS, median (IQR) | 8.0 (4.0–10.0) | 14.0 (4.8–26.8) | <0.01 |
| AIS abdomen >1, n (%) | 26 (10.4) | 27 (45.0) | <0.01 |
| Liver laceration, n (%) | 8 (3.2) | 5 (8.3) | 0.15 |
| Laboratory data | |||
| Positive BAC, n (%) | 63 (26.8) | 23 (45.1) | 0.02 |
| Positive Cannabis, n (%) | 36 (14.3) | 18 (36.0) | <0.01 |
| Positive PEth, n (%) | 136 (54.2) | 34 (58.6) | 0.64 |
| Hemoglobin (gm/dL), median (IQR) | 13.7 (12.4–14.7) | 13.4 (12.1–14.8) | 0.66 |
| Hospital characteristics | |||
| Length of stay, median (IQR) | 3 (1–7) | 9 (5–19) | <0.01 |
| ICU Length of stay, median (IQR) | 0.5 (0.0–3.0) | 3.0 (0.0–6.0) | <0.01 |
| Discharge status | |||
| Expired, n (%) | 0 (0) | 3 (5.0) | |
| Home, n (%) | 192 (76.5) | 37 (61.7) | <0.01 |
| Skilled Nursing/Rehab, n (%) | 49 (19.5) | 9 (15.0) | |
| Other | 10 (4.0) | 11 (18.3) |
Development/internal validation site at Level 1 Trauma Center of Loyola University Medical Center. External validation site at Level 1 Trauma Center of Zuckerberg San Francisco General Hospital & Trauma Center. Comorbidities except for unhealthy alcohol use based on diagnostic codes and billing codes. BP indicates blood pressure; PEth, phosphatidylethanol; PRBC, packed red blood cell transfused in first 24 h of arrival to trauma center; Other, against medical advice, psychiatry service, policy custody, nursing home; Unhealthy alcohol use, Alcohol Use Disorders Test (AUDIT) >5 for females and >8 for males; BAC, blood alcohol concentration; ISS, injury severity score; AIS Abdomen >1, abbreviated injury score with a score of at least mild injury; liver lacerations include any grade; ICU, intensive care unit.
Biomarker levels had highly skewed distributions so analyses included both raw values and natural log-transformed values. Missing data occurred in less than 7% of any single biomarker with BAC having the greatest frequency of missing data (n ¼ 16, 6.3%). Analyses included a multiply imputed and log-transformed dataset and raw values (complete case analysis). A bootstrapping and expectation-maximization algorithm produced 5 imputed datasets with estimates combined by Rubin rule.
The association of each biomarker with unhealthy alcohol use was assessed in univariate logistic regression with beta coefficients representing an increase of 1 standard deviation of each biomarker. To identify the best combination of biomarkers, variable screening was performed with the Least Absolute Squares Selection Operation (LASSO) across the log-transformed and imputed datasets. 21 CIs were developed with 100-iterations of a bootstrap procedure. The amount of shrinkage was tuned using 10-fold cross-validation. The general shrinkage averaging estimation was applied across the M imputed datasets to combine shrinkage estimators with different tuning parameters. Variable importance sums the weights w k of the candidate model M k that contain the relevant variable with the measure averaged over the M imputed data sets. The Variable Importance measure is a range between 0 (unimportant) and 1 (very important) 22 and was used to derive a multi-biomarker model. Multicollinearity among the biomarkers was also assessed using variance inflation factor (VIF) and correlation matrices.
Candidate biomarker(s) were compared by considering discrimination (AUROC), calibration, and decision curve analysis. AUROC comparisons between the models were performed using the DeLong method. 23 The net reclassification improvement (NRI) and integrated discrimination improvement (IDI) measures were used to examine the improvement in model performance with addition of biomarkers to the best baseline biomarker. Goodness-of-fit was formally assessed by the Hosmer-Lemeshow test and verified visually with calibration plots. For internal validation of the accuracy estimates, 200 bootstrap resamples were used to include a deflation factor for performance optimism.
Decision curve analysis was applied to examine the net benefit of the best derived biomarker against BAC. Net benefit is a decision analytic measure that puts benefits and harms on the same scale and is useful for clinical decisions. Net benefit is measured by sensitivity × prevalence - (1 - specificity) × (1 – prevalence) × w, where w is the odds at the threshold probability. 24 Net benefit is plotted against threshold probabilities to yield a decision curve to weigh the relative harms of false-positive and false-negative screens.
