Abstract
The NLRP3 inflammasome is a cytosolic multiprotein complex composed of the innate immune receptor protein NLRP3, adapter protein ASC, and inflammatory protease caspase-1 that responds to microbial infection, endogenous danger signals, and environmental stimuli. The assembled NLRP3 inflammasome can activate the protease caspase‐1 to induce gasdermin D-dependent pyroptosis and facilitate the release of IL-1β and IL-18, which contribute to innate immune defense and homeostatic maintenance. However, aberrant activation of the NLRP3 inflammasome is associated with the pathogenesis of various inflammatory diseases, such as diabetes, cancer, and Alzheimer’s disease. Recent studies have revealed that NLRP3 inflammasome activation contributes to not only pyroptosis but also other types of cell death, including apoptosis, necroptosis, and ferroptosis. In addition, various effectors of cell death have been reported to regulate NLRP3 inflammasome activation, suggesting that cell death is closely related to NLRP3 inflammasome activation. In this review, we summarize the inextricable link between NLRP3 inflammasome activation and cell death and discuss potential therapeutics that target cell death effectors in NLRP3 inflammasome-associated diseases.
Keywords: NLRP3 inflammasome, pyroptosis, apoptosis, necroptosis, ferroptosis
Subject terms: NOD-like receptors, Cell death and immune response
Introduction
The innate immune response initiated by pattern recognition receptors (PRRs) is the first line of defense against pathogen invasion and maintains homeostasis in the body [1]. NOD-like receptor (NLR) family pyrin domain-containing 3 (NLRP3) is an important PRR in the cytoplasm that has a tripartite domain organization consisting of a carboxy-terminal leucine-rich repeat (LRR) domain that has autoinhibitory functions and signal recognition capabilities, a central nucleotide-binding domain (NACHT) that has ATPase activity and mediates self-oligomerization, and an amino-terminal pyrin domain (PYD) that recruits apoptosis-associated speck-like protein containing a CARD (ASC) [2]. In response to a variety of stimuli, including pathogen-associated molecular patterns (PAMPs, such as viral RNAs, microbial toxins, and bacterial surface components) and danger-associated molecular patterns (DAMPs, such as uric acid crystals, ATP, aluminum adjuvant and β-amyloid peptide), NLRP3 acts as a sensor and undergoes self-oligomerization via homotypic NACHT domain interactions. Oligomerized NLRP3 recruits ASC via homotypic PYD–PYD domain interactions and induces the aggregation of ASC into a macromolecular focus, known as an ASC speck [3]. Subsequently, the assembled ASC recruits pro-caspase-1 through homotypic CARD–CARD domain interactions to form the NLRP3–ASC–caspase-1 protein complex, which is known as the NLRP3 inflammasome [4]. Once the NLRP3 inflammasome is activated, it induces pro-caspase-1 self-cleavage and activation, which results in the maturation of the proinflammatory cytokines interleukin 1β (IL-1β) and interleukin 18 (IL-18). In addition, activated caspase-1 also cleaves gasdermin D (GSDMD) and releases its N-terminal domain, which is transferred to the cell membrane and forms pores, mediating the release of cellular contents, including the inflammatory cytokines IL-1β and IL-18, and inducing the inflammatory cell death known as pyroptosis [5–7]. Therefore, activation of the NLRP3 inflammasome is crucial for host defense against pathogen invasion and maintaining homeostasis. However, excessive activation of the NLRP3 inflammasome also contributes to the progression of various inflammatory diseases, such as cryopyrin-associated periodic syndrome, arthritis, atherosclerosis, type 2 diabetes, Alzheimer’s disease, and cancers [8–10]. Therefore, activation of the NLRP3 inflammasome must be strictly regulated, and the comprehensive mechanism of its activation also needs to be further investigated.
For a long time, cell death has been considered an irreversible biological process in which cells cease to perform their functions and cause damage to the body. However, in recent decades, an increasing number of studies have shown that cell death is a normal physiological process in organisms and is essential for maintaining homeostasis; for example, cell death has been shown to eliminate damaged, harmful, and superfluous cells [11–13]. Depending on whether the process of cell death can be regulated, cell death can be divided into accidental cell death (ACD) or regulated cell death (RCD) [14]. ACD is an uncontrolled type of cell death that can be triggered by chemical, physical, or mechanical stress, such as extreme pH variations, high pressure, high temperature, and abnormal osmotic stress [15, 16], whereas RCD is a tightly regulated form of cell death induced by intricate molecular mechanisms. RCD was first discovered by Karl Vogt in 1842 when cell death was noticed in toads. The physiological form of RCD that occurs in the absence of any exogenous environmental disturbance is also known as programmed cell death (PCD) [17, 18]. In addition, RCD can be triggered in response to perturbations in the extracellular or intracellular microenvironment, and RCD is conducive to maintaining homeostasis under stress conditions by clearing damaged cells or releasing DAMPs [18–21]. Recently, the field of cell death has rapidly advanced, and multiple cell death pathways have been discovered, including apoptosis, necroptosis, pyroptosis, ferroptosis, and autophagy-dependent cell death [13, 22–24]. With further research on the molecular mechanism of cell death, studies have shown that a large number of effectors of cell death can regulate activation of the NLRP3 inflammasome, and NLRP3 inflammasome activation can lead to cell death through a variety of pathways, suggesting that cell death is closely related to activation of the NLRP3 inflammasome [25–28].
In this review, we discuss our current understanding of the inextricable link between NLRP3 inflammasome activation and cell death, including apoptosis, necroptosis, pyroptosis, and ferroptosis, and discuss potential therapeutics that target cell death effectors in NLRP3 inflammasome-associated diseases.
The mechanism of NLRP3 inflammasome activation
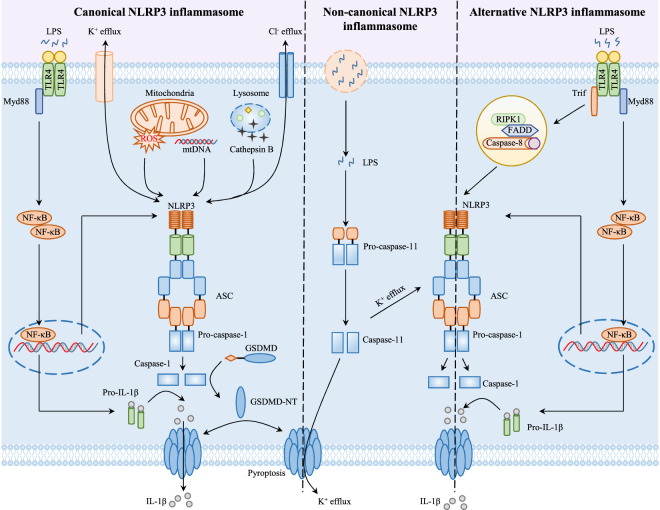
Because the NLRP3 inflammasome can be activated by multiple agonists, including PAMPs, such as viral RNAs, microbial toxins and bacterial surface components, and DAMPs, such as uric acid crystals, ATP, aluminum adjuvant, and β-amyloid peptide, its activation mechanism is extremely complex. To date, studies have shown that NLRP3 inflammasomes can be activated through three different signaling pathways: canonical NLRP3 inflammasome activation, noncanonical NLRP3 inflammasome activation, and alternative NLRP3 inflammasome activation, also known as one-step NLRP3 inflammasome activation [29, 30] (Fig. 1).
Fig. 1.
Mechanisms of canonical, noncanonical, and alternative NLRP3 inflammasome activation. (Left) Canonical NLRP3 inflammasome activation requires two steps: the priming step and the activation step. During the priming step, the ligands of toll-like receptors (TLRs), NOD-like receptors (NLRs), and cytokine receptors induce the expression of pro-IL-1β and NLRP3 via the myd88-NF-κB pathway. During the activation step, a wide variety of PAMPs or DAMPs promote NLRP3 inflammasome sassembly by multiple molecular and cellular events, such as K+ efflux, Cl− efflux, mitochondrial dysfunction, reactive oxygen species (ROS) release, mitochondrial DNA (mtDNA) production, and lysosomal disruption. Once the NLRP3 inflammasome is assembled and activated, it induces pro-caspase-1 self-cleavage and activation, which results in the maturation of the proinflammatory cytokines interleukin 1β (IL-1β) and interleukin 18 (IL-18). In addition, activated caspase-1 also cleaves gasdermin D (GSDMD) and releases its N-terminal domain, which transfers to the cell membrane and forms pores, mediating the release of cellular contents, including the inflammatory cytokines IL-1β and IL-18, and inducing a type of inflammatory cell death known as pyroptosis. (Middle) Noncanonical NLRP3 inflammasome activation is initiated by cytosolic LPS, which can be recognized by caspase-11 in mice (or caspase-4 and caspase-5 in human) via direct interactions, resulting in caspase-11 autoproteolysis and activation. Then, activated caspase-11 opens the pannexin-1 channel to induce K+ efflux, resulting in canonical NLRP3 inflammasome activation and IL-1β and IL-18 maturation. In parallel, activated caspase-11 also cleaves GSDMD to induce membrane pore formation and pyroptosis, which contribute to IL-1β and IL-18 release. (Right) Alternative NLRP3 inflammasome activation only requires a single signal. TLR ligands alone are sufficient to activate the NLRP3 inflammasome in human and porcine monocytes via the TLR4–TRIF–RIPK1–FADD–CASP8 signaling axis, which cannot induce K+ efflux, ASC speck formation, or pyroptosis
Canonical NLRP3 inflammasome activation
Currently, it is generally accepted that canonical NLRP3 inflammasome activation requires two steps: the priming step and the activation step. In the priming step, ligands (such as LPS, pam3csk4, IL-1, TNF-α, and MDP) of Toll-like receptors (TLRs, such as TLR2 and TLR4), cytokine receptors (such as IL-1 receptor and TNF-α receptor), or NLRs (such as NOD1 and NOD2) can induce the activation of the transcription factor NF-κB and promote the expression of NLRP3 and pro-IL-1β [31–34]. In addition to its role in transcriptional regulation, recent studies have shown that the priming step can also regulate posttranslational modifications (PTMs) of NLRP3, such as phosphorylation or ubiquitination. The JAMM domain-containing Zn2+ metalloprotease BRCC3 (BRCC36 in humans) is a deubiquitinating enzyme that can induce the deubiquitination of NLRP3 during the priming step and promote the activation of the NLRP3 inflammasome [35]. It has also been reported that c-Jun N-terminal kinase (JNK1)-mediated NLRP3 S194 phosphorylation during the priming step is essential for NLRP3 inflammasome assembly and activation [36]. These studies indicate that the priming step is crucial for NLRP3 inflammasome activation through transcriptional regulation and PTMs.
The activation step is involved in the recognition of NLRP3 inflammasome agonists and in inflammasome assembly and activation. In contrast to PRRs that can recognize only one or several structurally similar stimuli, NLRP3 has been reported to be activated by a wide variety of unrelated PAMPs and DAMPs, but there is no evidence that NLRP3 binds directly to these effectors. To date, multiple molecular and cellular events, including ion flux (such as K+ efflux, Cl− efflux, Na+ influx, and Ca2+ mobilization), mitochondrial dysfunction, the release of reactive oxygen species (ROS) and mitochondrial DNA (mtDNA), lysosomal disruption and trans-Golgi disassembly, induced by NLRP3 stimuli have been proposed to be upstream signals for the assembly and activation of inflammasomes [37–44]. Although NLRP3 agonists can activate inflammasomes through the aforementioned signaling pathways, none of these signaling pathways is applicable to all NLRP3 agonists, and there is no consistent pathway of NLRP3 activation. Therefore, the exact mechanism by which NLRP3 senses these stimuli and the mechanism by which the inflammasome is assembled and activated remain to be further elucidated.
Noncanonical NLRP3 inflammasome activation
In addition to describing the caspase-1-dependent canonical NLRP3 inflammasome, recent studies have shown that there is a caspase-11 (caspase-4 and caspase-5 in humans)-dependent noncanonical NLRP3 inflammasome signaling pathway [45, 46]. Typically, a priming signal is essential for noncanonical inflammasome activation in murine cells. During this step, the ligands of TLRs and cytokine receptors induce the activation of the transcription factor NF-κB and the production of type I interferons, which upregulate the expression of caspase-11 through the JAK/STAT pathway or the complement C3–C3aR axis [47, 48]. In contrast, the priming signal is not necessary for noncanonical NLRP3 inflammasome activation in human cells that constitutively expresses caspase-4 [49]. Moreover, type I interferons also promote the expression of a cluster of small interferon-inducible GTPases known as guanylate-binding proteins (GBPs) and the interferon-inducible protein IRGB10. It has been reported that after infection with gram-negative bacteria, IRGB10 directly targets the cell membrane of intracellular bacteria in a GBP-dependent manner, where it compromises bacterial structural integrity and releases LPS and lipid A into the cytoplasm [50, 51]. Recent studies have shown that LPS and lipid A directly bind to the CARD domain of murine caspase-11 or the human homologs caspase-4 and caspase-5, resulting in caspase oligomerization and autoproteolysis [49]. Activated caspase-4/5/11 proteolytically cleaves murine gasdermin D (GSDMD) at Asp276 (Asp275 in human GSDMD) and releases the N-terminal domain, which binds to cardiolipin, phosphatidylinositol phosphates, and phosphatidylserine in the plasma membrane and induces pore formation, pyroptosis [6, 7, 52], and subsequent potassium efflux-dependent canonical NLRP3 inflammasome activation [53]. In addition to gram-negative bacteria, self-encoded oxidized phospholipid 1-palmitoyl-2-arachidonoyl-sn-glycero-3-phosphorylcholine (oxPAPC) has been shown to bind directly to murine caspase-11 and orthologous human caspase-4 and activate the noncanonical NLRP3 inflammasome in dendritic cells (DCs) [54]. However, a recent study showed that oxPAPC inhibited the noncanonical NLRP3 inflammasome by competitively binding to caspase-11 and LPS in macrophages but not DCs and consequently protected the organism against septic shock [55]. The contradictory effects of oxPAPC on noncanonical NLRP3 inflammasome activation in different cells require further investigation.
Alternative NLRP3 inflammasome activation
In contrast to canonical and noncanonical NLRP3 inflammasome activation, which requires two steps, a novel NLRP3 inflammasome activation pathway, the alternative NLRP3 inflammasome pathway, was identified, and in this pathway, TLR ligands alone are insufficient to activate caspase-1 or induce the maturation and secretion of IL-1β in human and porcine monocytes [56, 57]. In addition, the alternative NLRP3 inflammasome is activated through the TLR4–TRIF–RIPK1–FADD–CASP8 signaling pathway, which is upstream of NLRP3. Although alternative NLRP3 inflammasome activation also requires NLRP3–ASC–caspase-1 signaling, this novel inflammasome lacks any characteristics of canonical and noncanonical NLRP3 inflammasome activation, including ASC speck formation, pyroptosis induction, or K+ efflux. Recent studies have shown that apolipoprotein C3 (ApoC3) also activates the caspase-8-dependent alternative NLRP3 inflammasome in human monocytes. ApoC3 interacts with Tlr2 and Tlr4 to induce their heterogenous dimerization and then facilitates Ca2+ influx, ROS production, and NADPH oxidase and caspase-8 activation through the TLR–SCIMP–Lyn–Syk–TRPM2 axis [58, 59]. Although caspase-8 is a key upstream molecule that activates the alternative NLRP3 inflammasome, the exact mechanism remains unknown. Therefore, the comprehensive link between NLRP3 and caspase-8 in alternative NLRP3 inflammasome activation needs to be further investigated.
