Abstract
The HRAS, NRAS, and KRAS genes are collectively mutated in a fifth of all human cancers. These mutations render RAS GTP-bound and active, constitutively binding effector proteins to promote signaling conducive to tumorigenic growth. To further elucidate how RAS oncoproteins signal, we mined RAS interactomes for potential vulnerabilities. Here we identify EFR3A, an adapter protein for the phosphatidylinositol kinase PI4KA, to preferentially bind oncogenic KRAS. Disrupting EFR3A or PI4KA reduces phosphatidylinositol-4-phosphate, phosphatidylserine, and KRAS levels at the plasma membrane, as well as oncogenic signaling and tumorigenesis, phenotypes rescued by tethering PI4KA to the plasma membrane. Finally, we show that a selective PI4KA inhibitor augments the antineoplastic activity of the KRASG12C inhibitor sotorasib, suggesting a clinical path to exploit this pathway. In sum, we have discovered a distinct KRAS signaling axis with actionable therapeutic potential for the treatment of KRAS-mutant cancers.
Subject terms: Proteins, Oncogenes
The lipid composition of the plasma membrane defines the localisation of KRAS and its oncogenic function. Here the authors show that EFR3A binds to active KRAS to recruit PI4KA and alters the lipid composition of the plasma membrane to promote KRAS oncogenic signalling and tumorigenesis.
Introduction
The three RAS genes HRAS, NRAS, and KRAS are collectively mutated in a fifth of human cancers1. These mutations primarily alter residues G12, G13, or Q612, which leave RAS in a constitutively active and oncogenic GTP-bound state3. Oncogenic RAS recruits effector proteins, the most studied being RAFs, PI3Ks, and RalGEFs3, to the plasma membrane where RAS resides to propagate oncogenic signaling3. Excitingly, small molecules specifically inhibiting the G12C-mutant variant of oncogenic KRAS have been shown to have therapeutic potential in human clinical trials4,5, with one, sotorasib, now approved for the treatment of lung cancer. Nevertheless, consistent with being a targeted therapy6,7, early indications suggest resistance arises through upregulating the EGFR pathway or by increasing RAS oncoprotein levels8,9. As such, there is a great clinical need to identify components of RAS signaling that could be leveraged to either augment the antineoplastic activity of, or combat resistance to these and future RAS inhibitors. As RAS is a signaling protein, the most logical source of such potential vulnerabilities are proteins in the immediate vicinity of the oncoprotein itself.
As a signaling protein, RAS is subjected to multiple levels of regulation10. One such level is the spatial-temporal control of the protein at membranes11,12. Each RAS isoform is prenylated at the C-terminus to foster membrane association10,13,14. Careful monitoring of the subcellular localization of RAS revealed that the protein cycles back and forth between internal membranes and the plasma membrane15–17, although the latter is considered to be the primary site of signaling18,19. While at the plasma membrane, activated RAS forms nanoclusters of six to seven RAS proteins11,19, which facilitate signaling by favoring the formation of oncoprotein signaling complexes19–22. Not surprisingly, the content of the plasma membrane affects the occupancy and nanoclustering of RAS in this locale, providing yet another layer of regulation23. Indeed, we previously found that a phosphatidylinositol-4-phosphate (PI(4)P) concentration gradient promotes and maintains KRAS localization and nanoclustering at the plasma membrane through an exchange with phosphatidylserine (PS) at contact sites between this membrane and the endoplasmic reticulum24. As such, it stands to reason that KRAS-associating proteins that affect PI(4)P metabolism may offer a unique way to not only regulate, but perhaps even to directly target the spatial-temporal regulation of this oncoprotein.
PI(4)P is typically generated by phosphorylating the D4 position in the inositol headgroup of phosphatidylinositol (PI) by PI(4)P kinases, one of which is PI4KA25,26. PI4KA is recruited to plasma membrane by the adapter protein EFR327,28, which has two isoforms, EFR3A and EFR3B29. These two proteins are evolutionarily conserved from mammals to yeast, and share 62% sequence identity29. EFR3A/B contain an N-terminal cysteine rich region that encodes palmitoylation sites for plasma membrane anchoring, followed by armadillo-like repeats that mediate protein-protein interactions29. EFR3A/B form a complex with PI4KA and two other proteins, FAM126A and TTC7A or TTC7B, which serve as scaffolding proteins stabilizing PI4KA at the plasma membrane30–32. While mutations in EFR3A have been associated with autism33 and neurogenesis34, no direct relationship between this protein and human cancers had been established. Although as adapter proteins EFR3A/B do not easily lend themselves to therapeutic intervention, the recruitment of PI4KA does. Lipid kinases can be inhibited with small molecules35,36, with a PI3K inhibitor approved for cancer treatment37. As such, a connection between PI(4)P and oncogenic KRAS could potentially be a druggable avenue to inhibit the regulation of the oncoprotein itself.
Critical to unmasking components involved in the spatial-temporal regulation of oncogenic RAS is identifying proteins associating with the oncoprotein, particularly those linked to the plasma membrane. While a number of specific protein-protein interactions had been documented over the years, which has greatly informed current understanding of RAS signaling3,38, a comprehensive characterization of RAS interactomes was lacking. To this end, BirA-mediated proximity labeling, which can identify proteins within 10 nm distance of a targeted protein39,40, had been used to define the interactome of oncogenic KRAS41. We42 and others41,43,44 similarly used this approach to define the interactomes of all three RAS oncoproteins. Not only did we identify 65 previously documented interacting proteins, but by comparing the interactomes of each RAS isoform, we identified the PI kinase PI5K1A as preferentially binding to and mediating oncogenic KRAS signaling and cellular proliferation42. One advantage of BirA-proximity labeling over biochemical purification of protein complexes is that transient and/or weak interactions can be captured, which are characteristic of regulatory proteins40. We thus sought to identify pristine interactions with RAS oncoproteins essential for oncogenesis. To this end, we report here that oncogenic RAS associates with EFR3A. Focusing on KRAS, as it is the most commonly mutated of the three RAS genes in human cancers1, we further show that that loss of EFR3A/B or PI4KA reduces the levels of PI(4)P and PS, as well as KRAS localization and nanoclustering at the plasma membrane. This is associated with reduced oncogenic KRAS signaling, transformation, and tumorigenesis. Importantly, epistatic analysis confirms these results, as tethering either PI4KA or RAS to the plasma membrane rescues these effects. Thus, EFR3A appears to form a unique positive-feedback circuit whereby activated RAS binds EFR3A, which in turn promotes signaling by the oncoprotein. Such a circuit opens up the possibility of pharmacologically augmenting the antineoplastic activity of RAS inhibitors with PI4KA inhibitors, and in agreement, we demonstrate that a selective PI4KA inhibitor is synergistic with the clinical KRASG12C inhibitor sotorasib.
Results
Identification of EFR3A from a RAS interactome screen
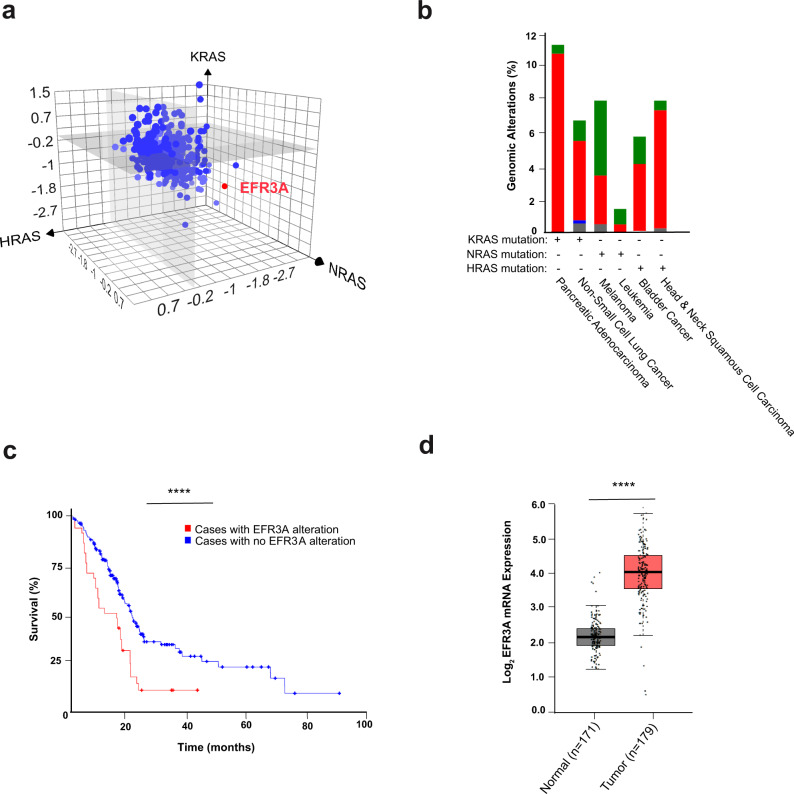
We previously performed BirA-mediated proximity labeling with each of the three oncogenic RAS isoforms (HRASG12V, NRASG12V, KRASG12V), identifying 474 ‘interactome’ proteins. Each of these proteins were then analyzed for their effect on RAS oncogenesis in a loss-of-function CRISPR/Cas9 screen using a panel of isogenic cell lines transformed by a different oncogenic RAS isoform. Comparing these two datasets uncovered PIP5K1A to specifically bind to and promote KRAS oncogenesis, validating the approach42. As noted above, a distinct advantage of BirA-mediated proximity labeling over conventional pull-down and other proteomic approaches is the identification of weak and/or transient interactions39,40, which are often characteristic of regulatory proteins40. Given this, we mined the two aforementioned datasets for interactome components that are particularly critical for oncogenesis, which identified EFR3A as the top candidate. In more detail, EFR3A was biotinylated by all three BirA-HRASG12V, BirA-NRASG12V, and BirA-KRASG12V fusion proteins, roughly at par with the known RAS effectors BRAF and CRAF (Supplementary Fig. 1a–c). Encouragingly, mining the BirA-KRASG12V dataset also revealed that EFR3B and FAM126A were biotinylated at a low level, as was PI4KA, although as only one peptide was recovered from the latter, PI4KA was excluded from our previous analysis42. EFR3A, PI4KA, FAM126A, and TTC7B were also variably biotinylated in most43,44, but not all41, other reported proximity labeling experiments using BirA-RAS oncoproteins. Furthermore, EFR3A sgRNAs were consistently the most negatively enriched of all targeted interactome candidates in cells transformed by oncogenic RAS in our dataset (Fig. 1a)42, and also negatively enriched in other similar datasets44. To put this into context, the average negative enrichment score for all five EFR3A sgRNAs in cells transformed by any RAS oncoprotein was almost twice that of BRAF sgRNAs, which again target a known RAS effector3 (Supplementary Fig. 1d). We note that CRAF sgRNA depletion was not as prominent as BRAF sgRNA depletion (Supplementary Fig. 1d), as seen on other screens45. Given these findings we explored EFR3A further.
Fig. 1. Identification of EFR3A as a potential vulnerability in KRAS-mutant pancreatic cancer.
a Mean log2 fold-enrichment of the 5 sgRNAs targeting each of the 474 genes encoding proteins of the RAS interactomes (determined by BirA-mediated proximity labeling) in the isogenic background of HEK-HT cells transformed by oncogenic (G12V) HRAS (x-axis), KRAS (y-axis), or NRAS (z-axis). b The frequency (%) that the EFR3A gene exhibits amplification and copy number variation (red bar), a point mutation (green bar), deletion (blue bar), or multiple alterations (gray bar) in 6 different RAS-mutant cancers from the cBiportal PanCan TCGA dataset. Only cancers with ≥2% genomic alterations are shown. c A Kaplan–Meier plot of overall survival of pancreatic adenocarcinoma cases with (n = 42) versus without (n = 327) a genomic alteration in EFR3A (p = 2.034e–4), as determined from 5 pancreatic cancer datasets (ICGC, Nature 2012; TCGA PanCancer Atlas; UTSW Pancreatic Cancer; TCGA Firehose Legacy; QCMG, and Nature 2016), p = 0.01504. d A boxplot of the log2 mRNA expression of EFR3A, normal max (3.1), min (1.3), center (2.2); tumor max (4.5), min (0.3), center (4.2) in 179 pancreatic adenocarcinoma versus 171 normal tissues based on GEPIA analysis of the PAAD dataset. Significance values calculated by one-sided logrank test for (c) and one-way ANOVA analysis for (d): ****p < 0.0001. Percentiles in the box plots pre-set at 50% (higher) and 50% (lower).