Test characteristics including sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) and their 95% CI were evaluated across all predicted probabilities for the complete case analyses. The optimal cutpoint for the final model was identified using a criterion for setting the minimum value for specificity at 80% and maximizing sensitivity. The final model and optimal cutpoint for the best biomarker(s) were externally validated in an independent Level I Trauma Center (UCSF). We followed the 2015 guideline for Transparent Reporting of a multivariable Prediction model for Individual Prognosis Or Diagnosis (Supplemental Table 1, http://links.lww.com/SLA/C957). 25 Statistical analyses were performed using R software version 3.3.1 (R Core Team).
Results
The development site enrolled 251 patients and 80 (31.9%) had unhealthy alcohol use (according to AUDIT scores) (Fig. 1). The external validation site enrolled 60 patients and 23 (38.3%) had unhealthy alcohol use. Differences between sites included an older cohort at LUMC with more blunt mechanism injuries and lower rates of co-substance use than UCSF (P < 0.05 for all comparisons). Further, patients from LUMC had a shorter length of stay and a greater proportion were discharged home (P < 0.05 for all comparisons) (Table 1). The UCSF group had a higher median injury severity score and a greater frequency with at least mild abdominal injury (P < 0.01). In the unhealthy alcohol group, the median PEth level at LUMC was 227.0 ng/mL (IQR: 94.8–565.0) whereas the median level at UCSF was 95.0ng/mL (IQR: 25.0–331.50).
Figure 1.
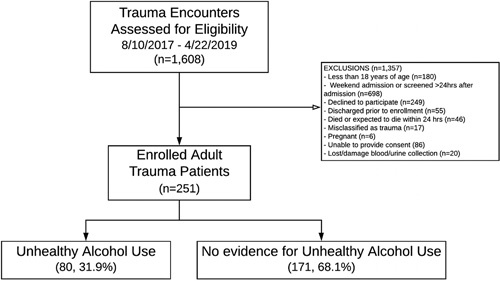
Patient flow diagram at development/internal validation site.
Within the development cohort, patients with unhealthy alcohol use had greater levels of all biomarkers than the group without unhealthy alcohol use (Table 2). There was a linear relationship between log-transformed biomarkers with log odds of unhealthy alcohol use (Supplemental Table 1, http://links.lww.com/SLA/ C957). The LASSO procedure identified a 4-variable model with PEth, BAC, CDT, and GGT. A 1-standard deviation increase in log-transformed PEth was strongly associated with unhealthy alcohol use (OR 8.82; 95% CI: 4.56–13.09) (Table 2). In a full main effects model with all biomarkers, urine EtG and Urine EtS had high measures of multicollinearity measured by VIF values greater than 16 and were strongly correlated to BAC and PEth (P < 0.01) (Supplemental Table 2, http://links.lww.com/SLA/C941). All other biomarkers had a VIF of less than 3. The variable importance measure was greatest for PEth and BAC among candidate biomarkers (Table 2).
Table 2.
Alcohol Biomarkers With Univariable Characteristics and Variable Importance at Development/Internal Validation Site
| Biomarker | Univariable Raw Median Values (IQR) and Odds Ratios (95% CI) | Multivariable LASSO Biomarker Model Odds Ratio (95% CI) | Variable Importance Measure | |||
|---|---|---|---|---|---|---|
| No Unhealthy Alcohol Use | Unhealthy Alcohol Use | P-value | Univariable Odds Ratio | |||
| PEth (ng/mL) | 0.0 (0.0–19.0) | 227.0 (94.8–565.5) | <0.01 | 15.12 (14.43–15.81) | 8.82 (4.56–13.09) | 1.0 |
| BAC (mg/dL) | 0.0 (0.0–0.0) | 98.0 (0.0–227.0) | <0.01 | 4.52 (4.41–4.90) | 1.27 (0.80–1.74) | 1.0 |
| CDT (%) | 1.7 (1.5–1.9) | 2.1 (1.7–3.1) | <0.01 | 2.49 (2.14–2.84) | 1.04 (0.96–1.13) | 0.91 |
| GGT (units/L) | 18.0 (12.3–27.5) | 31.0 (21.0–70.0) | <0.01 | 2.69 (2.33–3.05) | 1.30 (0.79–1.81) | 0.80 |
| Urine EtS (ng/mL) | 0.0 (0.0–0.0) | 7060.6 (530.0–24130.0) | <0.01 | 5.47 (5.10–5.85) | Not selected | 0 |
| Urine EtG (ng/mL) | 0.0 (0.0–0.0) | 8933.6 (688.9–90005.1) | <0.01 | 5.57 (5.19–5.95) | Not selected | 0 |
Variable importance sums up the weights w k of the candidate model M k that contain the relevant variable with the measure averaged over the M imputed data sets. The Variable Importance measure is a range between 0 (unimportant) and 1 (very important). Based on variable important a 4-variable model with PEth, BAC, CDT, and GGT was chosen. Multivariable model derived with Least Absolute Shrinkage and Selection Operator (LASSO) to represent odds ratios for selected biomarkers with odds ratios (OR) reported as each standard deviation increase in the biomarker of interest. The variables in the multivariable model were log-transformed and multiply imputed with Rubin rule.
