Abstract
Recently, Akkermansia muciniphila an anaerobic member of the gut microbiota, has been proposed as a next-generation probiotic. The aim of this study was evaluation of the effect of alive and pasteurized A. muciniphila on health status, intestinal integrity, immune response, lipid metabolism, and gut microbial composition in normal-diet fed mice as well as direct effects of the bacterium on Caco-2 cell line. A total of 30 mice were distributed into three different groups, control, alive, and pasteurized A. muciniphila-treated group. After acclimation, control and treatment groups were administrated with PBS and 109 CFU/200µL of bacterial suspension for 5 weeks, respectively. Besides, Caco-2 separately exposed to alive, pasteurized A. muciniphila and PBS for 24 h. The results showed that administration of A. muciniphila leads to reduction in body, liver, and white adipose weight. Histology data revealed both treatments had no adverse effects in colon, liver, and adipose tissues as well as induced better gut structure. Moreover, biochemical parameters and inflammatory biomarkers in plasma demonstrated that pasteurized A. muciniphila had more pronounce effect. Furthermore, alive A. muciniphia had better effects on the modulation of gene expression related to fatty acid synthesis, energy homeostasis, and immune response in the liver; meanwhile, these effects in the adipose was more in the pasteurized A. muciniphila administration. More importantly, the improvement of gut health by enhancing strengthen intestinal integrity and maintaining immune homeostasis was seen in both treatments; notably, pasteurized A. muciniphila had more effective. Similarly, treatment with the pasteurized form more effectively upregulated tight junction and regulated immune response-related genes in Caco-2 cell line. Both treatments triggered the improvement of microbiota communities, particularly the alive form. Therefore, both forms of A. muciniphila could modulate lipid and immune homeostasis, improved some gut microbiota, and promoted the overall health, while all these effects were dominantly observed in pasteurized form. In conclusion, pasteurized A. muciniphila can be considered as new medical supplement to maintain health state and prevent diseases in normal mice through different mechanisms.
Subject terms: Microbiology, Diseases
Introduction
Trillions of microorganism reside in the gastrointestinal (GI) tract, known as “intestinal microbiota”, that are involved in the regulation of food intake, motility of the gut, immune and metabolic pathways1. In addition, numerous studies show that intestinal microbiota also plays an important role in maintaining intestinal homeostasis by improvement of the gut barrier function and the inflammatory state2,3. As a bilateral relationship exists between the gut microbiota and intestinal immune system, the immune system playing a crucial role in maintaining the microbiota's homeostasis, as well as the intestinal microbiota affect immune system by influencing on T-reg cells. This two-way communication is accomplished through various mechanisms, including TLRs4.
Disruption of intestinal microbiota composition known as “dysbiosis” has been reported in many diseases and its return to normal pattern has been associated with the amelioration of diseases5, 6. One of the factors contribute to restoration of healthy condition is prebiotics, probiotics, and para-probiotics consumption7–9.
One of the next-generation probiotics considered to be a healthy biomarker bacterium in the human and animal intestines is Akkermansia muciniphila (A. muciniphila)10,11. Many researchers have confirmed a reduction in the bacterium frequency associated with several diseases12–14. On the other hand, a high abundance of A. muciniphila is linked to decreased blood lipids and improved metabolic features that ultimately lead to a healthy state in obese adults15. In addition, A. muciniphila can affect metabolic modulation, immune response regulation, serotonergic system, and gut health maintenance16–21. Increased A. muciniphila may be associated with increased intestinal integrity and normal mucus production in the intestines of healthy individuals22. Several lines of evidence have indicated that A. muciniphila, as a closer epithelial bacterium, have an impact on health promotion7,23.
In comparison with viable probiotics, para-probiotics are non-viable and have more effective properties9,17. Researchers reported that probiotics and para-probiotics have an important role in restoring health and ameliorating diseases12,17,18,24,25. But limited research has been performed on whether they can also be effective in maintaining health in normal subjects or animals and further studies are needed for detecting precise molecular mechanism in this area.
In the present study, the effects of alive and pasteurized A. muciniphila on the integrity of gut barrier, inflammation, and energy homeostasis were assessed in vitro and in vivo. Our research provided an insight into the beneficial effects of A. muciniphila on host health and the safety of its use in normal diet-fed mice.
Material and method
Bacterial culture and pasteurization
A. muciniphila MucT (ATCC BAA-835) was cultured in a synthetic medium under the anaerobic conditions as previously described17. After growth, the bacterium was inoculated into the broth with mild shaking under the above-mentioned conditions. After OD600 was reached 1, bacterial pellets were collected by centrifugation and then washed twice with an anaerobic PBS. After resuspended in PBS, the suspension used for the treatments was prepared freshly. For the preparation of pasteurized form, the bacteria suspension was heated at 70 °C for 30 min17.
Cell culture conditions, treatment, and quantitative real-time PCR
Human colorectal carcinoma cell line Caco-2 (ATCC® HTB-37) was cultured in DMEM (Gibco, UK), supplemented with 10% heat-inactivated FBS (Gibco) and 1% penicillin–streptomycin (Gibco) at 37 °C in 5% CO2. After 21 days of culture, Caco-2 monolayer was exposed to a live and pasteurized A. muciniphila at Multiplicity of infection (MOI) ratio of 100 (bacteria per cell) as well as an equal volume of PBS was used as a control for bacterial treatment. RNeasy Plus Mini kit (Qiagen, USA, Cat No. /ID: 74,134), PrimeScript RT Reagent Kit (Takara, Japan, Cat. # RR037A), and 2X SYBR Premix Ex Taq II (Tli RNase H, Plus Takara, Japan, Cat. #RR820L) were used for RNA extraction, cDNA synthesis, and real-time PCR, respectively. A sequence of primers used in this study is shown in Supplementary Table 1.
Experimental design and samples collection
All animal studies were carried out in compliance with the ARRIVE guidelines. All Animal procedures were approved by the Animal Experiment Committee of the Pasteur Institute of Iran (IR.PII.REC.1395.010) and confirming that all experiments were performed in this study in accordance with relevant guidelines and regulations. Eight-week-old mice purchased from Pasteur Institute of Iran (n = 30) and maintained on an equal condition including 12 h light/dark, 22 (± 2) °C and 40–60% humidity as well as received food and autoclaved water ad libitum. After acclimation with standard Normal Diet (ND) (A03, safe diet, France), mice were randomly divided into three groups as follows: The mice were treated for five weeks includes (1) ND + 200 µl PBS (C), (2) ND + 109 CFU /200 µl alive A. muciniphila (Am), and (3) ND + 109 CFU /200 µl pasteurized A. muciniphila (PAm).
The mice were individually housed in per cages and also body weight and average food were measured once a week. At the end of treatment, blood sample was collected by cardiac puncture at 12 h fasting condition and stored at − 80 °C for biochemical plasma analysis. In addition, stool samples were collected from individually housed mice and transferred to − 80 °C. All mice were sacrificed by cervical dislocation, and liver, epididymal adipose, and colon samples snap-frozen with liquid nitrogen stored in − 80 °C for real-time PCR. Moreover, the tissue specimens are saved for histological staining.
Plasma biochemical and cytokines analysis
Fasting blood glucose (Glu), total cholesterol (TC), triglycerides (TG), low-density lipoprotein (LDL), high-density lipoprotein (HDL), triglyceride (TG), alanine aminotransferase (ALT), and aspartate aminotransferase (AST) concentration were measured in plasma using a commercial kit (Bioclin-Quibasa, Belo Horizonte, MG, Brazil).
The concentration of IL-6, TNF-α, and IL-10 in the plasma of mice were determined by using ZellBio GmbH ELISA kit (Germany) according to the manufacturer’s instructions.
Histological evaluation
Four samples from each group were used for pathology examination. The colon, liver and adipose tissues were immersed in 10% buffered formalin. Then, tissues were dehydrated in ascending graded series of ethanol. They cleared in xylene and impregnated and embedded in paraffin. Paraffin blocks were cut using microtome at 5–7 µm thickness and mounted on glass slides. Histological sections were stained with hematoxylin and eosin (H&E). Then, histological slides were evaluated using a light microscope (Olympus SX-21) equipped with a digital Dino-Lite lens and Dino-capture 2 software (AnMo Electronics Corp., New Taipei City, Taiwan)26. Stained sections were evaluated by an expert pathologist, blind to study groups.