EFR3A is upregulated in pancreatic cancer
To explore EFR3A in the context of human cancers we surveyed the cBioportal cancer genomics datasets for genomic alterations in the EFR3A gene. Across all human cancers (n = 10,967 samples) there was a general bias towards EFR3A amplification, with ovarian, esophageal, breast, pancreatic, and liver cancers all exhibiting over 10% gene amplification (Supplementary Fig. 2a). Comparing EFR3A gene amplification in examples of cancers driven by a specific RAS isoform, we find EFR3A gene amplification is highest in pancreatic adenocarcinoma (Fig. 1b). Given that pancreatic adenocarcinoma has both the highest frequency of KRAS mutations and EFR3A amplification, we focused on this cancer. We find that pancreatic adenocarcinoma patients with an EFR3A genomic alteration (almost exclusively gene amplification, Fig. 1b) have a 26% reduction in overall median survival compared to patients without an EFR3A genomic alteration (i.e. 15.3 versus 20.8 months, Fig. 1c). This also manifested as a 53% reduction in disease-free progression in the same cohort (i.e. 9.6 versus 20.5 months, Supplementary Fig. 2b). At the gene expression level, GEPIA analysis of the PAAD cancer dataset revealed a significant 95% increase in relative EFR3A mRNA level in pancreatic adenocarcinoma tumors (n = 179) compared to matched normal (n = 171) tissues (Fig. 1d). Although a limited number of samples, differentiating KRAS mutation-positive (n = 64) from KRAS mutation-negative (n = 115) pancreatic adenocarcinoma samples revealed higher EFR3A expression in the former (Supplementary Fig. 2c). EFR3A gene amplification also tracked with KRAS-mutant pancreatic adenocarcinoma samples (Supplementary Fig. 2d). Finally, comparing the survival of pancreatic adenocarcinoma patients with high (n = 44) versus low (n = 46) EFR3A mRNA levels from the TGCA database revealed a 29% reduction in overall median survival of the former (Supplementary Fig. 2e). Pancreatic adenocarcinoma appears to be unique among RAS-driven cancers in this regard, as high EFR3A expression was not observed in either other KRAS-driven cancers examined, or cancers driven by mutations in other RAS-isoforms (Supplementary Fig. 3a). Given these findings, we explored whether expression of genes encoding the other components of the EFR3A signaling complex was increased in pancreatic adenocarcinoma, finding elevated mRNA levels for EFR3B (Supplementary Fig. 3b), PI4KA (Supplementary Fig. 3c), FAM126A (Supplementary Fig. 3d), and TTC7A (Supplementary Fig. 3e). In sum, the EFR3A complex appears to be preferentially upregulated in KRAS-mutant pancreatic adenocarcinoma, which is associated with a worse clinical outcome.
Loss of EFR3A inhibits oncogenic KRAS signaling and transformation
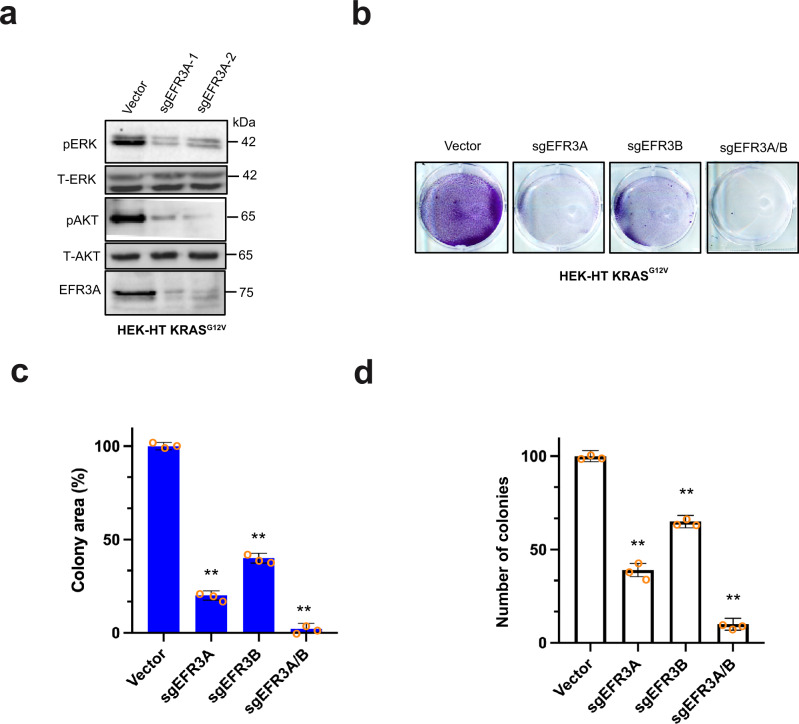
Given the labeling of multiple components of the EFR3-TTC7-FAM126-PI4KA complex by BirA-KRASG12V, the negative enrichment of EFR3A sgRNA in oncogenic KRAS-transformed cells, and the above correlation between increased EFR3A gene amplification and expression and pancreatic adenocarcinoma, we sought to experimentally test whether inhibiting EFR3A affects KRAS signaling and oncogenesis. To this end, we stably infected KRASG12V-transformed HEK-HT cells, which depend upon KRASG12V for tumorigenesis46, with a lentivirus encoding Cas9 in the absence (vector) or presence of an sgRNA targeting EFR3A (sgEFR3A), and confirmed by immunoblot reduction in detectable endogenous EFR3A protein in the latter cells (Fig. 2a). To assess the effect on oncogenic RAS signaling, we assayed the levels of phosphorylated ERK1/2 (P-ERK) and AKT (P-AKT) by immunoblot as a measure of oncogenic KRAS activation of the downstream MAPK and PI3K/AKT effector pathways3. This revealed a prominent reduction in oncogenic KRAS signaling upon the loss of EFR3A (Fig. 2a). These results were corroborated with a second independent sgRNA (sgEFR3A-2) targeting EFR3A (Fig. 2a). To evaluate the effect of EFR3A loss on KRAS oncogenesis, we measured colony formation, a known two-dimensional (2D)-transformed growth phenotype of oncogenic KRAS46, in the same cells, finding a reduction in this transformed phenotype upon the loss of EFR3A expression. We validated these results with an sgRNA targeting the EFR3B gene (sgEFR3B), and further show that the combination of sgEFR3A and sgEFR3B had the greatest reduction on colony formation (Fig. 2b, c). Finally, to evaluate the effect of EFR3A loss on a more aggressive transformed phenotype, we test and found that two independent sgEFR3As, sgEFR3B, or both sgEFR3A and sgEFR3B together all reduced three-dimensional (3D)-transformed growth, as assessed by colony formation in soft agar, of HEK-HT cells transformed with a codon-optimized KRASG12V (Fig. 2d). In sum, the loss of EFR3A inhibits oncogenic KRAS signaling and transformation.
Fig. 2. EFR3A loss inhibits oncogenic RAS signaling and transformed growth in isogenic cells.
a–d Analysis of the (a) levels of phosphorylated (P) and/or total (T) ERK, AKT, EFR3A, and actin (as a loading control), as assessed by immunoblot, b an example of colony formation, as assessed by crystal violet staining, which is (c) plotted as the mean ± SD total colony area, and (d) the mean ± SD number of colonies growing in soft agar of human HEK-HT cells transformed by oncogenic KRASG12V (codon-optimized in d) engineered to stably express Cas9 and a vector encoding no sgRNA (vector) or the indicated sgRNAs. Representative of 3 biological replicates tested in 3 independent experiments. Replicate experiments and full-length gels are provided in Supplementary Fig. 11. Significance values calculated by one-way ANOVA test for (c, d): **p < 0.01. Specific p values for (c) are 0.0078 (sgEFR3A), 0.0045 (sgEFR3B), and 0.0016 (sgEFR3A/B), and (d) are 0.0059 (sgEFR3A), 0.0083 (sgEFR3B), and 0.0026 (sgEFR3A/B).
Loss of EFR3A inhibits pancreatic tumorigenesis
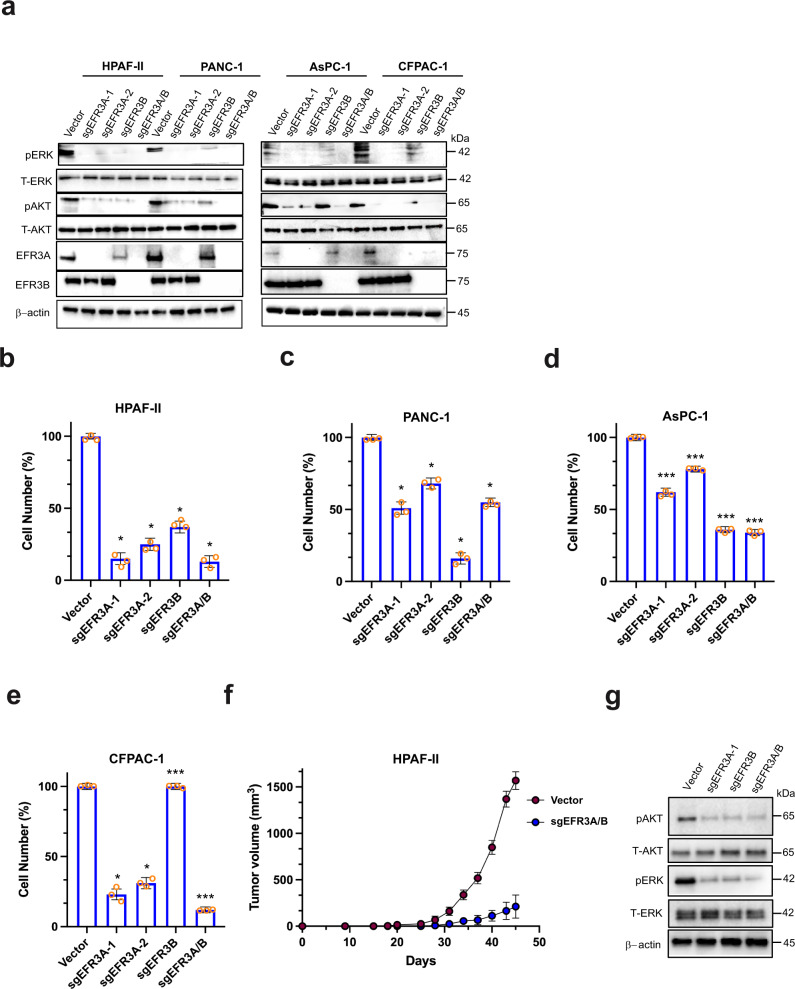
To evaluate the role of endogenous EFR3A in a cancer driven by an oncogenic mutation in the native KRAS gene, we targeted EFR3A, EFR3B, or both genes by CRISPR/Cas9-mediated gene inactivation as above in the KRAS-mutant human pancreatic adenocarcinoma cell line HPAF-II47. Immunoblot analysis confirmed the appropriate loss of endogenous protein expression of the targeted genes as well as a corresponding reduction in P-ERK and P-AKT levels (Fig. 3a). To independently validate these results, we repeated the experiment in the KRAS-mutant human pancreatic adenocarcinoma cell lines PANC-1, AsPC-1, and CFPAC-147. Again, targeting EFR3A, EFR3B, or both genes inhibited oncogenic KRAS signaling, with the loss of both isoforms having the greatest effect (Fig. 3a). To evaluate the effect of ERF3A loss on the pancreatic adenocarcinoma cell growth, these 20 cell lines were seeded at low density, after which the number of viable cells was determined over time using the CellTiter Glo reagent. In all cases, both EFR3A sgRNAs reduced cell counts compared to vector controls in all four cell lines, while the effect of EFR3B sgRNA was more variable, with the combined targeting of both isoforms being as or more potent than EFR3A sgRNA alone (Fig. 3b–e). As above, we validated these results in 3D-transformed growth, again finding that loss of EFR3A, EFR3B, or both reduced colony formation of all four pancreatic adenocarcinoma cells in soft agar (Supplementary Fig. 4a). Next, we evaluated the effect of EFR3A/B loss on the KRAS signaling and transformation in a rare KRAS mutation-negative human pancreatic adenocarcinoma cell line, finding that EFR3A and/or EFR3B sgRNA had little to no effect on the levels of P-ERK or P-AKT (Supplementary Fig. 4b), 2D- (Supplementary Fig. 4c, d), and 3D- (Supplementary Fig. 4a) transformed growth. To evaluate whether EFR3A was similarly required for KRAS-mutant pancreatic tumor growth, the aforementioned HPAF-II cells, in which the expression of EFR3A, EFR3B, or both genes was verified to be reduced (Fig. 3f), were injected subcutaneously into immunocompromised mice. Tumor growth was then compared over time to that of similarly injected vector control cells. Inactivating these genes individually or together potently inhibited tumor growth, as evidence by a 4.5-fold reduction at the experimental end-point compared to vector control cells (Fig. 3f and Supplementary Fig. 4e). We show that this reduction in tumor growth was, as in 2D- and 3D-transformed growth assays, associated with a reduction in oncogenic KRAS signaling, namely we observed a reduction in P-ERK and P-AKT levels in tumors in which EFR3A, EFR3B, or both genes were inactivated (Fig. 3g). In sum, loss of EFR3A (and/or EFR3B) expression inhibits oncogenic signaling in human KRAS-mutant pancreatic adenocarcinoma cells, which manifests as a reduction in transformed and tumorigenic growth.