%CDT indicates percent serum carbohydrate deficient transferrin; EtG, urine ethyl glucuronide normalized with urinary creatinine; EtS, urine ethyl sulfate normalized for urinary creatinine; GGT, serum gamma-glutamyl-transpeptidase; PEth, phosphatidylethanol 16:0/18:1.
PEth alone had an AUROC of 0.93 (95% CI: 0.90–0.96), and the addition of any single biomarker to PEth did not improve AUROC over PEth alone (Table 3). Reclassification as measured by NRI and IDI were marginally improved with the addition of BAC and the addition of urinary biomarkers to PEth. No improvement was shown in AUROC of the 4-variable model over PEth alone and marginal improvement was shown in NRI and IDI in the complete case analysis only (Table 3).
Table 3.
PEth as Optimal Baseline Biomarker and Comparisons to Models With Additional Biomarkers
| Biomarker Model | AUROC (95%CI) | Continuous NRI (95% CI) | Continuous IDI (95% CI) |
|---|---|---|---|
| PEth Only Model | |||
| Imputed/log-transformed | 0.93 (0.90–0.96) | — | — |
| Complete Case Analysis | 0.93 (0.90–0.96) | — | — |
| PEth and BAC Model | |||
| Imputed/log-transformed | 0.93 (0.90–0.97) | 0.39 (0.13–0.62)* | 0.03 (0.01–0.05)* |
| Complete Case Analysis | 0.93 (0.90–0.97) | 0.82 (0.58–1.06)* | 0.06 (0.020–10)* |
| PEth and GGT Model | |||
| Imputed/log-transformed | 0.93 (0.89–0.96) | 0.23 (–0.03–0.48) | –0.02 (–0.04–0.00) |
| Complete Case Analysis | 0.92 (0.88–0.95) | 0.07 (–0.15–0.29) | 0.01 (–0.01–0.03) |
| PEth and CDT Model | |||
| Imputed/log-transformed | 0.93 (0.90–0.96 | 0.19 (–0.06–0.43) | –0.02 (–0.04–0.00) |
| Complete Case Analysis | 0.92 (0.88–0.96) | –0.08 (–0.36–0.20) | 0.01 (–0.01–0.02) |
| PEth and Urine EtG Model | |||
| Imputed/log-transformed | 0.94 (0.92–0.97) | 0.79 (0.55–1.03)* | 0.02 (0.01–0.04)* |
| Complete Case Analysis | 0.94 (0.91–0.97) | 0.45 (0.24–0.67)* | 0.03 (–0.01–0.07) |
| PEth and Urine EtS Model | |||
| Imputed/log-transformed | 0.94 (0.91–0.97) | 0.54 (0.31-0.78)* | 0.01 (–0.01–0.02) |
| Complete Case Analysis | 0.94 (0.91–0.97) | 0.45 (0.24-0.67)* | 0.03 (–0.01–0.07) |
| Multivariable Model | |||
| PEth + BAC + GGT + CDT | |||
| Imputed/log-transformed | 0.93 (0.90–0.97) | –0.04 (–0.3–0.23) | 0.00 (–0.01–0.02) |
| Complete Case Analysis | 0.90 (0.85–0.95) | 0.67 (0.41–0.94)* | 0.07 (0.02–0.12)* |
P < 0.05.
%CDT, percent serum carbohydrate deficient transferrin. Complete case analysis of 4-variable model after cross-validated selection operation for selecting biomarkers across imputed and long-transformed datasets. Complete case analysis of 4-variable model with sample size n = 208. Multivariable model selected after LASSO performed on multiply imputed datasets and variable important averaged. Both EtG and EtS add nearly no importance and were excluded from the final selection of combination biomarkers for evaluation.