Cell death is an irreversible biological process in which living cells cease to perform their function. Various types of cell death have been identified by cell morphology, pathogenesis, and function [13]. In this review, we focus on summarizing the signaling pathways and functions of pyroptosis, apoptosis, necroptosis, and ferroptosis and their regulatory roles in NLRP3 inflammasome activation (Table 1).
Table 1.
Comparison of the main features of different cell death types
| Types | Pyroptosis | Apoptosis | Necroptosis | Ferroptosis |
|---|---|---|---|---|
| Initiators | DAMPs and PAMPs | Cell stress, radiation, DNA damage, growth factor withdrawal, mitotic defects, and hypoxia for intrinsic apoptosis; ligands of transmembrane receptor, such as TNFR1, FAS, DR4, and DR5 for extrinsic apoptosis | Ligands of TNFR1, FAS, TLR3, TLR4, and ZBP1 in the absence of active caspase-8 | Uptake of cysteine is inhibited or the GPX4-dependent antioxidant is blocked |
| Effectors | Caspase-1, -11 (or caspase-4, -5 in human) and mostly gasdermin family members | BAX/BAK, c-FLIP, Bcl-2, cytochrome C, AIF, XIAP, caspases-3, -6, -7, -8, -9, FADD, and TRADD | RIPK3, MLKL, ZBP1 | TXNRD1, GPX4, GSH, FSP1, NRF2 |
| Morphological characteristics | Chromatin condenses, nucleus remains intact, cellular swelling, and plasma-membrane rupture | Cell shrinkage, nuclear fragmentation, chromosomal DNA fragmentation, and plasma-membrane blebbing | Cell swelling, plasma-membrane rupture | Lipid peroxides accumulation, mitochondrial membrane rupture and condense, mitochondria cristae loss |
| Inflammatory response | Pro-inflammatory | Either anti-inflammatory or pro-inflammatory (context dependent) | Pro-inflammatory | Pro-inflammatory |
| Content releases | IL-1β, IL-18, IL-1α, HMGB1, and LDH | IL-1α, uric acid, ATP, and HMGB1 | HMGB1, long genomic DNA, and IL-6 | HMGB1 and lipid oxidation products, such as 4HNE, oxPLs, LTB4, LTC4, LTD4, and PGE2 |
| Functions | Against pathogens invasion and enhance antitumor immunity | Maintains homeostasis during development and aging | Defense to pathogens invasion and maintain tissue homeostasis | Tumor suppression, immune surveillance, and development |
| Related diseases | Sepsis, epilepsy, atherosclerosis, and Alzheimer’s disease | Atrophy, cancer, and neurodegenerative diseases | Pulmonary diseases, neurodegenerative diseases, cardiovascular disease, and cancer | Huntington’s disease, neuroferritinopathy, nonalcoholic steatohepatitis, chronic obstructive pulmonary disease |
The NLRP3 inflammasome in pyroptosis
Pyroptosis is a form of inflammatory PCD that is triggered by pathogen invasion and is dependent on caspase activation. In contrast to other forms of cell death, pyroptosis exhibits unique morphological and physical characteristics, including chromatin condensation, intact nuclei, cellular swelling, and plasma-membrane rupture [60]. Pyroptosis was originally observed by two separate research groups when they infected macrophages with Salmonella and Shigella and found a caspase-1-dependent cell death pathway. However, these two groups continue to contend that this discovery represents a novel form of apoptosis [61, 62]. The term pyroptosis was proposed by Cookson and Boise in 2001 to describe a particular type of proinflammatory PCD that is dependent on caspase-1 [63, 64]. In addition to caspase-1, recent findings indicate that pyroptosis can be induced by several other inflammatory caspases, such as caspase-3, -6, -7, and -8, murine caspase-11 and its human homologs caspase-4 and caspase-5 [6, 65, 66]. Upon activation, caspases specifically cleave gasdermin D (GSDMD) at the interdomain loop and release the N-terminal pore-forming domain, which is then translocated to the plasma membrane to form pores with inner diameters of 10–14 nm that consist of 16 symmetric protomers and induce pyroptosis [67]. Gasdermin D is a coexecutor of pyroptosis, and accumulating evidence indicates that other members of the gasdermin family, including GSDMA3, GSDMB, and GSDME, can also be cleaved and induce pyroptosis [6, 68–71]. It has been shown that the cleavage of GSDME by caspase-3 facilitates chemotherapy drug-induced pyroptosis in tumor cells [70, 72]. Moreover, granzyme B directly cleaves GSDME at the same site as caspase-3 to induce pyroptosis, enhancing antitumor immunity and acting as a tumor suppressor [68]. In addition, recent studies have shown that GSDMB can be cleaved by granzyme A to enhance antitumor immunity [69]. Although pyroptosis was originally thought to be a general innate immune response against pathogen invasion, it is currently thought to be involved in multiple diseases, including sepsis, epilepsy, atherosclerosis, and Alzheimer’s disease [73–76].
As a form of inflammatory PCD, pyroptosis facilitates the release of cell contents, including members of the IL-1 family of proinflammatory cytokines, and has long been known to be mediated by caspase-1, a protease that is activated by different inflammasomes in response to a variety of pathogenic bacterial infections and danger signals [60]. Recent studies have shown that in addition to caspase-1, caspase-11 and its corresponding proteases caspase-4 and caspase-5 in humans can trigger pyroptosis by cleaving pyroptosis executors in the gasdermin protein family [77]. These studies indicate that pyroptosis is dependent on gasdermin but not caspase-1. Therefore, pyroptosis has been recently redefined as gasdermin-mediated PCD [78].
The genes that encode the proteins of the gasdermin family are highly conserved in vertebrates. There are six genes in humans (GSDMA, GSDMB, GSDMC, GSDMD, GSDME, and DFNB59), and the gasdermin gene family in mice contains five paralogous genes but lacks Gsdmb [77]. Most gasdermin family members consist of a similar N-terminal domain and a conserved C-terminal domain, with the exception of DFNB59, which has a shorter and divergent C-terminal domain [79]. The gasdermin N-terminal domain has pore-forming activity, and it binds to the cell membrane to induce cell death upon activation, while the C-terminal domain has autoinhibitory activity that can suppress cell death by blocking the release of the N-terminal domain [67, 80].
GSDMD is the most widely studied protein in the gasdermin family and was first identified by three separate groups in 2015 as a substrate of inflammatory caspases (caspase-1 and -11 in mice and caspase-4 and -5 in humans) that initiates pyroptosis [6, 7, 81]. These groups found that GSDMD was required for pyroptosis and IL-1β release but not IL-1β maturation or caspase-1 activation via either the canonical or noncanonical NLRP3 inflammasome response pathways [6, 7]. Mechanistically, activated inflammatory caspases can directly cleave GSDMD at Asp276 (Asp275 in human GSDMD), resulting in the release of the N-terminal domain, which is subsequently transferred to the cell membrane to induce pyroptosis in a cell-intrinsic manner [6]. These studies suggest that GSDMD is a key target of inflammatory caspases and a critical executor of pyroptosis. However, the functional mechanism of action of gasdermin was unclear until a recent study showed that the N-terminal domain of GSDMD can bind to phosphoinositides and cardiolipin in membranes and form a pore with an inner diameter of 10–14 nm, which consists of 16 symmetric monomers [67]. However, by using cryo-electron and atomic force microscopy, another study showed that the diameters of GSDMD membrane pores were ~20 nm [82]. Regardless, the pore is large enough to allow the release of IL-1β (4.5 nm) and IL-18 (5.0 nm) prior to cell lysis [52]. In addition, the GSDMD membrane pores damage normal permeability and induce water entry into the cytosol, which causes an increase in cell volume and subsequent cell lysis [83]. Usually, if there are very few GSDMD pores in the cell membrane, compensatory mechanisms can reduce cell volume and repair the pores via the ESCRT complex, thus reestablishing the integrity of the cell membrane [84, 85]. Alternatively, if the number of GSDMD pores exceeds the membrane repair ability of the cell, lysis ensues, releasing large amounts of soluble cellular contents, such as lactate dehydrogenase and high mobility group box 1 [86].
In addition to GSDMD, accumulating evidence has indicated that most members of other gasdermin protein families can also induce pyroptosis [6]. It has been shown that alopecia and skin defects are associated with gain-of-function mutations in GSDMA3, which disrupt the inhibitory effect of the GSDMA3 C-terminal domain on its N-terminal domain, thus promoting pore formation and triggering pyroptosis [6]. Similarly, the amino acids encoded by SNPs related to asthma and inflammatory bowel disease also affect the structure and function of the GSDMB C-terminal domain [87]. Moreover, the apoptotic executioners caspase-3, -6, and -7 have been shown to cleave GSDMB at 88DNVD91 in the N-terminal domain [87]. In addition, Zhou et al. found that GSDMB can also be cleaved at Lys244 and Lys229, which are within the interdomain linker, by granzyme A released from cytotoxic T lymphocytes and natural killer cells, inducing pore formation and pyroptosis [69]. However, a recent study showed that GSDMB could not induce cell death alone but mediated pyroptosis by promoting the cleavage of GSDMD [88]. Mechanistically, GSDMB binds to the CARD domain of caspase-4 directly and facilitates its protease activity, which is required for GSDMD cleavage in the human monocyte cell line via noncanonical pyroptosis [88]. Consistent with GSDMB, GSDME has been shown to be cleaved by caspase-3 at Asp270 and convert apoptosis to pyroptosis during chemotherapy [70]. In addition, a recent study showed that granzyme B produced by natural killer and CD8+ T lymphocytes also induced pyroptosis by cleaving GSDME directly at the same site as caspase-3 [68]. Although the C-terminal domain of GSDMC has also been shown to induce pyroptosis [79], the specific mechanism of its function remains unclear, and how it is cleaved and activated needs to be further investigated.
Regulation of the NLRP3 inflammasome by apoptotic effectors
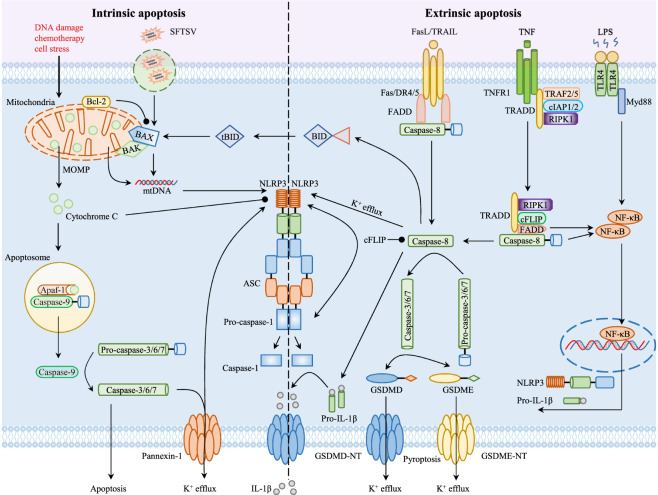
Apoptosis is a highly regulated form of PCD in which unwanted cells are discarded to avoid damaging neighboring cells and to maintain homeostasis during development and aging [89]. Although the principles and certain characteristics of apoptosis were proposed many years prior [90], in 1972, the term apoptosis was coined by Kerr, Wyllie, and Currie to describe a process of PCD with unique morphological features, including cell shrinkage, nuclear fragmentation, chromosomal DNA fragmentation, and plasma-membrane blebbing [91–94]. Apoptosis can be initiated through either the intrinsic or extrinsic pathway. The intrinsic apoptosis pathway is triggered by various nonreceptor-mediated stimuli, such as cell stress, radiation, DNA damage, growth factor withdrawal, mitotic defects, and hypoxia [95–97]. In response to these apoptotic stimuli, the proapoptotic proteins BAX and BAK form pores in the mitochondrial membrane, disrupting mitochondrial membrane potential and inducing the release of the proapoptotic proteins cytochrome C and apoptosis-inducing factor (AIF) into the cytosol [97–100]. Cytochrome C binds with apoptotic peptidase-activating factor 1 and pro-caspase-9 to form a complex known as the apoptosome, which is critical for pro-caspase-9 cleavage and activation. Activated caspase-9 can catalyze the proteolytic activation of the executioners caspase-3 and -7, which cleave various substrates, including cytoskeletal proteins and cytokeratins, to ultimately initiate apoptosis [101–104]. AIF is translocated to the nucleus to induce chromatin condensation and large-scale fragmentation of DNA (~50–300 kb) [105, 106]. The extrinsic apoptosis pathway is triggered by various transmembrane receptors, including tumor necrosis factor receptor superfamily member 1A, Fas cell surface death receptor (FAS), death receptor 4 (DR4), and DR5 [107–109]. Upon ligand stimulation, these receptors recruit corresponding adapter proteins to assemble a dynamic multiprotein complex known as the death-inducing signaling complex (DISC) [110]. The adapter of the Fas receptor, DR4, and DR5 is Fas-associated death domain protein (FADD), and the adapter of the TNF receptor is TNF receptor-associated death domain (TRADD), which also recruits FADD and RIPK1 in the absence of cIAP1/2. FADD then recruits pro-caspase-8 via the death effector domain and induces pro-caspase-8 activation by autoproteolytic cleavage [111–113]. Activated caspase-8 can directly cleave and activate effector caspases, such as caspase-3 and -7, to initiate apoptosis. In addition, caspase-8 can cleave BID to induce the release of cytochrome C and the subsequent activation of caspase-9, ultimately resulting in apoptosis through the activation of effector caspases [114, 115]. Although apoptosis is considered a vital biological phenomenon to maintain homeostasis and development, inappropriate apoptosis has been implicated in multiple diseases, including atrophy, cancer, and neurodegenerative diseases [116–118]. To date, various apoptosis effectors have been reported to be involved in NLRP3 inflammasome activation and IL-1β release (Fig. 2).
Fig. 2.