Tissue RNA isolation, cDNA synthesis, and real-time PCR
The liver, colon, and adipose were homogenized and RNA was extracted by Trizol reagent (Bio Basic, Canada). Genomic DNA removed from RNA by DNase I (Qiagen) then cDNA synthesis was performed using PrimeScript RT Reagent Kit (Takara). Real-time PCR was performed using SYBR Premix Ex Taq II (Takara). A sequence of primers used in this study is shown in Supplementary Table 1.
Bacterial DNA extraction and real-time quantitative PCR
Each stool sample was weighted at 180 mg and homogenized, then bacterial genomic DNA was extracted using a QIAamp Fast DNA Stool Mini Kit (Qiagen, USA, Cat No. /ID:51604) according to the manufacturer's instructions. Finally, gDNA of samples were stored at − 20 °C. Real-time PCR was carried out by RealQ Plus Master Mix Green (Amplicon, Denmark). The ΔCT method was used to measure each primer efficiency. Conversion of CT value to a percentage of bacterial communities was performed by using percentage formula as previously described27. A sequence of primers used in this experiment is shown in Supplementary Table 2.
Statistical analysis
Differences between groups were calculated using one-way analysis of variance (ANOVA) followed by Tukey’s post hoc test for multiple comparisons between more than two groups. The relative gene expression was analyzed by ΔΔCT method and internal controls were used in Caco-2, colon, liver, and adipose tissues are included gapdh, rpl-19, and hprt-1, respectively. For analysis of the relative difference of gene expression among treatments and control groups in both cell line and mice as well as generation of figures, GraphPad Prism 8.0 (GraphPad Software Inc, CA, USA) was used. All data are expressed as mean ± SD. In figures, data with * and ** are significantly different at p < 0.05 and p < 0.01, respectively. The non-parametric Kruskal–Wallis tests used for pairwise comparison of mean relative percentages of 16S rRNA genes between experimental groups, and p values less than 0.05 were taken as statically significant.
Results
A. muciniphila improved the weight of body and metabolic organs
Because the correlation between increased BMI and various diseases, prevention of body weight gain can play an effective role in promoting health status and inhibiting diseases, we evaluated the effects of alive and pasteurized A. muciniphila on body and metabolic organs (i.e. liver and eWAT) weight in study groups. Our finding revealed that the administration of pasteurized A. muciniphila caused a higher reduction in body weight gain in comparison with the alive form (p value 0.002 and 0.03, respectively), however, a significant difference was not observed between them. Mice were treated with both forms of A. muciniphila showed a slight decrease in food intake, which was not significant compared to control group. The adipose weight significantly reduced in both Am and PAm groups (p value 0.01 and 0.04, respectively), compared to C group. On the other hand, the liver weight of alive A. muciniphila-treated mice was significantly decreased (p value 0.0002), while it was not significant in PAm group) Table 1). Taken together, mice gavaged by alive and pasteurized A. muciniphila exhibited favorable effects on ameliorating body and metabolic organs weight, which indicates the beneficial role of this next generation probiotic candidate in preserving healthy status.
Table 1.
The effect of a live and pasteurized A. muciniphila administration on body, adipose and liver weight, food intake, and blood parameters in ND-fed mice after 5 weeks (n = 10 for each group).
| Variable | Study groups | P value | ||||
|---|---|---|---|---|---|---|
| C | Am | PAm | Am vs. C | PAm vs. C | Am vs. PAm | |
| Body weight gain (g) | 2.314 ± 0.414 | 1.800 ± 0.321 | 1.561 ± 0.293 | 0.031 | 0.002 | 0.42 |
| Daily food intake/mouse (g) | 3.65 ± 0.512 | 3.614 ± 0.241 | 3.714 ± 0.203 | 0.97 | 0.94 | 0.85 |
| Adipose weight (g) | 0.240 ± 0.053 | 0.170 ± 0.037 | 0.185 ± 0.023 | 0.01 | 0.04 | 0.74 |
| Liver weight (g) | 1.108 ± 0.037 | 0.908 ± 0.084 | 1.033 ± 0.086 | 0.0002 | 0.165 | 0.014 |
| Glu (mg/dl) | 78 ± 6.75 | 58 ± 5.56 | 47 ± 6.37 | < 0.0001 | < 0.0001 | 0.018 |
| TG (mg/dl) | 80.13 ± 6.61 | 67.86 ± 6.20 | 66.57 ± 6.70 | 0.006 | 0.002 | 0.92 |
| TC (mg/dl) | 71.79 ± 9.18 | 67.57 ± 5.12 | 45.14 ± 3.30 | 0.51 | < 0.0001 | < 0.0001 |
| LDL (mg/dl) | 13.9 ± 4.56 | 17.04 ± 3.46 | 7.32 ± 1.70 | 0.24 | 0.039 | 0.0003 |
| HDL (mg/dl) | 51.76 ± 9.36 | 105.7 ± 11.18 | 53.29 ± 9.55 | < 0.0001 | 0.95 | < 0.0001 |
| ALT (U/dl) | 65.00 ± 6.83 | 65.00 ± 9.73 | 24.86 ± 5.17 | > 0.999 | < 0.0001 | < 0.0001 |
| AST (U/dl) | 87.97 ± 5.29 | 48.14 ± 6.36 | 31.86 ± 4.67 | < 0.0001 | < 0.0001 | < 0.0001 |
| TNF-α (ng/L) | 162.3 ± 6.22 | 145.20 ± 5.76 | 128.40 ± 6.87 | 0.009 | < 0.0001 | 0.01 |
| IL-6 (pg/ml) | 177.60 ± 10.95 | 117.3 ± 12.43 | 89.95 ± 9.15 | < 0.0001 | < 0.0001 | 0.015 |
| IL-10 (pg/ml) | 349.80 ± 28.34 | 567.70 ± 54.69 | 426.50 ± 20.89 | < 0.0001 | 0.043 | 0.001 |
C; control, Am; A. muciniphila, PAm; pasteurized A. muciniphila, Glu; Glucose, TG; Triglyceride, TC; Total cholesterol, LDL; low-density lipoprotein, HDL; high-density lipoprotein, ALT; alanine aminotransferase, AST; aspartate aminotransferase, TNF-α; Tumour necrosis factor-α, IL-6; Interleukin-6, and IL-10; Interleukin-10. Bold P value are indicated statically significant.
A. muciniphila ameliorates the biochemical and inflammatory parameters
Due to the association of dysregulated lipids profile and metabolic parameters with various diseases and the importance of a balanced abovementioned markers in disease-resistant, we assessed the changing level of lipid profiles, glucose, and hepatic enzymes in the plasma of mice treated with A. muciniphila. A tendency to decrease biochemical parameters level after both treatments were observed, which accompanied by regulating lipid profiles i.e. reducing the level of TG, TC, and LDL and raising HDL as well as decreasing Glu, ALT, and AST. The significantly lower concentrations of TC, LDL, Glu, ALT, and AST were seen in PAm group (p value < 0.0001, 0.03, < 0.0001, < 0.0001, and < 0.0001, respectively). However, significant alterations in TC, LDL, and ALT levels not observed in Am group compared to C group. In addition, both treatments reduced TG concentration as compared to C group, but there was no difference between Am and PAm groups. In comparison with C group, increased HDL level was observed in Am group, while no change was seen following pasteurized A. muciniphila.
Both forms of A. muciniphila decreased TNF-a and IL-6 and also increased regulatory cytokines IL-10 level. As shown in Table 1, more reduction in concentrations of TNF-α and IL-6 was observed in PAm group (p value < 0.0001 and < 0.0001, respectively), while in case of IL-10, alive A. muciniphila had a better effect (p value < 0.0001). Overall, these results indicated that pasteurized A. muciniphila intervention promoted health and prevented the onset of metabolic disorders by lowering effects on lipid profiles, glucose, liver injury-related enzyme, and inflammatory biomarkers in the plasma of study groups.
