Abstract
Circular RNAs (circRNAs) regulate gene expression and participate in various biological and pathological processes. However, little is known about the effects of specific circRNAs on T helper cell 17 (Th17) differentiation and related autoimmune diseases, such as multiple sclerosis (MS). Here, using transcriptome microarray analysis at different stages of experimental autoimmune encephalomyelitis (EAE), we identified the EAE progression-related circINPP4B, which showed upregulated expression in Th17 cells from mice with EAE and during Th17 differentiation in vitro. Silencing of circINPP4B inhibited Th17 differentiation and alleviated EAE, characterized by less demyelination and Th17 infiltration in the spinal cord. Mechanistically, circINPP4B served as a sponge that directly targeted miR-30a to regulate Th17 differentiation. Furthermore, circINPP4B levels were associated with the developing phases of clinical relapsing-remitting MS patients. Our results indicate that circINPP4B plays an important role in promoting Th17 differentiation and progression of EAE by targeting miR-30a, which provides a potential diagnostic and therapeutic target for Th17-mediated MS.
Keywords: Circular RNA, Multiple sclerosis, Experimental autoimmune encephalomyelitis, T helper cell, miR-30a
Subject terms: Neuroimmunology, miRNA in immune cells
Introduction
Multiple sclerosis (MS), a chronic inflammatory autoimmune disease that affects millions worldwide [1], is characterized by demyelinating plaques in the central nervous system (CNS) due to myelin and oligodendrocyte injury [2]. Current studies have shown that the occurrence and development of MS is the result of multiple factors, such as autoimmunity, virus infection, genetic tendency, environmental effects and individual susceptibility, among which autoimmunity plays a key role, resulting in the infiltration of extensive immune cells into the CNS and causing an abnormal immune response [3,4,]. CD4+ T helper (Th) cells, especially IL-17-secreting Th cells 17 (Th17), are major initiators and participants in driving both MS and experimental autoimmune encephalomyelitis (EAE), a mouse model of MS [5–7]. Clinically, the ratio of Th17 cells in the cerebrospinal fluid (CSF) of MS patients increased significantly; in addition, the ratio of Th17 cells in MS patients at the relapse stage was higher than that at the remission stage [8]. Infusion of activated myelin-reactive CD4+ Th17 cells could induce EAE, and further injection of IL-17 resulted in worsening of this disease, while IL-17-deficient mice were resistant to EAE [9,10,]. Our previous results demonstrated that in immune-deficient Rag1−/− mice transferred with CD4+ naïve T cells (CD4+ Tn), inhibition of Th17 differentiation alleviated EAE, while promotion of Th17 differentiation aggravated EAE [7,11,]. Therefore, effective regulation of Th17 differentiation to maintain stability of the immune microenvironment is a possible strategy for the prevention and treatment of MS.
MicroRNAs (miRs) are endogenous small noncoding RNAs (ncRNAs) that act as powerful regulators of gene expression by mRNA degradation or translational repression under normal physiological and pathological conditions [12]. In recent years, many MS-related miRs have been identified from peripheral blood, plasma, CSF and the CNS as diagnostic biomarkers and therapeutic targets [13]. Our previous data showed that miR-30a dysfunction in vivo led to abnormal differentiation of Th17 cells and pathological processes of EAE. Overexpression of miR-30a could effectively inhibit the excessive activation of Th17 cells, reducing the inflammatory reaction in the demyelinating lesion area of the CNS to alleviate EAE [14]. However, the reasons for dysregulated expression of miR-30a in mice with EAE and MS patients are unclear.
Recent studies have shown that the levels and functions of many miRs in cells are regulated by another competing endogenous RNA—circular RNA (circRNA), which is widely distributed in eukaryotic cells and exosomes [15–18]. CircRNA, with a covalently closed continuous loop structure, is formed by backsplicing of exons or introns from precursor mRNA [19,20,]. An increasing number of studies have confirmed that circRNAs participate in the progression of many diseases, such as cancer, myocardial infarction, neurodegenerative disorder and autoimmune disease, by regulating cell proliferation, differentiation, apoptosis, invasion, and metastasis [15,21,22,]. Because circRNAs are rich in miRNA binding sites, they can act as a “molecular sponge” for miRs to regulate the expression of target genes [16,23,24,]. With the development of high-throughput sequencing and novel bioinformatics analysis, many previously unknown circRNAs have been verified to play key roles in various diseases. However, few circRNAs in MS have been reported, and the functions and mechanisms of circRNAs in MS remain largely unknown and need clarification.
In the present study, we identified the EAE progression-related circRNA circINPP4B in mice at different stages of EAE using a transcriptome microarray. Then, we designed a series of functional and molecular experiments to explore the effects and mechanism of circINPP4B and found that the circINPP4B/miR-30a/IL-21R axis could regulate Th17 cell differentiation both in vitro and in vivo. Finally, we discovered that circINPP4B levels were associated with MS progression at different clinical phases.
Results
Identification of EAE-related circRNAs via microarray analysis
To explore the specific circRNAs mediating EAE progression, we first established an EAE model and grouped the mice according to different EAE scores. The mice with EAE with increased scores (from 0 to 4) showed characteristic progressive clinical symptoms (Supplementary Movies S1–4), displaying aggravating inflammatory infiltration and demyelination in the spinal cord (Fig. 1A). Next, peripheral blood CD4+ lymphocytes from the mice with EAE with different identified scores (0, 1, 2, 3, 4) were obtained for the microarray assay. We analyzed the differentially expressed circRNAs (fold change > 2.0 or <0.5) and listed the top circRNAs with upregulated and downregulated expression (Supplementary Table S2) in a clustered heat map (Fig. 1B). When screening these differentially expressed circRNAs, we found that circ_3998 expression was not only significantly upregulated in the mice with EAE but also gradually upregulated as the EAE scores increased (Fig. 1B), suggesting that circ_3998 may play important roles in EAE progression.
Fig. 1.