The regulation of NLRP3 inflammasome activation by apoptotic effectors. (Left) Several intrinsic apoptotic effectors regulate NLRP3 inflammasome activation through different signaling pathways. BAX and BAK, two important intrinsic apoptotic effectors, induce NLRP3 inflammasome activation by promoting oxidized mitochondrial DNA (mtDNA) release and caspase-3/7-dependent K+ efflux in the context of severe fever with thrombocytopenia syndrome (SFTS) virus (SFTSV) infection, DNA damage, chemotherapy, or cellular stress. Cytochrome c, which is released from the inner mitochondrial membrane, impairs inflammasome activation by binding to the LRR domain of NLRP3 and reduces the interactions between NLRP3 and NEK7. Bcl-2, an antiapoptotic protein located in the outer mitochondrial membrane, restrains NLRP3 inflammasome activation by inhibiting Bax- and BAK-mediated mitochondrial homeostasis and VDAC activity upon Zika Virus infection or classical agonist stimulation. (Right) The extrinsic apoptosis pathway is triggered by various transmembrane receptors, many of which have been reported to regulate NLRP3 inflammasome activation. Cellular FADD-like IL-1β-converting enzyme (FLICE)-inhibitory protein (c-FLIP) is an antiapoptotic regulator that promotes NLRP3 inflammasome activation by binding to NLRP3 and pro-caspase-1 in a caspase-8-independent manner. FADD promotes inflammasome activation by inducing the transcription of NLRP3 and pro-IL-1β, as well as caspase-8. Caspase-8, an important effector of apoptosis, regulates NLRP3 inflammasome activation through multiple pathways, directly cleaves IL-1β as well as the channel-forming glycoprotein pannexin-1 to induce K+ efflux, and mediates the transcription of NLRP3 and pro-IL-1β during priming. Other caspases, such as caspase-3, -6, -7, and -9 promote NLRP3 inflammasome activation by inducing cell death and K+ efflux
BAX and BAK are two important apoptosis effectors that initiate the intrinsic apoptosis pathway by inducing mitochondrial outer membrane permeabilization (MOMP) and the release of proapoptotic factors in response to a diverse range of cellular stressors, such as hypoxia, DNA damage, infection, growth factor withdrawal, radiation, and chemotherapy [119]. It has been shown that BAX and BAK activate caspase-3 and -7 and subsequently induce NLRP3 inflammasome activation and IL-1β secretion by inducing potassium efflux [120]. In addition, recent research by Li et al. showed that severe fever with thrombocytopenia syndrome virus infection induced BAK- and BAX-dependent cytosolic release of oxidized mtDNA, which bound to and activated the NLRP3 inflammasome [121]. However, it should be noted that vioprolides, cyclic peptides isolated from myxobacteria, induce IL-1β release in both inflammasome-dependent and inflammasome-independent ways [122]. Mechanistically, vioprolides trigger BAX- and BAK-dependent MOMP by inhibiting Mcl-1 and B-cell lymphoma-2 (Bcl-2), which enables the activation of caspase-8, the main protease that catalyzes IL-1β maturation in the intrinsic apoptosis pathway [122]. Taken together, these studies indicate that Bax and Bak promote NLRP3 inflammasome activation by regulating mitochondrial homeostasis, but the detailed mechanism remains to be further explored.
Cellular FADD-like IL-1β-converting enzyme-inhibitory protein (c-FLIP) is a major antiapoptotic regulator that suppresses the activation of caspase-8 and -10, thereby preventing the initiation of downstream apoptotic cascades [123]. Wu et al. reported that c-FLIP promoted NLRP3 inflammasome activation by binding to NLRP3 and pro-caspase-1, which is required for NLRP3 mitochondrial localization and inflammasome assembly. This finding suggests that C-FLIP plays a caspase-8-independent role in regulating NLRP3 inflammasome activation [124]. In addition to c-FLIP, cellular inhibitor of apoptosis protein 1 (cIAP1) and cIAP2 have also been shown to facilitate NLRP3 inflammasome activation [125, 126]. Labbé et al. found that cIAPs were critical effectors of caspase-1 activation, and deficiency in cIAP1 (encoded by Birc2) or cIAP2 (encoded by Birc3) impaired NLRP3 inflammasome activation. Mechanistically, cIAP1 and cIAP2 physically interact with caspase-1 and mediate its activation and nondegradative K63-linked polyubiquitination [125]. In parallel, Suzuki et al. also showed that cIAP1 and cIAP2 acted as positive regulators of NLRP3 inflammasome activation during Shigella infection [126]. Thus, these results suggest that c-FLIP and cIAPs bind to and activate the NLRP3 inflammasome. However, it is not clear whether these regulators mediate apoptosis or NLRP3 inflammasome activation.
Fas-associated protein with death domain (FADD) is an adaptor protein that recruits pro-caspase-8 and -10 to form the DISC and induce apoptosis. Recent studies have shown that FADD promotes inflammasome activation by regulating the expression of NLRP3 during the priming step [127–129]. In addition, both apoptotic initiator caspases (such as caspase-9) and executioner caspases (such as caspase-3, -6, and -7) are important for NLRP3 inflammasome activation [120]. Caspase-3, -7, and -9 act upstream of both NLRP3 inflammasome activation and IL-1β secretion by inducing cell death and potassium efflux [120, 130]. Zheng et al. discovered that caspase-6 was required for ZBP1-mediated NLRP3 inflammasome activation by binding to receptor-interacting serine/threonine-protein kinase 3 (RIPK3) and enhanced the interaction between RIPK3 and ZBP1 during influenza A virus (IAV) infection [130]. In addition, this study showed that caspase-6 was a key regulator of ZBP1-mediated pyroptosis, apoptosis, and necroptosis (PANoptosis). However, it is unclear how caspase-6 coordinates these events, and the detailed mechanism needs to be further investigated.
In contrast to the promoting effect of these apoptosis effectors, several effectors have been shown to inhibit NLRP3 inflammasome activation, including cytochrome c, Bcl-2, and X-linked inhibitor of apoptosis protein (XIAP) [42, 131–136]. The release of cytochrome c from the inner mitochondrial membrane is critical for apoptosis. Shi et al. found that cytochrome c impaired inflammasome activation by binding to the LRR domain of NLRP3 and reducing the interactions between NLRP3 and NEK7 [134]. Bcl-2 is an antiapoptotic protein located in the outer mitochondrial membrane. Together with several other groups, we found that Bcl-2 inhibited classical agonist- and Zika virus infection-mediated NLRP3 inflammasome activation by regulating mitochondrial homeostasis and VDAC activity but had no effect on salmonella-mediated IPAF inflammasome activation [42, 135, 136]. XIAP is a key inhibitor of apoptosis that blocks cell death by binding to and inhibiting specific caspases, such as caspase-3, -7, or -9. A recent study showed that XIAP restricted TLR- and TNF-driven inflammasome activation and IL-1β secretion by regulating RIP1 ubiquitination and required RIP3 in DCs [131]. In addition, treatment of macrophages with an antagonist of the XIAP Smac mimetic triggered inflammasome activation, which depended on the kinase RIP3 and ROS production [132]. Moreover, XIAP regulated retinal pigment epithelial cell death by inhibiting NLRP3 inflammasome activation and thus contributed to age-related macular degeneration [133]. However, the exact mechanism by which XIAP inhibits the activation of the NLRP3 inflammasome remains unclear.
Notably, caspase-8, another apoptosis effector, was shown to have the dual functions of both promoting and inhibiting NLRP3 inflammasome activation [127, 137–142]. It has been reported that caspase-8 contributes to NLRP3 inflammasome activation and IL-1β release through the cleavage of GSDMD upon TAK1 or IkappaB kinase blockade by the Yersinia effector protein YopJ [137]. In parallel, caspase-8 was shown to cleave the channel-forming glycoprotein pannexin-1 to induce potassium efflux and subsequent NLRP3 inflammasome activation [138]. Moreover, caspase-8 provides a posttranslational priming signal to the NLRP3 inflammasome through its scaffolding function but not its catalytic function during Toll-like receptor 3 (TLR3) binding to dsRNA [139]. Consistent with this finding, Gurung et al. discovered that caspase-8 and FADD mediated transcriptional priming and posttranslational activation of the canonical and noncanonical NLRP3 inflammasome [127]. In addition to indirectly promoting inflammasome activation by inducing cell death, caspase-8 also plays a direct role in the cleavage of IL-1β. Maelfait et al. reported that caspase-8 blockade by RNAi or inhibitors impaired IL-1β processing in poly(I:C)- and LPS-induced macrophages [140]. Mechanistically, caspase-8 directly cleaved pro-IL-1β at the same site as caspase-1 [140]. Moreover, caspase-8 acted as a direct IL-1β-converting enzyme during NLRP3 inflammasome activation in the absence of caspase-1 upon sustained macrophage stimulation with nigericin [142]. In contrast, caspase-8 also has an inhibitory effect on NLRP3 inflammasome activation. Defects in caspase-8 in DCs facilitated LPS-induced NLRP3 inflammasome activation, which depended on the functions of RIPK1, RIPK3, MLKL, and phosphoglycerate mutase family member 5 (PGAM5) but did not cause cell death [141]. Collectively, these studies indicate that caspase-8 plays an opposing role in NLRP3 inflammasome activation, but our knowledge of the causes of these contradictions remains limited.
Necroptosis effectors in NLRP3 inflammasome activation
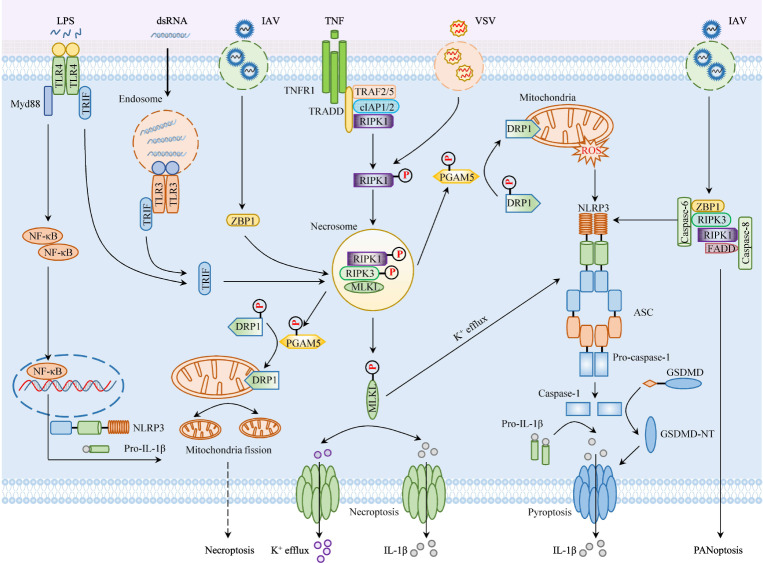
Necroptosis is a programmed form of inflammatory cell death with unique characteristics, including cell swelling and plasma-membrane rupture [143]. The necroptosis pathway is triggered by various specific death receptors, including the TNF receptor, FAS, TLR3, TLR4, and Z-DNA-binding protein 1 (ZBP1), to protect against pathogen infection and cellular damage [144–146]. The activation of mixed lineage kinase domain like pseudokinase (MLKL) and RIPK3 is well known to be a key step in the necroptosis pathway [147]. Upon stimulation by TNF signals, the TNF receptor recruits the adapters TRADD and RIPK1, as well as cIAPs and TRAF2/5. In the absence of caspase-8 and cIAPs, RIPK3 is phosphorylated and activated by RIPK1 via RIP homotypic interaction motif (RHIM)–RHIM interactions to form an amyloid-like filamentous signaling complex known as the necrosome complex [148]. In addition to RIPK1, RIPK3 can also be activated by RHIM-dependent interactions with other RHIM-containing proteins, such as TIR-domain-containing adapter-inducing interferon-β (TRIF), which is an adapter that is downstream of TLR3 (activated by double-stranded RNA (dsRNA) in endosomes), TLR4 (activated by lipopolysaccharide (LPS) or various PAMPs in the plasma membrane), and ZBP1, which acts as a sensor of Z nucleic acids [145, 146]. Activated RIPK3 phosphorylates the pseudokinase MLKL and facilitates its oligomerization and translocation to the plasma membrane, where it binds to phosphatidylinositol phosphate and cardiolipin, disrupting the integrity of the membrane and inducing necroptosis [149]. Previous studies have shown that, in addition to MLKL, PGAM5 is another downstream molecule of RIPK3 that induces the dephosphorylation of dynamin-related protein 1 (DRP1) and drives mitochondrial damage in necroptosis [150]. However, several subsequent studies have shown that the PGAM5-DRP1 axis is dispensable for necroptosis but promotes inflammation [151–154]. Therefore, the intricate role of PGAM5-DRP1 in necroptosis remains to be further investigated. Although necroptosis plays a key role in host defense against pathogen invasion and the maintenance of tissue homeostasis, dysregulation of necroptosis has been shown to be associated with a variety of diseases, including pulmonary diseases, neurodegenerative diseases, cardiovascular disease, and cancer [155, 156]. Currently, several necroptosis effectors have been reported to engage in crosstalk with the NLRP3 inflammasome to induce its activation, in addition to their roles in cell death. Here, we summarize the latest studies on the regulation of NLRP3 inflammasome activation by cell death effectors [157] (Fig. 3).
Fig. 3.
Necroptosis effectors contribute to NLRP3 inflammasome activation. Several necroptosis regulators have been reported to crosstalk with NLRP3 inflammasome activation, in addition to their roles in cell death. Mixed lineage kinase domain like pseudokinase (MLKL), a key executor of necroptosis, facilitates NLRP3 inflammasome activation by inducing K+ efflux. Although the functions of phosphoglycerate mutase family member 5 (PGAM5) and its substrate dynamin-related protein 1 (DRP1) in necroptosis have been challenged, their roles in NLRP3 inflammasome activation are widely accepted. In response to VSV infection, PGAM5 dephosphorylates DRP1 and drives mitochondrial fission, which then facilitates NLRP3 inflammasome activation by inducing ROS production in a cell death-independent manner. Z-DNA-binding protein 1 (ZBP1), a novel effector of necroptosis, triggers NLRP3 inflammasome activation and IL-1β release via the RIPK1–RIPK3–Caspase-8 axis during influenza virus (IAV) infection
RIPK3 is an essential effector of necroptosis, a lytic form of cell death that causes inflammation by releasing intracellular molecules [158]. RIPK3 induces NLRP3 inflammasome activation by activating caspase-8 and subsequently apoptosis in response to LPS in the absence of IAPs, and this process is independent of RIPK3 kinase and MLKL activity [159]. However, RIPK3 kinase and MLKL activity were shown to be essential for TLR-induced NLRP3 activation once both IAPs and caspase-8 were rendered defective [159]. Consistent with this finding, a recent study showed that RIPK3 formed a complex with MLKL to induce mitochondrial ROS (mROS) production and then triggered NLRP3 inflammasome activation via the mROS–AKT pathway to protect against S. pneumonia infection [160]. Moreover, RIPK3 has been shown to promote renal fibrosis by activating the NLRP3 inflammasome in a mouse model of folic acid‐induced nephropathy. Furthermore, dabrafenib, an inhibitor of RIPK3, effectively blocked the activation of the NLRP3 inflammasome to treat tubulointerstitial fibrosis [161]. In contrast, Moriwaki et al. found that RIPK3 had no effect on vesicular stomatitis virus-induced NLRP3 inflammasome activation or IL-1β secretion in bone-marrow-derived dendritic cells (BMDCs) [152]. Overall, the role of RIPK3 in NLRP3 inflammasome activation is complex, and the mechanism is also somewhat contradictory, as RIPK3-induced NLRP3 inflammasome activation could be caused by different stimuli in different cells.