A. muciniphila caused intestinal and immune homeostasis by improving intestinal barrier function and alleviating inflammation
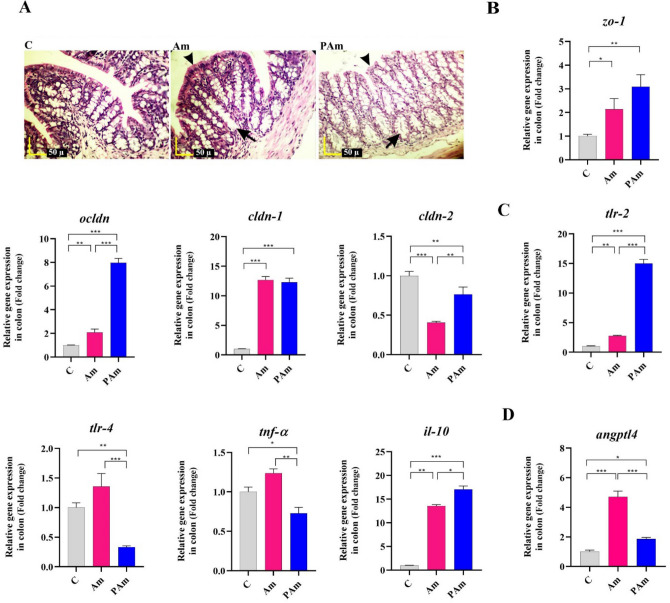
Since gut is connected to many organs in the whole body and the disruption of intestinal integrity causes inflammation and diseases, so maintaining intestinal homeostasis plays an important role in preventing the onset of various diseases. Therefore, we assessed the effects of alive and pasteurized A. muciniphila on morphology and intestinal barrier-related genes in the colon of ND-fed mice. The histopathological results indicated that the crypt depth and thickness of the mucous layer of the colon showed an increase in both treatment groups, compared to that in the control group. On the other hand, no inflammatory reactions were present in both study groups (Fig. 1A). Moreover, both treatments improved gut barrier function in mice through increasing tight junction proteins i.e. zo-1, ocldn, and cldn-1 and also decreasing cldn-2 mRNA level. The expression of zo-1 and cldn-1 genes significantly increased after both administrations. The significantly higher induction of down-regulating cldn-2 was seen in Am group (p value < 0.0001). In addition, pasteurized A. muciniphila induced the expression of ocldn significantly more than Am group (p value < 0.0001 and 0.006, respectively) (Fig. 1B).
Figure 1.
The assessment of a live and pasteurized A. muciniphila effects on mRNA expression of genes in the colon of ND-fed mice. Mice were gavaged with alive and pasteurized A. muciniphila (109 CFU) for 5 weeks. (A) Histopathology of colon (Black arrows: crypt depth, and black arrowheads: mucous thickness) (4 samples per group). Scale bar is 50 µm. Expression of (B) tight junction proteins (zo-1, ocldn, cldn-1, and cldn-2), (C) inflammation-related genes (tlr-2, tlr-4, tnf-α, and il-10), and (D) angptl4. *, **, *** 'P < 0.05, P < 0. 01, and P < 0. 001 were considered statistically significant, respectively. C: normal diet + PBS, Am: normal diet + A. muciniphila (109 CFU), PAm: normal diet + pasteurized A. muciniphila (109 CFU).
To determine whether para-probiotic and probiotic of A. muciniphila can affect intestinal immune homeostasis in non-inflammatory conditions, we were used mice undergoing standard diet. A significant alleviation in gene expression of tlr-4 and significant upregultion in tlr-2 were observed in the colon of pasteurized A. muciniphila-gavaged mice, compared to control group (p value 0.02 and < 0.0001, respectively). However, alive A. muciniphila consumption significantly increased the tlr-2 mRNA level (p value 0.003), it was lower than that of pasteurized form. A slight increase of tlr-4 mRNA level was observed in Am group in comparison with control group. The gavage with pasteurized A. muciniphila displayed a lower mRNA level of tnf-α, while alive form didn’t show significant change. Administrating mice with both treatments increased il-10 expression than ND-fed mice, while PAm group was more noticeable (p value < 0.0001) (Fig. 1C).
To investigate whether the next-generation probiotic A. muciniphila affects the digestion of intestinal lipids, we evaluated angptl4 mRNA level in the normal colon. A trend towards an increase in colonic angptl4 was observed in both treatment groups than control group. Interestingly, alive A. muciniphila significantly induced a higher upregulation of angptl4 in the healthy colon, compared to pasteurized form (p value < 0.0001 and 0.01, respectively) (Fig. 1D). Overall, these results showed that pasteurized A. muciniphila is involved in the host immunological and intestinal homeostasis at the gut by improving the function of gut barrier and regulating immune response.
Treatment with A. muciniphila affect tight junction proteins and inflammatory markers in Caco-2
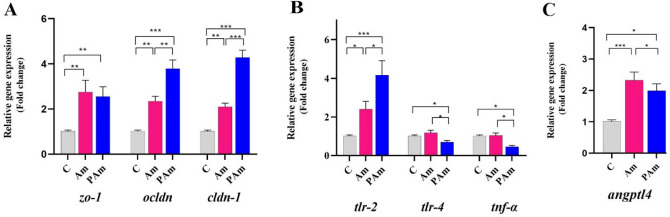
Caco-2 cell line was used to investigate direct effects of live and pasteurized A. muciniphila on the intestinal barrier function and inflammation in intestinal epithelial cells. Gene expression analysis showed tight junction proteins were upregulated, similar to mice's colon. Notably, ocldn and cldn-1 to be expressed at higher amounts in PAm than Am group. Compared to vehicle-treated Caco-2 cells, both treatments showed a significant increase in mRNA level of zo-1 (Fig. 2A). Similar to the in-vivo study, pasteurized A. muciniphila improved immune response, which was accompanied by down-regulating gene expression of tlr-4 and tnf-α as well as up-regulating tlr-2 genes (p value 0.02, 0.01, and 0.0005, respectively), while no change in tlr-4 and tnf-α expression was observed in Am group (Fig. 2B). In addition, the regulation of lipid metabolism by up-regulating angptl4 was observed after both treatments, whereas Am group was more noticeable (p value 0.0005) (Fig. 2C). Overall, these results suggested that alive and pasteurized A. muciniphila had a direct effect on the gene expression involved in integrity, inflammation, and lipid metabolism.
Figure 2.
The live and pasteurized A. muciniphila affects study's genes in Caco-2 cell line. Caco-2 monolayer were inoculated with A. muciniphila (MOI100) and pasteurized A. muciniphila (MOI100) for 24 h. The expression of genes: (A) tight junction proteins (zo-1, ocldn, and cldn-1), (B) inflammation-related genes (tlr-2, tlr-4, and tnf-α), and (C) angptl4. *, **, *** 'P < 0.05, P < 0. 01, and P < 0. 001 were considered statistically significant, respectively.
Administration of A. muciniphila improved liver health by reducing lipid metabolism and inflammation
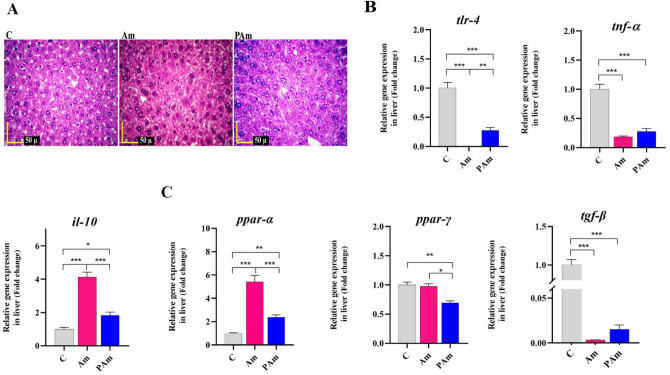
Considering the importance of the liver and its functions in the health, we evaluated the effects of alive and pasteurized A. muciniphila to improve liver homeostasis in study groups. In the liver, the hepatocytes and sinusoidal liver cells in Am and PAm groups were similar to control group. Indeed, no accumulation of lipids droplets was observed in both treatments, similar to that control groups (Fig. 3A). The gavage of alive A. muciniphila remarkably suppressed the hepatic expression of tlr-4 gene (p value < 0.0001). Moreover, the pasteurized form of the bacterium reduced tlr-4 expression (p value < 0.0001) in mice's liver, notably, the live form had more noticeable effect (p value < 0.0001). The expression of tnf-α significantly reduced following both treatments, however, there was no significant difference between both of them. Both treatments increased il-10 expression, while the live form had a greater effect on maintain liver health (p value < 0.0001) (Fig. 3B).
