Abstract
Recent double-blind, randomized, controlled trials reported that human umbilical cord-derived mesenchymal stem cell (MSC) infusions in COVID-19 patients with acute respiratory distress syndrome (ARDS) could diminish cytokine storm and lung damage. While these outcomes are significant, additional phase II/III trials are required to validate the efficacy of MSCs to improve the survival of COVID-19 patients with ARDS. Future studies also need to assess the efficacy of MSCs to prevent long COVID.
Subject terms: Respiratory distress syndrome, Mesenchymal stem cells
Coronavirus disease 2019 (COVID-19), a grave acute respiratory illness, is caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)1,2. SARS-CoV-2 infection is a worldwide pandemic currently with >219 million infected cases and >4.5 million deaths. Over 40 million people have already been infected in the United States, and >659,000 people have died of COVID-19 [Worldometers.info]. SARS-CoV-2 infection initially causes mild-to-moderate symptoms, which include fever, cough, loss of smell and taste, muscle aches, fatigue, and shortness of breath, but severe cases with acute respiratory distress syndrome (ARDS), cytokine storm, or pneumonia result in considerable mortality1,3,4. Older individuals and individuals with certain health conditions have a higher risk of developing severe COVID-19 and mortality5. Overactive immune response causing cytokine storm and immunothrombosis are believed to underlie severe COVID-196,7.
A widespread expression of the angiotensin-converting enzyme 2 (ACE2) receptor in the lung alveolar cells and capillary endothelial cells facilitates severe respiratory illness following SARS-CoV-2 infection8,9. Such infection results in a cytokine storm with elevations in multiple proinflammatory cytokines, leading to edema, air exchange dysfunction, ARDS, secondary infection, and death in many cases8. Because ACE2 expression is also present in other organs, including the heart, liver, kidney, and digestive system, infected patients develop other complications such as myocardial injury, arrhythmia, acute kidney injury, shock, and death from multiple organ dysfunction8,10. COVID-19 patients developing ARDS currently receive high-flow oxygen therapy, intensive care, and, frequently, mechanical ventilation6,11–14. Treating COVID-19 patients afflicted with ARDS, cytokine storm, or pneumonia is challenging despite several recent drug combinations showing variable efficacy15–17. On a positive note, vaccines against SARS-CoV-2 are now available, but it will take a significant amount of time to vaccinate the vast majority of the world population. While the vast majority of effort is currently aimed at reducing or eliminating infection through vaccination, there is also a need for reducing the mortality in severely ill patients with COVID-19. Therefore, safe and effective treatment for COVID-19 patients with severe complications such as ARDS, cytokine storm, or pneumonia is critical for saving lives.
Previously, studies from China and Mexico8,18,19 have shown that infusions of human umbilical cord-derived mesenchymal stem cells (UC-MSCs) into COVID-19 patients resulted in better functional outcomes8,18–22. Leng and associates showed that seven patients with COVID-19 pneumonia displayed improved functional outcomes and recovered after an intravenous administration of clinical-grade human UC-MSCs8. Among the patients who received UC-MSC infusions, one had a critically severe type, four had severe types, and the other two had common types of pneumonia8. This study provided the first evidence of the promise of MSC therapy for saving the lives of COVID-19 patients developing severe complications and has resulted in the commencement of many clinical trials23. A few subsequent studies also found that the infusion of UC-MSCs was safe in COVID-19 patients19,22. The study by Iglesias and colleagues enrolled five patients with severe ARDS who have not shown improvements in their clinical conditions with the prevailing medical care for 48 h and displayed arterial oxygen partial pressure (PaO2)/fractional inspired oxygen (FiO2) values <100 mmHg19. The study also reported that MSCs mediated anti-inflammatory effects in the lungs, evident from an improved respiratory function with higher PaO2/FiO2 values19. Treatment of MSCs resulted in the survival of three patients who were extubated 9 days post-infusion. However, the lack of double-blind, randomized, controlled trials using MSCs has raised questions about the efficacy of MSCs among skeptics.