Fig. 3. EFR3A loss inhibits pancreatic adenocarcinoma transformed and tumorigenic growth.
a–e Analysis of the (a) levels of phosphorylated (P) and/or total (T)- ERK, AKT, EFR3A, EFR3B, and actin (as a loading control), as assessed by immunoblot, and (b–e) the mean ± SD cell number (compared to vector control) over time, as assessed by the Titer Glo assay, of the indicated human KRAS mutation-positive pancreatic adenocarcinoma cell lines engineered to stably express Cas9 and a vector encoding no sgRNA (vector) or the indicated sgRNAs. f Mean ± SD tumor volume (mm3) versus time of HPAF-II cells engineered to stably express Cas9 and a vector encoding no sgRNA (vector) or the indicated sgRNAs after injected into mice. g Levels of (P) and/or (T) ERK, AKT, and actin (as a control), as assessed by immunoblot, in the indicated tumors from panel f. a: Representative of 3 biological replicates. b–e Representative of 3 biologic replicates assayed in triplicate. f The two cell lines were each injected into 5 mice. g Representative of 2 biological replicates. Replicate experiments and full-length gels are provided in Supplementary Fig. 11. Significance values calculated by one-way ANOVA test for (b–f): *p < 0.05 and ***p < 0.001. Specific p values for (b) are 0.0324 (sgEFR3A-1), 0.0227 (sgEFR3A-2), 0.0428 (sgEFR3B), and 0.0487 (sgEFR3A/B), (c) are 0.0241 (sgEFR3A-1), 0.0472 (sgEFR3A-2), 0.0281 (sgEFR3B), and 0.0307 (sgEFR3A/B), d are 0.00024 (sgEFR3A-1), 0.00062 (sgEFR3A-2), 0.00045 (sgEFR3B), and 0.00031 (sgEFR3A/B), e are 0.046 (sgEFR3A-1), 0.0381 (sgEFR3A-2), 0.00053 (sgEFR3B), and 0.00034 (sgEFR3A/B), and (f) is **p < 0.01.
Cellular EFR3A preferentially binds to active KRAS
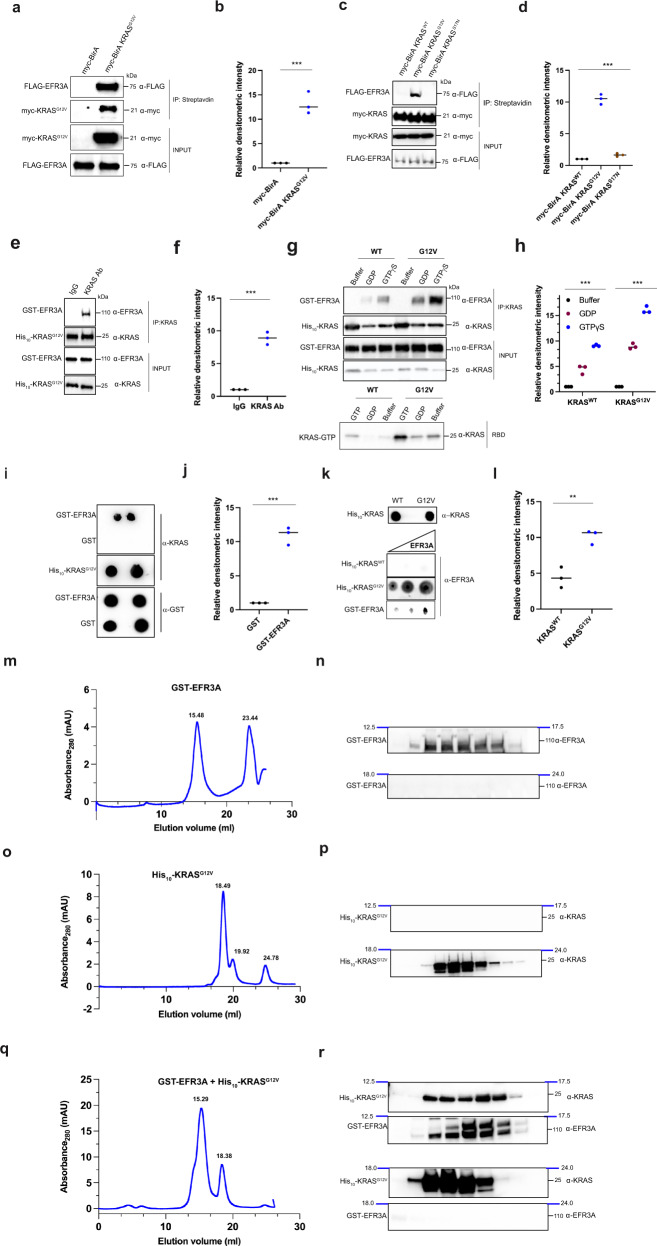
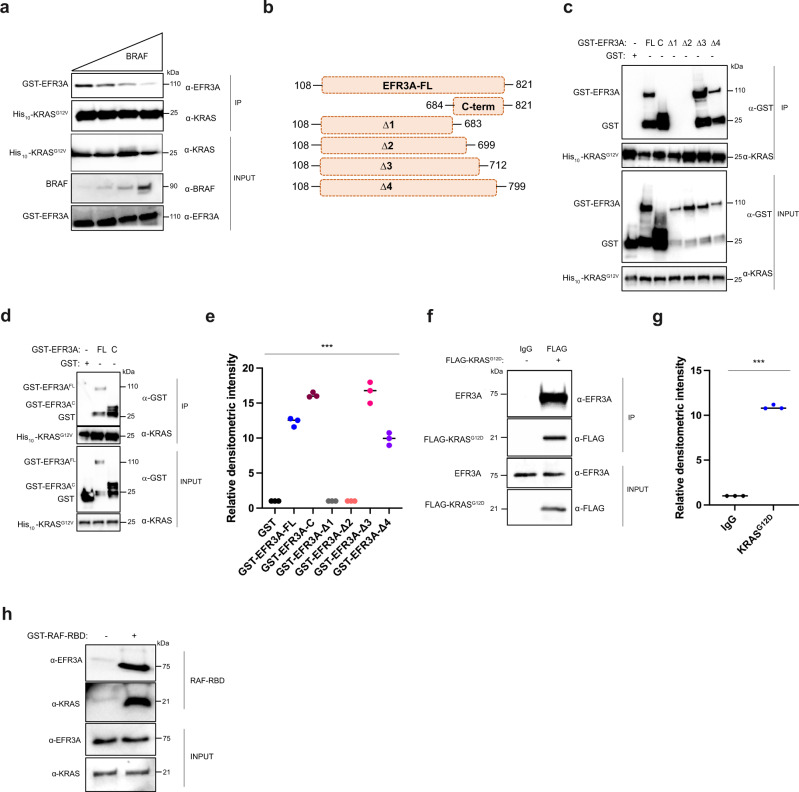
To elucidate the mechanism underpinning the effects of EFR3A on KRAS oncogenesis, we examined the association of EFR3A with oncogenic KRAS. We first validated the association of the two proteins by ectopically co-expressing FLAG epitope-tagged EFR3A (FLAG-EFR3A) with myc epitope-tagged BirA-KRASG12V (myc-BirA-KRASG12V) in human 293T cells. After treating these cells with biotin, proteins proximity-labeled by myc-BirA-KRASG12V were affinity purified with streptavidin and immunoblotted with FLAG- and myc-specific antibodies. FLAG-EFR3A was captured by streptavidin, suggesting that EFR3A lies within the immediate proximity (10 nm) of KRASG12V (Fig. 4a, b), a physiological distance that mediates protein-protein interactions39. To determine if this interaction was dependent on the activation state of KRAS, we repeated this experiment, except we compared the biotin labeling of FLAG-EFR3A by myc-BirA-KRAS with an oncogenic (G12V) mutation, with an inactivating (S17N) mutation48, or without a mutation (wild-type, WT). We confirmed the appropriate activation status of the three myc-BirA-KRAS proteins by affinity capturing GTP-bound RAS with the Ras Binding Domain (RBD) of the effector protein RAF1 (CRAF)49 fused to GST. Immunoblot analysis to detect the myc epitope-tag revealed a stepwise increase in amount of active myc-BirA-KRAS captured, from none (S17N), to low (WT), to very high (G12V) (Supplementary Fig. 5a). We next compared the amount of FLAG-EFR3A biotinylated by each of these versions of myc-BirA-KRAS as above, which revealed labeling primarily only by the G12V mutant (Fig. 4c, d), consistent with EFR3A associating with active KRAS in cells.
Fig. 4. EFR3A associates with oncogenic RAS.
a–d Analysis of (a, c) the levels and (b, d) quantification of biotin labeling of FLAG-EFR3A expressed in 293T cells by ectopic myc-BirA*-KRAS wild-type (WT), G12V, or S17N or by myc-BirA alone as a control, as assessed by streptavidin affinity capture (IP) of biotinylated proteins and immunoblotted with FLAG- and myc-specific antibodies. INPUT levels are shown as loading controls. e–h Analysis of (e, g) the levels and (f, h) quantification of recombinant GST-EFR3A detected by immunoblot with an EFR3A-specific antibody that co-immunoprecipitates (co-IP) WT or G12V recombinant His10-KRAS using a KRAS-specific antibody, and (g, h) preincubated with buffer, GDP, or GTPγS. INPUT levels are shown as loading controls. IgG serves as a negative control. i, j Analysis of (i) the levels and (j) quantification of recombinant His10-KRASG12V (preloaded with GTPγS) detected with a KRAS-specific antibody, that associates with recombinant GST-EFR3A versus control GST by far western. INPUT levels are shown as loading controls. k, l Analysis of (k) the levels and (l) quantification of GST-EFR3A detected with an EFR3A-specific antibody, that associate with recombinant wild-type (WT) versus His10-KRASG12V by far western. INPUT levels are shown as loading controls. m–r Analysis by (m, o, q) size-exclusion chromatography (SEC) profile and (n, p, r) immunoblot of the indicated fractions of recombinant (m, n) GST-EFR3A (peak 15.48: GST-EFR3A, o, p recombinant His10-KRASG12V preloaded with GTPγS (peak 18.49: His10-KRASG12V), and (q, r) a complex of recombinant GST-EFR3A and His10-KRASG12V preloaded with GTPγS (peak 15.29: complex). a–k Representative of 3 biological independent samples tested in 3 independent experiments. m–r Representative of 3 biological replicates. Replicate experiments and full-length gels are provided in Supplementary Fig. 12. Significance values calculated by one-sided student’s t test for (b, d, f, h, j, l): **p < 0.01 and ***p < 0.001. Specific p values for (b) is 0.00089, d are 0.00074 (G12V) and 0.00014 (S17N), f is 0.00058, (h) are 0.00048 (WTGDP), 0.00015 (WTGTP), 0.00031 (G12VGDP), and 0.00028 (G12VGTP), j is 0.00067, and l is 0.0069.
EFR3A directly binds to active KRAS through its C-terminus
To determine if the association between EFR3A and KRAS is direct, we performed in vitro co-immunoprecipitation assays with recombinant GST-EFR3A protein purified by affinity capture with a glutathione Sepharose 4B resin and recombinant His10-KRASG12V protein purified by affinity capture with a Ni-NTA resin. GST-EFR3A was then incubated with His10-KRASG12V, after which His10-KRASG12V was immunoprecipitated with a KRAS-specific antibody and immunoblotted with EFR3A- or KRAS-specific antibodies. This revealed that His10-KRASG12V co-immunoprecipitated with GST-EFR3A (Fig. 4e, f). This interaction was corroborated in the reverse direction. Namely, similarly purified recombinant GST alone as a negative control or the above GST-EFR3A were incubated with the above His10-KRASG12V protein. GST or GST-EFR3A protein was then immunoprecipitated with a GST-specific antibody followed by immunoblot with GST- or KRAS-specific antibodies. This revealed that His10-KRASG12V co-immunoprecipitated with GST-EFR3A, but not with GST (Supplementary Fig. 5b). To determine if this interaction depended upon the activation status of KRAS, we repeated this experiment, except using purified recombinant wild-type (WT) or oncogenic (G12V) His10-KRAS incubated with buffer, GDP, or non-hydrolysable GTP (GTPγS). As above, we confirmed the activation status in all six settings by RBD-affinity capture, which led to the predictable outcome, namely that oncogenic His10-KRASG12V was more active than His10-KRASWT in all conditions, and loading with GTPγS was more active than GDP or buffer with both proteins (Fig. 4g). These six versions of His10-KRAS were incubated with GST-EFR3A and again His10-KRASG12V or His10-KRASWT was immunoprecipitated with a KRAS-specific antibody and immunoblotted with EFR3A- or KRAS-specific antibodies. The results mirrored the activation status of the KRAS proteins, and ranged from GTPγS-loaded His10-KRASG12V capturing the most GST-EFR3A to buffer or GDP-loaded His10-KRASWT capturing the least (Fig. 4g, h). Thus, recombinant GST-EFR3A preferentially co-immunoprecipitates with GTP-bound or oncogenic recombinant His10-KRAS.
To validate these findings using a different detection platform, we repeated the analysis by far-western dot blot, again using purified recombinant proteins (Supplementary Fig. 5d, e). GST as a negative control or GST-EFR3A was affixed to membranes, incubated with His10-KRASG12V, and then immunoblotted with a KRAS-specific antibody. Levels of GST, GST-EFR3A, and His10-KRASG12V protein were validated by affixing these proteins to membranes and immunoblotting for their presence. We find that His10-KRASG12V directly bound to GST-EFR3A, but not GST (Fig. 4i, j). To address whether this association was dependent upon the oncogenic status of KRAS, we repeated the study at three concentrations using the above wild-type (WT) or oncogenic (G12V) recombinant His10-KRAS proteins with recombinant GST-EFR3A protein. We find that only His10-KRASG12V bound GST-EFR3A (Fig. 4k, l). Thus, far western analysis confirms a direct interaction of recombinant GST-EFR3A with recombinant oncogenic His10-KRAS.