Figure 3.
The effect of a live and pasteurized A. muciniphila on study's genes in the liver of normal mice. Mice were gavaged with a live and pasteurized A. muciniphila (109 CFU) for 5 weeks. (A) Histopathology of liver (White Arrows: accumulation of lipids droplets) (4 samples per group). Scale bar is 50 µm. Relative mRNA expression of (B) inflammation-related genes (e.g. tlr-4, tnf-α, and il-10) and (C) lipid metabolism-related genes (e.g. ppar-α, ppar-, and tgf-β) in the liver of normal diet-fed mice. *, **, *** 'P < 0.05, P < 0. 01, and P < 0. 001 were considered statistically significant, respectively. C: normal diet + PBS, Am: normal diet + A. muciniphila (109 CFU), PAm: normal diet + pasteurized A. muciniphila (109 CFU).
Supplementation with A. muciniphila significantly regulated the lipid metabolism-related gene in the liver of mice. These results were accompanied by up-regulated ppar-α and down-regulated ppar-γ and tgf-β genes. Alive A. muciniphila induced a higher mRNA level of ppar-α (p value < 0.0001), while pasteurized form had a better effect on lowering ppar-γ expression (p value < 0.0003). The down-regulation of tgf-β was observed after both treatments (Fig. 3C). Taken together, our study elicited that administration of alive and pasteurized A. muciniphila by mice resulted in improvement of the lipid metabolism and inflammatory markers in liver tissue.
Administration of A. muciniphila improved adipose health by reducing lipid metabolism and inflammation
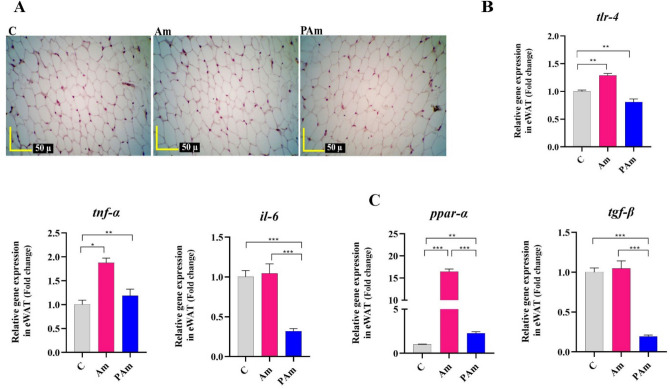
Since inflammation in white adipose tissue is a key factor in the onset of metabolic disorders, therefore, alive and pasteurized A. muciniphila were administrated to ND-fed mice to determine if they would control and prevent adipo-inflammation to preserve healthy adipose. In histopathology, no change in adipocyte size and no inflammatory infiltration in epididymal white adipose tissue (eWAT) was seen in both treatments, similar to control group (Fig. 4A). A reduction in tlr-4 and il-6 mRNA level (p value 0.002 and 0.0002, respectively) were seen in PAm group, compared to C group. However, alive A. muciniphila had no effect in il-6 mRNA expression and induced a slight increase in tlr-4 and tnf-α genes expression (Fig. 4B). Interestingly, the mRNA level of ppar-α following both treatments were significantly increased, whereas alive A. muciniphila induced remarkable upregulation (p value < 0.0001). Pasteurized A. muciniphila significantly decreased the expression of tgf-β (p value < 0.0001), while no change was observed in Am group (Fig. 4C). Altogether, probiotic and para-probiotic interventions have led to the maintenance of adipose health by regulating energy balance and immune homeostasis.
Figure 4.
The effect of a live and pasteurized A. muciniphila on study's genes in the liver of normal mice. Mice were gavaged with a live and pasteurized A. muciniphila (109 CFU) for 5 weeks. (A) Histopathology of eWAT of ND-fed mice. (4 samples per group). Scale bar is 50 µm. The mRNA expression of (B) inflammation-related genes (e.g. tlr-4, tnf-α, and il-6) and (C) lipid metabolism-related genes (e.g. ppar-α and tgf-β) in the eWAT of ND-fed mice. *, **, *** 'P < 0.05, P < 0. 01, and P < 0. 001 were considered statistically significant, respectively. C: normal diet + PBS, Am: normal diet + A. muciniphila (109 CFU), PAm: normal diet + pasteurized A. muciniphila (109 CFU).
Administration of A. muciniphila improved health by modulating some gut microbiota
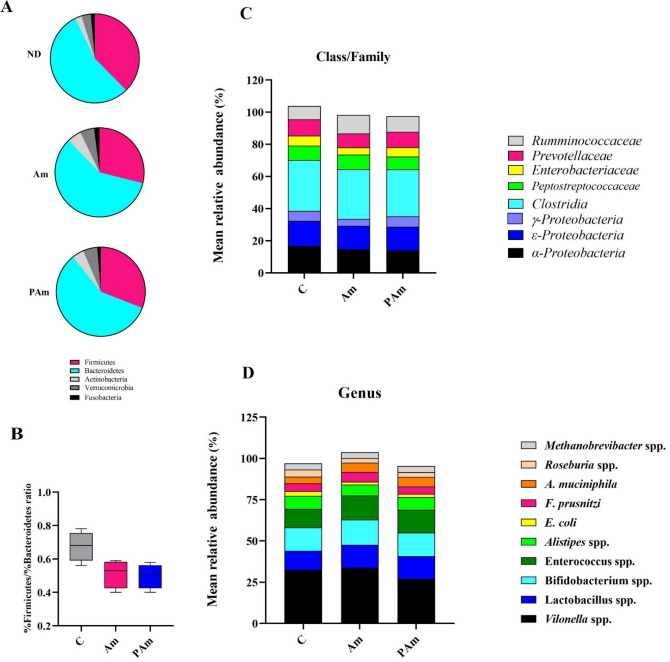
Since normal gut microbiota is essential for maintenance of health and intestinal dysbiosis is capable to inducing several diseases, therefore, the beneficial effects of alive and pasteurized A. muciniphila on the gut microbiota pattern was studied. The results showed that alive A. muciniphila significantly reduced the abundance of Firmicutes (p value 0.02) and increased Bacteroidetes (p value 0.01) and Verrucomicrobia (p value 0.01), while pasteurized A. muciniphila showed no significant change in Phylum level (Fig. 5A). Both treatments displayed some changes in Firmicutes/Bacteroidetes ratio; however, there was no statistical difference was observed (Fig. 5B). A decrease in Enterobacteriaceae (p value 0.006) and Prevotellaceae (p value 0.008) abundance in Am group as well as in α-Proteobacteria (p value 0.02) and Clostridia spp. (p value 0.005) abundance in PAm group were observed at the Class/Family level relative to control group (Fig. 5C).
Figure 5.
The alter of gut microbiota composition after treated with a live and pasteurized A. muciniphila in normal mice. The mean relative abundance at (A) phylum. (B) Firmicutes to Bacteroidetes ratio. The mean relative abundance at (C) Family/Class and (D) Genus. N = 10 per group. *p < 0.05. C: normal diet + PBS, Am: normal diet + A. muciniphila (109 CFU), PAm: normal diet + pasteurized A. muciniphila (109 CFU).