While the outcome of many more extensive double-blind clinical trials for severe COVID-19 patients is yet to be published, Lanzoni and colleagues’ study has been recently published by the journal Stem Cell Translational Medicine14. This study represents the first double-blind, phase I/IIa, randomized, controlled trial of MSC treatment for COVID-19 patients. Table 1 lists the significant outcomes of double-blind, randomized controlled trials performed so far using MSCs. The double-blind study by Lanzoni and associates showed that infusions of UC-MSCs were safe for COVID-19 patients with complications, such as ARDS and cytokine storm14. The study also did not find any serious adverse events in the 12 patients receiving UC-MSC infusions. The patients in the control and MSC treatment groups were matched for age and ARDS severity, which balanced the control and MSC treatment groups. The study also revealed that the cytokine storm could be restrained through MSC infusions in COVID-19 patients with ARDS. MSC infusions did not decrease the viral load but significantly dampened the concentration of multiple proinflammatory cytokines. This study’s critical takeaway message is that the survival of COVID-19 patients with ARDS could be improved substantially with MSC treatment. In all, 91% survival was seen in the MSC infusion group compared to 42% survival in the control group. This is the most impressive finding from this controlled trial. Since severe COVID-19 is widely believed to be due to the overactive immune response with cytokine storm causing other complications such as immunothrombosis, mini-strokes, and multiple organ failure, the ability of MSCs to modulate the immune response and improve the survival of patients with ARDS is a significant advance. Overall, this trial’s findings validate the results of previous trials8,19. The results of another randomized, double-blind, placebo-controlled phase II trial using UC-MSCs in COVID-19 patients with lung damage have been published recently24. UC-MSC infusions (~40 million/infusion, 3 infusions over 6 days) in 49 patients resulted in a reduced lung lesion volume compared to 25 patients receiving the placebo24. These findings suggest that UC-MSC infusions could be employed as an adjunct therapy to the standard care of COVID-19 patients with lung damage.
Table 1.
Double-blind, randomized, controlled trials using MSCs in COVID-19 patients.
| Type of MSCs administered | Study design and reference | No. of patients in whom MSC infusion efficacy has been measured | Major inclusion criteria | Measured parameters | Major outcomes |
|---|---|---|---|---|---|
| Umbilical cord MSCs | Phase I/IIa (single center, double blind, randomized, and controlled)14 | 12 | COVID-19 with ARDS and cytokine storm |
Survival Inflammation |
Improved survival Reduced proinflammatory cytokines |
| Umbilical cord MSCs | Phase II (multicenter, double blind, randomized, and controlled)24 | 49 | COVID-19 with confirmed lung damage |
Lung lesion volume 6-Min walk test |
Reduced lung lesion volume Increased distance traveled |
MSCs have been employed widely in cell therapy, which comprises numerous preclinical studies and a significant number of clinical trials25–27. The rationale for employing MSC infusions for COVID-19 complications is the safety and efficacy of MSCs for immune system-mediated inflammatory diseases, such as graft-versus-host disease28, type 2 diabetes29, and spinal cord injury30. Immunomodulatory effects of MSCs are believed to underlie improved function after MSC infusions in multiple disease conditions, as MSCs secrete multiple paracrine factors, which positively interact with immune cells, facilitating immunomodulation25–27,31,32. The better outcome in COVID-19 patients after MSC infusions also appeared to comprise the robust anti-inflammatory activity of MSCs suppressing the cytokine storm. MSCs typically accumulate in the lungs after intravenous infusion, which likely facilitates the secretion of multiple paracrine factors33, leading to significant protection and rejuvenation of alveolar epithelial cells, and improved lung function in COVID-19 patients. Figure 1 depicts the potential mechanisms by which MSCs improve outcomes in COVID-19 patients.
Fig. 1. Potential mechanisms by which mesenchymal stem cells (MSCs) can improve outcomes in COVID-19 patients.