To validate these results using a biophysical approach, we examined the interaction between GST-EFR3A and His10-KRASG12V by size-exclusion chromatography (SEC). Recombinantly purified GST-EFR3A and His10-KRASG12V were profiled using the Superose® 6 increase 10/300GL column with peak fractions assayed with EFR3A- or KRAS-specific antibodies to identify the desired proteins. We found that GST-EFR3A eluted as a monodisperse peak at 15.48 ml (Fig. 4m) as detected by immunoblot (Fig. 4n), and His10-KRASG12V eluted as a monodisperse peak at 18.49 ml (Fig. 3o) as detected by immunoblot (Fig. 4p). Consistent with the expected molecular weights of GST-EFR3A (106.7 kDa) and His10-KRASG12V (22 kDa), these proteins eluted at volumes close to those of the protein standards globulin (16.27 ml; 158 kDa) and myoglobin (18.64 ml, 17 kDa) (Supplementary Fig. 5c). Importantly, the SEC profile of a preincubated mixture of GST-EFR3A with a two-fold excess of His10-KRASG12V revealed two main peaks, one at the elution volume of 15.29 ml and the other at 18.38 ml (Fig. 4q). Immunoblot analysis with KRAS- and EFR3A-specific antibodies revealed that the first peak (15.29 ml) contained both His10-KRASG12V and GST-EFR3A, whereas the second peak (18.38 ml) contained only His10-KRASG12V (Fig. 4r). As His10-KRASG12V alone was exclusively detected at peak fractions at 18.49 ml, but not at 15.29 ml (Fig. 4o, p), our SEC analysis supports a direct interaction between GST-EFR3A and His10-KRASG12V and the formation of a stable protein complex.
To determine the specificity of EFR3A for active KRAS, we performed a competition assay with the known RAS effector BRAF using the above purified recombinant His10-KRASG12V and GST-EFR3A, and commercially purified recombinant BRAF. GST-EFR3A, His10-KRASG12V, and increasing concentrations of BRAF were incubated, His10-KRASG12V was then immunoprecipitated, followed by immunoblot to detect GST-EFR3A. As controls, we demonstrate by immunoblot constant levels of His10-KRASG12V and increasing levels of BRAF in the mixture. This analysis revealed a stepwise reduction in the amount of GST-EFR3A that co-immunoprecipitates with His10-KRASG12V (Fig. 5a). These data are consistent with EFR3A having an affinity for the conformation of KRAS that binds effectors.
Fig. 5. EFR3A associates with recombinant and endogenous oncogenic RAS.
a Analysis of levels of recombinant GST-EFR3A, as detected by immunoblot with an anti-EFR3A antibody, that co-immunoprecipitates (CO-IP) with recombinant His10-KRASG12V (preloaded with GTPγS) using an anti-KRAS antibody, in the presence of increasing levels of the RBD of BRAF, detected with and a BRAF-specific antibody. INPUT levels are shown as loading controls. b–e A (b) schematic of GST-EFR3A constructs used (FL: EFR3A base construct lacking 1-107 residues, C: C-terminal fragment, Δ: C-terminal truncations of FL) and (c, d) the levels and (e) quantification thereof of the indicated recombinant GST-EFR3A proteins, as detected by immunoblot with an EFR3A-specific antibody, co-immunoprecipitating (CO-IP) with recombinant His10-KRASG12V (preloaded with GTPγS) using a KRAS-specific antibody. INPUT and IP serve as loading controls. GST serves as a negative CO-IP control. f, g Analysis of the (f) levels and (g) quantification thereof of the endogenous EFR3A, as detected by immunoblot with an EFR3A-specific antibody, co-immunoprecipitating (CO-IP) with FLAG-KRASG12D using a FLAG-specific antibody when transiently expressed in 293T cells. h Levels of endogenous EFR3A bound to endogenous active KRAS, as assessed by RBD pull-down assay in AsPC-1 cells, as assessed by incubating AsPC-1 cell lysate with recombinant GST-RAF-RBD protein, followed by immunoblot with a KRAS-specific antibody to detect active KRAS and an EFR3A-specific antibody to detect endogenous EFR3A. INPUT and IP levels are shown as loading controls. a, c–h Representative of 3 biological replicates. Replicate experiments and full-length gels are provided in Supplementary Fig. 12. Significance values calculated by one-sided student’s t test for (e, g): ***p < 0.001. Specific p values for (e) are 0.00052 (FL), 0.00035 (C), 0.00011 (Δ1), 0.00014 (Δ2), 0.00056 (Δ3), and 0.00034 (Δ4) and (g) is 0.00087.
To map the region of EFR3A necessary to interact with KRASG12V, we generated four progressively larger C-terminal truncation mutants based on recombinant GST-EFR3A lacking the membrane targeting region (FL, Fig. 5b). Each truncation mutant was incubated with recombinant His10-KRASG12V, after which His10-KRASG12V was immunoprecipitated, followed by immunoblot with KRAS- and EFR3A-specific antibodies. This revealed that deletion mutants lacking the terminal 122 to 138 amino acids (Δ1 and Δ2) were expressed but failed to co-immunoprecipitate with His10-KRASG12V, while deletion mutants lacking the 22 to 109 C-terminal amino acids (Δ3 and Δ4) were co-immunoprecipitated (Fig. 5c, e). To address whether the C-terminus was alone sufficient to bind KRASG12V, we repeated the experiment with a GST-tagged polypeptide encoding the terminal 138 amino acids of EFR3A (mutant C, Fig. 5b), finding that this fragment co-immunoprecipitated with His10-KRASG12V (Fig. 5d, e). These data suggest that the region between amino acids 700 to 712 promotes the binding of GST-EFR3AFL to His10-KRASG12V in vitro, supporting the contention of a specific association of EFR3A to active KRAS.
Endogenous EFR3A co-immunoprecipitates with endogenous active KRAS
To address whether endogenous EFR3A binds active KRAS, we ectopically expressed FLAG epitope-tagged KRASG12D (FLAG-KRASG12D) in human 293T cells. FLAG-KRASG12D was then immunoprecipitated with a FLAG-specific antibody followed by immunoblot with an EFR3A-specific antibody. This revealed the co-immunoprecipitation of endogenous EFR3A with oncogenic KRAS (Fig. 5f, g). To confirm that this interaction is dependent upon the activation status of endogenous KRAS, we used the aforementioned RBD polypeptide to affinity capture endogenous GTP-bound RAS from lysates isolated from the KRAS mutation-positive human pancreatic adenocarcinoma cell line AsPC-1. Immunoblot with an EFR3A-specific antibody detected endogenous EFR3A (Fig. 5h). Thus, endogenous EFR3A co-immunoprecipitates with endogenous GTP-bound KRAS in cells. The specificity of this interaction was validated by demonstrating a temporal enrichment of EFR3A in the affinity-captured RAS-GTP pool after EGF stimulation of the KRAS mutation-negative BxPC3 cell line (Supplementary Fig. 5f). Collectively, these results support the contention that EFR3A directly binds KRAS in the active GTP-bound state in a fashion resembling how activated RAS engages with its effectors.
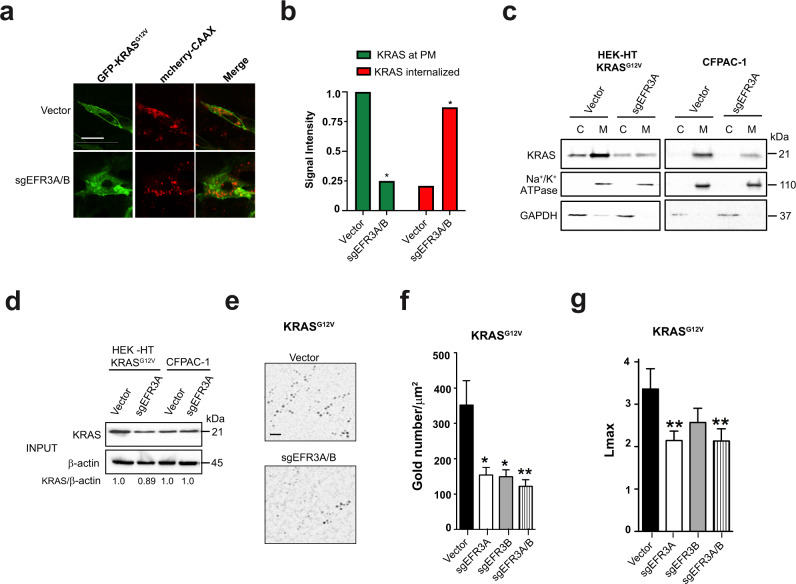
Loss of EFR3A reduces KRAS plasma membrane localization and nanoclustering
Two observations suggest a direct effect of EFR3A on KRAS itself. First, as noted above, EFR3A bound to activated KRAS. Second, the loss of EFR3A reduced not only PI3K/AKT signaling—not unexpected as the protein recruits PI4K to the plasma membrane28,29—but also MAPK signaling. Such a finding suggests that EFR3A functions where these two pathways converge, namely at the KRAS oncoprotein. As KRAS must reside at the plasma membrane to signal50, the very membrane whereby EFR3A resides29, we explored the effect of EFR3A loss on the localization of KRAS at this membrane. Specifically, we transiently transfected vector or sgEFR3A/B HEK-HT cells with a vector encoding both GFP-KRASG12V to visualize the subcellular localization of KRAS, and mCherry-CAAX, which marks endomembranes24. Confocal microscopy imaging revealed the expected distribution of GFP-KRASG12V preferentially at the plasma membrane rather than endomembranes marked by mCherry-CAAX, while loss of EFR3A/B reversed this localization, with GFP-KRASG12V preferentially detected on endomembrane (Fig. 6a). Quantification revealed a significant 3.4-fold shift of GFP-KRASG12V from the plasma membrane to internal membranes (Fig. 6b). To independently validate these results with a completely different detection platform, we turned to biochemical fractionation. Specifically, KRASG12V-transformed HEK-HT cells in which EFR3A was knocked out, as confirmed by immunoblot (Fig. 2a), were subjected to membrane fractionation to isolate the plasma membrane fraction, as confirmed by the presence of the Na+/K+ ATPase by immunoblot51, from the cytosolic fraction, as confirmed by the presence of GAPDH52 (Fig. 6c). Immunoblot analysis revealed that the normal enrichment of KRAS in the membrane fraction was substantially reduced upon loss of EFR3A (Fig. 6c). To validate these results at the endogenous level, we repeated the experiment in the KRAS-mutant pancreatic adenocarcinoma cell line CFPAC-1, except we immunoblotted for endogenous KRAS protein. Again, in cells lacking EFR3A (Fig. 3a), KRAS was mis-localized from the membrane to cytosolic fraction (Fig. 6c). We ruled out that the loss of KRAS at the plasma membrane was due to an overall reduction in KRAS protein, as immunoblot quantification revealed a negligible change in the level of ectopic or endogenous KRAS protein upon loss of EFR3A (Fig. 6d).
Fig. 6. EFR3A promotes the localization and nanoclustering of KRAS at the plasma membrane.
a Representative confocal images showing the distribution of ectopically expressed GFP-KRASG12V, mCherry-CAAX, or both protein (merge) in HEK-HT cells stably infected with a lentiviral vector encoding Cas9 and no sgRNA (vector) or sgEFR3A/B. Scale bar: 20 μm. b Mean ± SD signal intensity of GFP-KRASG12V at the plasma membrane (KRAS at PM) versus internalized (KRAS internalized) in the same two cell lines. c Levels of KRAS and Na+/K + ATPase (PM marker), and GAPDH (cytosolic marker), as assessed by immunoblot analysis, in the cytosolic (C) versus membrane (M) fraction of KRASG12V-transformed human HEK-HT cells or the human pancreatic adenocarcinoma cell line CFPAC-1 stably infected with a lentivirus encoding Cas9 and no sgRNA (vector) or sgEFR3A. d Levels of KRAS versus actin as a loading control in the same cells from the endomembrane fraction. e–g A (e) representative EM micrograph showing gold conjugated, GFP-specific antibody (black dots) bound to GFP-KRASG12V, (f) mean ± SEM of gold particles per 1 μm2 of plasma membrane sheets representative of the amount of KRAS on the plasma membrane, and (g) the extent of nanoclustering of KRAS on the plasma membrane as measured by Lmax from human HEK-HT cells transiently expressing GFP-KRASG12V and stably infected with a lentivirus(es) encoding Cas9 with no sgRNA (vector) or the indicated sgRNAs. Scale bar: 100 nm. a–d Representative of 3 biological replicates. e, f EM analysis is based on 30 images. Replicate experiments and full-length gels are provided in Supplementary Fig. 13. Significance values were calculated by one-sided student’s t test for (b) and two-sided bootstrap test for (f, g): *p < 0.05 and **p < 0.01. Specific p values for (b) are 0.011 (sgEFR3A/B KRAS at PM) and 0.024 (sgEFR3A/B KRAS internalized), f are 0.0138698 (sgEFR3A), 0.0117821 (sgEFR3B), and 0.005275 (sgEFR3A/B), g are 0.001 (sgEFR3A), 0.424 (sgEFR3B), and 0.001 (sgEFR3A/B).