AUROC indicates area under the receiver operating characteristic curve; EtG, urine ethyl glucuronide normalized with urinary creatinine; EtS, urine ethyl sulfate normalized for urinary creatinine; GGT, serum gamma-glutamyl-transpeptidase; IDI, integrated discrimination index; NRI, net reclassification index; PEth, phosphatidylethanol 16:0/18:1.
An optimal cutpoint of 25 ng/dL was identified for PEth and demonstrated a sensitivity and specificity of 95.0% (95% CI: 88.0%–99.0%) and 80.0% (95% CI: 73.0%–85.0%), respectively. The PPV and NPV were 68.5% (95% CI: 65.2%–77.3%) and 97.1% (95% CI: 93.3%–99.0%), respectively. At this cutpoint, PEth had a 1.6% (n = 4) false-negative rate and 13.9% (n = 35) false-positive rate. In the false-positive cases, 34.3% (n = 12) had a detectable BAC and 22.9% (n = 8) had BAC levels above 80 mg/dL. A table of cutpoints across a range of predicted probabilities is shown in Supplemental Table 2 (http://links.lww.com/SLA/C941). In sensitivity analysis, patients that received packed red blood cell transfusion (n = 13, 5.2%) were removed from analysis and no change in AUROC was found. At a cutpoint of 25 ng/dL, sensitivity and specificity were 95.5% (95% CI: 87.0%–99.0%) and 80.0% (95% CI: 73.0%–86.0%), respectively. The PPV and NPV were 69.0% (95% CI: 59.0%–78.8%) and 97.0% (95% CI: 93.0%–99.0%).
BAC was worse than PEth at discriminating unhealthy alcohol use with an AUROC of 0.81 (95% CI: 0.76–0.87, P < 0.01). Decision curve analysis showed better performance of PEth over BAC across most predicted probability thresholds, with greatest net benefit in borderline cases (Fig. 2). Urine EtG and EtS carried the next highest AUROC after PEth but were significantly lower with an AUROC of 0.86 (95% CI: 0.81–0.91, P ¼ 0.01) and 0.86 (95% CI: 0.81-0.91, P = 0.02), respectively.
Figure 2.
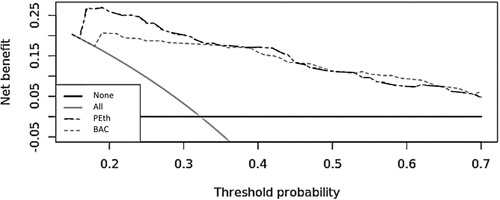
Decision curve analysis between PEth and blood alcohol concentration for screening unhealthy alcohol use. Decision curve analysis was applied to examine the net benefit of the best derived biomarker against BAC. Net benefit is a decision analytic measure that puts benefits and harms on the same scale and is useful for clinical decisions. Net benefit is measured by sensitivity × prevalence - (1 – specificity) × (1 – prevalence) × w, where w is the odds at the threshold probability. Net benefit is plotted against threshold probabilities to yield a decision curve to weigh the relative harms of false-positive and false-negative screens. The diagram shows the scenarios for all screened (grey line) and none screened (dark black line) as well. BAC indicates blood alcohol concentration;PEth, phosphatidylethanol.
Internal validation from LUMC with bootstrap optimism demonstrated an AUROC of 0.93 (95% CI: 0.92–0.93). The PEth model fit the data well (P = 0.98). A plot of calibration also demonstrates good fit (Supplemental Figure 1a, http://link-s.lww.com/SLA/C942) with a calibration slope of 1.0 (95% CI: 0.77–1.29) for log-transformed PEth.
In external validation, the AUROC for PEth was 0.83 (95% CI: 0.72–0.94) with a calibration slope of 1.0 (95% CI: 0.54–1.58) and a calibration plot that demonstrates the model fit the data well (P ¼ 0.35) (Supplemental Figure 1b, http://links.lww.com/SLA/C942). The AUROC of PEth remained greater than BAC, which had an AUROC of 0.67 (95% CI: 0.52–0.82, P < 0.01). At the optimal cutpoint of 25 ng/mL, the sensitivity and specificity were 76.0% (95% CI: 53.0%–92.0%) and 73.0% (95% CI: 56.0%–86.0%), respectively. The PPV and NPV were 62.0% (95% CI: 41.0%– 80.0%) and 84.0% (95% CI: 67.0%–95.0%), respectively. Approximately 80% (n = 8) of the false-positives had detectable BAC levels and all but 2 were above 80 mg/dL.