MLKL has been identified as a key component downstream of RIPK3 and has been suggested to be a terminal executor of necroptosis and mediator of the inflammatory response [149, 162]. To date, several studies have shown that MLKL facilitates NLRP3 inflammasome activation [129, 139, 163, 164]. Previous studies showed that MLKL induces NLRP3 inflammasome activation in a cell-intrinsic manner, which requires the death effector four-helical bundle and self-oligomerization, as well as translocation to cellular membranes [163]. Mechanistically, activated MLKL induces necroptosis and directly disrupts the plasma membrane via the formation of pores that drive lytic cell death and mediate potassium efflux. Moreover, activated MLKL mediates activation of the NLRP3 inflammasome and IL-1β secretion independent of the pyroptotic effector gasdermin D (GSDMD) [135]. Consistent with this finding, Gutierrez et al. demonstrated that MLKL was an endogenous activator of the NLRP3 inflammasome, and MLKL activation of MLKL by a small-molecule ligand was sufficient to induce the release of mature IL-1β in a GSDMD-independent manner [164]. Moreover, MLKL, which is downstream of TLR3, has also been shown to regulate NLRP3 inflammasome activation by inducing potassium efflux in response to dsDNA in the context of caspase-1 deficiency or blockade [139]. In addition to regulating potassium efflux, MLKL also promotes inflammasome activation by inducing ASC speck formation and NF-κB-dependent NLRP3 transcription [129]. However, the detailed mechanisms by which MLKL regulates ASC speck formation and NLRP3 transcription need to be further investigated.
In addition to MLKL, the mitochondrial phosphatase PGAM5 has also been reported to act as a functional necroptosis executor downstream of RIPK3 [152]. Although the functions of PGAM5 and its substrate DRP1 in necroptosis have been challenged, various studies have reported that these factor play important roles in NLRP3 inflammasome activation [141, 152, 154, 165–170]. Previous research by our group and others showed that RNA viruses induced the assembly of the RIP1/RIP3 complex, which subsequently promoted NLRP3 inflammasome activation in a DRP1-dependent but an MLKL-independent manner [154]. Mechanistically, the RIP1/RIP3 complex induces DRP1 activation and promotes its translocation to mitochondria to drive mitochondrial damage [154, 167]. Consistent with our findings, Zhou et al. discovered that DRP1, which is downstream of the RIP1/RIP3 complex, promoted NLRP3 inflammasome activation by causing mitochondrial dysfunction and mtROS release during subarachnoid hemorrhage-induced brain injury [165]. More recently, three inhibitors of DRP1 were shown to block NLRP3 inflammasome activation and thus alleviate inflammasome-associated disease [168–170]. In parallel, it has been shown that PGAM5 functions independently of RIPK3-mediated necroptosis to induce ROS production and promote ASC polymerization and inflammasome activation in the context of infection with vesicular stomatosis virus [152]. Moreover, PGAM5 also facilitated LPS-induced assembly and activation of the NLRP3 inflammasome in caspase-8-deficient BMDCs in a cell death-independent manner [141]. However, it is unclear how PGAM5 induces NLRP3 inflammasome activation. One possible mechanism is that PGAM5 regulates mitochondrial homeostasis.
As a newly identified necroptosis effector, ZBP1, also known as DNA-dependent activator of IFN-regulatory factors, has been reported in two separate studies to be involved in the regulation of NLRP3 inflammasome activation [28, 171–174]. Kuriakose et al. found that ZBP1 was a sensor of influenza virus (IAV) nucleoprotein and polymerase subunit PB1 and triggered NLRP3 inflammasome activation and proinflammatory cytokine production via the RIPK1–RIPK3–Caspase-8 axis [28]. In addition, ZBP1 was found to be essential for the release of IL-1β after infection with IAV [173]. It has been shown that in C. albicans and A. fumigatus infection, ZBP1 and its Zα2 domain were required for inflammasome activation during fungal infection, similar to infection with IAV [174]. Taken together, these findings indicate that ZBP1 is a critical regulator of NLRP3 inflammasome activation in host defense against IAV and certain kinds of fungal infections. However, our previous study revealed that ZBP1 was indispensable for defense against other RNA viruses, such as VSV-induced NLRP3 inflammasome activation [154]. Thus, the detailed mechanism that explains this discrepancy needs to be further explored.
Ferroptosis effectors in NLRP3 inflammasome activation
Ferroptosis, a novel type of PCD, was first coined by Brent R. Stockwell and Scott J. Dixon in 2012, when they discovered a unique iron-dependent form of nonapoptotic cell death triggered by the oncogenic RAS-selective lethal small-molecule erastin [175]. Ferroptosis differs from apoptosis and necroptosis morphologically and biochemically, depending on intracellular iron and exhibiting unique characteristics, including lipid peroxide accumulation, mitochondrial cristae loss, and mitochondrial membrane rupture and condensation [176]. Ferroptosis is triggered by high levels of iron- and ROS-dependent lipid peroxidation accumulation [176]. Specifically, cystine is transported intracellularly from the extracellular space by the xc− cystine-glutamate antiporter and is subsequently transformed into cysteine by thioredoxin reductase 1 (TXNRD1), which contributes to the biosynthesis of glutathione (GSH). GSH is an important cofactor of glutathione peroxidase 4 (GPX4) and promotes the GPX4-mediated reduction of phospholipid hydroperoxides into alcohols, ultimately reducing ROS accumulation and iron-dependent cell death. Ferroptosis is initiated when cysteine uptake is inhibited or GPX4-dependent antioxidants are blocked [175, 177]. Although the inhibitory effect of the system xc−–GSH–GPX4 pathway on ferroptosis has been widely accepted, recent studies have identified several other GPX4-independent protective pathways [178–181]. Two independent studies found that the CoQ oxidoreductase ferroptosis suppressor protein 1 (FSP1) acted in parallel with GPX4 to inhibit ferroptosis. FSP1 mediated the reduction of ubiquinone to ubiquinol, as well as the conversion of the oxidized α-tocopheryl radical (vitamin E) to oxidized α-tocopheryl as a nonradical form, both of which function as antioxidants to inhibit lipid peroxidation [178, 179]. In addition, squalene and di-/tetrahydrobiopterin (BH2/BH4) also act as antioxidants to inhibit lipid peroxidation [180, 181]. Ferroptosis, as an important mode of cell death, occurs in not only mammals but also in plants, protozoa, and fungi [177]. More recently, it has been shown that ferroptosis has a physiological role in tumor suppression and immune surveillance and is thus a promising target for anticancer therapy, but further studies are required to investigate its exact mechanism [182].
GPX4 is an important negative effector of ferroptosis and has recently been shown to inhibit caspase-11-dependent pyroptosis and IL-1β release [183]. The loss of GPX4 in BMDMs failed to affect the priming signal for inflammasome activation but increased caspase-11 activation and IL-1β maturation following Escherichia coli infection or LPS electroporation. Moreover, conditional knockout of Gpx4 in myeloid lineage cells facilitated susceptibility to polymicrobial infection in a GSDMD- and caspase-11-dependent manner [183]. Following this finding, another recent study reported that a small molecule called compound C (6-[4-(2-piperidin-1-yl-etoxy)-phenyl]-3-pyridin-4-yl-pyrazolo[1,5-a] pyrimidine) protected mice against HFD-induced obesity and hepatic steatosis by suppressing NLRP3 inflammasome activation. In addition, compound C promoted GPX4 expression at the mRNA level. These results suggest that GPX4 may have an inhibitory effect on NLRP3 inflammasome activation [184]. Overall, these studies indicate that GPX4 inhibits NLRP3 inflammasome activation and is as an important target in inflammasome-related diseases. However, the exact mechanism by which GPX4 inhibits NLRP3 inflammasome activation needs to be further explored.
Ubiquinone is a major substrate of FSP1, and its mitochondria-targeted derivative MitoQ has been shown to ameliorate experimental murine colitis by suppressing NLRP3 inflammasome activation [185]. In addition to ubiquinone, GSH was shown to strongly inhibit NLRP3 inflammasome activation in vitro and in vivo by modulating redox homeostasis [186]. However, Alberts et al. showed that TXNRD1 had no effect on NLRP3 inflammasome activation and that IL-1β secretion proceeded independent of oxidative stress in monocytes isolated from gout patients [187]. In conclusion, these findings indicate that redox homeostasis has opposite effects on NLRP3 inflammasome activation under different conditions, and the reason for these discrepancies may be due to differences in cell types.
In contrast to the inhibitory effect of the aforementioned regulators, several other ferroptosis regulators have been reported to promote NLRP3 inflammasome activation [188–190]. Desbien et al. found that squalene was an adjuvant to the TLR4 agonist GLA and potentiated GLA activity via caspase-1/11 and inflammasomes [188]. Coincidentally, inflammasome activation and the subsequent release of IL-1β have been found to be central effectors of squalene activity. Moreover, defects in ASC, NLRP3, or IL-1R activity completely ablated the effect of squalene [189], suggesting that squalene is a positive regulator of NLRP3 inflammasome activation. In addition, nuclear factor E2-related factor 2 (NRF2) is a recently identified inhibitor of ferroptosis that induces the expression of its target gene FSP1 [177]. Recent research has shown that NLRP3 activators fail to induce IL-1β secretion by inhibiting ASC speck formation in Nrf2-deficient BMDMs and human monocytes/macrophages [190]. In another proposed mechanism, NRF2 mediates the secretion of NLRP3 and IL-β by controlling phagocytosis [190]. However, it remains unclear how NRF2 regulates ASC speck formation and phagocytosis.
Targeting cell death effectors to treat NLRP3 inflammasome-associated diseases
The NLRP3 inflammasome is an important part of the body’s innate immunity and plays a critical role in defending against pathogen invasion and maintaining homeostasis. However, aberrant and uncontrolled activation of the NLRP3 inflammasome contributes to the pathogenesis of various inflammatory diseases, such as gouty, type 2 diabetes, atherosclerosis, tumors, and Alzheimer’s disease. Since various cell death effectors have been reported to regulate the activation of the NLRP3 inflammasome, their targeted inhibitors can be used to treat NLRP3-associated diseases (Table 2).
Table 2.
Targeted small molecules of cell death effectors for the treatment of NLRP3 inflammasome-associated diseases
| Pharmacological small molecules | Targets | Mechanisms | NLRP3 inflammasome-associated diseases | |
|---|---|---|---|---|
| Disulfiram | GSDMD (+) | Pyroptosis effectors | K+ efflux, DAMPs, and cytokines release | Sepsis, peritonitis |
| Necrosulfonamide | ||||
| MLKL (+) | Necroptosis effectors | K+ efflux, priming | Pulmonary damage | |
| Dabrafenib, GSK872 | RIPK3 (+) | mtROS, RIP3–NLRP3 interactions | Diabetic nephropathy, acute lung damage | |
| β-hydroxybutyrate, Mdivi-1, Metformin, resveratrol | DRP1 (+) | Mitochondrial dysfunction, mtROS | Subarachnoid hemorrhage (SAH)-induced brain injury, diabetic, acute kidney injury, sepsis | |
| Kaempferol 3-O-rutinoside | BAX/BAK (+) | Apoptosis effectors | K+ efflux, mtDNA | Severe fever with thrombocytopenia syndrome |
| Baicalein | c-FLIP (+) | Interacts with NLRP3 and pro-caspase-1 | Liver damage | |
| ASTX660, Birinapant | cIAP1/2 (+) | Interacts with pro-caspase-1 | Colorectal cancer | |
| NSC47147 | FADD (+) | Priming | Lymphoproliferative disease, endotoxemia | |
| Smac mimetic | XIAP (−) | mtROS, bind to caspase-3, -7, and -9 | Age-related macular degeneration | |
| Z-IETD-FMK, Berberine chloride | Caspase-8 (?) | K+ efflux, cleave pro-IL-1β and GSDMD, priming, mtROS, RIPK1–RIPK3–MLKL | Cryptococccosis, Alzheimer’s disease, Liver damage, obesity | |
| Compound C | GPX4 (−) | Ferroptosis effectors | mtROS | Obesity and hepatosteatosis |
| Oleoylethanolamide | NRF2 (+) | ASC polymerization, phagocytosis | Acute liver damage | |
Symbols: (+), promote; (−), inhibit; (?), dual functions of both promote and inhibit NLRP3 inflammasome activition
Necrosulfonamide (NSA)
NSA is a specific inhibitor of MLKL that binds to the active domain of MLKL to inhibit its activation, thereby preventing plasma-membrane rupture and necroptosis. Previous studies showed that NSA had a strong protective effect against pulmonary ischemia-reperfusion injury, Alzheimer’s disease, and psoriatic and neurological impairments by inhibiting necroptosis. In addition, the role of NSA in NLRP3 inflammasome activation has also been reported. Kitur et al. showed that NSA could inhibit IL-1β release by blocking MLKL‐mediated necroptosis. Moreover, NSA efficiently ameliorated NLRP3 inflammasome-dependent inflammatory pneumonia and pulmonary damage during Staphylococcus aureus (SA) infection [191]. Interestingly, another study discovered that NSA also acted as a targeted inhibitor of gasdermin D by covalently binding to a unique residue of human GSDMD, Cys191 (Cys192 in mice), and could therefore inhibit NLRP3 inflammasome-mediated pyroptosis and IL-1β release in primary cells [192]. More importantly, NSA treatment in vivo improved the survival of sepsis model mice [192]. However, a recent study by Rashidi et al. showed that the loss of GSDMD or MLKL neither inhibited MSU crystal-mediated release of bioactive IL-1β nor prevented MSU crystal-mediated peritonitis, whereas the inhibitor NSA blocked IL-1 production and cell death [193]. These results indicate that NSA inhibits NLRP3 inflammasome activation and related diseases in a GSDMD- and MLKL-independent manner, but the specific mechanism remains to be further explored.