At the genus level, the amount of E. coli (p value 0.02) tended to decrease in Am group, whereas A. muciniphila (p value 0.01) and Lactobacillus spp. (p value 0.03) level were significantly increased. Pasteurized A. muciniphila induced a significant decrease in Roseburia spp. level (p value 0.04) (Fig. 5D). However, no significant change in abundance of other genera was observed in both treatment groups. The significant change in the mean relative abundance of gut microbiota between treatment and control groups was shown in Table 2. Overall, results showed that alive A. muciniphila treatment can maintain healthy gut microbiota pattern and promote health.
Table 2.
The The significant change in the mean relative abundance of gut microbiota between treatment and control groups (n = 10 per group).
| Microbiota (%) | Study groups | P value | ||||
|---|---|---|---|---|---|---|
| C | Am | PAm | Am vs. C | PAm vs. C | Am vs. PAm | |
| Firmicutes | 37.82 ± 0.590 | 28.77 ± 0.555 | 29.43 ± 0.843 | 0.02 | 0.13 | > 0.999 |
| Bacteroidetes | 55.79 ± 0.67 | 58.53 ± 0.57 | 56.76 ± 0.78 | 0.01 | 0.83 | 0.2 |
| Verrucomicrobia | 3.58 ± 0.45 | 5.06 ± 0.37 | 4.84 ± 0.51 | 0.01 | 0.14 | > 0.999 |
| α- proteobacteria | 16.9 ± 0.41 | 14.53 ± 0.49 | 13.38 ± 0.44 | 0.53 | 0.02 | 0.52 |
| Enterobacteriaceae | 6.42 ± 6.75 | 4.53 ± 0.44 | 5.64 ± 0.45 | 0.006 | 0.38 | 0.38 |
| Clostridia | 31.64 ± 0.34 | 30.60 ± 0.46 | 29.29 ± 0.46 | 0.35 | 0.005 | 0.35 |
| Prevotellaceae | 10.44 ± 0.60 | 8.53 ± 0.46 | 9.49 ± 0.44 | 0.008 | 0.46 | 0.34 |
| E. coli | 2.94 ± 0.42 | 1.65 ± 0.26 | 1.81 ± 0.28 | 0.02 | 0.11 | > 0.999 |
| Lactobacillus spp. | 11.45 ± 0.42 | 13.59 ± 0.54 | 13.43 ± 0.40 | 0.03 | 0.08 | > 0.999 |
| Roseburia spp. | 4.83 ± 0.56 | 2.65 ± 0.31 | 2.64 ± 0.30 | 0.06 | 0.04 | > 0.999 |
| A. muciniphila | 4.09 ± 0.37 | 5.65 ± 0.39 | 5.41 ± 0.28 | 0.01 | 0.14 | > 0.999 |
C: control, Am: A. muciniphila, and PAm: pasteurized A. muciniphila. Bold P value are indicated statically significant.
Discussion
The gut microbiota modulates the intestinal immune system, while the immune system affects the composition of the intestinal microbiota. Due to the interaction between the host and the intestinal microbiota, the probiotic bacteria can have significant effects and help the homeostasis of the immune system28. Among next-generation probiotics, A. muciniphila is widely used for its positive roles in the treatment of various diseases and also for animal and human health preservation7,17,18,24,25,29. Moreover in recent years, the propensity towards using non-viable bacterial strains as alternative products to prevent the potential risks of probiotics has increased, especially in high-risk individuals such as infants, the elderly and immunocompromised patients30. We carried out a comparative study that compared the effects of alive and pasteurized A. muciniphila on normal mice's overall health performance through biochemical, pathological, and molecular techniques. Although several studies addressed the beneficial effects of pasteurized A. muciniphila on obesity and metabolic disorders17, 25, 31, no research has been performed to identify pharmacological applications of pasteurized A. muciniphila for preserving health conditions. This study will help to comprehend the mechanisms and possibility using of pasteurized A. muciniphila as an effective para-probiotic in health condition.
Health benefits of probiotics are reported by reducing body and metabolic tissues weight gain as well as preventing fat mass development and liver injury32,33. In our study, we demonstrated lowering-body, -liver, and -eWAT weight impact of both forms of A. muciniphila in study groups. In consistent with our research, normal mice treated with alive A. muciniphila showed a decrease in body and adipose weight gain7,18. In addition, several studies showed that alive, pasteurized A. muciniphila, its extracellular vesicles, and other derivatives cause a decrease in body weight, metabolism homeostasis, and combating obesity17,18,34,35. Besides, normal morphology and structure of vital tissues exist in a healthy body and very important to determine the safety of probiotic administration, while the morphology of the tissues are changed in onset of HFD-induced obesity and metabolic disorders36,37, therefore, maintenance of tissue morphology at a normal state seems critical for promoting health. The findings of this research revealed that both forms of A. muciniphila could play a positive role in intestinal, liver, and adipose homeostasis, indicating their beneficial effects on health maintenance. Thus, improved metabolic vital tissues structure in A. muciniphila-fed mice can be due to positive effects of the probiotic and para-probiotic on digestion, absorption, nutrient usage, and metabolism. In agreement with our pathological results, recent studies confirmed the beneficial health effects of alive A. muciniphila on the morphology of liver7,24, muscle7, colon12,18, and adipose tissue of mice undergoing standard diet18. In addition, reversing effects of alive or pasteurized A. muciniphila on HFD-induced abnormal morphology of tissues revealed in animal models17,18,24,29. Taken together, these observations in our and other experiments have shown that the administration of A. muciniphila provided some health benefits in multiple tissues, which are correlated with lowering the risk of various diseases.
Increased plasma lipid profiles and inflammatory markers are a major risk factors of cardiovascular disease38 and lead to change onset of metabolic disorders39,40, therefore, balancing these blood biochemical factors via probiotics or paraprobiotics may help maintain health41 and even protect people from diseases42,43. In our experiment, we demonstrated that both forms of A. muciniphila had corrective properties on lipid, glucose, and liver injury-related enzymes, while the pasteurized form had better effects. These findings are consistent with other recent research demonstrated beneficial lipid-lowering effects of alive A. muciniphila in normal mice7. Moreover, previous in vivo studies reported the reduction of glucose18,24, ALT, and AST after alive A. muciniphila administration in ND-fed mice24. Furthermore, it is demonstrated that supplementation of Lactobacillus and Bifidobacterium reduced lipid profiles in healthy adults41,44.
One consideration in evaluating the safety of probiotics is the recognition of undesirable changes in immune parameters45, owing to emerging evidence that probiotics or their derivatives may have immunomodulatory impacts. The main finding of our cytokine analysis was that A. muciniphila induced immunomodulatory effects. Higher level of anti-inflammatory cytokine (IL-10) and lower levels of the pro-inflammatory cytokine (TNF-α and IL-6) were found in the plasma of Am and PAm group, respectively. Regarding, recent investigations exhibited that probiotics improved to maintain balance immunity and inflammation-related cytokines in healthy mice46 and subjects47. It has been also reported that the live multi-strain probiotics were more effective than the non-viable probiotic strains in in improving insulin resistance associated with intestinal microbiota alteration, butyrate production, and il-10 induction48. Thus, the possible reasons of better effect of alive A. muciniphila on increasing il-10 could be due to the production of different components and interactions with other intestinal symbionts. The observations of these investigations support the notion that the consumption of the probiotic and para-probiotic can modify plasma metabolic/inflammation-related profiles intensely and reduce risk of metabolic disorders.
The first tissue exposed to diet-derived nutrients is the gastrointestinal tract, which has cross-talks with other organs by impacting different signaling pathways49. Therefore, the improvement of intestinal and immune hemostasis can inhibit inflammatory disease onset, which accompanied by increasing intestinal integrity and preventing passing bacterial components into lamina propria as well as peripheral tissues50. Our data demonstrated that alive and pasteurized A. muciniphila had a substantial link with intestinal and immune homeostasis, which highlighted their key role in health promotion. We found pasteurized A. muciniphila had more beneficial effects on intestinal integrity and no inflammatory adverse effects was seen in non-inflamed Caco-2 and colon of mice. These parameters are critical for determining the intestinal health and immune status of normal mice. In these regards, A. muciniphila, which grown in mucin-based medium, induced an increase in intestinal integrity and modulated immune response in ND mice and cell line18,20,51. In addition, the beneficial effects of pasteurized A. muciniphila17 and its extracellular vesicles18,35 have been reported on strengthening intestinal integrity in obese mice, which indicates the positive role of non-viable form of this bacterium. Additionally, the upregulation of colonic il-1052 and increased IL-10 and decreased TNF-α levels in intestinal fluid were observed in probiotics treated-normal animals53. Collectively, viable and non-viable forms of probiotics can promote gut health in normal state, while non-viable form was more effective.