Identification of EAE-related circRNAs via microarray analysis. A H&E staining and LFB staining of spinal cord sections from the WT mice and the mice with EAE with different identified scores (1–4). The numbers in brackets indicate the EAE scores. Blue arrows show lymphocyte infiltration. Black arrows show demyelination. Scale bar, 200 μm. B Clustered heat map showing the differentially expressed circRNAs (|log2FC| > 10.0) in the peripheral blood CD4+ lymphocytes between the mice with EAE with different scores and the WT mice. WT-1, WT-2, and WT-3 indicate three replicates. Red arrow shows the target circRNA in this study
Circular characteristics of circINPP4B in CD4+ T cells
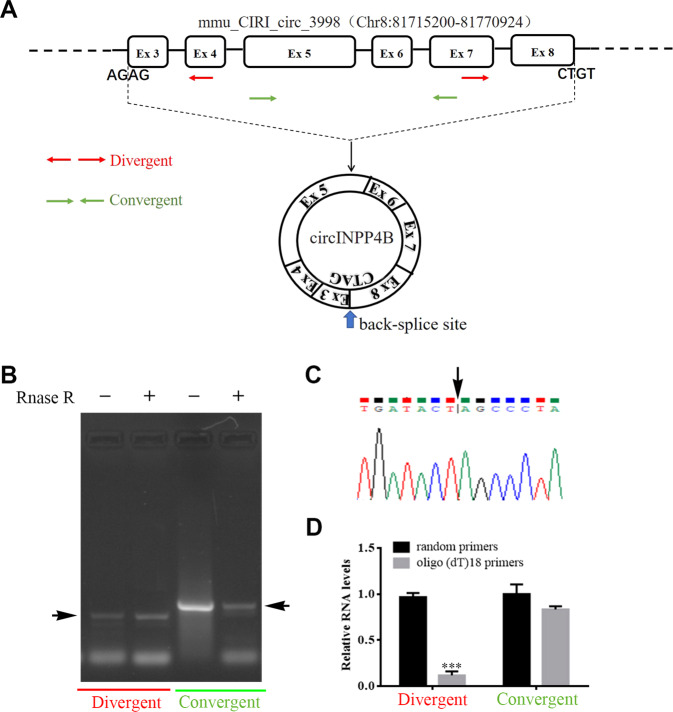
According to the microarray data and bioinformatics analysis, we found that circ_3998 was located on chromosome 8, consisting of 6 exons (exons 3–8) from the INPP4B gene, which is 684 bp in length (Fig. 2A). To confirm the circular characteristics of circ_3998 (circINPP4B), we designed convergent and divergent (covering the backsplice site) primers for PCR analysis in CD4+ T cells, and the RNase R resistance assay showed that circINPP4B (PCR product of divergent primers) could resist RNase R, while linear INPP4B mRNA (PCR product of convergent primers) was degraded by RNase R (Fig. 2B). Then, Sanger sequencing of the PCR product of divergent primers confirmed the presence of a backsplice site in circINPP4B (Fig. 2C). Furthermore, more circINPP4B products were obtained when using random hexamers in reverse transcription experiments than when oligo (dT)18 primers were used, while there was no obvious difference when linear INPP4B mRNA was produced using these two kinds of primers (Fig. 2D), proving that circINPP4B had no poly-A tail. Collectively, these findings demonstrated that circINPP4B was a circular and stable transcript.
Fig. 2.
Circular characteristics of circINPP4B in CD4+ T cells. A Scheme illustrating the formation of circ_3998 (circINPP4B). The binding sites of PCR primers used to detect circINPP4B (red arrows) and linear INPP4B mRNA (green arrows) are presented. The backsplice site is shown by the blue arrow. B The existence of circINPP4B was validated by RT-PCR. CircINPP4B (product of divergent primer) could resist RNase R, while linear INPP4B mRNA (product of convergent primer) was degraded by RNase R. C The backsplice site (black arrow) of circINPP4B was tested by Sanger sequencing. D Random hexamer or oligo (dT)18 primers were used in the reverse transcription experiments. The relative RNA levels were measured by RT-qPCR. Data are presented as the mean ± SEM. ***P < 0.001 compared with the random primer group
CircINPP4B was associated with Th17 cell differentiation in vitro
To explore the circINPP4B expression level and specificity during EAE progression, we measured its expression in CD4+ T cells from the mice with EAE with different identified scores and in different kinds of CD4+ T cells. The results showed that the expression level of circINPP4B in CD4+ T cells was significantly increased as the severity of EAE increased (Fig. 3A). In addition, circINPP4B displayed substantially higher expression in CD4+IL-17+ T cells and slightly higher expression in CD4+IFN-γ+ cells and CD4+CXCR5+ cells than in CD4+ Tn cells (Fig. 3B). To further investigate the role of circINPP4B in CD4+IL-17+ T cells, we induced CD4+ Tn cells to differentiate into Th17 cells in vitro and found that circINPP4B expression was upregulated within days of the increase (Fig. 3C). FISH assay results also showed that circINPP4B was highly expressed in the cytoplasm of Rorγt+ Th17 cells (Fig. 3D). Next, we designed and synthesized circINPP4B siRNA at the backsplice site to knock down circINPP4B expression (Fig. 3E). During Th17 cell differentiation in vitro, silencing circINPP4B led to a lower percentage of induced IL-17+ cells (Fig. 3F, G). Consistently, the expression of the Th17 lineage marker gene Rorγt (Fig. 3H) and the secretion of Th17-related cytokines (Fig. 3I) were also decreased when circINPP4B was knocked down. These results indicated that circINPP4B regulated Th17 differentiation in vitro.
Fig. 3.
CircINPP4B was associated with Th17 cell differentiation in vitro. A The expression of circINPP4B was detected by RT-qPCR in peripheral blood CD4+ lymphocytes from the mice with EAE with different scores and the WT mice. B The levels of circINPP4B were measured in the different kinds of CD4+ lymphocytes from the mice with EAE. CD4+ Tn cells (naïve) were isolated from splenocytes, and other kinds of CD4+ lymphocytes were isolated from peripheral blood mononuclear cells by flow cytometry. C RT-qPCR analysis of circINPP4B expression on different days during Th17 cell differentiation in vitro. D FISH assay showed the localization of circINPP4B in Tn and Th17 cells. DAPI was used to stain the nuclei. Scale bar, 20 μm. E The expression of circINPP4B was detected by PCR (left) and qPCR (right) after circINPP4B silencing. NC, negative control infection. siRNA, circINPP4B siRNA infection. F, G Flow cytometric analysis of IL-17+ and Rorγt+ cells in the CD4+ gate three days after induction of Th17 polarization. H RT-qPCR analysis of Rorγt expression in cultured cells. I ELISAs of IL-17A, IL-17F, IL-21, TNF-α and GM-CSF in culture supernatants. Data are presented as the mean ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001
Silencing of circINPP4B alleviated EAE in vivo
Based on the data of increased circINPP4B expression during EAE progression, to explore whether circINPP4B was involved in the development of EAE, we knocked down circINPP4B via siRNA to decrease its level in CD4+ lymphocytes in mice (Fig. 4A). After MOG35–55 immunization, the circINPP4B-silenced mice developed alleviated EAE with a delayed onset and reduced EAE peak scores (Fig. 4B). Histological analysis of spinal cord sections showed that the circINPP4B-silenced mice had less inflammatory infiltration (Fig. 4C1) and demyelination (Fig. 4C2), which was further supported by the increased myelin basic protein (MBP) expression (Fig. 4C3), more myelinated axons and greater myelin thickness (Fig. 4C4). Compared with those of the control mice, the proportions of IL-17+ lymphocytes from the peripheral blood, spinal cord, and lymph nodes were much lower in the circINPP4B-silenced mice (Fig. 4D, E). Accordingly, the concentrations of Th17-related cytokines in the peripheral blood serum were significantly decreased (Fig. 4F).
Fig. 4.