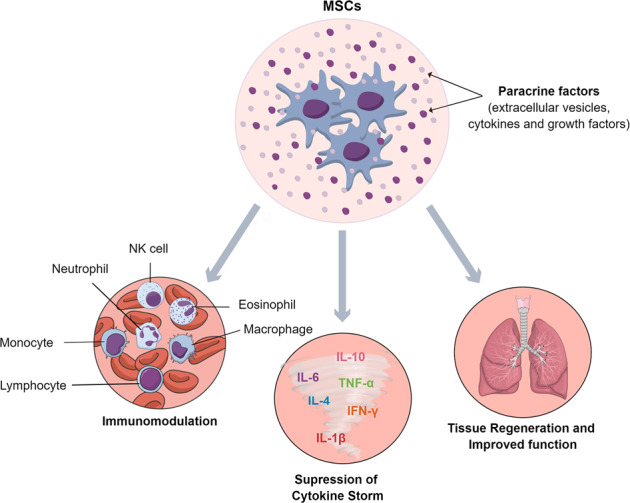
MSCs can directly modulate a variety of immune cells from their proinflammatory states into non-inflammatory or anti-inflammatory phenotypes, suppress cytokine storm, and promote lung regeneration.
While the new clinical trial results provide further support for considering MSC infusions for COVID-19 complications, a few critical issues need to be addressed in future MSC therapy trials for COVID-19 patients with ARDS. The numbers of patients recruited in one of the trials were low (24 subjects; out of that, 12 patients received MSC infusions, and the other 12 were controls)14. However, another trial has validated some of the beneficial effects of MSC infusions in 49 COVID-19 patients24. Since UC-MSCs were used in both studies, as in the previous study8, it will be necessary to ascertain whether similar efficacy could be obtained from MSCs from other sources such as those derived from the bone marrow or adipose tissue. The average age of patients employed in these trials was ~59–60 years14,24. Therefore, it remains to be determined whether MSC infusions would be efficacious in much older COVID-19 patients (e.g., ≥65 years). Furthermore, the dose employed was 40–100 million per infusion, with infusions separated by 72 h apart. Dose–response studies are needed in the future to improve the efficacy further. Since the primary endpoint was safety, efficacy measures in these studies were restricted to patient survival and cytokine levels in the blood14 or lung lesion volume and a 6-min walk test24. Future studies need to assess whether MSC infusions in COVID-19 patients with ARDS or other complications would prevent the symptoms of long COVID. Patients with long COVID (i.e., patients with symptoms persisting for >6 months post-infection) experience persistent brain-related problems, including brain fog typified by cognitive impairments, post-exertional malaise, and chronic fatigue, mimicking chronic multisymptom conditions, such as fibromyalgia34,35, chronic fatigue syndrome36, or Gulf War Illness37. Additional phase II/III trials evaluating the outcomes of MSC infusions for reducing mortality and preventing long COVID symptoms are needed. Also, while the advent of efficacious vaccines has reduced SARS-CoV-2 infections in many nations, SARS-CoV-2-infected survivors experiencing long COVID symptoms might benefit from MSC therapy because of the ability of MSCs to ease neuroinflammation and promote regeneration in organs, such as the lungs, brain, and heart.
Acknowledgements
The authors are supported by grants from the National Institute of Neurological Disorders and Stroke (1R01NS106907-01 to A.K.S.) and the Department of Defense (W81XWH-14-1-0572 and W81XWH-16-1-0480 to A.K.S.). G.Z. is supported by a postdoctoral fellowship from Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Government of Brazil.
Author contributions
All authors contributed to manuscript writing and editing and approved the final version of the manuscript.