We independently validated these results at the resolution of electron microscopy (EM). Specifically, vector, sgEFR3A, sgEFR3B, or sgEFR3A/B HEK-HT cells were transiently transfected with GFP-KRASG12V. Intact basal plasma membrane sheets were prepared from these cells and labeled with a GFP-specific antibody directly coupled to gold nanoparticles and imaged by a transmission electron microscopy (TEM). Loss of either or both EFR3A or EFR3B significantly decreased the amount of immunogold-labeled KRASG12V quantified by measuring gold particle density (Fig. 6e and Supplementary Fig. 6a), indicative of KRAS mis-localization from the inner plasma membrane. Specifically, there was a ~57% reduction in KRASG12V at the plasma membrane in EFR3A- or EFR3B- knockout cells, and a 65% reduction in EFR3A/B double-knockout cells (Fig. 6f and Supplementary Fig. 6a). In addition to localizing to the plasma membrane, KRAS molecules form nanoclusters to promote signaling19. We therefore extended the analysis of the immunogold-labeled plasma membrane sheets to include spatial mapping of the nanogold point patterns. 20 to 30 images per sample were analyzed using the univariate K-function expressed as L(r)-r. Values of L(r)-r greater than 1 (=99% CI for a random pattern) indicate significant clustering, and the peak value of the function termed Lmax serves as a useful summary statistic that quantifies the extent of clustering23. Loss of one or more EFR3 isoforms significantly reduced KRASG12V nanoclustering, as evidenced by the reduction in Lmax values (Fig. 6g). Thus, by three independent assays, at both the ectopic and endogenous level, and by knocking out either or both EFR3 isoforms, we demonstrate that EFR3A promotes KRAS plasma membrane localization, nanoclustering, and engagement with effectors.
The above findings predict that other components of the EFR3A complex should similarly co-cluster with KRAS. To this end, 293T cells were transiently co-transfected with GFP-KRASG12V and mCherry-EFR3A, mCherry-EFR3B, mCherry-TTC7A, or mCherry-FAM126A. 293T cells co-transfected with GFP-KRASG12V and RFP-KRASG12V, or GFP-KRASG12V and RFP-tH (the minimal C-terminal anchor of HRAS) served as positive and negative controls for proteins that extensively co-cluster or spatially segregate, respectively. Intact basal plasma membrane sheets prepared from these cells and attached to gold EM grids were fixed and co-labeled with GFP-specific and RFP-specific antibodies directly conjugated to 6 nm and 2 nm gold nanoparticles, respectively. The plasma membrane sheets were imaged in by TEM and co-localization between the 6 nm and the 2 nm gold particle distributions was quantified using bivariate K-functions expressed as Lbiv(r)-i. As a summary parameter to quantify the extent of co-clustering, we use a defined integral of the Lbiv(r)-r function termed Lbiv-Integrated (LBI). LBI values <100 (the 95% C.I.) indicate no significant co-clustering, whereas the higher the LBI value the more extensive the co-localization of the 2 nm and 6 nm gold distributions11,23. This analysis revealed that EFR3A, EFR3B, TTC7A, and FAM126A all co-cluster with KRASG12V to an extent that is similar to GFP-KRASG12V co-clustering with RFP-KRASG12V (Supplementary Fig. 7). Conversely, the low LBI value for RFP-KRASG12V and GFP-tH illustrates that proteins ectopically expressed on the plasma membrane do not always co-localize on the nanoscale. Collectively these data support the recruitment of EFR3A to activated KRAS increasing the retention and nanoclustering of KRAS at the plasma membrane.
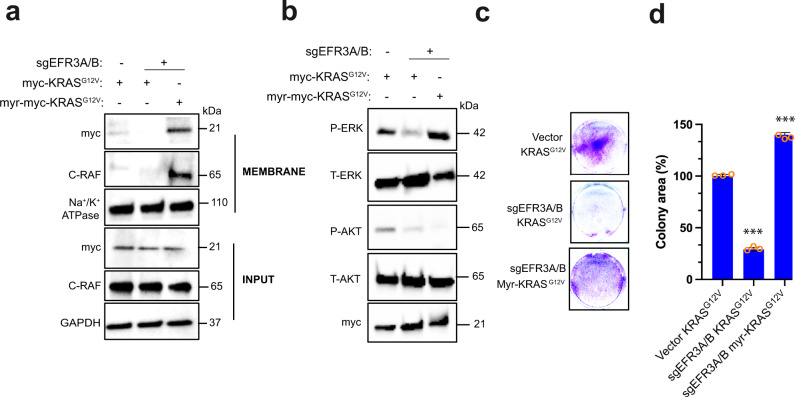
Targeting KRAS to the plasma membrane by an alternative anchor rescues the loss of EFR3A
The above observations are consistent with a model whereby activated KRAS binds EFR3A, which retains KRAS at the plasma membrane to signal. A direct test of this hypothesis is that maintaining KRAS at the plasma membrane should rescue the defect in oncogenic KRAS signaling and transformation due to a loss of EFR3A. Localization of KRAS to the plasma membrane is mediated by a combination of a polybasic domain and prenylation of the C-terminal CAAX sequence53. As loss of EFR3A reduces the residency of KRAS at the plasma membrane, we queried whether targeting KRAS to the plasma membrane by other means overcomes this effect. To this end, we note that artificial N-terminal myristoylation can tether KRAS to the plasma membrane in the absence of prenylation54. We thus fused an N-terminal myristoylation sequence (myr)55 to a SAAX-mutant (which blocks prenylation)54 of oncogenic (G12V) myc epitope-tagged KRAS, creating myr-myc-KRASG12V. We note here that this construct still retains the polybasic domain of KRAS(4B) but lacks prenylation sites, which is known to be important for binding PS23 that, as discussed below, is critical for retention of KRAS at the plasma membrane11,23. sgEFR3A/B HEK-HT cells were stably infected with a lentivirus expressing myr-myc-KRASG12V. As controls, HEK-HT cells with and without sgEFR3A/B were infected with a lentivirus expressing myc-KRASG12V. The three cell lines were then subjected to the same biochemical fractionation and immunoblot analysis as above. This revealed that myr-myc-KRASG12V was enriched in the membrane fraction in the absence of EFR3A/B beyond that of control myc-KRASG12V (Fig. 7a), and thus could be used for epistatic analysis. To next address whether KRAS retention at the plasma membrane in this manner could rescue the loss of EFR3A/B, we analyzed these three cell lines for oncogenic KRAS signaling. Immunoblot analysis revealed that the decrease in P-ERK levels upon loss of EFR3A/B were restored by expressing myr-myc-KRASG12V, but not in the control myc-KRASG12V, back to the level of positive-control cells (Fig. 7b). We ascribe this to recruiting RAS effectors to the plasma membrane, as C-RAF was enriched in the membrane fraction in cells lacking EFR3A/B upon specifically expressing myr-myc-KRASG12V (Fig. 7a). Interestingly however, we note that myr-myc-KRASG12V did not restore P-AKT levels, perhaps reflecting an inability of this protein to completely replicate the normal membrane localization of KRAS, the likelihood of a different lipid composition around myristoylated KRAS clusters, or the additional importance of recruiting EFR3A to generate PI(4)P required for PI3K activity. Finally, to address whether myr-myc-KRASG12V could rescue the loss of transformation, these three cell lines were assayed for 2D-transformed growth, which revealed that the reduction of colony formation due the loss of EFR3A/B was rescued to the level of the control KRASG12V-transformed cells upon expressing myr-myc-KRASG12V but not myc-KRASG12V (Fig. 7c, d). To evaluate the effect on 3D-transformed growth, these three cell lines were assayed for growth in soft agar, which similarly revealed restoration of anchorage-independent colony formation by myr-myc-KRASG12V (Supplementary Fig. 8). Thus, epistatic analysis supports EFR3A promoting oncogenic KRAS signaling and transformation by, in large part, retaining the oncoprotein at the plasma membrane, likely through specific lipid species.
Fig. 7. KRAS membrane anchoring rescues signaling and transformation defects in EFR3A-null cells.
a–d Immunoblot analysis of (a) membrane fraction for myc-KRASG12V, myr-myc-KRASG12V, C-RAF, Na+/K+ ATPase, and GAPDH, and (b) phosphorylated (P) and total (T) ERK and AKT, and (c) an example of colony formation, as assessed by crystal violet staining, which is (d) plotted as mean ± SD of colony area, for human HEK-HT cells stably infected with a lentivirus encoding Cas9 encoding no sgRNA (vector) or sgEFR3A/B in the absence and presence of ectopic myc-KRASG12V or when targeted to the PM by an N-terminal myristoylation sequence (myr-myc-KRASG12V). INPUT levels of indicated proteins serve as loading controls. a, b Representative of 3 biological replicates. c, d Representative of 3 biological replicates tested in triplicate. Replicate experiments and full-length gels are provided in Supplementary Fig. 13. Significance values were calculated by one-sided student’s t test for (d): ***p < 0.001. Specific p values for (d) are 0.00027 (KRASG12VsgEFR3A/B) and 0.00022 (myr-KRASG12VsgEFR3A/B).
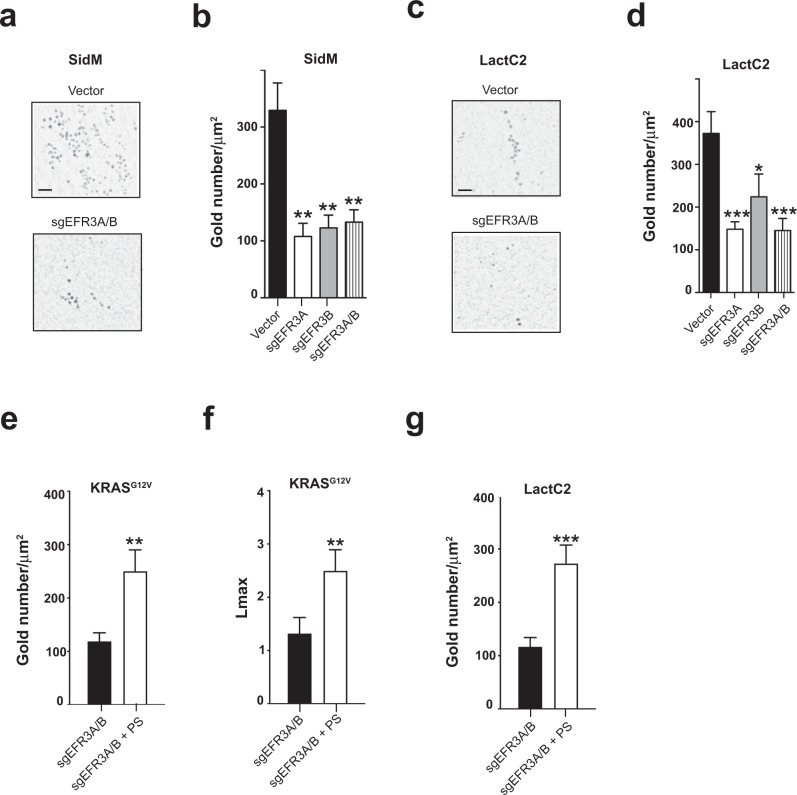
Loss of EFR3A alters the phosphatidylserine content, KRAS localization, and nanoclustering at the plasma membrane
KRAS nanoclustering and plasma membrane localization are abrogated by reducing the PS content at the plasma membrane23,56. PS is required for both the localization of KRAS to this membrane and nanoclustering, which is mediated by the exquisite binding specificity of the KRAS C-terminal membrane anchor for PS. PS is transported to the plasma membrane by the ORP5 and ORP8 lipid transporters that exchange PS for PI(4)P generated by PI4KA at the plasma membrane24,26,57. Since EFR3A functions to recruit PI4KA28 to the plasma membrane29 and knockout of EFR3A reduced KRAS plasma membrane localization and nanoclustering, we addressed whether these effects were mediated by PI(4)P and PS levels on the plasma membrane. Vector, sgEFR3A, sgEFR3B, and sgEFR3A/B HEK-HT cells were transiently transfected with GFP-P4M-SidM. GFP-P4M-SidM is a PI(4)P biosensor comprised of GFP for detection purposes fused in-frame with the PI(4)P binding domain of the protein SidM58 that specifically recognizes the plasma membrane pool of PI(4)P. As above, derived plasma membrane sheets were incubated with a GFP-specific antibody directly coupled to gold nanoparticles and imaged by TEM, which revealed approximately a 64% reduction of immunogold-labeled GFP-P4M-SidM upon the loss of one or both ERF3 isoforms (Fig. 8a, b and Supplementary Fig. 6b). Since PI(4)P is essential for PS localization to the plasma membrane59,60, we addressed whether EFR3A ablation also reduced plasma membrane PS levels. The same HEK-HT cells were transiently transfected with the PS biosensor GFP-LactC2 in which GFP is fused in-frame the C2 domain encoding the PS-binding activity of the protein lactadherin56. Again, immuno-EM analysis revealed a decrease in immunogold-labeled GFP-LactC2 in the plasma membrane of cells lacking one or both EFR3 isoforms (Fig. 8c, d and Supplementary Fig. 6c). Importantly, addition of exogenous PS to cells prior to plasma membrane sheet preparation rescued the plasma membrane localization (Fig. 8e) and nanoclustering (Fig. 8f) of GFP-KRASG12V expressed in sgEFR3A/B HEK-HT cells. We further confirm that this increase in KRASG12V plasma membrane localization and nanoclustering was indeed a result of increased plasma membrane PS levels, as addition of PS to sgEFR3A/B HEK-HT cells transiently expressing GFP-LactC2 increased the amount of plasma membrane-localized GFP-LactC2 visualized by immuno-EM (Fig. 8g). Thus, we suggest that the loss of EFR3A reduces plasma membrane PI(4)P and PS levels leading to reduced plasma membrane localization and nanoclustering of KRAS.