Silencing of circINPP4B alleviated EAE in vivo. A RT-qPCR analysis of circINPP4B expression in CD4+ peripheral blood lymphocytes (PBLs), splenocytes (SPs) and lymph node lymphocytes (LNs) from negative control (NC) or circINPP4B siRNA (siRNA)-infected mice 18 days after MOG immunization. B Clinical score analysis for the mice with EAE infected with NC or siRNA (n = 5 per group per trial). (C1–C4) H&E staining, LFB staining, MBP immunofluorescence staining and TEM observation of spinal cord sections from NC- or siRNA-infected mice 18 days after MOG immunization. Scale bars, 300 μm in B1, B2, and B3 and 2 μm in B4. D–F PBLs, spinal cord-infiltrated lymphocytes (SCIs) and LNs from NC- or siRNA-infected mice 18 days after MOG immunization were stimulated in vitro. Flow cytometric analysis of IL-17+ cells among CD4+-gated cells is shown in (D and E). ELISAs of IL-17A, IL-17F, IL-21, TNF-α and GM-CSF in culture supernatants are presented in (F). G–J Rag1−/− mice were transferred with NC-infected or siRNA-infected CD4+ Tn cells and then immunized with MOG for EAE induction. On day 14, the numbers of CD4+ cells in the PBL were assessed by flow cytometry and are presented in (G). Clinical score analysis for EAE is shown in (H) (n = 5 per group per trial). Relative quantification of IL-17+ cells and IFN-γ+ cells in PBL, SCI and LN lymphocytes is shown in (I and J). Data are presented as the mean ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001
To further clarify the role of circINPP4B in the differentiation of Th17 cells in vivo, we transferred circINPP4B-silenced CD4+ Tn cells into age-matched Rag1−/− immunodeficient mice, followed by EAE induction. Although the number of CD4+ cells was equal in the peripheral blood from the circINPP4B-silenced and control CD4+ Tn-transferred mice (Fig. 4G), the Rag1−/− mice receiving circINPP4B-silenced CD4+ Tn exhibited a lower EAE score (Fig. 4H) and fewer IL-17+ lymphocytes (Fig. 4I). However, the proportion of IFN-γ+ lymphocytes did not change significantly (Fig. 4J). Taken together, these data indicated that silencing circINPP4B might inhibit Th17 cell differentiation in vivo, leading to mild EAE.
CircINPP4B acted as a sponge of miR-30a
The finding that circINPP4B was localized in the cytoplasm indicated that circINPP4B may serve as a miRNA sponge. To identify the downstream miRNAs of circINPP4B, we performed a miRNA microarray of peripheral blood CD4+ lymphocytes from the mice with EAE with different scores (0, 1, 2, 3, 4). We analyzed the differentially expressed miRNAs (fold change > 2.0 or <0.5) and listed the top mRNAs with upregulated and downregulated expression (Supplementary Table S3) in Fig. 5A. According to the related expression trend of circINPP4B, we focused on the miRNAs with downregulated expression and increasing EAE scores and screened 9 miRNAs (Fig. 5A). Based on the microarray results of both circRNAs and miRNAs, correlation analysis showed that there was a strong relationship between circINPP4B and miR-30a (Fig. 5B). In addition, we used bioinformatics analysis tools to predict the miRNAs that could bind to circINPP4B (Fig. 5C, Supplementary Table S4). The above results indicated that miR-30a may be a direct target of circINPP4B.
Fig. 5.
CircINPP4B acted as a sponge of miR-30a. A Clustered heat map showing the differentially expressed miRNAs (fold change > 10.0 or <0.1) in the peripheral blood CD4+ lymphocytes between the mice with EAE with different scores and the WT mice. Those in red boxes were the miRNAs with downregulated expression and increasing EAE scores. B Correlation analysis displaying the relationship between miR-30a and related circRNAs. The depth of the line color represents the strength of the relationship. Red arrow shows the target circRNA in this study. C Schematic model illustrating the putative binding sites of candidate miRNAs correlated with circINPP4B through miRWalk and miRDB. Red underline showing the target miRNA in this study. D RT-qPCR analysis of circINPP4B and miR-30a expression on different days during Th17 cell differentiation in vitro compared with Day 0. E The expression of miR-30a was detected in peripheral blood CD4+ lymphocytes after circINPP4B silencing in mice. NC, negative control infection. siRNA, circINPP4B siRNA infection. F Colocalization of circINPP4B and miR-30a was measured using FISH in the cells three days after Th17 polarizing induction. G RIP experiments were performed using an antibody against AGO2 on extracts from CD4+ cells. H RT-qPCR analysis of miR-30a levels in CD4+ cell lysates incubated with circINPP4B probe or NC probe using an RNA pulldown assay. I Schematic of the putative binding sites of miR-30a on the wild-type or mutant circINPP4B sequence. J A luciferase reporter assay was performed to detect luciferase activity after cotransfection with wild-type circINPP4B (wt-circINPP4B) and miR-30a or miR-NC vectors. K Luciferase activity was measured after cotransfection with mutant circINPP4B (mut-circINPP4B) and miR-30a or miR-NC vectors. Data are presented as the mean ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001
To clarify the interaction between circINPP4B and miR-30a, we first found that circINPP4B expression was gradually upregulated, while miR-30a expression was downregulated during Th17 cell differentiation in vitro (Fig. 5D). When circINPP4B was knocked down in mice, the miR-30a level was increased (Fig. 5E). The FISH assay confirmed the colocalization of circINPP4B and miR-30a in the cytoplasm (Fig. 5F). Next, we conducted an RIP assay with an antibody against AGO2 and found that both circINPP4B and miR-30a were significantly enriched by the AGO2 antibody (Fig. 5G). In addition, the results of the RNA pulldown assay indicated that miR-30a was enriched using a biotin-coupled circINPP4B probe (Fig. 5H). Finally, a dual-luciferase reporter assay was performed, and the data revealed that miR-30a observably attenuated the luciferase activity of wild-type (WT) circINPP4B compared with the scrambled control (Fig. 5I, J), while miR-30a did not affect the luciferase activity of mutant circINPP4B (Fig. 5K). Above all, these results indicated that circINPP4B could function as a sponge by targeting miR-30a.
CircINPP4B regulated Th17 cell differentiation via miR-30a
Our previous results proved that miR-30a inhibited Th17 differentiation both in vitro and in vivo by targeting IL-21R [14]. Here, to evaluate the effect of the circINPP4B/miR-30a axis on the differentiation of Th17 cells, we performed a rescue assay to co-overexpress circINPP4B and miR-30a, as well as mutant circINPP4B and miR-30a. Our results showed that during Th17 induction in vitro, miR-30a suppressed Th17 generation, while circINPP4B promoted Th17 cell differentiation by increasing the proportion of IL-17+ cells (Fig. 6A, B), secretion of Th17-related cytokines (Fig. 6C), and expression of Rorγt and IL-21R (Fig. 6D). Moreover, circINPP4B could partially reverse the inhibitory effects of miR-30a, as indicated by the results that compared with miR-30a overexpression, the co-overexpression of circINPP4B and miR-30a remarkably increased Th17 cell differentiation-associated markers (Fig. 6A–D) as mentioned above. However, there were no significant differences between the miR-30a group and the group with co-overexpression of mutant circINPP4B and miR-30a (Fig. 6A–D).