Another host factor is involved in the regulation of lipid metabolism and inflammation in the intestine and inversely associated with obesity is Angptl454. Increased Angptl4 can play a key role in maintaining intestinal lipid homeostasis. We found that both treatments had a beneficial effect on lipid metabolism in the colon of mice, similar to Caco-2. It is noteworthy that alive A. muciniphila had a greater effect on it, which may be due to generating enzymes or short-chain fatty acids (SCFAs), in addition to surface proteins. Similarly, the greater effect of alive A. muciniphila and Lactobacillus rhamnosus CNCMI-4317 on angptl4 expression demonstrated in comparison with A. municipia's EVs and heat-killed L. rhamnosus, respectively18,55. These results suggested that intestinal lipid metabolism is more regulated by viable probiotics. Overall, in our present research and previous studies, these findings support a strong connection between A. muciniphila and immune, lipid, and intestinal homeostasis, suggesting direct and indirect effects of the bacterium on the improvement of gut health.
One of the peripheral organs proved that have a strong association with gut is liver, which is in many respects a reflection of a person's health56 and has multiple functions and cross-talking with other organs. The improvement of gut health by using probiotics is associated with the restoration and maintenance of liver homeostasis, thereby preventing NAFLD57. Recently, probiotics and para-probiotics are widely used for their beneficial role in health and immunity58,59. We found that orally administrated both forms of A. muciniphila triggered hepatic immune response-/lipid metabolism-related genes in study groups, which indicated improving liver health. Notably, alive A. muciniphila had better immunomodulatory and lipid-regulating effects on the liver of mice. In line with these findings, oral daily A. muciniphila administration improved liver health by reducing lipid accumulation, downregulating FA oxidation-related genes, and reducing chronic low-grade inflammation in normal mice7. Treatment with Bifidobacterium animalis induced lipid metabolism increase, while inflammatory genes in healthy rats were unchanged60. Moreover, the beneficial hepatic lipid-lowering capability of A. muciniphila was demonstrated in ND-fed mice24. Altogether, these observations demonstrated the remarkable role of alive and pasteurized A. muciniphila in the health of mice's liver, which represents the importance of probiotics and para-probiotics in the maintenance of hepatic lipid and immune homeostasis.
Adipose tissue is an immunological organ that plays a key role in the homeostasis of energy and stores extra lipids61. In metabolic diseases, adipose tissue is the first peripheral tissue to be affected by HFD49, therefore, restoration and maintenance of healthy adipose can help to maintain metabolic homeostasis. Our results showed oral administration of pasteurized A. muciniphila had better effects on the alleviation of adipo-inflammation, while alive form of the probiotic resulted in better effects on lipid homeostasis in the normal adipose tissue. In agreement with our research, the positive role of A. muciniphila in the adipose health and immune status in normal18 and obese mice18,62 were reported. In addition, specific strains of Lactobacillus modulate cytokines secretion in mouse adipocyte cell line63. These findings may provide new evidence for the administration of probiotics and para-probiotics to metabolic normal mice and also suggested a new strategy to prevent the onset of metabolic diseases such as obesity.
Positive regulation of the gut microbiota has been proposed as one of the mechanisms underlying probiotic effects64; nevertheless, contradictory data exists regarding probiotics ability to modulate intestinal microbiota in healthy hosts. For instance, proper effects of probiotics on modulation of beneficial microbiota was shown in previous study65, while another study was reported no significant change in gut microbiota compositions after probiotics consuming by healthy individuals66. Although previous studies addressed the supplementation of A. muciniphila did not induce major changes in gut microbiome in obese human25 and HFD-induced obese mice12, no data was reported in case of A. muciniphila's effects on gut microbiota pattern of ND-fed mice. In our study, we demonstrated that alive A. muciniphila was more effective in improving gut microbiota composition, compared to the pasteurized form. In Am group, there was a decrease in amount of Firmicutes, Enterobacteriaceae, Prevotellaceae, and E. coli as well as increased abundance of Bacteroidetes, Verrucomicrobia, Lactobacillus spp. and A. muciniphila. On the other hand, the significant reduction in α-Proteobacteria, Clostridia, and Roseburia spp. abundance was observed in PAm group. As a result, daily supplementation of A. muciniphila generated a reduction in harmful bacteria and an increased population of beneficial microbiota that could be correlated with improving the immune and lipid homeostasis of mice in this study. In these regards, 10-strain probiotic cocktail (i.e. 5 Lactobacillus and 5 Enterococcus strains) modulate gut microbiota in ND-fed mice by increasing SCFA-producers bacteria67. Moreover, we demonstrated that the Firmicutes/ Bacteroidetes (F/B) ratio did not have a significant change following both treatments, while contradictory data have been reported on the F/B ratio, which may be due to many lifestyle-associated factors or methodological differences68. Overall, these data demonstrated that consuming probiotics can play a vital role in fostering health via improvement of gut microbiota composition.
In conclusion, the above our outcomes displayed that A. muciniphila improved body weight, plasma biochemical and inflammatory markers, and morphology of vital tissues. The administration of both alive and pasteurized A. muciniphila, significantly promote gut, adipose, and liver health by modulating immune response and lipid metabolism as well as intestinal homeostasis by improving gut barrier functions and intestine microbiota composition in the study groups. According to better health effects of pasteurized A. muciniphila, use of the pasteurized form as a new strategy seems to be a valid, safe, and potentially more cost-effective medication to improve the host's health and reduce the risk of metabolic disorders. This study will help to comprehend the probiotic and para-probiotic mechanism and possibility of using pasteurized A. muciniphila as a potent para-probiotic bacterial combination in the health promotion of normal mice.
Supplementary Information
Author contributions
S.D. and F.A. designed the project and experiments. F.A. performed the most of experiments, analyzed data and wrote the manuscript. AS performed bacterial culture. A.B. performed cell culture. F.A., A.S., and S.K. carried out animal and other experiments. R.Y. performed labratory experiments. A.L. analyzed data. H.R.M. dissected, sampled and histologically evaluated the mice. S.D. supervised the study. S.D., S.K., M.D.O., F.V., and A.M. revised the manuscript. All authors have read and approved the final manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Fatemeh Ashrafian and Shahrbanoo Keshavarz Azizi Raftar.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-021-95738-5.