Fig. 8. EFR3A regulates plasma membrane lipid content to promote RAS oncogenesis.
a, c Representative EM micrographs of plasma membrane (PM) sheets from human HEK-HT cells stably infected with a lentivirus(es) encoding Cas9 with no sgRNA (vector) or the indicated sgRNAs and transiently transfected with (a) GFP-P4M-SidM (a marker of PM PI(4)P) or (c) GFP-LactC2 (a marker of PM phosphatidylserine). Gold-conjugated GFP-antibodies seen as black dots are bound to the probe of interest. Scale bar: 100 nm. b, d The corresponding mean ± SEM of the number of gold particles per 1 μm2 of plasma membrane sheets representative of amount of (b) PI(4)P and (d) PS on the plasma membrane. e, f Mean ± SEM of (e) gold particles per 1 μm2 of plasma membrane sheets representative of the amount of KRAS on the plasma membrane and the (f) the extent of nanoclustering of KRAS on the plasma membrane as measured by Lmax from human HEK-HT cells transiently expressing GFP-KRASG12V and stably infected with a lentiviruses encoding Cas9 and sgEFR3A/B in the absence and presence of exogenous phosphatidylserine (PS). g Mean ± SEM of gold particles per 1 μm2 of the plasma membrane sheets from human HEK-HT cells transiently transfected with GFP-LactC2 and stably infected with a lentiviruses encoding Cas9 and sgEFR3A/B in the absence and presence of exogenous phosphatidylserine (PS). a–g EM analysis is based on 30 images. Significance values were calculated by the two-sided bootstrap test for (b, d–g): *p < 0.05, **p < 0.01, and ***p < 0.001. Specific p values for (b) are 0.00062274 (sgEFR3A), 0.00111426 (sgEFR3B), 0.00167654 (sgEFR3A/B), d are 0.000436671 (sgEFR3A), 0.056486805 (sgEFR3B), 0.000536135 (sgEFR3A/B), e is 0.00680191 (sgEFR3A/B + PS), f is 0.001 (sgEFR3A/B + PS), and (g) is 0.00051866 (sgEFR3A/B + PS).
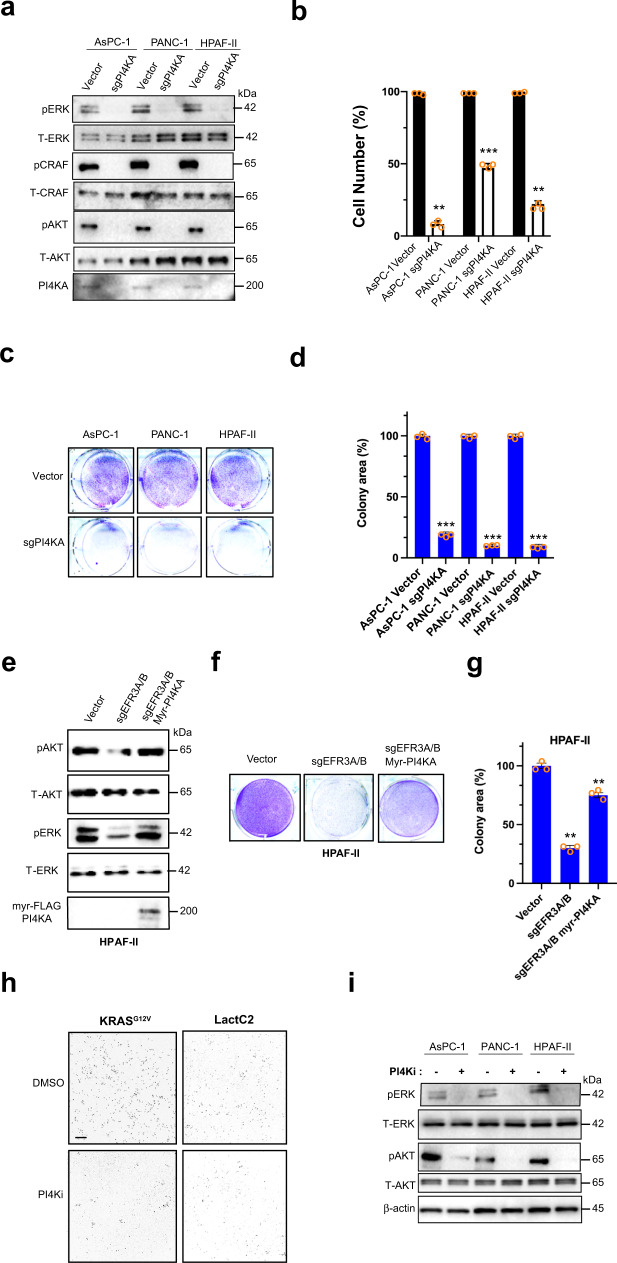
EFR3A promotes KRAS oncogenesis through PI4KA
The above findings argue that EFR3A promotes oncogenic KRAS signaling and transformation by recruiting PI4KA to alter the local lipid composition of the plasma membrane. EFR3A forms a complex with TTC7A or B and FAM126A in addition to PI4KA28. To therefore determine if PI4KA acts downstream of EFR3A, we tested whether the loss of PI4KA affects oncogenic KRAS signaling and transformation. To this end, KRAS-mutant human pancreatic adenocarcinoma cell lines AsPC-1, PANC-1, and HPAF-II were stably transduced with a vector encoding Cas9 in the absence (vector control) and presence of an sgRNA targeting the PI4KA gene (sgPI4KA), which was confirmed by immunoblot to reduce endogenous PI4KA levels (Fig. 9a). The loss of PI4KA also reduced P-AKT, P-ERK, and P-CRAF levels (Fig. 9a), cell number over time (Fig. 9b), 2D- (Fig. 9c, d), and 3D- (Supplementary Fig. 9a) transformed growth in all three cell lines. Given these results, we next performed epistatic analysis by testing whether tethering PI4KA to the plasma membrane could rescue the loss of endogenous oncogenic KRAS signaling and transformation in EFR3A/B-knockout cells. To this end, HPAF-II cells in which EFR3A/B were knocked out (Fig. 3a) were engineered to stably express PI4KA fused to the previously described N-terminal myristoylation sequence (myr-PI4KA), a strategy that has been used to localize other PI-kinases to the plasma membrane61. Vector encoding no sgRNA or sgEFR3A/B served as positive and negative controls, respectively. Consistent with the above model, myr-PI4KA rescued P-ERK and P-AKT (Fig. 9e), 2D- (Fig. 9f, g), and 3D- (Supplementary Fig. 9b) transformed growth of HPAF-II cells lacking EFR3A/B to the levels observed in positive control cells. Thus, recruiting PI4KA to the plasma membrane in the absence of EFR3A stimulates oncogenic KRAS signaling and transformation. To pharmacologically affirm the role of PI4KA in KRAS signaling, we first confirmed that the selective PI4KA inhibitor C762 does indeed reduce PS and KRAS at the plasma membrane. As expected, plasma membrane sheets imaged by immunogold-EM analysis revealed a reduction of PS (as assessed with the biomarker Lact-C2) and KRASG12V (GFP-KRASG12V) at the plasma membrane in MDCK cells (chosen for ease of imaging) treated with C7 at a dose of 30 µM for 48 h compared to cells treated with vehicle alone (Fig. 9h). Given this, we next assayed the effect of C7 on endogenous oncogenic KRAS signaling in KRAS-mutant human pancreatic adenocarcinoma cell lines. Specifically, the aforementioned AsPC-1, PANC-1, and HPAF-II cell lines were treated with C7 at a dose of 20 µM for 4 h or vehicle alone as a control, followed by immunoblot analysis, which revealed a robust reduction in P-AKT and P-ERK in cells treated with C7 (Fig. 9i). Collectively, these data are consistent with a feedback circuit whereby activated KRAS binds EFR3A, which in turn recruits PI4KA and thereby increases plasma membrane levels of PI(4)P and PS to promote the localization and nanoclustering of oncogenic KRAS at the plasma membrane, stimulating oncogenic signaling and transformation.
Fig. 9. PI4KA promotes plasma membrane association of oncogenic RAS.
a–d Analysis of the (a) level of phosphorylated (P) and/or total (T) ERK, AKT, and PI4KA, as assessed by immunoblot, b mean ± SD cell number over time in culture, as assessed by the Titer Glo assay, c an example of colony formation, as assessed by crystal violet staining, which is (d) plotted as the mean ± SD total colony area, in the KRAS-mutant human pancreatic adenocarcinoma cell lines AsPC-1, PANC-1, and HPAF-II stably infected with a lentivirus encoding Cas9 and no sgRNA (vector) or sgPI4KA. e–g Analysis of the (e) level of phosphorylated (P) and/or total (T) ERK, AKT, and PI4KA with an N-terminal myristoylation sequence and FLAG epitope-tag (myr-FLAG-PI4KA), as assessed by immunoblot, and (f) an example of colony formation, as assessed by crystal violet staining, which is (g) plotted as the mean ± SD total colony area, of HPAF-II cells stably infected with a lentivirus(es) encoding Cas9 and no sgRNA (vector) or sgEFR3A/B in the absence or presence of myr-FLAG-PI4KA. h A representative EM micrograph showing gold conjugated, anti-GFP antibody (black dots) reactivity in plasma membrane sheets from MDCK cells ectopically expressing GFP-KRASG12V or GFP-LactC2 and treated with 30 μM C7 (PI4Ki) or vehicle (DMSO) for 48 h. Scale bar: 100 nm. i Levels of (P) and (T) ERK, AKT, and actin (as a loading control), as assessed by immunoblot analysis, in the human pancreatic adenocarcinoma cell lines AsPC-1, PANC-1, and HPAF-II after treatment with control DMSO or 20 μM of C7 (PI4Ki) for 4 h. a, e, i Representative of 3 biological replicates. b–d, f, g Representative of 3 biologic replicates tested in triplicate. h EM analysis is based on 30 images. Replicate experiments and full-length gels are provided in Supplementary Fig. 14. Significance values were calculated by one-sided student’s t test (b, d, g): *p < 0.05, **p < 0.01, ***p < 0.001, and ****p < 0.0001. Specific p values for (b) are 0.0036 (AsPC-1sgPI4KA), 0.0003 (PANC-1sgPI4KA), and 0.0045 (HPAF-IIsgPI4KA), d are 0.00063 (AsPC-1sgPI4KA), 0.00052 (PANC-1sgPI4KA), and 0.00021 (HPAF-IIsgPI4KA), and (g) are 0.0017 (sgEFR3A/B) and 0.0065 (sgEFR3A/B myr-PI4KA).
Combined inhibition of oncogenic KRAS and PI4KA is synergistic
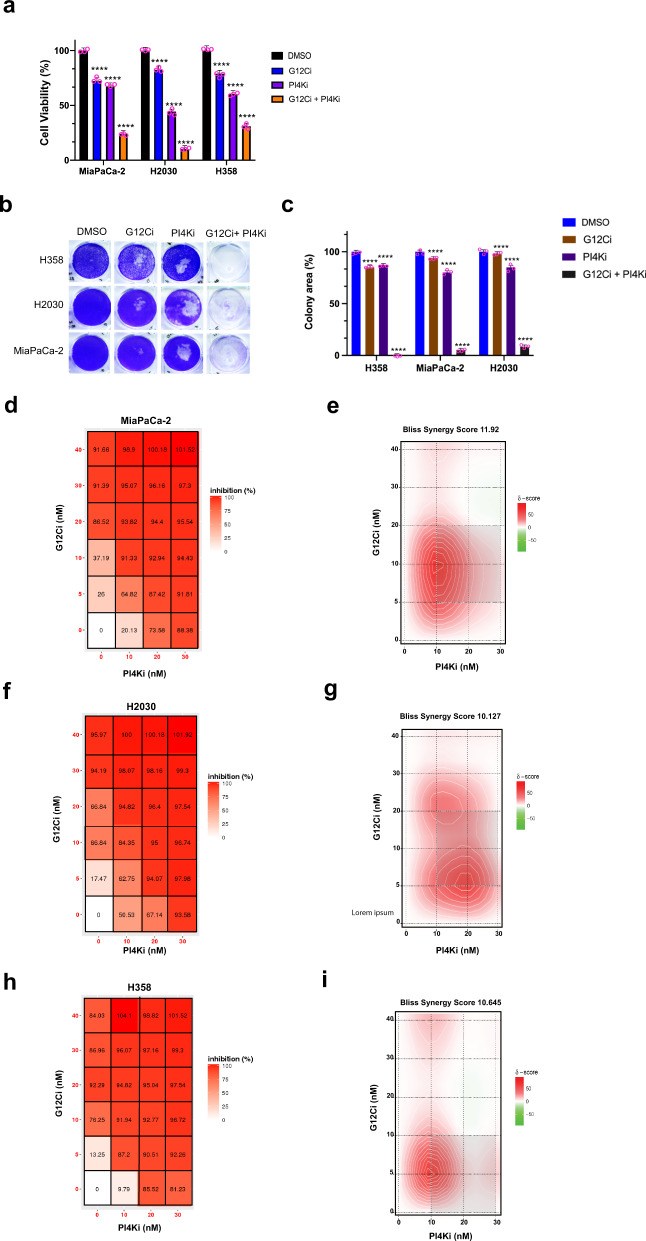
The finding that EFR3A recruits PI4KA and binds oncogenic KRAS suggests the possibility of targeting this signaling circuit at the level of the kinase for the treatment of KRAS-mutant cancers. One challenge of pharmacologically targeting phosphatidylinositol kinases is the toxicity of inhibiting this class of enzymes63, and PI4KA is no exception to this63. Indeed, monotreatment with a PI4KA inhibitor developed for the treatment of Hepatitis C infection exhibited acute toxicity in mice63. However, combining a PI4K inhibitor with radiation was found to decrease xenograft tumor growth64,65. We thus suggest that a PI4KA inhibitor may have a therapeutic window in situations whereby oncogenic KRAS signaling is already reduced, rendering cancer cells hypersensitive to any further loss of KRAS signaling8,63. In this regard, we note that inhibitors targeting KRASG12C are showing promise in the clinic, with sotorasib now approved for the treatment of lung cancer, but that drug combinations may be required for durable responses8,9. Given this, we treated three different KRASG12C-mutant human cancer cell lines (MiaPaCa-2, H2030, and H358) in triplicate with the vehicle DMSO as a control, the KRASG12C inhibitor AMG510 (sotorasib)4 at a fixed dose of 5 nM, which corresponds to an IC20 to IC30 depending on the cell line, and the PI4KA inhibitor C762 at a fixed dose of 10 nM, which corresponds to the approximate IC30 to IC55, depending on the cell line, or both compounds at these concentrations. 72 h later the number of viable cells was calculated by the Titer Glo assay and normalized to those of the vehicle-treated control cells, which was expressed as the percent of viable cells. This revealed that the combination of the KRASG12C and PI4KA inhibitors was uniformly more inhibitory, reducing the number of viable cells by 80% to 95% (Fig. 10a). To assess the effect of protracted inhibitor treatment, we repeated the experiment, but at the lower IC10 dose and for 12 consecutive days using colony formation as metric of 2D-transformed growth. This analysis revealed potent synergy between the two inhibitors, leading to an almost complete loss of colony formation (Fig. 10b, c). This is unlikely a general effect of disrupting PI4K activity, as reducing EFR3A/B expression (Supplementary Fig. 10a) was synergistic with AMG150 in two of these three lines (Supplementary Fig. 10b). Given these results, we expanded the doses to 0, 5, 10, 20, and 40 nM of AMG510 and 0, 10, 20, and 30 nM of C7 to derive a dose inhibition matrix66. This again revealed a synergistic effect across at a wide range of doses, but particularly at low doses that may be better tolerated clinically (Fig. 10d, f, h). To quantitate the degree of synergy we calculated the Bliss synergy score across all doses, which was 11.92 for MiaPaca-2, 10.13 for H2030, and 10.65 for H358 (Fig. 10e, g, i). Thus, low doses of a PI4KA inhibitor greatly augment the antineoplastic activity of a KRASG12C inhibitor, suggesting a potential clinical application.