Fig. 6.
CircINPP4B regulated Th17 cell differentiation via miR-30a. A–D CD4+ Tn cells were infected with miR-NC, miR-30a, wt-circINPP4B or mut-circINPP4B before induction of Th17 polarization. Three days later, flow cytometric analysis of the stimulated IL-17+ cells was performed and is shown in (A and B). ELISAs of IL-17A, IL-17F, IL-21, TNF-α and GM-CSF in culture supernatants is presented in (C). RT-qPCR analysis of Rorγt and IL-21R expression in the cultured cells is displayed in (D). E, F CD4+ Tn cells were infected with negative control (NC) or circINPP4B siRNA and then induced under Th17 polarizing conditions with or without IL-21. Three days later, IL-17+ cells in the CD4+ gate were analyzed by flow cytometry. Data are presented as the mean ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001
Figure 3I shows that the level of IL-21 was significantly decreased after circINPP4B silencing. To determine whether the decreased IL-21 was one of the reasons leading to inhibition of Th17 cell differentiation, we added exogenous IL-21 to the induction medium, and the data showed that IL-21 could not raise the proportion of Th17 cells when circINPP4B was knocked down (Fig. 6E, F). Thus, the above results suggested that circINPP4B might promote Th17 differentiation partially by impairing the inhibitory effect of miR-30a on IL-21R.
CircINPP4B levels were associated with the clinical characteristics of MS
To further investigate whether circINPP4B expression was correlated with clinical MS prognosis, we examined the circINPP4B level in peripheral blood lymphocytes from 18 patients with relapsing-remitting MS (RRMS). Remarkably, the test results showed that circINPP4B expression was significantly upregulated, while miR-30a expression was downregulated in MS patients at the relapse stage compared with the age- and sex-matched healthy controls (Table 1). Moreover, when these MS patients were in remission, circINPP4B and miR-30a expression returned to normal levels, with an obvious difference compared to that of patients in relapse (Table 1). These findings implied a possible correlation between circINPP4B level and RRMS progression.
Table 1.
Characteristics of patients and the relative expression of circINPP4B and miR-30a in MS
| Group | Sample number & Sex | Age | EDSS | Clinical stage | Relative circINPP4B expression | Relative miR-30a expression |
|---|---|---|---|---|---|---|
| Control |
20 (15 F, 5 M) |
40.1 ± 15.5 | – | – | 1.0 ± 0.6 | 1.0 ± 0.4 |
| RRMS |
18 (14 F, 4 M) |
42.3 ± 17.4 | 5.5 ± 2.6 | Relapsing | 2.2 ± 0.9a | 0.5 ± 0.3a |
| Remitting | 1.5 ± 0.8b | 0.8 ± 0.3b |
Data are presented as the mean ± standard deviation. “–” indicates “not applicable”
EDSS expanded disability status scale, F female, M male, RRMS relapsing remitting multiple sclerosis
aP < 0.001 compared to the control
bP < 0.05 compared to relapsing
Discussion
MS is a kind of autoimmune disease mainly caused by abnormal inflammation in the CNS with spatial and temporal multiplicity. Disturbance of the immune microenvironment in the CNS of MS patients and mice with EAE results in diffuse infiltration of inflammatory cells, abnormal activation of microglia and astrocytes, and damage to the myelin sheath due to attacks by the immune response [3,25,]. At present, although the clinical application of immunosuppressants or immunomodulatory drugs can alleviate MS [26,27,], the lack of effective therapeutic targets, as well as biomarkers for rapid diagnosis and prognosis, are major problems in the prevention and treatment of MS. Current studies have shown that some circRNAs have the potential to be biomarkers or prognostic factors in tumors because of structural stability, sequence conservation, and cell- or tissue-specificity [28–30]. However, the functions of circRNAs in MS remain largely unknown. In this study, to explore EAE progression-related circRNAs, we screened differentially expressed circRNAs in mice at different stages of EAE using a transcriptome microarray and identified circINPP4B, which could regulate Th17 differentiation and EAE development by sponging miR-30a (Fig. 7).
Fig. 7.
Schematic model of the circINPP4B/miR-30a/IL-21R axis regulating Th17 cell differentiation during the progression of experimental autoimmune encephalomyelitis (EAE). The expression of miR-30a, a negative regulator in Th17 differentiation, is sponged by circINPP4B. Red star denotes our previous results. The red question mark represents the research focus of this study
CircRNA was first considered a byproduct of abnormal splicing without regulatory function. In recent years, with the rapid development of RNA sequencing technology and bioinformatics analysis, circRNAs and their functions have received increasing attention [15]. More than 10,000 candidate circRNAs have been identified in the human transcriptome. The circRNA/miR axis participates in the progression of cancer, myocardial infarction, and neurodegenerative diseases by regulating cell proliferation, differentiation, apoptosis, invasion and metastasis and has gradually become a biomarker for the diagnosis of tumors and neuroinflammatory diseases [17,18,21,22,]. In addition, circRNAs are closely related to the occurrence and development of autoimmune diseases, such as systemic lupus erythematosus (SLE) and MS. Overexpression of circPOLR2A in peripheral blood-derived T lymphocytes of SLE patients could reduce the secretion of interferon [31]. The analysis of circRNA levels in peripheral blood mononuclear cells from MS patients showed that the circRNAs circ_0005402 and circ_0035560, located in the ANXA2 gene, might be potential biomarkers of MS [32]. Through bioinformatics analysis, the Paraboschi group identified 18 circRNAs from MS-related genes, suggesting that they may be related to susceptibility to MS [33]. Zurawska et al. reported in their review that circRNAs were differentially expressed in immune cells of MS patients, and specific circRNAs might participate in the progression of MS by regulating the polarization of Th17 cells and the expression of STAT3, a key transcription factor mediating the inflammatory response [34,35,]. Our study showed that circINPP4B not only regulated the differentiation of Th17 cells and participated in the process of EAE but also that its expression levels were closely related to the course of clinical MS.
It has been proven that more than half of the DNA in the human genome is transcribed, while <2% of the DNA is translated into protein. Therefore, there are many ncRNAs in cells. MiR, as one of the most widely studied ncRNAs, is involved in various cell activities during ontogenetic development and disease progression by post-transcriptional regulation of expression. To date, several miRs, such as miR-20b [36], miR-155 [37], miR-326 [38], miR-448 [39], miR-15b [40], miR-140 [41], miR-374c [42] and miR-30a [14,43,], have been identified as regulators of Th17 differentiation during EAE and MS development [44]. In 2016, for the first time, our group, together with another research team, found that the expression of miR-30a in peripheral blood CD4+ T lymphocytes of MS patients and mice with EAE was significantly decreased, and overexpression of miR-30a could effectively inhibit the abnormal overdifferentiation of Th17 cells and reduce the inflammatory reaction in the demyelinating area of the CNS to alleviate EAE [14,43,]. Moreover, administration of diphenhydramine hydrochloride and disulfiram reversed the decrease in miR-30a to inhibit Th17 cell differentiation and alleviate EAE [43]. In the next few years, miR-30a was further proven to regulate CD4+ T lymphocyte development [45], and a series of clinical studies showed that the levels of hsa-mir-30a in peripheral blood and CSF of MS patients were significantly abnormal [46–48], suggesting that miR-30a might be used as a potential biomarker for MS diagnosis [47]. In this study, through miRNA microarray, bioinformatics analysis and a series of functional and molecular experiments, we confirmed the relationship between circINPP4B and miR-30a. This study elucidated one of the reasons for the abnormal decrease in miR-30a in MS patients.