Discussion
In our study, we tested alcohol biomarkers individually and in combination for screening unhealthy alcohol misuse. PEth alone proved to be the single best biomarker for screening with excellent discrimination and calibration. A cutoff of 25 ng/mL had a sensitivity above 75% in both internal and external validation. Many of the false positives were likely due to underreporting from the AUDIT, and PEth showed improvement over the existing BAC screen. PEth collection with a dried blood spot from a lancet stick or vascular access is feasible for SBI programs at trauma centers.
PEth variability and range varied between trauma sites. We ascribe some of the variability to differences in trauma center characteristics. Additional variability may be attributed to patientlevel characteristics with the formation and elimination of PEth. 26 Although the PPV was around 65% at both sites, we noted many of the false-positives had BACs above the level for legal intoxication. One study described individuals who reported 30-day abstinence but still had detectable PEth levels, suggestive of underreported drink-ing. 27 PEth may capture cases of unhealthy alcohol use that are not captured during self-report. 28,29
PEth carried one of the highest variable importance measures in deriving a multivariable model. Among the candidate biomarkers, PEth was strongly associated with unhealthy alcohol use with an odds ratio nearly 8-fold higher than the next highest biomarker. In decision curve analysis, PEth performed better than our current screening program using BAC. More cases were detected in the lower predicted probabilities where BAC may be undetectable. As an ethanol metabolite, PEth is detectable after alcohol intake, where it has demonstrated a dose dependent correlation with single use. 30,31 In healthy volunteers, PEth could detect moderate alcohol use (16 grams of ethanol) with better performance at discriminating between abstinence and moderate consumption than CDT or GGT. 32 Other studies have supported the superiority of PEth over CDT and GGT. 33,34 Indirect biomarkers like CDT and GGT can be affected by liver dysfunction, but PEth has not been shown to be affected by liver dysfunction 16 since its formation is in red blood cells and independent of liver function.
Urinary EtG and EtS are available at commercial laboratories and even include rapid dipstick analysis with a qualitative immunoas-say, but results can be affected by acute kidney injury. 35,36 Urine EtG and EtS have shown benefit in detecting unhealthy alcohol use in patients who arrive to the ED with undetectable BAC, 37 but our results show a lower AUROC than PEth. One study showed potential benefit in EtG and EtS over PEth but metrics at reclassification were not applied and improvement for screening rates were not addressed. 38 Our data suggest the urinary biomarkers are highly correlated with PEth and BAC and do not offer improvement in discrimination nor added value in a multi-biomarker model. Our LASSO approach derived a 4-variable model with PEth, CDT, GGT, and BAC but showed no improvement in AUROC over PEth alone and little improvement in reclassification. Similar to our results, other studies have shown little added benefit of additional biomarkers to PEth. 12,39
Cutpoints for PEth have varied by clinical settings where the severity of unhealthy alcohol use may differ. 3,40 Higher cutpoints than those we propose have previously been reported; however, they have not been evaluated in the trauma setting. 10,41 A national laboratory in Sweden set 210 ng/mL and another study in a mixed cohort of critically ill and alcohol use disorder patients set 250 ng/mL as the cutpoint for unhealthy alcohol use. 41,42 Lower PEth levels in acute care settings have also been described, and reports of cutpoints between 20 and 80 ng/mL have been proposed but none provided external validation for a screening tool. 10,13,43 We opted for a lower cutpoint of 25 ng/mL to maximize sensitivity in preference for an optimal screening test, and this continued to perform well in external validation.
Several limitations occur in our study. First, PEth is comprised of a group of phospholipids formed in the presence of alcohol by the enzyme phospholipase D and multiple homologs exist. Others have shown that certain PEth homologs have different pharmacokinetics and the combination of PEth homologs may provide more information than the sing le 16:0/18:1 homolog used in this study. 44–47 Red blood cell transfusions in trauma settings may also affect PEth levels; however, patients enrolled in our study had minimal red blood cell transfusion requirements and sensitivity analysis showed PEth performance did not change when patients that received blood transfusions were excluded from analysis. Effects on hemoglobin from chronic disease have also been suggested to influence results, but prior evidence has not confirmed an effect on PEth's performance, 26 and our comparison groups had no major differences in hemoglobin levels. The sensitivity of PEth has greatly improved with recent development and validation of the liquid chromatography-mass spectrometry assay. 48 This assay is not routinely available in clinical laboratories so point-of-care screening may not be pragmatic for some centers. Lastly, while we demonstrate predictive validity for PEth as a screening tool, we acknowledge prospective studies are needed to examine its role in SBI programs to provide meaningful health outcomes.