Disulfiram (DSF)
DSF is an established drug that was approved by the Food and Drug Administration for the treatment of alcoholism in 1951. Since it has been widely used in the clinic for 70 years with no apparent side effects, DSF has been proven relatively safe for use by most patients [194]. The pharmacological mechanism of DSF in the treatment of alcoholism involves inhibiting the conversion of aldehyde dehydrogenase to acetate, which promotes alcohol consumption [195]. Increasing evidence indicates that DSF has great potential for the treatment of a variety of diseases in addition to alcoholism, especially NLRP3 inflammasome-related diseases [194–197]. Deng et al. showed that DSF could effectively inhibit NLRP3 inflammasome activation and IL-1β release [197]. More importantly, treatment with DSF showed notable therapeutic effects on LPS-induced septicopyemia and MSU-induced peritonitis. Mechanistically, DSF prevents lysosomal rupture and the subsequent release of cathepsin B into the cytoplasm [197]. In addition, DSF also inhibits ROS production, and ROS and cathepsin B are essential upstream signals of NLRP3 inflammasome activation [197]. However, the specific target of DSF was unknown until a recent study showed that it covalently modified GSDMD at the same site as NSA to block membrane pore formation [196]. Consistent with previous studies, DSF improved the survival of model mice with LPS-induced sepsis in an NLRP3 inflammasome-GSDMD-dependent manner [196]. In general, DSF can effectively inhibit NLRP3 inflammasome-associated diseases, although the exact mechanism remains to be further explored.
Other small-molecule compounds
In addition to the aforementioned inhibitors, some other antagonists of cell death regulators have also been shown to control the occurrence of disease by regulating NLRP3 inflammasome activation. It has been reported that the caspase-8 inhibitor Z-IETD-FMK blocks NLRP3 inflammasome activation in caspase-1/11-deficient BMDCs and suppresses cryptococcosis induced by Ab-opsonized Cryptococcus neoformans [198]. Compound C-induced GPX4 expression significantly alleviated HFD-induced obesity and nonalcoholic fatty liver disease by inhibiting NLRP3 inflammasome activation [184]. As a selective inhibitor of RIPK3, GSK872 has also been observed to significantly ameliorate LPS-induced acute lung injury by inhibiting the interaction between NLRP3 and RIP3 and inflammasome activation [199]. In addition, although originally identified as a B-raf inhibitor, dabrafenib was recently shown to be a potent RIPK3 inhibitor [200]. Shi et al. reported that dabrafenib treatment attenuated tubulointerstitial fibrosis and increased the survival rate by suppressing NLRP3 inflammasome activation in a mouse model of diabetic nephropathy [201]. In summary, these results suggest that Z-IETD-FMK, compound C, GSK872, and dabrafenib are potential therapeutic drugs for NLRP3-associated inflammatory diseases. However, the therapeutic effects of small-molecule compounds that target other cell death regulators on NLRP3 inflammasome-associated diseases are unclear and need to be further investigated.
Concluding remarks and future perspectives
The NLRP3 inflammasome is a cytosolic multiprotein complex consisting of NLRP3, ASC, and pro-caspase-1. Although the NLRP3 inflammasome plays a critical role in the host immune response, abnormal inflammasome activation has been implicated in the pathogenesis of various inflammatory diseases, such as diabetes, cancers, and Alzheimer’s disease. In the past decade, understanding of the mechanisms of NLRP3 inflammasome activation and regulation has advanced rapidly. Recent studies have begun to reveal the inextricable link between NLRP3 inflammasome activation and cell death, including pyroptosis, apoptosis, necroptosis, and ferroptosis. A large number of cell death effectors, such as c-FLIP, caspase-8, MLKL, and GPX4, have been shown to directly or indirectly regulate NLRP3 inflammasome activation and IL-1β release in response to a wide variety of PAMPs and DAMPs. Moreover, various agonists and inhibitors of cell death effectors have also been shown to be effective in treating NLRP3 inflammasome-associated diseases. However, these modulatory molecules have multiple functions in regulating the NLRP3 inflammasome in different cell types and tissues, creating major challenges for the clinical application of small molecules that target cell death effectors, and these problems will not be solved until the exact mechanisms by which these compounds regulate NLRP3 inflammasome activation are elucidated. Moreover, why these effectors have roles in NLRP3 inflammasome activation in addition to their roles in cell death, how numerous cell death pathways coordinate NLRP3 inflammasome activation, and the role of the NLRP3 inflammasome in regulating cell death including pyroptosis, apoptosis, necroptosis, and ferroptosis need to be further examined. Overall, understanding the inextricable link between NLRP3 inflammasome activation and cell death will not only advance our knowledge of the inflammatory response but will also provide new therapeutic targets and strategies for NLRP3 inflammasome-related diseases.
Acknowledgements
This work was supported by the Fundamental Research Funds for the Central Universities (WK2070000191, WK9110000037), the fellowship of China National Postdoctoral Program for Innovative Talents (BX20200325), and the Natural Science Foundation of Anhui province (1808085QH244).
Competing interests
The authors declare no competing interests.
Footnotes
These authors contributed equally: Yi Huang, Wen Xu.
References
- 1.Mukhopadhyay S, Pluddemann A, Gordon S. Macrophage pattern recognition receptors in immunity, homeostasis and self tolerance. Adv Exp Med Biol. 2009;653:1–14. doi: 10.1007/978-1-4419-0901-5_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.He Y, Hara H, Nunez G. Mechanism and regulation of NLRP3 inflammasome activation. Trends Biochem Sci. 2016;41:1012–21. doi: 10.1016/j.tibs.2016.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lu A, Magupalli VG, Ruan J, Yin Q, Atianand MK, Vos MR, et al. Unified polymerization mechanism for the assembly of ASC-dependent inflammasomes. Cell. 2014;156:1193–206. doi: 10.1016/j.cell.2014.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Martinon F, Burns K, Tschopp J. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol. Cell. 2002;10:417–26. doi: 10.1016/s1097-2765(02)00599-3. [DOI] [PubMed] [Google Scholar]
- 5.Lei Q, Yi T, Chen C. NF-kappaB-Gasdermin D (GSDMD) axis couples oxidative stress and NACHT, LRR and PYD Domains-Containing Protein 3 (NLRP3) inflammasome-mediated cardiomyocyte pyroptosis following myocardial infarction. Med Sci Monit. 2018;24:6044–52. doi: 10.12659/MSM.908529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shi J, Zhao Y, Wang K, Shi X, Wang Y, Huang H, et al. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature. 2015;526:660–5. doi: 10.1038/nature15514. [DOI] [PubMed] [Google Scholar]
- 7.He WT, Wan H, Hu L, Chen P, Wang X, Huang Z, et al. Gasdermin D is an executor of pyroptosis and required for interleukin-1beta secretion. Cell Res. 2015;25:1285–98. doi: 10.1038/cr.2015.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang Z, Zhang S, Xiao Y, Zhang W, Wu S, Qin T, et al. NLRP3 inflammasome and inflammatory diseases. Oxid Med Cell Longev. 2020;2020:4063562. doi: 10.1155/2020/4063562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tourkochristou E, Aggeletopoulou I, Konstantakis C, Triantos C. Role of NLRP3 inflammasome in inflammatory bowel diseases. World J Gastroenterol. 2019;25:4796–804. doi: 10.3748/wjg.v25.i33.4796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abderrazak A, Syrovets T, Couchie D, El Hadri K, Friguet B, Simmet T, et al. NLRP3 inflammasome: from a danger signal sensor to a regulatory node of oxidative stress and inflammatory diseases. Redox Biol. 2015;4:296–307. doi: 10.1016/j.redox.2015.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Conrad M, Angeli JP, Vandenabeele P, Stockwell BR. Regulated necrosis: disease relevance and therapeutic opportunities. Nat Rev Drug Discov. 2016;15:348–66. doi: 10.1038/nrd.2015.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fuchs Y, Steller H. Live to die another way: modes of programmed cell death and the signals emanating from dying cells. Nat Rev Mol Cell Biol. 2015;16:329–44. doi: 10.1038/nrm3999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Galluzzi L, Vitale I, Aaronson SA, Abrams JM, Adam D, Agostinis P, et al. Molecular mechanisms of cell death: recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death Differ. 2018;25:486–541. doi: 10.1038/s41418-017-0012-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Galluzzi L, Bravo-San Pedro JM, Vitale I, Aaronson SA, Abrams JM, Adam D, et al. Essential versus accessory aspects of cell death: recommendations of the NCCD 2015. Cell Death Differ. 2015;22:58–73. doi: 10.1038/cdd.2014.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Radi ZA, Stewart ZS, O’Neil SP. Accidental and programmed cell death in investigative and toxicologic pathology. Curr Protoc Toxicol. 2018;76:e51. doi: 10.1002/cptx.51. [DOI] [PubMed] [Google Scholar]
- 16.Lin X, Sun T, Cai M, Shen P. Cell-death-mode switch from necrosis to apoptosis in hydrogen peroxide treated macrophages. Sci China Life Sci. 2010;53:1196–203. doi: 10.1007/s11427-010-4075-4. [DOI] [PubMed] [Google Scholar]
- 17.Ameisen JC. On the origin, evolution, and nature of programmed cell death: a timeline of four billion years. Cell Death Differ. 2002;9:367–93. doi: 10.1038/sj.cdd.4400950. [DOI] [PubMed] [Google Scholar]
- 18.Fuchs Y, Steller H. Programmed cell death in animal development and disease. Cell. 2011;147:742–58. doi: 10.1016/j.cell.2011.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jorgensen I, Rayamajhi M, Miao EA. Programmed cell death as a defence against infection. Nat Rev Immunol. 2017;17:151–64. doi: 10.1038/nri.2016.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Korsmeyer SJ. Programmed cell death and the regulation of homeostasis. Harvey Lect. 1999;95:21–41. [PubMed] [Google Scholar]
- 21.Godlewski M, Kobylinska A. Programmed cell death—strategy for maintenance cellular organisms homeostasis. Postepy Hig Med Dosw (Online) 2016;70:1229–44. [PubMed] [Google Scholar]
- 22.Duprez L, Wirawan E, Vanden Berghe T, Vandenabeele P. Major cell death pathways at a glance. Microbes Infect. 2009;11:1050–62. doi: 10.1016/j.micinf.2009.08.013. [DOI] [PubMed] [Google Scholar]
- 23.Uversky AV, Xue B, Peng Z, Kurgan L, Uversky VN. On the intrinsic disorder status of the major players in programmed cell death pathways. F1000Res. 2013;2:190. doi: 10.12688/f1000research.2-190.v1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lamkanfi M, Dixit VM. Manipulation of host cell death pathways during microbial infections. Cell Host Microbe. 2010;8:44–54. doi: 10.1016/j.chom.2010.06.007. [DOI] [PubMed] [Google Scholar]
- 25.Vince JE, Silke J. The intersection of cell death and inflammasome activation. Cell Mol Life Sci. 2016;73:2349–67. doi: 10.1007/s00018-016-2205-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gaidt MM, Hornung V. The NLRP3 inflammasome renders cell death pro-inflammatory. J Mol Biol. 2018;430:133–41. doi: 10.1016/j.jmb.2017.11.013. [DOI] [PubMed] [Google Scholar]
- 27.Zheng M, Williams EP, Malireddi R, Karki R, Banoth B, Burton A, et al. Impaired NLRP3 inflammasome activation/pyroptosis leads to robust inflammatory cell death via caspase-8/RIPK3 during coronavirus infection. J Biol Chem. 2020;295:14040–52. doi: 10.1074/jbc.RA120.015036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kuriakose T, Man SM, Malireddi RK, Karki R, Kesavardhana S, Place DE, et al. ZBP1/DAI is an innate sensor of influenza virus triggering the NLRP3 inflammasome and programmed cell death pathways. Sci Immunol. 2016;1. 10.1126/sciimmunol.aag2045. [DOI] [PMC free article] [PubMed]
- 29.Swanson KV, Deng M, Ting JP. The NLRP3 inflammasome: molecular activation and regulation to therapeutics. Nat Rev Immunol. 2019;19:477–89. doi: 10.1038/s41577-019-0165-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kelley N, Jeltema D, Duan Y, He Y. The NLRP3 inflammasome: an overview of mechanisms of activation and regulation. Int J Mol Sci. 2019;20. 10.3390/ijms20133328. [DOI] [PMC free article] [PubMed]
- 31.Bauernfeind FG, Horvath G, Stutz A, Alnemri ES, MacDonald K, Speert D, et al. Cutting edge: NF-kappaB activating pattern recognition and cytokine receptors license NLRP3 inflammasome activation by regulating NLRP3 expression. J Immunol. 2009;183:787–91. doi: 10.4049/jimmunol.0901363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Franchi L, Eigenbrod T, Nunez G. Cutting edge: TNF-alpha mediates sensitization to ATP and silica via the NLRP3 inflammasome in the absence of microbial stimulation. J Immunol. 2009;183:792–6. doi: 10.4049/jimmunol.0900173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lin KM, Hu W, Troutman TD, Jennings M, Brewer T, Li X, et al. IRAK-1 bypasses priming and directly links TLRs to rapid NLRP3 inflammasome activation. Proc Natl Acad Sci U S A. 2014;111:775–80. doi: 10.1073/pnas.1320294111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xing Y, Yao X, Li H, Xue G, Guo Q, Yang G, et al. Cutting edge: TRAF6 mediates TLR/IL-1R signaling-induced nontranscriptional priming of the NLRP3 inflammasome. J Immunol. 2017;199:1561–6. doi: 10.4049/jimmunol.1700175. [DOI] [PubMed] [Google Scholar]