In conclusion, our study has the following major findings: 1. We identified a novel circRNA, circINPP4B, which was closely related to the progression of EAE and MS. 2. We verified that this circINPP4B was formed from exons 3 to 8 of INPP4B mRNA. 3. We demonstrated that circINPP4B could regulate Th17 cell differentiation both in vitro and in vivo. 4. We showed that silencing of circINPP4B alleviated EAE. 5. We confirmed that circINPP4B functioned as a sponge by directly targeting miR-30a. Therefore, this study provides a better understanding of functional circRNAs in MS and suggests a potential diagnostic and therapeutic target for Th17-mediated MS disease.
Materials and methods
Clinical subjects
Eighteen patients who were diagnosed with MS were recruited from the Affiliated Hospital of Xuzhou Medical University. All participants were examined by neurologists for expanded disability status scale measures [49]. Peripheral blood samples were obtained from MS patients as well as age- and sex-matched healthy volunteers. Informed consent was obtained from each participant. This study was approved by the Clinical Research Ethnics Committee of Affiliated Hospital of Xuzhou Medical University.
Mouse samples
C57BL/6 WT mice were obtained from the Experimental Animal Center of Xuzhou Medical University. Rag1−/− mice were purchased from the Model Animal Research Center of Nanjing University (Nanjing, China). All experiments were performed in accordance with the Provisions and General Recommendations of the Chinese Experimental Animal Administration Legislation, as well as institutional approval from the Experimental Animal Ethics Committee of Xuzhou Medical University.
EAE induction
Eight-week-old female mice were immunized subcutaneously with 100 μg MOG35–55 (MEVGWYRSPFSRVVHLYRNGK) (GL Biochem, Shanghai, China) in complete Freund’s adjuvant (Sigma-Aldrich, St. Louis, MO, USA) containing 4 mg/mL heat-killed Mycobacterium tuberculosis H37Ra (BD Bioscience, San Jose, CA, USA). Each mouse was intraperitoneally (i.p.) administered 200 ng pertussis toxin (Sigma-Aldrich, USA) on the day of immunization and 48 h later. Clinical assessment of EAE was performed daily according to the following criteria: 0, no clinical signs; 1, paralyzed tail; 2, paresis (weakness, incomplete paralysis of one or two hindlimbs); 3, paraplegia (complete paralysis of both hindlimbs); 4, paraplegia with forelimb weakness or paralysis; and 5, moribund state or death.
CD4+ Tn cell purification and Th17 induction
As described in our previous study [14], purification of CD4+ Tn cells from splenocytes of 5- to 6-week-old mice was achieved by depletion of magnetically labeled non-naïve CD4+ T cells and CD44+ memory T cells following the manufacturer’s instructions (Miltenyi Biotec, Bergisch Gladbach, Germany). The phenotype of isolated CD4+ Tn was CD4+CD25−CD44−CD62L+. For adoptive transfer studies, each recipient Rag1−/− mouse was injected intravenously in the tail veins with 5 × 106 CD4+ Tn cells on the day before EAE immunization.
For Th17 induction, purified CD4+ Tn cells were cultured for 3 days under Th17 cell-polarizing conditions: RPMI-1640 containing 10% FBS, 1 mM glutamine, 0.1 mM β-mercaptoethanol, 1% nonessential amino acids (Sigma-Aldrich, USA), anti-CD3 plus anti-CD28-coated beads (Invitrogen, CA, USA), 20 ng/mL IL-6, 5 ng/mL TGF-β, 10 ng/mL IL-23, 2 μg/mL anti-IL-4, and 2 μg/mL anti-IFN-γ (BD Bioscience, USA). In some experiments, 10 ng/mL IL-21 (BD Bioscience, USA) was added to the medium.
Transcriptome microarray
Total RNA extracted from CD4+ T cells was measured for transcriptome microarray analysis by Majorbio Biopharm Technology Co., Ltd. (Shanghai, China). The data were analyzed on the free online platform of the Majorbio Cloud Platform (www.majorbio.com). All data were uploaded to the Sequence Read Archive database (PRJNA725306, PRJNA725350).
Plasmid construction, lentivirus preparation and administration
CircINPP4B cDNA and siRNA (CUGGUUUUAGGGCUAGUAUC) were synthesized and cloned into the pLV-ciR and pGPU6 vectors (GenePharma, Shanghai, China). MiR-30a cDNA (TGTAAACATCCTCGACTGGAAG) was cloned into the H1-MCS-CMV-EGFP vector (GeneChem, Shanghai, China). Plasmids were transfected into 293T cells to package lentivirus. CD4+ Tn cells were infected with lentivirus according to the user’s manual before Th17 polarizing induction. For administration in mice, ~2 × 107 lentiviruses were delivered into the tail vein 7 days before EAE induction.
Histological analyses
After anesthesia with pentobarbital, mice were perfused with buffered 4% paraformaldehyde. The paraffin-embedded spinal cord sections were stained with hematoxylin and eosin (H&E) and Luxol fast blue (LFB).
For immunofluorescence staining, cryosections were incubated with an antibody specific for MBP (Abcam, Cambridge, UK). Images were acquired using a fluorescence microscope system (Olympus, Tokyo, Japan).
For transmission electron microscopy analysis, the ultrathin sections were stained with uranyl acetate and lead citrate. Electron micrographs were captured using an FEI Tecnai G2 T12 transmission electron microscope (Thermo Fisher Scientific, Waltham, MA, USA) and analyzed by TEM Imaging and Analysis (TIA) software.
RNA isolation and quantitative RT-PCR
Total RNA was isolated using TRIzol (Invitrogen, CA, USA) according to the manufacturer’s protocol. For RNase R treatment, total RNA was incubated for 15 min at 37 °C with or without 3 U/μg RNase R (Epicentre, Madison, WI, USA). cDNA was synthesized using a QuantScript RT kit (Tiangen, Beijing, China) and miRcute Plus miRNA First-Strand cDNA Kit (Tiangen, China) and then examined with a SYBR Green real-time PCR kit (Roche, Basel, Switzerland) and miRcute Plus miRNA qPCR Kit (Tiangen, China). In certain reverse transcription experiments to confirm the circRNA, random hexamer or oligo (dT)18 primers were used. Relative expression of circRNA, mRNA or miRNA was evaluated by the 2−ΔΔCt method in a LightCycler® 480 System (Roche, Switzerland) and normalized to the expression of β-actin or U6. The primers are listed in Supplementary Table S1.