Conclusions
In this study, we examine the predictive performance of PEth over existing alcohol biomarkers for screening unhealthy alcohol use across 2 Level I trauma centers. Using an optimal cutpoint of 25 ng/ mL in PEth can overcome existing barriers to screening, and help identify patients at-risk for deleterious health outcomes from unhealthy alcohol use.
Supplementary Material
Acknowledgments
The authors would also like to thank the trauma registrars Holly Molloy (LUMC) and Brenda Nunez-Garci (UCSF) forproviding the trauma variables for analyses.
Footnotes
Our study includes more than 8 authors because this was a multi-center study with co-investigators that are on the data use agreement between sites. In addition, we have included our study coordinators who provided major effort for screening, consenting, and recording patient data. Lastly, this project was a multi-PI effort supported by multiple NIH grants so all senior authors have been involved with study design.
Funding for this study was supported by NIH/NIAAA K23 AA024503 (MA) and NIH/NIDA R01 DA051464 (MA) and NIH/NIA R01 AG018859 (EJK) and NIH/NHLBI K23 HL133495 (CH).
The authors report no conflicts of interest.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s Web site (www.annalsofsurgery.com).
This is an open access article distributed under the Creative Commons Attribution License 4.0 (CCBY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
REFERENCES
- 1. Stahre M, Roeber J, Kanny D, et al. Contribution of excessive alcohol consumption to deaths and years of potential life lost in the United States. Prev Chronic Dis. 2014;11:E109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. White AM, Castle IJP., Hingson RW, et al. Using death certificates to explore changes in alcohol-related mortality in the United States, 1999 to 2017. Alcohol Clin Exp Res. 2020;44:178–187. [DOI] [PubMed] [Google Scholar]
- 3. White AM, Slater ME, Ng G, et al. Trends in alcohol-related emergency department visits in the United States: results from the nationwide emergency department sample, 2006 to 2014. Alcohol Clin Exp Res. 2018;42:352–359. [DOI] [PubMed] [Google Scholar]
- 4. US Department of Health and Human Services. Alcohol Screening and Brief Intervention (SBI) for Trauma Patients. Committee on Trauma Quick Guide Chicago: ASCOT; 2007. [Google Scholar]
- 5. Gentilello LM, Rivara FP, Donovan DM, et al. Alcohol interventions in a trauma center as a means of reducing the risk of injury recurrence. Ann Surg. 1999;230:473–480. discussion 480–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gentilello LM, Ebel BE, Wickizer TM, et al. Alcohol interventions for trauma patients treated in emergency departments and hospitals: a cost benefit analysis. Ann Surg. 2005;241:541–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Daeppen JB, Gaume J, Bady P, et al. Brief alcohol intervention and alcohol assessment do not influence alcohol use in injured patients treated in the emergency department: a randomized controlled clinical trial. Addiction. 2007;102:1224–1233. [DOI] [PubMed] [Google Scholar]
- 8. Curry SJ, Krist AH, Owens DK, et al. Screening and behavioral counseling interventions to reduce unhealthy alcohol use in adolescents and adults: US preventive services task force recommendation statement. JAMA. 2018;320:1899–1909. [DOI] [PubMed] [Google Scholar]
- 9. Hannuksela ML, Liisanantti MK, Nissinen AE, et al. Biochemical markers of alcoholism. Clin Chem Lab Med. 2007;45:956–961. [DOI] [PubMed] [Google Scholar]
- 10. Wurst FM, Thon N, Yegles M, et al. Ethanol metabolites: their role in the assessment of alcohol intake. Alcohol Clin Exp Res. 2015;39:2060–2072. [DOI] [PubMed] [Google Scholar]