- 35.Py BF, Kim MS, Vakifahmetoglu-Norberg H, Yuan J. Deubiquitination of NLRP3 by BRCC3 critically regulates inflammasome activity. Mol Cell. 2013;49:331–8. doi: 10.1016/j.molcel.2012.11.009. [DOI] [PubMed] [Google Scholar]
- 36.Song N, Liu ZS, Xue W, Bai ZF, Wang QY, Dai J, et al. NLRP3 phosphorylation is an essential priming event for inflammasome activation. Mol Cell. 2017;68:185–97.e6. doi: 10.1016/j.molcel.2017.08.017. [DOI] [PubMed] [Google Scholar]
- 37.Muñoz-Planillo R, Kuffa P, Martínez-Colón G, Smith BL, Rajendiran TM, Núñez G. K(+) efflux is the common trigger of NLRP3 inflammasome activation by bacterial toxins and particulate matter. Immunity. 2013;38:1142–53. doi: 10.1016/j.immuni.2013.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Murakami T, Ockinger J, Yu J, Byles V, McColl A, Hofer AM, et al. Critical role for calcium mobilization in activation of the NLRP3 inflammasome. Proc Natl Acad Sci U S A. 2012;109:11282–7. doi: 10.1073/pnas.1117765109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tang T, Lang X, Xu C, Wang X, Gong T, Yang Y, et al. CLICs-dependent chloride efflux is an essential and proximal upstream event for NLRP3 inflammasome activation. Nat Commun. 2017;8:202. doi: 10.1038/s41467-017-00227-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Domingo-Fernandez R, Coll RC, Kearney J, Breit S, O’Neill LAJ. The intracellular chloride channel proteins CLIC1 and CLIC4 induce IL-1beta transcription and activate the NLRP3 inflammasome. J Biol Chem. 2017;292:12077–87. doi: 10.1074/jbc.M117.797126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schorn C, Frey B, Lauber K, Janko C, Strysio M, Keppeler H, et al. Sodium overload and water influx activate the NALP3 inflammasome. J Biol Chem. 2011;286:35–41. doi: 10.1074/jbc.M110.139048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhou R, Yazdi AS, Menu P, Tschopp J. A role for mitochondria in NLRP3 inflammasome activation. Nature. 2011;469:221–5. doi: 10.1038/nature09663. [DOI] [PubMed] [Google Scholar]
- 43.Halle A, Hornung V, Petzold GC, Stewart CR, Monks BG, Reinheckel T, et al. The NALP3 inflammasome is involved in the innate immune response to amyloid-beta. Nat Immunol. 2008;9:857–65. doi: 10.1038/ni.1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen J, Chen ZJ. PtdIns4P on dispersed trans-Golgi network mediates NLRP3 inflammasome activation. Nature. 2018;564:71–6. doi: 10.1038/s41586-018-0761-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kayagaki N, Warming S, Lamkanfi M, Vande Walle L, Louie S, Dong J, et al. Non-canonical inflammasome activation targets caspase-11. Nature. 2011;479:117–21. doi: 10.1038/nature10558. [DOI] [PubMed] [Google Scholar]
- 46.Casson CN, Yu J, Reyes VM, Taschuk FO, Yadav A, Copenhaver AM, et al. Human caspase-4 mediates noncanonical inflammasome activation against gram-negative bacterial pathogens. Proc Natl Acad Sci U S A. 2015;112:6688–93. doi: 10.1073/pnas.1421699112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schauvliege R, Vanrobaeys J, Schotte P, Beyaert R. Caspase-11 gene expression in response to lipopolysaccharide and interferon-gamma requires nuclear factor-kappa B and signal transducer and activator of transcription (STAT) 1. J Biol Chem. 2002;277:41624–30. doi: 10.1074/jbc.M207852200. [DOI] [PubMed] [Google Scholar]
- 48.Napier BA, Brubaker SW, Sweeney TE, Monette P, Rothmeier GH, Gertsvolf NA, et al. Complement pathway amplifies caspase-11-dependent cell death and endotoxin-induced sepsis severity. J Exp Med. 2016;213:2365–82. doi: 10.1084/jem.20160027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shi J, Zhao Y, Wang Y, Gao W, Ding J, Li P, et al. Inflammatory caspases are innate immune receptors for intracellular LPS. Nature. 2014;514:187–92. doi: 10.1038/nature13683. [DOI] [PubMed] [Google Scholar]
- 50.Man SM, Karki R, Sasai M, Place DE, Kesavardhana S, Temirov J, et al. IRGB10 liberates bacterial ligands for sensing by the AIM2 and Caspase-11-NLRP3 inflammasomes. Cell. 2016;167:382–96.e17. doi: 10.1016/j.cell.2016.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Meunier E, Dick MS, Dreier RF, Schürmann N, Kenzelmann Broz D, Warming S, et al. Caspase-11 activation requires lysis of pathogen-containing vacuoles by IFN-induced GTPases. Nature. 2014;509:366–70. doi: 10.1038/nature13157. [DOI] [PubMed] [Google Scholar]
- 52.Liu X, Zhang Z, Ruan J, Pan Y, Magupalli VG, Wu H, et al. Inflammasome-activated gasdermin D causes pyroptosis by forming membrane pores. Nature. 2016;535:153–8. doi: 10.1038/nature18629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ruhl S, Broz P. Caspase-11 activates a canonical NLRP3 inflammasome by promoting K(+) efflux. Eur J Immunol. 2015;45:2927–36. doi: 10.1002/eji.201545772. [DOI] [PubMed] [Google Scholar]
- 54.Zanoni I, Tan Y, Di Gioia M, Broggi A, Ruan J, Shi J, et al. An endogenous caspase-11 ligand elicits interleukin-1 release from living dendritic cells. Science. 2016;352:1232–6. doi: 10.1126/science.aaf3036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chu LH, Indramohan M, Ratsimandresy RA, Gangopadhyay A, Morris EP, Monack DM, et al. The oxidized phospholipid oxPAPC protects from septic shock by targeting the non-canonical inflammasome in macrophages. Nat Commun. 2018;9:996. doi: 10.1038/s41467-018-03409-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gaidt MM, Ebert TS, Chauhan D, Schmidt T, Schmid-Burgk JL, Rapino F, et al. Human monocytes engage an alternative inflammasome pathway. Immunity. 2016;44:833–46. doi: 10.1016/j.immuni.2016.01.012. [DOI] [PubMed] [Google Scholar]
- 57.Netea MG, Nold-Petry CA, Nold MF, Joosten LA, Opitz B, van der Meer JH, et al. Differential requirement for the activation of the inflammasome for processing and release of IL-1beta in monocytes and macrophages. Blood. 2009;113:2324–35. doi: 10.1182/blood-2008-03-146720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zewinger S, Reiser J, Jankowski V, Alansary D, Hahm E, Triem S, et al. Apolipoprotein C3 induces inflammation and organ damage by alternative inflammasome activation. Nat Immunol. 2020;21:30–41. doi: 10.1038/s41590-019-0548-1. [DOI] [PubMed] [Google Scholar]
- 59.Gong T, Zhou R. ApoC3: an ‘alarmin’ triggering sterile inflammation. Nat Immunol. 2020;21:9–11. doi: 10.1038/s41590-019-0562-3. [DOI] [PubMed] [Google Scholar]
- 60.Bergsbaken T, Fink SL, Cookson BT. Pyroptosis: host cell death and inflammation. Nat Rev Microbiol. 2009;7:99–109. doi: 10.1038/nrmicro2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hersh D, Monack DM, Smith MR, Ghori N, Falkow S, Zychlinsky A. The Salmonella invasin SipB induces macrophage apoptosis by binding to caspase-1. Proc Natl Acad Sci U S A. 1999;96:2396–401. doi: 10.1073/pnas.96.5.2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hilbi H, Moss JE, Hersh D, Chen Y, Arondel J, Banerjee S, et al. Shigella-induced apoptosis is dependent on caspase-1 which binds to IpaB. J Biol Chem. 1998;273:32895–900. doi: 10.1074/jbc.273.49.32895. [DOI] [PubMed] [Google Scholar]
- 63.Boise LH, Collins CM. Salmonella-induced cell death: apoptosis, necrosis or programmed cell death? Trends Microbiol. 2001;9:64–7. doi: 10.1016/s0966-842x(00)01937-5. [DOI] [PubMed] [Google Scholar]
- 64.Cookson BT, Brennan MA. Pro-inflammatory programmed cell death. Trends Microbiol. 2001;9:113–4. doi: 10.1016/s0966-842x(00)01936-3. [DOI] [PubMed] [Google Scholar]
- 65.Aglietti RA, Estevez A, Gupta A, Ramirez MG, Liu PS, Kayagaki N, et al. GsdmD p30 elicited by caspase-11 during pyroptosis forms pores in membranes. Proc Natl Acad Sci U S A. 2016;113:7858–63. doi: 10.1073/pnas.1607769113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sarhan J, Liu BC, Muendlein HI, Li P, Nilson R, Tang AY, et al. Caspase-8 induces cleavage of gasdermin D to elicit pyroptosis during Yersinia infection. Proc Natl Acad Sci U S A. 2018;115:E10888–97. doi: 10.1073/pnas.1809548115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ding J, Wang K, Liu W, She Y, Sun Q, Shi J, et al. Pore-forming activity and structural autoinhibition of the gasdermin family. Nature. 2016;535:111–6. doi: 10.1038/nature18590. [DOI] [PubMed] [Google Scholar]
- 68.Zhang Z, Zhang Y, Xia S, Kong Q, Li S, Liu X, et al. Gasdermin E suppresses tumour growth by activating anti-tumour immunity. Nature. 2020;579:415–20. doi: 10.1038/s41586-020-2071-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhou Z, He H, Wang K, Shi X, Wang Y, Su Y, et al. Granzyme A from cytotoxic lymphocytes cleaves GSDMB to trigger pyroptosis in target cells. Science. 2020;368. 10.1126/science.aaz7548. [DOI] [PubMed]
- 70.Wang Y, Gao W, Shi X, Ding J, Liu W, He H, et al. Chemotherapy drugs induce pyroptosis through caspase-3 cleavage of a gasdermin. Nature. 2017;547:99–103. doi: 10.1038/nature22393. [DOI] [PubMed] [Google Scholar]
- 71.Wang Q, Wang Y, Ding J, Wang C, Zhou X, Gao W, et al. A bioorthogonal system reveals antitumour immune function of pyroptosis. Nature. 2020;579:421–6. doi: 10.1038/s41586-020-2079-1. [DOI] [PubMed] [Google Scholar]
- 72.Yu J, Li S, Qi J, Chen Z, Wu Y, Guo J, et al. Cleavage of GSDME by caspase-3 determines lobaplatin-induced pyroptosis in colon cancer cells. Cell Death Dis. 2019;10:193. doi: 10.1038/s41419-019-1441-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gao YL, Zhai JH, Chai YF. Recent advances in the molecular mechanisms underlying pyroptosis in sepsis. Mediat Inflamm. 2018;2018:5823823. doi: 10.1155/2018/5823823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tan CC, Zhang JG, Tan MS, Chen H, Meng DW, Jiang T, et al. NLRP1 inflammasome is activated in patients with medial temporal lobe epilepsy and contributes to neuronal pyroptosis in amygdala kindling-induced rat model. J Neuroinflammation. 2015;12:18. doi: 10.1186/s12974-014-0233-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Duewell P, Kono H, Rayner KJ, Sirois CM, Vladimer G, Bauernfeind FG, et al. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature. 2010;464:1357–61. doi: 10.1038/nature08938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tan MS, Tan L, Jiang T, Zhu XC, Wang HF, Jia CD, et al. Amyloid-beta induces NLRP1-dependent neuronal pyroptosis in models of Alzheimer’s disease. Cell Death Dis. 2014;5:e1382. doi: 10.1038/cddis.2014.348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Taylor RC, Cullen SP, Martin SJ. Apoptosis: controlled demolition at the cellular level. Nat Rev Mol Cell Biol. 2008;9:231–41. doi: 10.1038/nrm2312. [DOI] [PubMed] [Google Scholar]
- 78.Kerr JF. A histochemical study of hypertrophy and ischaemic injury of rat liver with special reference to changes in lysosomes. J Pathol Bacteriol. 1965;90:419–35. doi: 10.1002/path.1700900210. [DOI] [PubMed] [Google Scholar]
- 79.Kerr JF, Wyllie AH, Currie AR. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer. 1972;26:239–57. doi: 10.1038/bjc.1972.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Elmore S. Apoptosis: a review of programmed cell death. Toxicol Pathol. 2007;35:495–516. doi: 10.1080/01926230701320337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hacker G. The morphology of apoptosis. Cell Tissue Res. 2000;301:5–17. doi: 10.1007/s004410000193. [DOI] [PubMed] [Google Scholar]
- 82.Zucker RM, Hunter ES, 3rd, Rogers JM. Confocal laser scanning microscopy of morphology and apoptosis in organogenesis-stage mouse embryos. Methods Mol Biol. 2000;135:191–202. doi: 10.1385/1-59259-685-1:191. [DOI] [PubMed] [Google Scholar]
- 83.Pihan P, Carreras-Sureda A, Hetz C. BCL-2 family: integrating stress responses at the ER to control cell demise. Cell Death Differ. 2017;24:1478–87. doi: 10.1038/cdd.2017.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Roos WP, Thomas AD, Kaina B. DNA damage and the balance between survival and death in cancer biology. Nat Rev Cancer. 2016;16:20–33. doi: 10.1038/nrc.2015.2. [DOI] [PubMed] [Google Scholar]
- 85.Nuñez G, London L, Hockenbery D, Alexander M, McKearn JP, Korsmeyer SJ. Deregulated Bcl-2 gene expression selectively prolongs survival of growth factor-deprived hemopoietic cell lines. J Immunol. 1990;144:3602–10. [PubMed] [Google Scholar]
- 86.Czabotar PE, Lessene G, Strasser A, Adams JM. Control of apoptosis by the BCL-2 protein family: implications for physiology and therapy. Nat Rev Mol Cell Biol. 2014;15:49–63. doi: 10.1038/nrm3722. [DOI] [PubMed] [Google Scholar]
- 87.Tait SW, Green DR. Mitochondria and cell death: outer membrane permeabilization and beyond. Nat Rev Mol Cell Biol. 2010;11:621–32. doi: 10.1038/nrm2952. [DOI] [PubMed] [Google Scholar]
- 88.Saelens X, Festjens N, Vande Walle L, van Gurp M, van Loo G, Vandenabeele P. Toxic proteins released from mitochondria in cell death. Oncogene. 2004;23:2861–74. doi: 10.1038/sj.onc.1207523. [DOI] [PubMed] [Google Scholar]
- 89.Chinnaiyan AM. The apoptosome: heart and soul of the cell death machine. Neoplasia. 1999;1:5–15. doi: 10.1038/sj.neo.7900003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hill MM, Adrain C, Duriez PJ, Creagh EM, Martin SJ. Analysis of the composition, assembly kinetics and activity of native Apaf-1 apoptosomes. EMBO J. 2004;23:2134–45. doi: 10.1038/sj.emboj.7600210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Li P, Nijhawan D, Budihardjo I, Srinivasula SM, Ahmad M, Alnemri ES, et al. Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell. 1997;91:479–89. doi: 10.1016/s0092-8674(00)80434-1. [DOI] [PubMed] [Google Scholar]