Flow cytometry
Peripheral blood lymphocytes from mice and clinical participants were collected using lymphocyte separation medium (Dakewe, Shenzhen, China). Fluorescent-labeled antibodies, such as CD4, CD25, and CXCR5 (Miltenyi Biotec, Bergisch Gladbach, Germany), were used to label cells for isolation by flow cytometry (BD Bioscience, San Jose, CA, USA).
Peripheral blood, lymph nodes, and type I collagenase-digested spinal cords from mice were collected to prepare lymphocytes by Percoll gradient centrifugation. For detection of intracellular cytokine levels and transcription factors, cells were incubated with Cell Stimulation Cocktail (eBioscience, USA) for 5 h, resuspended in fixation/permeabilization solution (BD Pharmingen, USA) and stained with IFN-γ, IL-4, IL-17 (Miltenyi Biotec, Germany) and Rorγt antibodies (eBioscience, USA). Labeled cells were isolated by flow cytometry or measured on MACSQuantTM Flow Cytometers (Miltenyi Biotec, Germany) and analyzed with FlowJo software.
Fluorescence in situ hybridization
CD4+ cells were fixed and then tested using a fluorescence in situ hybridization kit (GenePharma, Shanghai, China) according to the manufacturer’s instructions. A FAM-labeled circINPP4B probe (5′-CUGGUUUUAGGGCUAGUAUC-3′) and a Cy3-labeled miR-30a probe (5′-CUUCCAGUCGAGGAUGUUUACA-3′) were designed and synthesized by GenePharma. In some experiments, cells were coincubated with an antibody specific for Rorγt (CST, MA, USA). The cell nucleus was counterstained with 4′,6-diamidino-2-phenylindole (DAPI, KeyGEN, Nanjing, China). Images were acquired on a fluorescence microscope system (Olympus, Tokyo, Japan).
Cytokine analysis
Levels of IL-17A, IL-17F, IL-21, TNF-α, and GM-CSF in the culture supernatants and mouse peripheral blood serum were assayed by enzyme-linked immunosorbent assay (ELISA) kits according to the manufacturer’s instructions (Jianglaibio, Shanghai, China). The measurement of each sample was repeated three times.
RNA-binding protein immunoprecipitation (RIP) assay
The RIP assay was performed using an RNA-Binding Protein Immunoprecipitation Kit (Millipore, Billerica, MA, USA) according to the manufacturer’s protocol. Briefly, CD4+ T cells were collected and lysed with RIP lysis buffer and then incubated with magnetic beads conjugated with anti-AGO2 antibody or control IgG (Abcam, Cambridge, UK). Next, the immunoprecipitated RNA was extracted and measured by RT-qPCR.
RNA pulldown assay
CD4+ T cells were harvested, lysed, sonicated, and then incubated with circINPP4B probe (biotin- CUGGUUUUAGGGCUAGUAUC) and NC probe (biotin-UUCUCCGAACGUGUCACGUTT) (RiboBio, Guangzhou, China)-coated magnetic beads (LandMBio, Guangzhou, China). The bound RNAs were washed and purified for RT-qPCR analysis.
Luciferase reporter assay
The sequence of WT circINPP4B or mutant circINPP4B was cloned into the pGL4.20[luc2Puro] vector (Promega, Madison, WI, USA). Recombinant vectors were cotransfected with miR-30a or miR-NC into Jurkat cells using Transfect-Mate (GenePharma, Shanghai, China). After 48 h of transfection, cells were harvested, and relative luciferase activities were measured using the Dual-Luciferase Reporter Assay System (Promega, USA).
Statistical analysis
Statistical analysis was carried out with GraphPad Prism. Each experiment was repeated five times. All results are summarized and presented as the mean ± standard error of the mean (SEM). Independent sample t-tests were used to evaluate the differences between groups. One-way analysis of variance (ANOVA) followed by Bonferroni’s post hoc test was used for multiple comparisons. EAE clinical scores were evaluated by two-way repeated-measures ANOVA. A P value of 0.05 or less was considered statistically significant.
Supplementary information
Video S1. Clinical symptoms of EAE mice with score 1
Video S2. Clinical symptoms of EAE mice with score 2
Video S3. Clinical symptoms of EAE mice with score 3
Video S4. Clinical symptoms of EAE mice with score 4
Acknowledgements
We thank the Affiliated Hospital of Xuzhou Medical University for the clinical samples. We thank the Majorbio Cloud Platform for microarray data analysis. This research was supported by the National Natural Science Foundation of China (81771337, 81271345), The National Key R&D Program of China (2017YFA0104202), The Natural Science Foundation of Jiangsu Province (BK20161174), the 333 Project of Jiangsu Province, The Xuzhou Basic Research Science and Technology Project (KC19059) and Xuzhou Medical University Scientific Research Fund for Talents.
Author contributions
JJH: designed the research, performed the experiments, analyzed the data, and wrote the paper. WZ: performed the experiments and analyzed the data. WHF: performed the experiments and analyzed the data. FXD: performed the experiments and analyzed the data. FH: designed the research and analyzed the data. RQY: designed the research and analyzed the data. XBQ designed the research, performed the experiments, analyzed the data, and wrote the paper.
Data availability
All data are available in the main text or the supplementary materials.
Competing interests
The authors declare no competing interests.
Footnotes
These authors contributed equally: Jingjing Han, Wei Zhuang, Wanhua Feng.
Contributor Information
Ruiqin Yao, Email: wenxi_yao@163.com.
Xuebin Qu, Email: 100002012036@xzhmu.edu.cn.
Supplementary information
The online version contains supplementary material available at 10.1038/s41423-021-00748-y.