Data availability
All data needed to evaluate the conclusions of this commentary are present in the paper.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Munster VJ, Koopmans M, van Doremalen N, van Riel D, de Wit E. A novel coronavirus emerging in China - key questions for impact assessment. N. Engl. J. Med. 2020;382:692–694. doi: 10.1056/NEJMp2000929. [DOI] [PubMed] [Google Scholar]
- 2.Zhou P, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Neumann-Podczaska A, et al. COVID 19 - clinical picture in the elderly population: a qualitative systematic review. Aging Dis. 2020;11:988–1008. doi: 10.14336/AD.2020.0620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sohrabi C, et al. World Health Organization declares Global Emergency: a review of the 2019 novel coronavirus (COVID-19) Int. J. Surg. 2020;76:71–76. doi: 10.1016/j.ijsu.2020.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barzilai N, et al. Geroscience in the age of COVID-19. Aging Dis. 2020;11:725–729. doi: 10.14336/AD.2020.0629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berlin DA, Gulick RM, Martinez FJ. Severe Covid-19. N. Engl. J. Med. 2020;383:2451–2460. doi: 10.1056/NEJMcp2009575. [DOI] [PubMed] [Google Scholar]
- 7.Kox M, Waalders NJB, Kooistra EJ, Gerretsen J, Pickkers P. Cytokine levels in critically ill patients with COVID-19 and other conditions. JAMA. 2020;324:1565–1567. doi: 10.1001/jama.2020.17052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leng Z, et al. Transplantation of ACE2- mesenchymal stem cells improves the outcome of patients with COVID-19 pneumonia. Aging Dis. 2020;11:216–228. doi: 10.14336/AD.2020.0228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hoffmann M, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271.e8–280.e8. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hamming I, et al. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J. Pathol. 2004;203:631–637. doi: 10.1002/path.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wiersinga WJ, Rhodes A, Cheng AC, Peacock SJ, Prescott HC. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): a review. JAMA. 2020;324:782–793. doi: 10.1001/jama.2020.12839. [DOI] [PubMed] [Google Scholar]
- 12.Wu C, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan. China JAMA Intern. Med. 2020;180:934–943. doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Demoule A, et al. High-flow nasal cannula in critically iii patients with severe COVID-19. Am. J. Respir. Crit. Care Med. 2020;202:1039–1042. doi: 10.1164/rccm.202005-2007LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lanzoni G, et al. Umbilical cord mesenchymal stem cells for COVID-19 acute respiratory distress syndrome: a double-blind, phase 1/2a, randomized controlled trial. Stem Cells Transl. Med. 2021;10:660–673. doi: 10.1002/sctm.20-0472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fauci AS, Lane HC, Redfield RR. Covid-19 – navigating the uncharted. N. Engl. J. Med. 2020;382:1268–1269. doi: 10.1056/NEJMe2002387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li H, et al. Updated approaches against SARS-CoV-2. Antimicrob. Agents Chemother. 2020;64:e00483–20. doi: 10.1128/AAC.00483-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Peng Y, Tao H, Satyanarayanan SK, Jin K, Su H. A comprehensive summary of the knowledge on COVID-19 treatment. Aging Dis. 2021;12:155–191. doi: 10.14336/AD.2020.1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liang B, et al. Clinical remission of a critically ill COVID-19 patient treated by human umbilical cord mesenchymal stem cells: a case report. Medicine. 2020;99:e21429. doi: 10.1097/MD.0000000000021429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Iglesias M, et al. Mesenchymal stem cells for the compassionate treatment of severe acute respiratory distress syndrome due to COVID 19. Aging Dis. 2021;12:360–370. doi: 10.14336/AD.2020.1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Metcalfe SM. Mesenchymal stem cells and management of COVID-19 pneumonia. Med. Drug Discov. 