- 92.Slee EA, Adrain C, Martin SJ. Executioner caspase-3, -6, and -7 perform distinct, non-redundant roles during the demolition phase of apoptosis. J Biol Chem. 2001;276:7320–6. doi: 10.1074/jbc.M008363200. [DOI] [PubMed] [Google Scholar]
- 93.Susin SA, Lorenzo HK, Zamzami N, Marzo I, Snow BE, Brothers GM, et al. Molecular characterization of mitochondrial apoptosis-inducing factor. Nature. 1999;397:441–6. doi: 10.1038/17135. [DOI] [PubMed] [Google Scholar]
- 94.Joza N, Susin SA, Daugas E, Stanford WL, Cho SK, Li CY, et al. Essential role of the mitochondrial apoptosis-inducing factor in programmed cell death. Nature. 2001;410:549–54. doi: 10.1038/35069004. [DOI] [PubMed] [Google Scholar]
- 95.Locksley RM, Killeen N, Lenardo MJ. The TNF and TNF receptor superfamilies: integrating mammalian biology. Cell. 2001;104:487–501. doi: 10.1016/s0092-8674(01)00237-9. [DOI] [PubMed] [Google Scholar]
- 96.Wajant H. The Fas signaling pathway: more than a paradigm. Science. 2002;296:1635–6. doi: 10.1126/science.1071553. [DOI] [PubMed] [Google Scholar]
- 97.Fleten KG, Flørenes VA, Prasmickaite L, Hill O, Sykora J, Mælandsmo GM, et al. hvTRA, a novel TRAIL receptor agonist, induces apoptosis and sustained growth retardation in melanoma. Cell Death Discov. 2016;2:16081. doi: 10.1038/cddiscovery.2016.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kischkel FC, Hellbardt S, Behrmann I, Germer M, Pawlita M, Krammer PH, et al. Cytotoxicity-dependent APO-1 (Fas/CD95)-associated proteins form a death-inducing signaling complex (DISC) with the receptor. EMBO J. 1995;14:5579–88. doi: 10.1002/j.1460-2075.1995.tb00245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hsu H, Xiong J, Goeddel DV. The TNF receptor 1-associated protein TRADD signals cell death and NF-kappa B activation. Cell. 1995;81:495–504. doi: 10.1016/0092-8674(95)90070-5. [DOI] [PubMed] [Google Scholar]
- 100.Kallenberger SM, Beaudouin J, Claus J, Fischer C, Sorger PK, Legewie S, et al. Intra- and interdimeric caspase-8 self-cleavage controls strength and timing of CD95-induced apoptosis. Sci Signal. 2014;7:ra23. doi: 10.1126/scisignal.2004738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Oberst A, Pop C, Tremblay AG, Blais V, Denault JB, Salvesen GS, et al. Inducible dimerization and inducible cleavage reveal a requirement for both processes in caspase-8 activation. J Biol Chem. 2010;285:16632–42. doi: 10.1074/jbc.M109.095083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Li H, Zhu H, Xu CJ, Yuan J. Cleavage of BID by caspase 8 mediates the mitochondrial damage in the Fas pathway of apoptosis. Cell. 1998;94:491–501. doi: 10.1016/s0092-8674(00)81590-1. [DOI] [PubMed] [Google Scholar]
- 103.Micheau O, Tschopp J. Induction of TNF receptor I-mediated apoptosis via two sequential signaling complexes. Cell. 2003;114:181–90. doi: 10.1016/s0092-8674(03)00521-x. [DOI] [PubMed] [Google Scholar]
- 104.Hague A, Paraskeva C. Apoptosis and disease: a matter of cell fate. Cell Death Differ. 2004;11:1366–72. doi: 10.1038/sj.cdd.4401497. [DOI] [PubMed] [Google Scholar]
- 105.Solano-Galvez SG, Abadi-Chiriti J, Gutiérrez-Velez L, Rodríguez-Puente E, Konstat-Korzenny E, Álvarez-Hernández DA, et al. Apoptosis: activation and inhibition in health and disease. Med Sci. 2018;6. 10.3390/medsci6030054. [DOI] [PMC free article] [PubMed]
- 106.Sinkovics JG, Horvath JC. Role of apoptosis in health and disease. JAMA. 1998;279:1699. [PubMed] [Google Scholar]
- 107.Dhuriya YK, Sharma D. Necroptosis: a regulated inflammatory mode of cell death. J Neuroinflammation. 2018;15:199. doi: 10.1186/s12974-018-1235-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Vercammen D, Vandenabeele P, Beyaert R, Declercq W, Fiers W. Tumour necrosis factor-induced necrosis versus anti-Fas-induced apoptosis in L929 cells. Cytokine. 1997;9:801–8. doi: 10.1006/cyto.1997.0252. [DOI] [PubMed] [Google Scholar]
- 109.Kaiser WJ, Sridharan H, Huang C, Mandal P, Upton JW, Gough PJ, et al. Toll-like receptor 3-mediated necrosis via TRIF, RIP3, and MLKL. J Biol Chem. 2013;288:31268–79. doi: 10.1074/jbc.M113.462341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Upton JW, Kaiser WJ, Mocarski ES. DAI/ZBP1/DLM-1 complexes with RIP3 to mediate virus-induced programmed necrosis that is targeted by murine cytomegalovirus vIRA. Cell Host Microbe. 2012;11:290–7. doi: 10.1016/j.chom.2012.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Rodriguez DA, Weinlich R, Brown S, Guy C, Fitzgerald P, Dillon CP, et al. Characterization of RIPK3-mediated phosphorylation of the activation loop of MLKL during necroptosis. Cell Death Differ. 2016;23:76–88. doi: 10.1038/cdd.2015.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Vanden Berghe T, Hassannia B, Vandenabeele P. An outline of necrosome triggers. Cell Mol Life Sci. 2016;73:2137–52. doi: 10.1007/s00018-016-2189-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Sun L, Wang H, Wang Z, He S, Chen S, Liao D, et al. Mixed lineage kinase domain-like protein mediates necrosis signaling downstream of RIP3 kinase. Cell. 2012;148:213–27. doi: 10.1016/j.cell.2011.11.031. [DOI] [PubMed] [Google Scholar]
- 114.Wang Z, Jiang H, Chen S, Du F, Wang X. The mitochondrial phosphatase PGAM5 functions at the convergence point of multiple necrotic death pathways. Cell. 2012;148:228–43. doi: 10.1016/j.cell.2011.11.030. [DOI] [PubMed] [Google Scholar]
- 115.Lu W, Sun J, Yoon JS, Zhang Y, Zheng L, Murphy E, et al. Mitochondrial protein PGAM5 regulates mitophagic protection against cell necroptosis. PLoS ONE. 2016;11:e0147792. doi: 10.1371/journal.pone.0147792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Moriwaki K, Farias Luz N, Balaji S, De Rosa MJ, O'Donnell CL, Gough PJ, et al. The mitochondrial phosphatase PGAM5 is dispensable for necroptosis but promotes inflammasome activation in macrophages. J Immunol. 2016;196:407–15. doi: 10.4049/jimmunol.1501662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Moujalled DM, Cook WD, Murphy JM, Vaux DL. Necroptosis induced by RIPK3 requires MLKL but not Drp1. Cell Death Dis. 2014;5:e1086. doi: 10.1038/cddis.2014.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Wang X, Jiang W, Yan Y, Gong T, Han J, Tian Z, et al. RNA viruses promote activation of the NLRP3 inflammasome through a RIP1-RIP3-DRP1 signaling pathway. Nat Immunol. 2014;15:1126–33. doi: 10.1038/ni.3015. [DOI] [PubMed] [Google Scholar]
- 119.Choi ME, Price DR, Ryter SW, Choi AMK. Necroptosis: a crucial pathogenic mediator of human disease. JCI Insight. 2019;4. 10.1172/jci.insight.128834. [DOI] [PMC free article] [PubMed]
- 120.Khoury MK, Gupta K, Franco SR, Liu B. Necroptosis in the pathophysiology of disease. Am J Pathol. 2020;190:272–85. doi: 10.1016/j.ajpath.2019.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Dixon SJ, Lemberg KM, Lamprecht MR, Skouta R, Zaitsev EM, Gleason CE, et al. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell. 2012;149:1060–72. doi: 10.1016/j.cell.2012.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Latunde-Dada GO. Ferroptosis: role of lipid peroxidation, iron and ferritinophagy. Biochim Biophys Acta Gen Subj. 2017;1861:1893–900. doi: 10.1016/j.bbagen.2017.05.019. [DOI] [PubMed] [Google Scholar]
- 123.Jiang X, Stockwell BR, Conrad M. Ferroptosis: mechanisms, biology and role in disease. Nat Rev Mol Cell Biol. 2021. 10.1038/s41580-020-00324-8. [DOI] [PMC free article] [PubMed]
- 124.Bersuker K, Hendricks JM, Li Z, Magtanong L, Ford B, Tang PH, et al. The CoQ oxidoreductase FSP1 acts parallel to GPX4 to inhibit ferroptosis. Nature. 2019;575:688–92. doi: 10.1038/s41586-019-1705-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Doll S, Freitas FP, Shah R, Aldrovandi M, da Silva MC, Ingold I, et al. FSP1 is a glutathione-independent ferroptosis suppressor. Nature. 2019;575:693–8. doi: 10.1038/s41586-019-1707-0. [DOI] [PubMed] [Google Scholar]
- 126.Sainani GS, Edul NC, Phadke MA, Khedkar VA. Cell mediated immunity in rheumatic fever. Indian Heart J. 1981;33:270–3. [PubMed] [Google Scholar]
- 127.Garcia-Bermudez J, Baudrier L, Bayraktar EC, Shen Y, La K, Guarecuco R, et al. Squalene accumulation in cholesterol auxotrophic lymphomas prevents oxidative cell death. Nature. 2019;567:118–22. doi: 10.1038/s41586-019-0945-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Yuan H, Pratte J, Giardina C. Ferroptosis and its potential as a therapeutic target. Biochem Pharm. 2021;186:114486. doi: 10.1016/j.bcp.2021.114486. [DOI] [PubMed] [Google Scholar]
- 129.Broz P, Pelegrin P, Shao F. The gasdermins, a protein family executing cell death and inflammation. Nat Rev Immunol. 2020;20:143–57. doi: 10.1038/s41577-019-0228-2. [DOI] [PubMed] [Google Scholar]
- 130.Shi J, Gao W, Shao F. Pyroptosis: Gasdermin-mediated programmed necrotic cell death. Trends Biochem Sci. 2017;42:245–54. doi: 10.1016/j.tibs.2016.10.004. [DOI] [PubMed] [Google Scholar]
- 131.Liu X, Xia S, Zhang Z, Wu H, Lieberman J. Channelling inflammation: gasdermins in physiology and disease. Nat Rev Drug Discov. 2021. 10.1038/s41573-021-00154-z. [DOI] [PMC free article] [PubMed]
- 132.Ruan J, Xia S, Liu X, Lieberman J, Wu H. Cryo-EM structure of the gasdermin A3 membrane pore. Nature. 2018;557:62–7. doi: 10.1038/s41586-018-0058-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Kayagaki N, Stowe IB, Lee BL, O'Rourke K, Anderson K, Warming S, et al. Caspase-11 cleaves gasdermin D for non-canonical inflammasome signalling. Nature. 2015;526:666–71. doi: 10.1038/nature15541. [DOI] [PubMed] [Google Scholar]
- 134.Sborgi L, Rühl S, Mulvihill E, Pipercevic J, Heilig R, Stahlberg H, et al. GSDMD membrane pore formation constitutes the mechanism of pyroptotic cell death. EMBO J. 2016;35:1766–78. doi: 10.15252/embj.201694696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Kovacs SB, Miao EA. Gasdermins: effectors of pyroptosis. Trends Cell Biol. 2017;27:673–84. doi: 10.1016/j.tcb.2017.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Hoffmann EK, Lambert IH, Pedersen SF. Physiology of cell volume regulation in vertebrates. Physiol Rev. 2009;89:193–277. doi: 10.1152/physrev.00037.2007. [DOI] [PubMed] [Google Scholar]
- 137.Rühl S, Shkarina K, Demarco B, Heilig R, Santos JC, Broz P. ESCRT-dependent membrane repair negatively regulates pyroptosis downstream of GSDMD activation. Science. 2018;362:956–60. doi: 10.1126/science.aar7607. [DOI] [PubMed] [Google Scholar]
- 138.Frank D, Vince JE. Pyroptosis versus necroptosis: similarities, differences, and crosstalk. Cell Death Differ. 2019;26:99–114. doi: 10.1038/s41418-018-0212-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Chao KL, Kulakova L, Herzberg O. Gene polymorphism linked to increased asthma and IBD risk alters gasdermin-B structure, a sulfatide and phosphoinositide binding protein. Proc Natl Acad Sci U S A. 2017;114:E1128–37. doi: 10.1073/pnas.1616783114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Chen Q, Shi P, Wang Y, Zou D, Wu X, Wang D, et al. GSDMB promotes non-canonical pyroptosis by enhancing caspase-4 activity. J Mol Cell Biol. 2019;11:496–508. doi: 10.1093/jmcb/mjy056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Strasser A, Vaux DL. Viewing BCL2 and cell death control from an evolutionary perspective. Cell Death Differ. 2018;25:13–20. doi: 10.1038/cdd.2017.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Vince JE, De Nardo D, Gao W, Vince AJ, Hall C, McArthur K, et al. The mitochondrial apoptotic effectors BAX/BAK activate Caspase-3 and -7 to trigger NLRP3 inflammasome and Caspase-8 driven IL-1beta activation. Cell Rep. 2018;25:2339–53.e4. doi: 10.1016/j.celrep.2018.10.103. [DOI] [PubMed] [Google Scholar]
- 143.Li S, Li H, Zhang YL, Xin QL, Guan ZQ, Chen X, et al. SFTSV infection induces BAK/BAX-dependent mitochondrial DNA release to trigger NLRP3 inflammasome activation. Cell Rep. 2020;30:4370–85.e7. doi: 10.1016/j.celrep.2020.02.105. [DOI] [PubMed] [Google Scholar]
- 144.Chauhan D, Bartok E, Gaidt MM, Bock FJ, Herrmann J, Seeger JM, et al. BAX/BAK-induced apoptosis results in Caspase-8-dependent IL-1beta maturation in macrophages. Cell Rep. 2018;25:2354–68.e5. doi: 10.1016/j.celrep.2018.10.087. [DOI] [PubMed] [Google Scholar]
- 145.Safa AR. c-FLIP, a master anti-apoptotic regulator. Exp Oncol. 2012;34:176–84. [PMC free article] [PubMed] [Google Scholar]
- 146.Wu YH, Kuo WC, Wu YJ, Yang KT, Chen ST, Jiang ST, et al. Participation of c-FLIP in NLRP3 and AIM2 inflammasome activation. Cell Death Differ. 2014;21:451–61. doi: 10.1038/cdd.2013.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Labbe K, McIntire CR, Doiron K, Leblanc PM, Saleh M. Cellular inhibitors of apoptosis proteins cIAP1 and cIAP2 are required for efficient caspase-1 activation by the inflammasome. Immunity. 2011;35:897–907. doi: 10.1016/j.immuni.2011.10.016. [DOI] [PubMed] [Google Scholar]