- 11. Bakhireva LN, Leeman L, Savich RD, et al. The validity of phosphatidyle-thanol in dried blood spots of newborns for the identification of prenatal alcohol exposure. Alcohol Clin Exp Res. 2014;38:1078–1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Neumann J, Beck O, Helander A, et al. Performance of PEth compared with other alcohol biomarkers in subjects presenting for occupational and pre-employment medical examination. Alcohol Alcohol. 2020;55:401–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Stewart SH, Koch DG, Willner IR, et al. Validation of blood phosphatidyle-thanol as an alcohol consumption biomarker in patients with chronic liver disease. Alcohol Clin Exp Res. 2014;38:1706–1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Saunders JB, Aasland OG, Babor TF, et al. Development of the alcohol use disorders identification test (AUDIT): WHO collaborative project on early detection of persons with harmful alcohol consumption-II. Addiction. 1993;88:791–804. [DOI] [PubMed] [Google Scholar]
- 15. Friedmann PD, Saitz R, Gogineni A, et al. Validation of the screening strategy in the NIAAA “Physicians’ Guide to Helping Patients with Alcohol Problems”. J Studies Alcohol. 2001;62:234–238. [DOI] [PubMed] [Google Scholar]
- 16. Neumann T, Neuner B, Gentilello LM, et al. Gender differences in the performance of a computerized version of the alcohol use disorders identification test in subcritically injured patients who are admitted to the emergency department. Alcohol Clin Exp Res. 2004;28:1693–1701. [DOI] [PubMed] [Google Scholar]
- 17. Donovan DM, Dunn CW, Rivara FP, et al. Comparison of trauma center patient self-reports and proxy reports on the Alcohol Use Identification Test (AUDIT). J Trauma. 2004;56:873–882. [DOI] [PubMed] [Google Scholar]
- 18. Jones J, Jones M, Plate C and Lewis D. The detection of 1-palmitoyl-2-oleoyl-sn-glycero-3-phophoethanol and ethyl glucuronide in human umbilical cord. Anal. Methods. 2011; 3:1101–1106. [Google Scholar]
- 19. Rosalki SB, Tarlow D. Optimized determination of gamma-glutamyltransfer-ase by reaction-rate analysis. Clin Chem. 1974;20:1121–1124. [PubMed] [Google Scholar]
- 20. Schellenberg F, Schwan R, Mennetrey L, et al. Dose-effect relation between daily ethanol intake in the range 0-70 grams and% CDT value: validation of a cut-off value. Alcohol Alcohol. 2005;40:531–534. [DOI] [PubMed] [Google Scholar]
- 21. Schomaker M, Heumann C. Model averaging and model selection after multiple imputation using the R-package MAMI. Last Modified. 2017;12: 1–26. [Google Scholar]
- 22. Burnham KP. and Others. dr AnderSon. Model Selection and Multimodel Inference: A Practical Information-Theoretic Approach. New York, New York, USA: Ecological Modelling Springer Science & Business Media; 2002. [Google Scholar]
- 23. DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated received operating characteristic curves: a nonpara-metric approach. Biometrics. 1988;44:837–845. [PubMed] [Google Scholar]
- 24. Vickers AJ, Van Calster B, Steyerberg EW. Net benefit approaches to the evaluation of prediction models, molecular markers, and diagnostic tests. BMJ. 2016;352:i6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Moons KGM, Altman DG, Reitsma JB, et al. Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD): explanation and elaboration. Ann Intern Med. 2015;162:W1–73. [DOI] [PubMed] [Google Scholar]
- 26. Afshar M, Burnham EL, Joyce C, et al. Optimal cut-points for phosphatidy-lethanol vary by clinical setting: response to Nguyen and Seth’s (2018) letter to the editor. Alcohol Clin Exp Res. 2018;42:2064–2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Adong J, Fatch R, Emenyonu NI, et al. Social desirability bias impacts self-reported alcohol use among persons with HIV in Uganda. Alcohol Clin Exp Res. 2019;43:2591–2598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bracero LA, Maxwell S, Nyanin A, et al. Improving screening for alcohol consumption during pregnancy with phosphatidylethanol. Reprod Toxicol. 2017;74:104–107. [DOI] [PubMed] [Google Scholar]
- 29. Bajunirwe F, Haberer JE, Boum Y, et al. Comparison of self-reported alcohol consumption to phosphatidylethanol measurement among HIV-infected patients initiating antiretroviral treatment in Southwestern Uganda. PLoS One. 2014;9:e113152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gnann H, Thierauf A, Hagenbuch F, et al. Time dependence of elimination of different PEth homologues in alcoholics in comparison with social drinkers. Alcohol Clin Exp Res. 2014;38:322–326. [DOI] [PubMed] [Google Scholar]
- 31. Gnann H, Weinmann W, Thierauf A. Formation of phosphatidylethanol and its subsequent elimination during an extensive drinking experiment over 5 days. Alcohol Clin Exp Res. 2012;36:1507–1511. [DOI] [PubMed] [Google Scholar]