2020;5:100019. doi: 10.1016/j.medidd.2020.100019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shetty AK. Mesenchymal stem cell infusion shows promise for combating coronavirus (COVID-19)-induced pneumonia. Aging Dis. 2020;11:462–464. doi: 10.14336/AD.2020.0301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guo Z, et al. Administration of umbilical cord mesenchymal stem cells in patients with severe COVID-19 pneumonia. Crit. Care. 2020;14:420. doi: 10.1186/s13054-020-03142-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang SH, Shetty AK, Jin K, Zhao RC. Combating COVID-19 with mesenchymal stem/stromal cell therapy: promise and challenges. Front. Cell. Dev. Biol. 2021;8:627414. doi: 10.3389/fcell.2020.627414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shi L, et al. Effect of human umbilical cord-derived mesenchymal stem cells on lung damage in severe COVID-19 patients: a randomized, double-blind, placebo-controlled phase 2 trial. Signal Transduct. Target. Ther. 2021;6:58. doi: 10.1038/s41392-021-00488-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Prockop DJ. The exciting prospects of new therapies with mesenchymal stromal cells. Cytotherapy. 2017;19:1–8. doi: 10.1016/j.jcyt.2016.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Connick P, et al. Autologous mesenchymal stem cells for the treatment of secondary progressive multiple sclerosis: an open-label phase 2a proof-of-concept study. Lancet Neurol. 2012;11:150–156. doi: 10.1016/S1474-4422(11)70305-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wilson JG, et al. Mesenchymal stem (stromal) cells for treatment of ARDS: a phase 1 clinical trial. Lancet Respir. Med. 2015;3:24–32. doi: 10.1016/S2213-2600(14)70291-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fisher SA, et al. Mesenchymal stromal cells as treatment or prophylaxis for acute or chronic graft-versus-host disease in haematopoietic stem cell transplant (HSCT) recipients with a haematological condition. Cochrane Database Syst. Rev. 2019;1:CD009768. doi: 10.1002/14651858.CD009768.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Päth G, Perakakis N, Mantzoros CS, Seufert J. Stem cells in the treatment of diabetes mellitus - focus on mesenchymal stem cells. Metabolism. 2019;90:1–15. doi: 10.1016/j.metabol.2018.10.005. [DOI] [PubMed] [Google Scholar]
- 30.Shende P, Subedi M. Pathophysiology, mechanisms and applications of mesenchymal stem cells for the treatment of spinal cord injury. Biomed. Pharmacother. 2017;91:693–706. doi: 10.1016/j.biopha.2017.04.126. [DOI] [PubMed] [Google Scholar]
- 31.Borlongan MC, Borlongan MC, Sanberg PR. The disillusioned comfort with COVID-19 and the potential of convalescent plasma and cell therapy. Cell Transpl. 2020;29:963689720940719. doi: 10.1177/0963689720940719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Park YJ, et al. Fighting the war against COVID-19 via cell-based regenerative medicine: lessons learned from 1918 Spanish flu and other previous pandemics. Stem Cell Rev. Rep. 2021;17:9–32. doi: 10.1007/s12015-020-10026-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee RH, et al. Intravenous hMSCs improve myocardial infarction in mice because cells embolized in lung are activated to secrete the anti-inflammatory protein TSG-6. Cell Stem Cell. 2009;5:54–63. doi: 10.1016/j.stem.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Davis, H. E. et al. Characterizing long COVID in an international cohort: 7 months of symptoms and their impact. EClinicalMedicine10.1016/j.eclinm.2021.101019 (2021). [DOI] [PMC free article] [PubMed]
- 35.Bair MJ, Krebs EE. Fibromyalgia. Ann. Intern Med. 2020;172:ITC33–ITC48. doi: 10.7326/AITC202003030. [DOI] [PubMed] [Google Scholar]
- 36.Mohabbat AB, Mohabbat NML, Wight EC. Fibromyalgia and chronic fatigue syndrome in the age of COVID-19. Mayo Clin. Proc. Innov. Qual. Outcomes. 2020;4:764–766. doi: 10.1016/j.mayocpiqo.2020.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dickey B, Madhu LN, Shetty AK. Gulf War Illness: mechanisms underlying brain dysfunction and promising therapeutic strategies. Pharmacol. Ther. 2020;24:107716. doi: 10.1016/j.pharmthera.2020.107716. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data needed to evaluate the conclusions of this commentary are present in the paper.