- 148.Suzuki S, Suzuki T, Mimuro H, Mizushima T, Sasakawa C. Shigella hijacks the glomulin-cIAPs-inflammasome axis to promote inflammation. EMBO Rep. 2018;19:89–101. doi: 10.15252/embr.201643841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Gurung P, Anand PK, Malireddi RK, Vande Walle L, Van Opdenbosch N, Dillon CP, et al. FADD and caspase-8 mediate priming and activation of the canonical and noncanonical Nlrp3 inflammasomes. J Immunol. 2014;192:1835–46. doi: 10.4049/jimmunol.1302839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Allam R, Lawlor KE, Yu EC, Mildenhall AL, Moujalled DM, Lewis RS, et al. Mitochondrial apoptosis is dispensable for NLRP3 inflammasome activation but non-apoptotic caspase-8 is required for inflammasome priming. EMBO Rep. 2014;15:982–90. doi: 10.15252/embr.201438463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Zhang X, Fan C, Zhang H, Zhao Q, Liu Y, Xu C, et al. MLKL and FADD are critical for suppressing progressive lymphoproliferative disease and activating the NLRP3 inflammasome. Cell Rep. 2016;16:3247–59. doi: 10.1016/j.celrep.2016.06.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Zheng M, Karki R, Vogel P, Kanneganti TD. Caspase-6 is a key regulator of innate immunity, inflammasome activation, and host defense. Cell. 2020;181:674–87.e13. doi: 10.1016/j.cell.2020.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Yabal M, Müller N, Adler H, Knies N, Groß CJ, Damgaard RB, et al. XIAP restricts TNF- and RIP3-dependent cell death and inflammasome activation. Cell Rep. 2014;7:1796–808. doi: 10.1016/j.celrep.2014.05.008. [DOI] [PubMed] [Google Scholar]
- 154.Vince JE, Wong WW, Gentle I, Lawlor KE, Allam R, O'Reilly L, et al. Inhibitor of apoptosis proteins limit RIP3 kinase-dependent interleukin-1 activation. Immunity. 2012;36:215–27. doi: 10.1016/j.immuni.2012.01.012. [DOI] [PubMed] [Google Scholar]
- 155.Gao J, Cui JZ, Wang A, Chen H, Fong A, Matsubara JA. The reduction of XIAP is associated with inflammasome activation in RPE: implications for AMD pathogenesis. J Neuroinflammation. 2019;16:171. doi: 10.1186/s12974-019-1558-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Shi CS, Kehrl JH. Cytochrome c negatively regulates NLRP3 inflammasomes. PLoS ONE. 2016;11:e0167636. doi: 10.1371/journal.pone.0167636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Shimada K, Crother TR, Karlin J, Dagvadorj J, Chiba N, Chen S, et al. Oxidized mitochondrial DNA activates the NLRP3 inflammasome during apoptosis. Immunity. 2012;36:401–14. doi: 10.1016/j.immuni.2012.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Liu T, Tang L, Tang H, Pu J, Gong S, Fang D, et al. Zika virus infection induces acute kidney injury through activating NLRP3 inflammasome via suppressing Bcl-2. Front Immunol. 2019;10:1925. doi: 10.3389/fimmu.2019.01925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Orning P, Weng D, Starheim K, Ratner D, Best Z, Lee B, et al. Pathogen blockade of TAK1 triggers caspase-8-dependent cleavage of gasdermin D and cell death. Science. 2018;362:1064–9. doi: 10.1126/science.aau2818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Chen KW, Demarco B, Heilig R, Shkarina K, Boettcher A, Farady CJ, et al. Extrinsic and intrinsic apoptosis activate pannexin-1 to drive NLRP3 inflammasome assembly. EMBO J. 2019;38. 10.15252/embj.2019101638. [DOI] [PMC free article] [PubMed]
- 161.Kang S, Fernandes-Alnemri T, Rogers C, Mayes L, Wang Y, Dillon C, et al. Caspase-8 scaffolding function and MLKL regulate NLRP3 inflammasome activation downstream of TLR3. Nat Commun. 2015;6:7515. doi: 10.1038/ncomms8515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Maelfait J, Vercammen E, Janssens S, Schotte P, Haegman M, Magez S, et al. Stimulation of Toll-like receptor 3 and 4 induces interleukin-1beta maturation by caspase-8. J Exp Med. 2008;205:1967–73. doi: 10.1084/jem.20071632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Kang TB, Yang SH, Toth B, Kovalenko A, Wallach D. Caspase-8 blocks kinase RIPK3-mediated activation of the NLRP3 inflammasome. Immunity. 2013;38:27–40. doi: 10.1016/j.immuni.2012.09.015. [DOI] [PubMed] [Google Scholar]
- 164.Antonopoulos C, Russo HM, El Sanadi C, Martin BN, Li X, Kaiser WJ, et al. Caspase-8 as an effector and regulator of NLRP3 inflammasome signaling. J Biol Chem. 2015;290:20167–84. doi: 10.1074/jbc.M115.652321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Speir M, Lawlor KE. RIP-roaring inflammation: RIPK1 and RIPK3 driven NLRP3 inflammasome activation and autoinflammatory disease. Semin Cell Dev Biol. 2021;109:114–24. doi: 10.1016/j.semcdb.2020.07.011. [DOI] [PubMed] [Google Scholar]
- 166.Moriwaki K, Chan FK. RIP3: a molecular switch for necrosis and inflammation. Genes Dev. 2013;27:1640–9. doi: 10.1101/gad.223321.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Lawlor KE, Khan N, Mildenhall A, Gerlic M, Croker BA, D'Cruz AA, et al. RIPK3 promotes cell death and NLRP3 inflammasome activation in the absence of MLKL. Nat Commun. 2015;6:6282. doi: 10.1038/ncomms7282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Huang HR, Cho SJ, Harris RM, Yang J, Bermejo S. Sharma L, et al. RIPK3 activates MLKL-mediated necroptosis and inflammasome signaling during streptococcus infection. Am J Respir Cell Mol Biol. 2021. 10.1165/rcmb.2020-0312OC. [DOI] [PMC free article] [PubMed]
- 169.Shi Y, Huang C, Yi H, Cao Q, Zhao Y, Chen J, et al. RIPK3 blockade attenuates kidney fibrosis in a folic acid model of renal injury. FASEB J. 2020;34:10286–98. doi: 10.1096/fj.201902544RR. [DOI] [PubMed] [Google Scholar]
- 170.Zhao J, Jitkaew S, Cai Z, Choksi S, Li Q, Luo J, et al. Mixed lineage kinase domain-like is a key receptor interacting protein 3 downstream component of TNF-induced necrosis. Proc Natl Acad Sci U S A. 2012;109:5322–7. doi: 10.1073/pnas.1200012109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Conos SA, Chen KW, De Nardo D, Hara H, Whitehead L, Núñez G, et al. Active MLKL triggers the NLRP3 inflammasome in a cell-intrinsic manner. Proc Natl Acad Sci U S A. 2017;114:E961–9. doi: 10.1073/pnas.1613305114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Gutierrez KD, Davis MA, Daniels BP, Olsen TM, Ralli-Jain P, Tait SW, et al. MLKL activation triggers NLRP3-mediated processing and release of IL-1beta independently of Gasdermin-D. J Immunol. 2017;198:2156–64. doi: 10.4049/jimmunol.1601757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Zhou K, Shi L, Wang Z, Zhou J, Manaenko A, Reis C, et al. RIP1-RIP3-DRP1 pathway regulates NLRP3 inflammasome activation following subarachnoid hemorrhage. Exp Neurol. 2017;295:116–24. doi: 10.1016/j.expneurol.2017.06.003. [DOI] [PubMed] [Google Scholar]
- 174.Holley CL, Schroder K. The rOX-stars of inflammation: links between the inflammasome and mitochondrial meltdown. Clin Transl Immunol. 2020;9:e01109. doi: 10.1002/cti2.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Park HS, Liu G, Liu Q, Zhou Y. Swine influenza virus induces RIPK1/DRP1-mediated interleukin-1 beta production. Viruses. 2018;10. 10.3390/v10080419. [DOI] [PMC free article] [PubMed]
- 176.Li A, Zhang S, Li J, Liu K, Huang F, Liu B. Metformin and resveratrol inhibit Drp1-mediated mitochondrial fission and prevent ER stress-associated NLRP3 inflammasome activation in the adipose tissue of diabetic mice. Mol Cell Endocrinol. 2016;434:36–47. doi: 10.1016/j.mce.2016.06.008. [DOI] [PubMed] [Google Scholar]
- 177.Guo M, Wang X, Zhao Y, Yang Q, Ding H, Dong Q, et al. Ketogenic diet improves brain ischemic tolerance and inhibits NLRP3 inflammasome activation by preventing Drp1-mediated mitochondrial fission and endoplasmic reticulum stress. Front Mol Neurosci. 2018;11:86. doi: 10.3389/fnmol.2018.00086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Liu R, Wang SC, Li M, Ma XH, Jia XN, Bu Y, et al. An inhibitor of DRP1 (Mdivi-1) alleviates LPS-induced septic AKI by inhibiting NLRP3 inflammasome activation. Biomed Res Int. 2020;2020:2398420. doi: 10.1155/2020/2398420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Malireddi RKS, Kesavardhana S, Kanneganti TD. ZBP1 and TAK1: master regulators of NLRP3 inflammasome/pyroptosis, apoptosis, and necroptosis (PAN-optosis) Front Cell Infect Microbiol. 2019;9:406. doi: 10.3389/fcimb.2019.00406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Zheng M, Kanneganti TD. The regulation of the ZBP1-NLRP3 inflammasome and its implications in pyroptosis, apoptosis, and necroptosis (PANoptosis) Immunol Rev. 2020;297:26–38. doi: 10.1111/imr.12909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Thapa RJ, Ingram JP, Ragan KB, Nogusa S, Boyd DF, Benitez AA, et al. DAI senses influenza A virus genomic RNA and activates RIPK3-dependent cell death. Cell Host Microbe. 2016;20:674–81. doi: 10.1016/j.chom.2016.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Banoth B, Tuladhar S, Karki R, Sharma BR, Briard B, Kesavardhana S, et al. ZBP1 promotes fungi-induced inflammasome activation and pyroptosis, apoptosis, and necroptosis (PANoptosis) J Biol Chem. 2020;295:18276–83. doi: 10.1074/jbc.RA120.015924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Kang R, Zeng L, Zhu S, Xie Y, Liu J, Wen Q, et al. Lipid peroxidation drives Gasdermin D-mediated pyroptosis in lethal polymicrobial sepsis. Cell Host Microbe. 2018;24:97–108.e4. doi: 10.1016/j.chom.2018.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Wang F, Liu Y, Yuan J, Yang W, Mo Z. Compound C protects mice from HFD-induced obesity and nonalcoholic fatty liver disease. Int J Endocrinol. 2019;2019:3206587. doi: 10.1155/2019/3206587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Dashdorj A, Jyothi KR, Lim S, Jo A, Nguyen MN, Ha J, et al. Mitochondria-targeted antioxidant MitoQ ameliorates experimental mouse colitis by suppressing NLRP3 inflammasome-mediated inflammatory cytokines. BMC Med. 2013;11:178. doi: 10.1186/1741-7015-11-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Zhang T, Tsutsuki H, Islam W, Ono K, Takeda K, Akaike T, et al. ATP exposure stimulates glutathione efflux as a necessary switch for NLRP3 inflammasome activation. Redox Biol. 2021;41:101930. doi: 10.1016/j.redox.2021.101930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Alberts BM, Bruce C, Basnayake K, Ghezzi P, Davies KA, Mullen LM. Secretion of IL-1beta from monocytes in gout is redox independent. Front Immunol. 2019;10:70. doi: 10.3389/fimmu.2019.00070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Desbien AL, Reed SJ, Bailor HR, Dubois Cauwelaert N, Laurance JD, Orr MT, et al. Squalene emulsion potentiates the adjuvant activity of the TLR4 agonist, GLA, via inflammatory caspases, IL-18, and IFN-gamma. Eur J Immunol. 2015;45:407–17. doi: 10.1002/eji.201444543. [DOI] [PubMed] [Google Scholar]
- 189.Seydoux E, Liang H, Dubois Cauwelaert N, Archer M, Rintala ND, Kramer R, et al. Effective combination adjuvants engage both TLR and inflammasome pathways to promote potent adaptive immune responses. J Immunol. 2018;201:98–112. doi: 10.4049/jimmunol.1701604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Jhang JJ, Yen GC. The role of Nrf2 in NLRP3 inflammasome activation. Cell Mol Immunol. 2017;14:1011–2. doi: 10.1038/cmi.2017.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Kitur K, Parker D, Nieto P, Ahn DS, Cohen TS, Chung S, et al. Toxin-induced necroptosis is a major mechanism of Staphylococcus aureus lung damage. PLoS Pathog. 2015;11:e1004820. doi: 10.1371/journal.ppat.1004820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Rathkey JK, Zhao J, Liu Z, Chen Y, Yang J, Kondolf HC, et al. Chemical disruption of the pyroptotic pore-forming protein gasdermin D inhibits inflammatory cell death and sepsis. Sci Immunol. 2018;3. 10.1126/sciimmunol.aat2738. [DOI] [PMC free article] [PubMed]
- 193.Rashidi M, Simpson DS, Hempel A, Frank D, Petrie E, Vince A, et al. The pyroptotic cell death effector Gasdermin D is activated by gout-associated uric acid crystals but is dispensable for cell death and IL-1beta release. J Immunol. 2019;203:736–48. doi: 10.4049/jimmunol.1900228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012. [PubMed]
- 195.Lu C, Li X, Ren Y, Zhang X. Disulfiram: a novel repurposed drug for cancer therapy. Cancer Chemother Pharm. 2021;87:159–72. doi: 10.1007/s00280-020-04216-8. [DOI] [PubMed] [Google Scholar]
- 196.Hu JJ, Liu X, Xia S, Zhang Z, Zhang Y, Zhao J, et al. FDA-approved disulfiram inhibits pyroptosis by blocking gasdermin D pore formation. Nat Immunol. 2020;21:736–45. doi: 10.1038/s41590-020-0669-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Deng W, Yang Z, Yue H, Ou Y, Hu W, Sun P. Disulfiram suppresses NLRP3 inflammasome activation to treat peritoneal and gouty inflammation. Free Radic Biol Med. 2020;152:8–17. doi: 10.1016/j.freeradbiomed.2020.03.007. [DOI] [PubMed] [Google Scholar]
- 198.Chen M, Xing Y, Lu A, Fang W, Sun B, Chen C, et al. Internalized cryptococcus neoformans activates the canonical Caspase-1 and the noncanonical Caspase-8 inflammasomes. J Immunol. 2015;195:4962–72. doi: 10.4049/jimmunol.1500865. [DOI] [PubMed] [Google Scholar]
- 199.Chen J, Wang S, Fu R, Zhou M, Zhang T, Pan W, et al. RIP3 dependent NLRP3 inflammasome activation is implicated in acute lung injury in mice. J Transl Med. 2018;16:233. doi: 10.1186/s12967-018-1606-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Li JX, Feng JM, Wang Y, Li XH, Chen XX, Su Y, et al. The B-Raf(V600E) inhibitor dabrafenib selectively inhibits RIP3 and alleviates acetaminophen-induced liver injury. Cell Death Dis. 2014;5:e1278. doi: 10.1038/cddis.2014.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Shi Y, Huang C, Zhao Y, Cao Q, Yi H, Chen X, et al. RIPK3 blockade attenuates tubulointerstitial fibrosis in a mouse model of diabetic nephropathy. Sci Rep. 2020;10:10458. doi: 10.1038/s41598-020-67054-x. [DOI] [PMC free article] [PubMed] [Google Scholar]