References
- 1.Honda K, Littman DR. The microbiota in adaptive immune homeostasis and disease. Nature. 2016;535:75–84. doi: 10.1038/nature18848. [DOI] [PubMed] [Google Scholar]
- 2.Cani PD, et al. Changes in gut microbiota control inflammation in obese mice through a mechanism involving GLP-2-driven improvement of gut permeability. Gut. 2009;58:1091–1103. doi: 10.1136/gut.2008.165886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hayes CL, et al. Commensal microbiota induces colonic barrier structure and functions that contribute to homeostasis. Sci. Rep. 2018;8:1–14. doi: 10.1038/s41598-018-32366-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Valentini M, et al. Immunomodulation by gut microbiota: Role of Toll-like receptor expressed by T cells. J. Immunol. Res. 2014;2014:586939. doi: 10.1155/2014/586939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carding S, Verbeke K, Vipond DT, Corfe BM, Owen LJ. Dysbiosis of the gut microbiota in disease. Microb. Ecol. Health Dis. 2015;26:26191. doi: 10.3402/mehd.v26.26191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brüssow H. Problems with the concept of gut microbiota dysbiosis. Microb. Biotechnol. 2020;13:423–434. doi: 10.1111/1751-7915.13479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhao S, et al. Akkermansia muciniphila improves metabolic profiles by reducing inflammation in chow diet-fed mice. J. Mol. Endocrinol. 2017;58:1–14. doi: 10.1530/JME-16-0054. [DOI] [PubMed] [Google Scholar]
- 8.McFarland LV. Use of probiotics to correct dysbiosis of normal microbiota following disease or disruptive events: A systematic review. BMJ Open. 2014;4:e005047. doi: 10.1136/bmjopen-2014-005047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Taverniti V, Guglielmetti S. The immunomodulatory properties of probiotic microorganisms beyond their viability (ghost probiotics: Proposal of paraprobiotic concept) Genes Nutr. 2011;6:261–274. doi: 10.1007/s12263-011-0218-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Derrien, et al. M Derrien EE Vaughan CM Plugge WM Vos de 2004 Akkermansia muciniphila gen nov, sp nov, a human intestinal mucin-degrading bacterium. Int. J. Syst. Evol. Microbiol. 2004;54:1469–1476. doi: 10.1099/ijs.0.02873-0. [DOI] [PubMed] [Google Scholar]
- 11.Cani PD, de Vos WM. Next-generation beneficial microbes: The case of Akkermansia muciniphila. Front. Microbiol. 2017;8:1765. doi: 10.3389/fmicb.2017.01765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Everard A, et al. Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc. Natl. Acad. Sci. 2013;110:9066–9071. doi: 10.1073/pnas.1219451110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Raftar SKA, et al. Assessment of fecal Akkermansia muciniphila in patients with osteoporosis and osteopenia: A pilot study. J. Diabetes Metab. Disord. 2021;20:1–6. doi: 10.1007/s40200-020-00539-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dehghanbanadaki H, et al. Global scientific output trend for Akkermansia muciniphila research: A bibliometric and scientometric analysis. BMC Med. Inform. Decis. Mak. 2020;20:1–12. doi: 10.1186/s12911-020-01312-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dao MC, et al. Akkermansia muciniphila and improved metabolic health during a dietary intervention in obesity: Relationship with gut microbiome richness and ecology. Gut. 2016;65:426–436. doi: 10.1136/gutjnl-2014-308778. [DOI] [PubMed] [Google Scholar]
- 16.Zhai Q, Feng S, Arjan N, Chen W. A next generation probiotic, Akkermansia muciniphila. Crit. Rev. Food Sci. Nutr. 2019;59:3227–3236. doi: 10.1080/10408398.2018.1517725. [DOI] [PubMed] [Google Scholar]
- 17.Plovier H, et al. A purified membrane protein from Akkermansia muciniphila or the pasteurized bacterium improves metabolism in obese and diabetic mice. Nat. Med. 2017;23:107. doi: 10.1038/nm.4236. [DOI] [PubMed] [Google Scholar]
- 18.Ashrafian F, et al. Akkermansia muciniphila-derived extracellular vesicles as a mucosal delivery vector for amelioration of obesity in mice. Front. Microbiol. 2019;10:2155. doi: 10.3389/fmicb.2019.02155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yaghoubfar R, et al. Effects of Akkermansia muciniphila and Faecalibacterium prausnitzii on serotonin transporter expression in intestinal epithelial cells. J. Diabetes Metab. Disord. 2021;20:1–5. doi: 10.1007/s40200-020-00539-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yaghoubfar R, et al. Modulation of serotonin signaling/metabolism by Akkermansia muciniphila and its extracellular vesicles through the gut-brain axis in mice. Sci. Rep. 2020;10:1–12. doi: 10.1038/s41598-020-79171-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yaghoubfar R, et al. Effect of Akkermansia muciniphila, Faecalibacterium prausnitzii, and their extracellular vesicles on the serotonin system in intestinal epithelial cells. Probiotics Antimicrob. Proteins. 2021;13:1–11. doi: 10.1007/s12602-020-09640-z. [DOI] [PubMed] [Google Scholar]
- 22.Collado MC, et al. Intestinal integrity and Akkermansia muciniphila, a mucin-degrading member of the intestinal microbiota present in infants, adults, and the elderly. Appl. Environ. Microbiol. 2007;73:7767–7770. doi: 10.1128/AEM.01477-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jayachandran M, Chung SSM, Xu B. A critical review of the relationship between dietary components, the gut microbe Akkermansia muciniphila, and human health. Crit. Rev. Food Sci. Nutr. 2020;60:2265–2276. doi: 10.1080/10408398.2019.1632789. [DOI] [PubMed] [Google Scholar]
- 24.Kim, S. et al. Akkermansia muciniphila Prevents fatty liver disease, decreases serum triglycerides, and maintains gut homeostasis. Appl. Environ. Microbiol.86, e03004-19 (2020). [DOI] [PMC free article] [PubMed]
- 25.Depommier C, et al. Supplementation with Akkermansia muciniphila in overweight and obese human volunteers: A proof-of-concept exploratory study. Nat. Med. 2019;25:1096–1103. doi: 10.1038/s41591-019-0495-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Erben U, et al. A guide to histomorphological evaluation of intestinal inflammation in mouse models. Int. J. Clin. Exp. Pathol. 2014;7:4557. [PMC free article] [PubMed] [Google Scholar]
- 27.Yang Y-W, et al. Use of 16S rRNA gene-targeted group-specific primers for real-time PCR analysis of predominant bacteria in mouse feces. Appl. Environ. Microbiol. 2015;81:6749–6756. doi: 10.1128/AEM.01906-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kang H-J, Im S-H. Probiotics as an immune modulator. J. Nutr. Sci. Vitaminol. 2015;61:S103–S105. doi: 10.3177/jnsv.61.S103. [DOI] [PubMed] [Google Scholar]
- 29.Yang M, et al. Beneficial effects of newly isolated Akkermansia muciniphila strains from the human gut on obesity and metabolic dysregulation. Microorganisms. 2020;8:1413. doi: 10.3390/microorganisms8091413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barros CP, et al. Paraprobiotics and postbiotics: Concepts and potential applications in dairy products. Curr. Opin. Food Sci. 2020;32:1–8. doi: 10.1016/j.cofs.2019.12.003. [DOI] [Google Scholar]
- 31.Depommier C, et al. Pasteurized Akkermansia muciniphila increases whole-body energy expenditure and fecal energy excretion in diet-induced obese mice. Gut Microbes. 2020;11:1–15. doi: 10.1080/19490976.2020.1737307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Borgeraas H, Johnson L, Skattebu J, Hertel J, Hjelmesaeth J. Effects of probiotics on body weight, body mass index, fat mass and fat percentage in subjects with overweight or obesity: A systematic review and meta-analysis of randomized controlled trials. Obes. Rev. 2018;19:219–232. doi: 10.1111/obr.12626. [DOI] [PubMed] [Google Scholar]
- 33.Loman BR, Hernández-Saavedra D, An R, Rector RS. Prebiotic and probiotic treatment of nonalcoholic fatty liver disease: A systematic review and meta-analysis. Nutr. Rev. 2018;76:822–839. doi: 10.1093/nutrit/nuy031. [DOI] [PubMed] [Google Scholar]
- 34.Yoon HS, et al. Akkermansia muciniphila secretes a glucagon-like peptide-1-inducing protein that improves glucose homeostasis and ameliorates metabolic disease in mice. Nat. Microbiol. 2021;6:563–573. doi: 10.1038/s41564-021-00880-5. [DOI] [PubMed] [Google Scholar]