References
- 1.Dolati S, Babaloo Z, Jadidi-Niaragh F, Ayromlou H, Sadreddini S, Yousefi M. Multiple sclerosis: therapeutic applications of advancing drug delivery systems. Biomed Pharmacother. 2017;86:343–53. doi: 10.1016/j.biopha.2016.12.010. [DOI] [PubMed] [Google Scholar]
- 2.Popescu BF, Lucchinetti CF. Pathology of demyelinating diseases. Annu Rev Pathol. 2012;7:185–217. doi: 10.1146/annurev-pathol-011811-132443. [DOI] [PubMed] [Google Scholar]
- 3.Duffy SS, Lees JG, Moalem-Taylor G. The contribution of immune and glial cell types in experimental autoimmune encephalomyelitis and multiple sclerosis. Mult Scler Int. 2014;2014:285245. doi: 10.1155/2014/285245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ramaglia V, Rojas O, Naouar I, Gommerman JL. The Ins and Outs of Central Nervous System Inflammation-Lessons Learned from Multiple Sclerosis. Annu Rev Immunol. 2021;39:199–226. doi: 10.1146/annurev-immunol-093019-124155. [DOI] [PubMed] [Google Scholar]
- 5.Matsui M. [Immunology for understanding the pathogenesis of multiple sclerosis] Rinsho Shinkeigaku. 2013;53:898–901. doi: 10.5692/clinicalneurol.53.898. [DOI] [PubMed] [Google Scholar]
- 6.Moser T, Akgün K, Proschmann U, Sellner J, Ziemssen T. The role of TH17 cells in multiple sclerosis: therapeutic implications. Autoimmun Rev. 2020;19:102647. doi: 10.1016/j.autrev.2020.102647. [DOI] [PubMed] [Google Scholar]
- 7.Qu X, Han J, Zhang Y, Wang X, Fan H, Hua F, et al. TLR4-RelA-miR-30a signal pathway regulates Th17 differentiation during experimental autoimmune encephalomyelitis development. J Neuroinflammation. 2019;16:183. doi: 10.1186/s12974-019-1579-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brucklacher-Waldert V, Stuerner K, Kolster M, Wolthausen J, Tolosa E. Phenotypical and functional characterization of T helper 17 cells in multiple sclerosis. Brain: J Neurol. 2009;132:3329–41. doi: 10.1093/brain/awp289. [DOI] [PubMed] [Google Scholar]
- 9.Paradowska A, Maślińiski W, Grzybowska-Kowalczyk A, Łacki J. The function of interleukin 17 in the pathogenesis of rheumatoid arthritis. Arch Immunol Ther Exp (Warsz.) 2007;55:329–34. doi: 10.1007/s00005-007-0032-8. [DOI] [PubMed] [Google Scholar]
- 10.Quinn JL, Kumar G, Agasing A, Ko RM, Axtell RC. Role of TFH Cells in Promoting T Helper 17-Induced Neuroinflammation. Front Immunol. 2018;9:382. doi: 10.3389/fimmu.2018.00382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Qu X, Han J, Zhang Y, Wang Y, Zhou J, Fan H, et al. MiR-384 Regulates the Th17/Treg Ratio during Experimental Autoimmune Encephalomyelitis Pathogenesis. Front Cell Neurosci. 2017;11:88. doi: 10.3389/fncel.2017.00088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ma X, Zhou J, Zhong Y, Jiang L, Mu P, Li Y, et al. Expression, regulation and function of microRNAs in multiple sclerosis. Int J Med Sci. 2014;11:810–8. doi: 10.7150/ijms.8647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dolati S, Marofi F, Babaloo Z, Aghebati-Maleki L, Roshangar L, Ahmadi M, et al. Dysregulated Network of miRNAs Involved in the Pathogenesis of Multiple Sclerosis. Biomed Pharmacother. 2018;104:280–90. doi: 10.1016/j.biopha.2018.05.050. [DOI] [PubMed] [Google Scholar]
- 14.Qu X, Zhou J, Wang T, Han J, Ma L, Yu H, et al. MiR-30a inhibits Th17 differentiation and demyelination of EAE mice by targeting the IL-21R. Brain Behav Immun. 2016;57:193–9. doi: 10.1016/j.bbi.2016.03.016. [DOI] [PubMed] [Google Scholar]
- 15.Han B, Chao J, Yao H. Circular RNA and its mechanisms in disease: from the bench to the clinic. Pharmacol Ther. 2018;187:31–44. doi: 10.1016/j.pharmthera.2018.01.010. [DOI] [PubMed] [Google Scholar]
- 16.Verduci L, Strano S, Yarden Y, Blandino G. The circRNA-microRNA code: emerging implications for cancer diagnosis and treatment. Mol Oncol. 2019;13:669–80. doi: 10.1002/1878-0261.12468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang C, Hu J, Yu Y. CircRNA Is a Rising Star in Researches of Ocular Diseases. Front Cell Dev Biol. 2020;8:850. doi: 10.3389/fcell.2020.00850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li R, Jiang J, Shi H, Qian H, Zhang X, Xu W. CircRNA: a rising star in gastric cancer. Cell Mol Life Sci. 2020;77:1661–80. doi: 10.1007/s00018-019-03345-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Memczak S, Jens M, Elefsinioti A, Torti F, Krueger J, Rybak A, et al. Circular RNAs are a large class of animal RNAs with regulatory potency. Nature. 2013;495:333–8. doi: 10.1038/nature11928. [DOI] [PubMed] [Google Scholar]
- 20.Hansen TB, Jensen TI, Clausen BH, Bramsen JB, Finsen B, Damgaard CK, et al. Natural RNA circles function as efficient microRNA sponges. Nature. 2013;495:384–8. doi: 10.1038/nature11993. [DOI] [PubMed] [Google Scholar]
- 21.Zheng Q, Bao C, Guo W, Li S, Chen J, Chen B, et al. Circular RNA profiling reveals an abundant circHIPK3 that regulates cell growth by sponging multiple miRNAs. Nat Commun. 2016;7:11215. doi: 10.1038/ncomms11215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.D’Ambra E, Capauto D, Morlando M. Exploring the Regulatory Role of Circular RNAs in Neurodegenerative Disorders. Int J Mol Sci. 2019;20:5477. doi: 10.3390/ijms20215477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang R, Zhang S, Chen X, Li N, Li J, Jia R, et al. EIF4A3-induced circular RNA MMP9 (circMMP9) acts as a sponge of miR-124 and promotes glioblastoma multiforme cell tumorigenesis. Mol Cancer. 2018;17:166. doi: 10.1186/s12943-018-0911-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bi J, Liu H, Dong W, Xie W, He Q, Cai Z, et al. Circular RNA circ-ZKSCAN1 inhibits bladder cancer progression through miR-1178-3p/p21 axis and acts as a prognostic factor of recurrence. Mol Cancer. 2019;18:133. doi: 10.1186/s12943-019-1060-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fischer MT, Sharma R, Lim JL, Haider L, Frischer JM, Drexhage J, et al. NADPH oxidase expression in active multiple sclerosis lesions in relation to oxidative tissue damage and mitochondrial injury. Brain:J Neurol. 2012;135:886–99. doi: 10.1093/brain/aws012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Casella G, Rasouli J, Boehm A, Zhang W, Xiao D, Ishikawa L, et al. Oligodendrocyte-derived extracellular vesicles as antigen-specific therapy for autoimmune neuroinflammation in mice. Sci Transl Med. 2020;12:eaba0599. doi: 10.1126/scitranslmed.aba0599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ishihara A, Ishihara J, Watkins EA, Tremain AC, Nguyen M, Solanki A, et al. Prolonged residence of an albumin-IL-4 fusion protein in secondary lymphoid organs ameliorates experimental autoimmune encephalomyelitis. Nat Biomed Eng. 2021;5:387–98. doi: 10.1038/s41551-020-00627-3. [DOI] [PubMed] [Google Scholar]