- 32. Kechagias S, Dernroth DN, Blomgren A, et al. Phosphatidylethanol compared with other blood tests as a biomarker of moderate alcohol consumption in healthy volunteers: a prospective randomized study. Alcohol Alcohol. 2015;50:399–406. [DOI] [PubMed] [Google Scholar]
- 33. Walther L, de Bejczy A, Löf E, et al. Phosphatidylethanol is superior to carbohydrate-deficient transferrin and g-glutamyltransferase as an alcohol marker and is a reliable estimate of alcohol consumption level. Alcohol Clin Exp Res. 2015;39:2200–2208. [DOI] [PubMed] [Google Scholar]
- 34. Hahn JA, Anton RF, Javors MA. The formation, elimination, interpretation, and future research needs of phosphatidylethanol for research studies and clinical practice. Alcohol Clin Exp Res. 2016;40:2292–2295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Leickly E, McDonell MG, Vilardaga R, et al. High levels of agreement between clinic-based ethyl glucuronide (EtG) immunoassays and laboratory-based mass spectrometry. Am J Drug Alcohol Abuse. 2015;41:246–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hoiseth G, Nordal K, Pettersen E, et al. Prolonged urinary detection times of EtG and EtS in patients with decreased renal function. Alcohol Clin Exp Res. 2012;36:1148–1151. [DOI] [PubMed] [Google Scholar]
- 37. Bogstrand ST, Høiseth G, Rossow I, et al. Prevalence of ethyl glucuronide and ethyl sulphate among patients injured when driving or at work. Alcohol Alcohol. 2015;50:68–73. [DOI] [PubMed] [Google Scholar]
- 38. Kummer N, Wille SMR, Poll A, et al. Quantification of EtG in hair, EtG and EtS in urine and PEth species in capillary dried blood spots to assess the alcohol consumption in driver’s licence regranting cases. Drug Alcohol Depend. 2016;165:191–197. [DOI] [PubMed] [Google Scholar]
- 39. Reisfield GM, Teitelbaum SA, Large SO, et al. The roles of phosphatidyle-thanol (PEth), ethyl glucuronide (EtG), and ethyl sulfate (EtS) in identifying alcohol consumption among participants in professionals' health programs. Drug Test Anal. 2020;12:1102–1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Roscón B, Monte R, Gamallo R, et al. Prevalence and routine assessment of unhealthy alcohol use in hospitalized patients. Eur J Intern Med. 2010;21:458–464. [DOI] [PubMed] [Google Scholar]
- 41. Afshar M, Burnham EL, Joyce C, et al. Cut-point levels of phosphatidylethanol to identify alcohol misuse in a mixed cohort including critically ill patients. Alcohol Clin Exp Res. 2017;41:1745–1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Helander A, Hansson T. National harmonization of the alcohol biomarker PEth. Lakartidningen. 2013;110:1747–1748. [PubMed] [Google Scholar]
- 43. Lowery EM, Walsh M, Yong M, et al. Use of alcohol biomarkers to identify alcohol misuse in organ donors. Alcohol. 2018;73:67–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Hill-Kapturczak N, Dougherty DM, Roache JD, et al. Differences in the synthesis and elimination of phosphatidylethanol 16:0/18:1 and 16:0/18:2 after acute doses of alcohol. Alcohol Clin Exp Res. 2018;42:851–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Helander A, Böttcher M, Dahmen N, et al. Elimination characteristics of the alcohol biomarker phosphatidylethanol (PEth) in blood during alcohol detoxification. Alcohol Alcohol. 2019;54:251–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Javors MA, Hill-Kapturczak N, Roache JD, et al. Characterization of the pharmacokinetics of phosphatidylethanol 16:0/18:1 and 16:0/18:2 in human whole blood after alcohol consumption in a clinical laboratory study. Alcohol Clin Exp Res. 2016;40:1228–1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Lopez-Cruzan M, Roache JD, Hill-Kapturczak N, et al. Pharmacokinetics of phosphatidylethanol 16:0/20:4 in human blood after alcohol intake. Alcohol Clin Exp Res. 2018;42:2094–2099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Schröck A, Thierauf A, Wurst FM, et al. Progress in monitoring alcohol consumption and alcohol abuse by phosphatidylethanol. Bioanalysis. 2014;6:2285–2294. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