- 35.Chelakkot C, et al. Akkermansia muciniphila-derived extracellular vesicles influence gut permeability through the regulation of tight junctions. Exp. Mol. Med. 2018;50:450. doi: 10.1038/emm.2017.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Revelo XS, Luck H, Winer S, Winer DA. Morphological and inflammatory changes in visceral adipose tissue during obesity. Endocr. Pathol. 2014;25:93–101. doi: 10.1007/s12022-013-9288-1. [DOI] [PubMed] [Google Scholar]
- 37.Xie Y, et al. Impact of a high-fat diet on intestinal stem cells and epithelial barrier function in middle-aged female mice. Mol. Med. Rep. 2020;21:1133–1144. doi: 10.3892/mmr.2020.10932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.DiRienzo DB. Effect of probiotics on biomarkers of cardiovascular disease: Implications for heart-healthy diets. Nutr. Rev. 2014;72:18–29. doi: 10.1111/nure.12084. [DOI] [PubMed] [Google Scholar]
- 39.Allam-Ndoul B, et al. Association between metabolite profiles, metabolic syndrome and obesity status. Nutrients. 2016;8:324. doi: 10.3390/nu8060324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Magri-Tomaz L, et al. Two weeks of high-fat feeding disturb lipid and cholesterol molecular markers. Cell Biochem. Funct. 2018;36:387–393. doi: 10.1002/cbf.3358. [DOI] [PubMed] [Google Scholar]
- 41.Bjerg AT, et al. The effect of Lactobacillus paracasei subsp. paracasei L. casei W8® on blood levels of triacylglycerol is independent of colonisation. Beneficial Microbes. 2015;6:263–269. doi: 10.3920/BM2014.0033. [DOI] [PubMed] [Google Scholar]
- 42.Shimizu M, Hashiguchi M, Shiga T, Tamura H-O, Mochizuki M. Meta-analysis: Effects of probiotic supplementation on lipid profiles in normal to mildly hypercholesterolemic individuals. PLoS One. 2015;10:e0139795. doi: 10.1371/journal.pone.0139795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nataraj BH, Ali SA, Behare PV, Yadav H. Postbiotics-parabiotics: The new horizons in microbial biotherapy and functional foods. Microb. Cell Fact. 2020;19:1–22. doi: 10.1186/s12934-020-01426-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sadrzadeh-Yeganeh H, et al. The effects of probiotic and conventional yoghurt on lipid profile in women. Br. J. Nutr. 2010;103:1778–1783. doi: 10.1017/S0007114509993801. [DOI] [PubMed] [Google Scholar]
- 45.Sanders ME, et al. Safety assessment of probiotics for human use. Gut Microbes. 2010;1:164–185. doi: 10.4161/gmic.1.3.12127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li A, et al. Probiotics isolated from yaks improves the growth performance, antioxidant activity, and cytokines related to immunity and inflammation in mice. Microb. Cell Fact. 2019;18:1–12. doi: 10.1186/s12934-018-1049-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Harbige LS, Pinto E, Allgrove J, Thomas LV. Immune response of healthy adults to the ingested probiotic Lactobacillus casei Shirota. Scand. J. Immunol. 2016;84:353–364. doi: 10.1111/sji.12495. [DOI] [PubMed] [Google Scholar]
- 48.Li X, et al. A comparative study of the antidiabetic effects exerted by live and dead multi-strain probiotics in the type 2 diabetes model of mice. Food Funct. 2016;7:4851–4860. doi: 10.1039/C6FO01147K. [DOI] [PubMed] [Google Scholar]
- 49.Bleau C, Karelis AD, St-Pierre DH, Lamontagne L. Crosstalk between intestinal microbiota, adipose tissue and skeletal muscle as an early event in systemic low-grade inflammation and the development of obesity and diabetes. Diabetes Metab. Res. Rev. 2015;31:545–561. doi: 10.1002/dmrr.2617. [DOI] [PubMed] [Google Scholar]
- 50.O’Callaghan AA, Corr SC. Establishing boundaries: The relationship that exists between intestinal epithelial cells and gut-dwelling bacteria. Microorganisms. 2019;7:663. doi: 10.3390/microorganisms7120663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ashrafian F, Behrouzi A. Comparative study of effect of Akkermansia muciniphila and its extracellular vesicles on toll-like receptors and tight junction. Gastroenterol. Hepatol. Bed Bench. 2019;12:163. [PMC free article] [PubMed] [Google Scholar]
- 52.Chunchai T, et al. Decreased microglial activation through gut-brain axis by prebiotics, probiotics, or synbiotics effectively restored cognitive function in obese-insulin resistant rats. J. Neuroinflammation. 2018;15:11. doi: 10.1186/s12974-018-1055-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dogi C, García G, Moreno De, de LeBlanc A, Greco C, Cavaglieri L. Lactobacillus rhamnosus RC007 intended for feed additive: Immune-stimulatory properties and ameliorating effects on TNBS-induced colitis. Beneficial Microbes. 2016;7:539–547. doi: 10.3920/BM2015.0147. [DOI] [PubMed] [Google Scholar]
- 54.Phua T, et al. Angiopoietin-like 4 mediates colonic inflammation by regulating chemokine transcript stability via tristetraprolin. Sci. Rep. 2017;7:1–16. doi: 10.1038/srep44351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jacouton E, et al. Lactobacillus rhamnosus cncmi-4317 modulates fiaf/angptl4 in intestinal epithelial cells and circulating level in mice. PloS one. 2015;10:e0138880. doi: 10.1371/journal.pone.0138880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Marcellin P, Kutala BK. Liver diseases: A major, neglected global public health problem requiring urgent actions and large-scale screening. Liver Int. 2018;38:2–6. doi: 10.1111/liv.13682. [DOI] [PubMed] [Google Scholar]
- 57.Meroni M, Longo M, Dongiovanni P. The role of probiotics in nonalcoholic fatty liver disease: A new insight into therapeutic strategies. Nutrients. 2019;11:2642. doi: 10.3390/nu11112642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Piqué N, Berlanga M, Miñana-Galbis D. Health benefits of heat-killed (Tyndallized) probiotics: An overview. Int. J. Mol. Sci. 2019;20:2534. doi: 10.3390/ijms20102534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Klaenhammer TR, Kleerebezem M, Kopp MV, Rescigno M. The impact of probiotics and prebiotics on the immune system. Nat. Rev. Immunol. 2012;12:728–734. doi: 10.1038/nri3312. [DOI] [PubMed] [Google Scholar]
- 60.Yan Y, et al. Probiotic Bifidobacterium lactis V9 attenuates hepatic steatosis and inflammation in rats with non-alcoholic fatty liver disease. AMB Express. 2020;10:1–11. doi: 10.1186/s13568-019-0926-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Choe SS, Huh JY, Hwang IJ, Kim JI, Kim JB. Adipose tissue remodeling: Its role in energy metabolism and metabolic disorders. Front. Endocrinol. 2016;7:30. doi: 10.3389/fendo.2016.00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shin N-R, et al. An increase in the Akkermansia spp. population induced by metformin treatment improves glucose homeostasis in diet-induced obese mice. Gut. 2014;63:727–735. doi: 10.1136/gutjnl-2012-303839. [DOI] [PubMed] [Google Scholar]
- 63.Fabersani E, et al. Specific strains of lactic acid bacteria differentially modulate the profile of adipokines in vitro. Front. Immunol. 2017;8:266. doi: 10.3389/fimmu.2017.00266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hemarajata P, Versalovic J. Effects of probiotics on gut microbiota: Mechanisms of intestinal immunomodulation and neuromodulation. Ther. Adv. Gastroenterol. 2013;6:39–51. doi: 10.1177/1756283X12459294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Khalesi S, et al. A review of probiotic supplementation in healthy adults: Helpful or hype? Eur. J. Clin. Nutr. 2019;73:24–37. doi: 10.1038/s41430-018-0135-9. [DOI] [PubMed] [Google Scholar]
- 66.Brown AC, Shovic A, Ibrahim S, Holck P, Huang A. A non-dairy probiotic’s (poi) influence on changing the gastrointestinal tract’s microflora environment. Altern. Ther. Health Med. 2005;11:58. [PMC free article] [PubMed] [Google Scholar]
- 67.Nagpal R, et al. Human-origin probiotic cocktail increases short-chain fatty acid production via modulation of mice and human gut microbiome. Sci. Rep. 2018;8:1–15. doi: 10.1038/s41598-018-30114-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Magne F, et al. The firmicutes/bacteroidetes ratio: A relevant marker of gut dysbiosis in obese patients? Nutrients. 2020;12:1474. doi: 10.3390/nu12051474. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.