- 28.Chen J, Li Y, Zheng Q, Bao C, He J, Chen B, et al. Circular RNA profile identifies circPVT1 as a proliferative factor and prognostic marker in gastric cancer. Cancer Lett. 2017;388:208–19. doi: 10.1016/j.canlet.2016.12.006. [DOI] [PubMed] [Google Scholar]
- 29.Kumar L, Shamsuzzama, Haque R, Baghel T, Nazir A. Circular RNAs: the Emerging Class of Non-coding RNAs and Their Potential Role in Human Neurodegenerative Diseases. Mol Neurobiol. 2017;54:7224–34. doi: 10.1007/s12035-016-0213-8. [DOI] [PubMed] [Google Scholar]
- 30.Shen L, Bai Y, Han B, Yao H. Non-coding RNA and neuroinflammation: implications for the therapy of stroke. Stroke Vasc Neurol. 2019;4:96–8. doi: 10.1136/svn-2018-000206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu CX, Li X, Nan F, Jiang S, Gao X, Guo SK, et al. Structure and Degradation of Circular RNAs Regulate PKR Activation in Innate Immunity. Cell. 2019;177:865–80. doi: 10.1016/j.cell.2019.03.046. [DOI] [PubMed] [Google Scholar]
- 32.Iparraguirre L, Muñoz-Culla M, Prada-Luengo I, Castillo-Triviño T, Olascoaga J, Otaegui D. Circular RNA profiling reveals that circular RNAs from ANXA2 can be used as new biomarkers for multiple sclerosis. Hum Mol Genet. 2017;26:3564–72. doi: 10.1093/hmg/ddx243. [DOI] [PubMed] [Google Scholar]
- 33.Paraboschi EM, Cardamone G, Soldà G, Duga S, Asselta R. Interpreting Non-coding Genetic Variation in Multiple Sclerosis Genome-Wide Associated Regions. Front Genet. 2018;9:647. doi: 10.3389/fgene.2018.00647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zurawska A, et al. Dominant role of circular RNA in miRNA circuit in multiple sclerosis. Mult Scler J. 2018;24:P1074. [Google Scholar]
- 35.Zurawska A, Mycko MP, Selmaj KW. Circular RNAs as a novel layer of regulatory mechanism in multiple sclerosis. J Neuroimmunol. 2019;334:576971. doi: 10.1016/j.jneuroim.2019.576971. [DOI] [PubMed] [Google Scholar]
- 36.Zhu E, Wang X, Zheng B, Wang Q, Hao J, Chen S, et al. miR-20b suppresses Th17 differentiation and the pathogenesis of experimental autoimmune encephalomyelitis by targeting RORgammat and STAT3. J Immunol. 2014;192:5599–609. doi: 10.4049/jimmunol.1303488. [DOI] [PubMed] [Google Scholar]
- 37.Mycko MP, Cichalewska M, Cwiklinska H, Selmaj KW. miR-155-3p Drives the Development of Autoimmune Demyelination by Regulation of Heat Shock Protein 40. J Neurosci: Off J Soc Neurosci. 2015;35:16504–15. doi: 10.1523/JNEUROSCI.2830-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Du C, Liu C, Kang J, Zhao G, Ye Z, Huang S, et al. MicroRNA miR-326 regulates TH-17 differentiation and is associated with the pathogenesis of multiple sclerosis. Nat Immunol. 2009;10:1252–9. doi: 10.1038/ni.1798. [DOI] [PubMed] [Google Scholar]
- 39.Wu R, He Q, Chen H, Xu M, Zhao N, Xiao Y, et al. MicroRNA-448 promotes multiple sclerosis development through induction of Th17 response through targeting protein tyrosine phosphatase non-receptor type 2 (PTPN2) Biochem Biophys Res Commun. 2017;486:759–66. doi: 10.1016/j.bbrc.2017.03.115. [DOI] [PubMed] [Google Scholar]
- 40.Liu R, Ma X, Chen L, Yang Y, Zeng Y, Gao J, et al. MicroRNA-15b Suppresses Th17 Differentiation and Is Associated with Pathogenesis of Multiple Sclerosis by Targeting O-GlcNAc Transferase. J Immunol. 2017;198:2626–39. doi: 10.4049/jimmunol.1601727. [DOI] [PubMed] [Google Scholar]
- 41.Liu R, Li Y, Zhou H, Wang H, Liu D, Wang H, et al. OIP5-AS1 facilitates Th17 differentiation and EAE severity by targeting miR-140-5p to regulate RhoA/ROCK2 signaling pathway. Life Sci. 119108 (2021). [DOI] [PubMed]
- 42.Guan D, Li Y, Cui Y, Guo Y, Dong N, Li G, et al. Down-regulated miR-374c and Hsp70 promote Th17 cell differentiation by inducing Fas expression in experimental autoimmune encephalomyelitis. Int J Biol Macromol. 2020;154:1158–65. doi: 10.1016/j.ijbiomac.2019.11.147. [DOI] [PubMed] [Google Scholar]
- 43.Zhao M, Sun D, Guan Y, Wang Z, Sang D, Liu M, et al. Disulfiram and Diphenhydramine Hydrochloride Upregulate miR-30a to Suppress IL-17-Associated Autoimmune Inflammation. J Neurosci: Off J Soc Neurosci. 2016;36:9253–66. doi: 10.1523/JNEUROSCI.4587-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gao Y, Han D, Feng J. MicroRNA in multiple sclerosis. Clin Chim Acta; Int J Clin Chem. 2021;516:92–9. doi: 10.1016/j.cca.2021.01.020. [DOI] [PubMed] [Google Scholar]
- 45.Schiavinato J, Haddad R, Saldanha-Araujo F, Baiochi J, Araujo AG, Santos Scheucher P, et al. TGF-beta/atRA-induced Tregs express a selected set of microRNAs involved in the repression of transcripts related to Th17 differentiation. Sci Rep. 2017;7:3627. doi: 10.1038/s41598-017-03456-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yang Q, Pan W, Qian L. Identification of the miRNA-mRNA regulatory network in multiple sclerosis. Neurol Res. 2017;39:142–51. doi: 10.1080/01616412.2016.1250857. [DOI] [PubMed] [Google Scholar]
- 47.Quintana E, Ortega FJ, Robles-Cedeño R, Villar ML, Buxó M, Mercader JM, et al. miRNAs in cerebrospinal fluid identify patients with MS and specifically those with lipid-specific oligoclonal IgM bands. Mult Scler. 2017;23:1716–26. doi: 10.1177/1352458516684213. [DOI] [PubMed] [Google Scholar]
- 48.Teymoori-Rad M, Mozhgani SH, Zarei-Ghobadi M, Sahraian MA, Nejati A, Amiri MM, et al. Integrational analysis of miRNAs data sets as a plausible missing linker between Epstein-Barr virus and vitamin D in relapsing remitting MS patients. Gene. 2019;689:1–10. doi: 10.1016/j.gene.2018.12.004. [DOI] [PubMed] [Google Scholar]
- 49.Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS) Neurology. 1983;33:1444–52. doi: 10.1212/WNL.33.11.1444. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Video S1. Clinical symptoms of EAE mice with score 1
Video S2. Clinical symptoms of EAE mice with score 2
Video S3. Clinical symptoms of EAE mice with score 3
Video S4. Clinical symptoms of EAE mice with score 4
Data Availability Statement
All data are available in the main text or the supplementary materials.