Fig. 10. A PI4KA inhibitor synergized with a clinical RASG12C inhibitor.
a Mean ± SD cell viability, as assessed by the Cell Titer Glo assay, of the KRASG12C-mutant human cancer cell lines MiaPaCa-2, H2030, and H358 treated with a fixed concentration of vehicle (DMSO), 5 nM of the RASG12C inhibitor (G12Ci) AMG510, 10 nM of the PI4KA inhibitor (PI4Ki) C7, or both drugs at these concentrations. b, c An example of (b) colony formation, as detected by crystal violet staining, which is (c) plotted as the mean ± SD of colony area, of MiaPaCa-2, H2030, and H358 cells treated with the indicated drugs at a IC10 dose for 12 days. d, f, h Dose response inhibition matrix and (e, g, i) BLISS synergy plot of (b, c) MiaPaCa-2, d, e H2030, and (f, g) H358 cells treated with the indicated drug concentrations for 72 h. a–i Representative of 3 biological replicates assayed in triplicate. Replicate experiments are provided in Supplementary Fig. 15. Significance values were calculated by two-sided Welch’s t-test (a, c): ****p < 0.0001. Specific p values for (a) are 0.000021 (G12Ci), 0.000036 (PI4Ki), and 0.000047 (G12Ci + -PI4Ki) for MiaPaCa-2 cells, 0.000043 (G12Ci), 0.000034 (PI4Ki), and 0.000038 (G12Ci + PI4Ki) for H2030 cells, and 0.000013 (G12Ci), 0.000021 (PI4Ki), and 0.000019 (G12Ci + PI4Ki) for H358 cells, for c are 0.000021 (G12Ci), 0.000036 (PI4Ki), and 0.000032 (G12Ci + -PI4Ki) for MiaPaCa-2 cells, 0.000025 (G12Ci), 0.000016 (PI4Ki), and 0.000033 (G12Ci + PI4Ki) for H2030 cells, and 0.000038 (G12Ci), 0.000024 (PI4Ki), and 0.000017 (G12Ci + PI4Ki) for H358 cells.
Discussion
Here we identify EFR3A as a component of the RAS interactome mediating a positive-feedback circuit that promotes oncogenic KRAS signaling and tumorigenesis, unearthing a potential vulnerability to enhance the antineoplastic activity of KRASG12C-inhibitors for the treatment of pancreatic and possibly other KRAS-mutant cancers. Mechanistically, we propose that activated KRAS binds EFR3A, recruiting PI4KA and increasing the local concentration of PI(4)P and in turn PS, which maintains oncogenic KRAS localization and nanoclustering at the plasma membrane, leading to sustained recruitment of KRAS effectors and signaling that promotes tumorigenesis (Supplementary Fig. 16).
The first step of this model, EFR3A preferentially binds KRAS in the active state, is supported by multiple experiments. Namely, we show that recombinant EFR3A binds to recombinant GTP-bound and/or oncogenic, but not GDP-bound and/or mutant-inactive KRAS, as assessed by co-immunoprecipitation and far western analysis. This association is disrupted by a RAS effector, and an EFR3A/KRASG12V complex is captured by analytical size-exclusion chromatography. We also mapped the binding region on EFR3A to 12 amino acid stretch in the C-terminus. Of note, the C-terminus is also responsible for binding TTC7A, which stabilizes the EFR3/TTC7/FAM126/PI4KA complex on the plasma membrane30–32. The association of EFR3A with active KRAS was also captured in cells. Namely, we show a stepwise increase in biotin labeling of EFR3A by progressively more active BirA-KRAS in cells, and further, endogenous GTP-bound KRAS was associated endogenous EFR3A. Collectively, these data suggest that EFR3A is recruited to active GTP-bound KRAS in a manner reminiscent of effector proteins.
The second step of this model is that EFR3A recruits PI4KA, thereby increasing the local concentration of PI(4)P and PS. In this regard, we note that it is well established that EFR3A recruits PI4KA to the plasma membrane. Indeed, we see co-localization of all components of the EFR3A complex (including PI4KA) with KRASG12V at the plasma membrane, and that a loss of EFR3A or PI4KA reduces PI(4)P levels at the plasma membrane, as assessed by immuno-EM. Further, we previously demonstrated that depleting PI(4)P on the plasma membrane results in the loss of the exchange of PI(4)P with PS in the endoplasmic reticulum, thereby reducing PS at the plasma membrane24. Again, we show that loss of EFR3A or PI4KA also reduces the level of PS at the plasma membrane.
The third step of this model is that the accumulation of PI(4)P and PS promotes active KRAS accumulation and nanoclustering at the plasma membrane23,24. In support, we previously demonstrated that the KRAS membrane anchor selectively interacts with symmetric unsaturated PS species (such as 16:1/18:1) to promote plasma membrane binding and asymmetric unsaturated PS (such as 16:0/18:1) to form nanoclusters23. Here we show that loss of EFR3A, EFR3B, and/or PI4KA reduces the amount of KRASG12V at the plasma membrane, as assessed by immuno-fluorescence, biochemical fractionation, and/or immuno-EM. Similarly, we show that the loss of any of these proteins reduces KRASG12V nanoclustering. Finally, we show that tethering PI4KA to the plasma membrane or providing PS to cells restores KRASG12V localization and nanoclustering at the plasma membrane in the absence of EFR3A.
The fourth step is that increasing the localization and nanoclustering of KRAS on the plasma membrane enhances oncogenic signaling to promote tumorigenesis. Here we show that the that loss of EFR3A, EFR3B, or PI4KA all reduce P-ERK and P-AKT levels, as assessed by immunoblot, effects rescued by tethering KRASG12V or PI4KA to the plasma membrane. Moreover, we show that loss of EFR3A, EFR3B, or PI4KA all reduce short-term (Titer-Glo analysis) and long-term (colony formation) 2D-transformed growth, as well as 3D growth-transformed growth (soft agar) in not only oncogenic KRAS-transformed HEK-HT cells, but also in multiple human pancreatic adenocarcinoma cell lines characterized by an endogenous mutant KRAS allele. Again, these phenotypes were rescued by tethering KRASG12V or PI4KA to the plasma membrane. Furthermore, loss of EFR3A, EFR3B, or both reduced tumor growth of a human pancreatic adenocarcinoma cell line in vivo.
We recognize the possibility that loss of EFR3A, EFR3B, or PI4KA may reduce oncogenic signaling due to a general decrease in PI(4)P and PS levels at the plasma membrane, and in turn, KRAS localization and nanoclustering. However, the finding that EFR3A specifically binds KRAS in the active GTP-bound state and that sgEFR3A did not affect the growth of HEK-HT cells in the absence of oncogenic KRAS or in the KRAS wild-type pancreatic adenocarcinoma cell line BxPC3 supports, but admittedly does not prove, that it is the recruitment of EFR3A to KRAS that is a key step in the ability of EFR3A to promote oncogenic KRAS signaling. We also recognize the possibility that EFR3A may promote KRAS signaling through other proteins. However, the loss of EFR3A was phenocopied by the loss of PI4KA and rescued by tethering PI4KA to the plasma membrane, arguing against this possibility. Perhaps more likely, PI4KA may have effects beyond those described, especially given the role of phosphatidylinositol metabolism in membrane trafficking25, there could be other mechanism fostering KRAS nanoclustering beyond a PI(4)P/PS exchange, or the elevation in PI(4)P recruits unknown proteins regulating RAS signaling.
We suggest that this unique signaling circuit has the potential to be therapeutically targeted. First, as noted above, we show that loss of EFR3A, EFR3B, both proteins, or PI4KA reduced oncogenic KRAS signaling, transformation, and/or tumorigenesis, both at the ectopic and endogenous level in multiple cell backgrounds. Interestingly, components of EFR3A signaling are upregulated in human pancreatic adenocarcinoma, but not other tested RAS-mutant (KRAS, NRAS, or HRAS) cancers. Whether this reflects a hyper-dependency on KRAS mutations in pancreatic adenocarcinoma, or some unique feature of pancreatic tumorigenesis remains to be elucidated. While EFR3A is not easily druggable, the kinase it associates with is. Indeed, an interplay of RAS and another class of PI4 kinases (PI5P4K) was uncovered as a product of the dual-inhibitory compound a13167. However, dosing PI4KA inhibitors can be challenging, as evidenced by testing different PI4K inhibitors developed for the treatment of Hepatitis C viral infection, that have exhibited a rather variable range of toxicity63,64. Here we show that low doses of the PI4KA-specific inhibitor C7 are synergistic with the clinical KRASG12C-specific inhibitor sotorasib, resulting in near complete ablation of the transformed growth of KRASG12C-mutant human cancer cell lines. Interestingly, as noted above, while components of the EFR3A complex were upregulated in KRAS mutation-positive pancreatic tumors, we nevertheless show synergy of C7 with sotorasib in KRASG12C-mutant human lung adenocarcinoma cell lines. Such a finding bodes well for the possible deployment of a PI4KA inhibitor to either enhance the antineoplastic activity of, or prevent resistance to KRASG12C inhibitors in multiple cancer types. In conclusion, we uncover a component of the KRAS interactome that induces a unique positive-feedback circuit promoting KRAS oncogenesis that could be leveraged for the treatment of KRAS mutation-positive cancers.
Methods
Plasmids
pWZL-Blast-myc-KRASG12V was generated by PCR cloning myc epitope-tagged KRASG12V (4B splice variant) cDNA (Thermofisher Scientific) into the plasmid pWZL-Blast (a kind gift of Jay Morgenstern). pWZL-Blast-myc-KRASG12V(op) was generated in the same fashion except codon optimized. mycBioID-pBabePuro-myc-BirA-KRASG12V was previously described42,68. pcDNA3.1-FLAG-EFR3A was generated by cloning FLAG-EFR3A cDNAs (Genescript) into pcDNA3.1. pWZL-Neo-myr-myc-KRASG12V-SAAX and myr-FLAG-PI4KA were generated by introducing a myristoylation signal sequence (5′-ATGGGGTCTTCAAAATCTAAACCAAAGGACCCCAGCCAGCGCCGGCGCAGGATCCGAGGTTACCTT-3′)55 at the N-terminus and a of KRAS-SAAX (ThermoFisher Scientific) and PI4KA cDNAs (Genescript) by PCR, which was then inserted into pWZL-Neo (Cell Biolabs). pET21a-His10-KRASWT was generated by incorporating an N-terminal 10x His sequence in frame into the 5′ end of KRAS cDNA (ThermoFisher Scientific), which was then inserted into pET21a (EMD Millipore). pET21a-His10-KRASG12V was generated in the same manner based on KRASG12V cDNA (Thermofisher Scientific). pGEX-6P2-GST-EFR3A was generated by PCR amplification of EFR3A cDNA (Genescript) and subcloned into pGEX-6P2 (GE Healthcare). LentiCRISPR-v2.0-Puro-sgEFR3A-1 and -2 were generated by cloning sgEFR3A-1 (5′-TGAGAGGCTCATCCGTGACG-3′) or sgEFR3A-2 (5′-GAAGTTTGCCAACATCGAGG-3′) into LentiCRISPR v2.0-Puro (Addgene vector 52961). LentiCRISPR-v2.0-Blast-sgEFR3B and sgPI4KA were generated by cloning sgEFR3B-1 (5′-TGAGAGGCTCATCCGTGACG-3′) or sgPI4KA (5′-ATGTCTAAGAAAACCAACCG-3′) into LentiCRISPR-v2.0-Blast (a gift from Sina Ghaemmaghani, University at Rochester). pCDH-GFP-KRASG12V-IRES-mCherry-CAAX, GFP-pCDH-GFP-LactC2/mCherry-CAAX, and pEGFP-SidM/mCherry-CAAX were as previously described24. plenti6.3-EFR3A-mCherry was generated by PCR amplification of EFR3A-mCherry fragment from pcDNA3.1 EFR3A-mCherry was then subcloned into plenti6.3. EFR3A cDNA (Genescript) was cloned into the N-terminus of the mCherry fragment of pcDNA3.1-mCherry. The EFR3A-mCherry fragment was then subcloned into plenti6.3 (Invitrogen). Similarly, plenti6.3-EFR3B-mCherry and plenti6.3-FAM126A-mCherry were generated by PCR amplification of EFR3B-mCherry and FAM126A-mCherry fragments from the respective pcDNA3.1-EFR3B-mCherry and -FAM126A-mCherry plasmids. pcDNA3.1-TTC7A-mCherry was generated by PCR amplification of TTC7A cDNA (Genescript) and cloned into the N-terminus of the mCherry fragment of pcDNA3.1-mCherry. All plasmids were confirmed by sequencing.
Cell culture
HEK-HT human embryonic kidney epithelial cells stably expressing hTERT and proteins encoded by the SV40 early region46 were confirmed to be free of mycoplasma infections by the Duke Cell Culture Facility using MycoAlert PLUS test (Lonza). MDCK cells were previously described24. The human cell line 293T, the human pancreatic adenocarcinoma cell lines CFPAC-1, AsPC-1, HPAF-II, PANC-1, and BxPC3 and the human lung adenocarcinoma cell lines H358 and H2030 were purchased from Duke University Cell Culture Facility and similarly certified to be mycoplasma-free as above and also authenticated by STR analysis. All cell cultures were maintained at 5% CO2 and cultured in media supplemented with 10% FBS (fetal bovine serum) and 1% pen/strep antibiotic mixture. HEK-HT and 293T cells were cultured in Dulbecco’s Modified Eagle Medium (DMEM). MDCK cells were cultured in Minimum Essential Medium Eagle (MEM). CFPAC-1 cells were cultured in Iscove’s Modified Dulbecco’s Medium (IMDM). AsPC-1, BxPC3, H358, and H2030 cells were cultured in RPMI medium. HPAF-II cells were cultured in Minimum Eagle’s Medium (MEM) supplemented with 1% NEAA and 1% sodium pyruvate. PANC-1 cells were cultured in DMEM. MiaPaCa-2 cells were cultured in DMEM supplemented with 2.5% equine serum. For EGF stimulation, BxPC3 cells were serum starved in 0.1% FBS for 24 h and then treated with EGF (50 ng/ml) for 5, 10, and 20 min.
Cell lines
Transient transfections were performed with 6 μg DNA and 18 μl of Fugene6 reagent (Promega Corporation) incubated in serum-free medium for 25 min according to the manufacturer’s protocol. 48 h later the cells were analyzed. For lentivirus infection, 293T cells were seeded onto 10 cm plates and 24 h later transfected with a mixture containing 0.3 μg VSVg, 3 μg psPAX2, 3 μg DNA and 18 μl Fugene6 in OptiMEM. The following day, the medium was exchanged with DMEM medium containing 30% FBS. 48 h later, the virus was filtered through a 0.45 μm filter and then frozen at −80 °C until further use. 100 μl of the virus was used to transduce 20,000 cells seeded onto 6-well plates and selected on antibiotic medium for 7 days before being analyzed. Cell lines for evaluating the loss of EFR3A/B on RAS signaling were created by stably transducing KRASG12V-transformed HEK-HT, HPAF-II, PANC-1, AsPC-1, CFPAC-1, MiaPaCa-2, H358, and H2030 with lentiviruses encoding either vector (Cas9), sgEFR3A-1, sgEFR3A-2, sgEFR3B, or both sgEFR3A-1 and sgEFR3B-1. For co-immunoprecipitation experiments of biotinylated proteins, 293T cells were infected with retroviruses derived from pBabePuro-myc-BirA-KRASG12V, -KRASWT, -or -KRASS17N, after which stable cells were transiently transfected with pcDNA3.1-FLAG-EFR3A plasmid68. For visualization of KRAS, HEK-HT cells stably expressing vector (Cas9) or sgEFR3A-1 and sgEFR3B-1 were transduced with lentiviruses encoding GFP-KRASG12V-IRES-mCherry-CAAX. Cells for membrane fractionation studies were generated by stably transducing KRASG12V-transformed HEK-HT, CFPAC-1, and HPAF-II cells with lentiviruses encoding sgEFR3A-1, or both sgEFR3A-1 and sgEFR3B-1. For rescue experiments, KRASG12V-transformed HEK-HT or HPAF-II cells were infected with lentiviruses encoding sgEFR3A-1 and sgEFR3B-1, after which stably infected cells were infected with retrovirus encoding either pWZL-Neo-myr-myc-KRASG12V-SAAX or pWZL-Neo-myr-FLAG-PI4KA.
Cell proliferation
2 × 105 cells from the indicated cell lines per well were seeded in triplicates in 6-well plates. 16 to 18 h later the cells were washed twice in PBS and refed with media supplemented with 0.5% FBS. Five days later the cells were stained with crystal violet using standard procedures42.
Soft agar growth
Wells of a 6-well plate were seeded with 1.0 ×106 of HEK-HT cells stably infected with a retrovirus derived from pWZL-Blast-myc-KRASG12V(op) or 2.0 ×105 of the indicated pancreatic adenocarcinoma cells with the indicated perturbations in 1 ml of 0.3% bacto-agar (BD Bioscience) containing DMEM or RPMI solution. The bottom layer was prepared in 2 ml of 0.6% bacto-agar. Cells were fed with 500 µl of medium every three days. Colonies >20 cells in number were counted after 28 days in the case of HEK-HT cells. Colonies >50 cells in number were counted after 21 days in the case of pancreatic adenocarcinoma cells.
Xenograft tumor assay
1 × 107 of the indicated HPAF-II cells were resuspended in 100 μl of 1:1 PBS/matrigel and subcutaneously injected into flanks of six-week-old female SCID/beige mice (Charles River). The injection site was measured twice weekly until tumors were palpable (~200 mm3), after which measurements were increased to daily until reaching a maximum tumor volume (1,500 mm3). All studies with vertebrate animals were performed under a protocol approved by Duke IACUC.
Immunoblotting
Cells were lysed in the lysis buffer containing 1% NP-40, 100 mM Tris HCl pH 8.0, 50 mM NaCl, 1 mM EDTA, 1 mM PMSF, and protease inhibitors (Roche). Protein extracts were separated on 10-15% gradient SDS-PAGE gels (BioRAD) and transferred onto PVDF membranes using BioRAD Turbo blot system. Membranes were blocked in 5% dry milk or BSA (bovine serum albumin), incubated with the primary (see below) and then appropriate secondary antibodies, before being processed for signal detection using enhanced chemiluminescence reagent (ThermoFisher Scientific). Images were digitally retrieved using Chemi Doc Imager (BioRad). Primary antibodies used detected EFR3A (ThermoFisher Scientific #PA5-54694; diluted 1:100), EFR3B (ThermoFisher Scientific #PA5-107118; diluted 1:250), PI4KA (ThermoFisher Scientific #PA5-28570; diluted 1:50) myc (Cell Signaling #2276; diluted 1:1000), β-actin (Cell Signaling #3700; diluted 1:5000), FLAG (Sigma #F3165; diluted 1:1000), KRAS (Sigma #WH0003845M1; diluted 1:1000), P-ERK1/2T202,Y204 (Cell Signaling #9101; diluted 1:1000), P-AKT308 (Cell Signaling #9271; 1:500), AKT (Cell Signaling #9272; diluted 1:1000), ERK (Cell Signaling #9102; diluted 1:1000), BRAF (Cell Signaling #14814; diluted 1:5000), CRAF (Cell signaling #53745; diluted 1:500) and P-CRAF (Cell Signaling #9427; diluted1:500), GST (Santacruz #sc-138; diluted at 1:1000), Membrane fraction WB cocktail (Abcam #: diluted 1:1000) and Strep-HRP (ThermoFisher Scientific # SA10001; diluted 1:20000). Secondary antibodies used were goat anti-rabbit IgG (H + L) HRP (Thermo Fisher Scientific, #65-6120, 1:3000) or goat anti-mouse IgG (H + L) HRP (Life Technologies, #G21040, 1:5000). Different exposure times were used to optimize detection of each protein.
RBD affinity capture
Affinity capture of RAS-GTP by the RBD pull-down method was performed as per the manufacturer’s instructions (Cell Signaling #8821). Cell lysates were incubated with 10 µl of glutathione beads and 100 µg GST-RBD for 1 h at 4 °C with end-to-end rotation. The beads were washed five times with manufacturer-supplied wash buffer. Bound proteins were eluted in SDS loading buffer heated 95 °C for 5 min and then resolved by SDS-PAGE. GTP-bound KRAS was detected by immunoblot with a KRAS4B-specific antibody. The same assay was performed with recombinant proteins, except recombinant KRAS proteins were first loaded with either GTPγS or GDP. Briefly, >0.5 mg of the recombinant proteins were incubated with 20 µl of 0.5 M EDTA and 2 mM of GTPγS or GDP for 45 min at room temperature with gentle rotation. The reactions were stopped by the addition of 60 µl of 1 M MgCl2.
Protein purification and size-exclusion chromatography
BL21 Rosetta cells were transformed with expression vectors encoding full-length His10-KRASWT, full-length His10-KRASG12V, and GST-EFR3A (108-821). We note that full-length EFR3A could not be expressed in bacteria. Thus, based on previous studies demonstrating that deletion of the N-terminal membrane targeting region permitted the yeast version to be expressed, we generated a similar N-terminal deletion protein. Transformed cells were grown in LB media to a OD600 of 0.6, then induced with 0.5 mM isopropyl β-D-1-thiogalactopyranoside at 16 °C and harvested 16 h later. Cell pellets were resuspended in Buffer A (20 mM HEPES pH 7.5, 200 mM NaCl, and 2 mM β-mercaptoethanol) supplemented with 1 mM PMSF and protease inhibitor cocktail (Roche). The cells were lysed using sonication (10 seconds; 30 pulses) and clarified by centrifugation. With regards to His10-KRASWT and His10-KRASG12V proteins, the clarified lysates were passed over Ni-NTA resin that was pre-equilibrated with Buffer B (20 mM HEPES pH 7.5, 150 mM NaCl, 2 mM β-mercaptoethanol, and 1 mM PMSF) containing 5 mM imidazole. The column was washed with Buffer B with increasing concentrations of imidazole and eluted with Buffer B containing 250 mM imidazole. With regards to GST-EFR3A protein, the clarified lysate was purified using Glutathione sepharose 4B resin (GE Healthcare) pre-equilibrated with Buffer A. The column was washed with ten column volume of Buffer A and eluted with Buffer A containing 10 mM reduced glutathione. Protein concentrations were measured using NanoDrop 1000 (Thermo Fisher Scientific). The purified proteins were aliquoted, flash frozen in liquid nitrogen, and stored at −80 °C. Purified proteins were subjected to size-exclusion chromatography (SEC) using Superose® 6 Increase 10/300 GL (Cytiva) pre-equilibrated with Buffer C (20 mM HEPES pH 7.5, 150 mM NaCl, 1 mM DTT) at a flowrate of 0.2 ml per minute. To test for an EFR3A-KRAS interaction, 16 µM SEC-purified His10-KRASG12V was loaded with 2 mM GTPγS and incubated with 12.8 µM SEC-purified EFR3A for 1 h at 4 °C with end-to-end rotation. The mixture was centrifuged for 10 min at 12,000 g and subjected to SEC exactly as above. Fractions from individual GST-EFR3A, His10-KRASG12V, and GST-EFR3A + His10-KRASG12V runs were analyzed by immunoblot.
Far western analysis
Recombinant proteins were derived as above, except in one experiment (Fig. 4k) GST-EFR3A was purchased from a commercial vendor (Novus Biologicals, H00023167). 150 μg/ml of recombinant GST-EFR3A was dot blotted on a nitrocellulose membrane, which was then blocked with 5% BSA and incubated with 10 μg/ml recombinant His10-KRASWT and His10-KRASG12V in 5% BSA for 2 h at room temperature. Membranes were washed at least three times with 1X TBST containing 5% BSA at room temperature and then incubated with KRAS4B- and EFR3A-specific antibodies at 4 °C overnight. Membranes were washed at least three times with 1X TBST and incubated with secondary antibody for 1 h at room temperature followed by three additional washes with 1X TBST. Resultant blots were developed using standard chemiluminescence detection.
in vitro coimmunoprecipitation
50 μg of purified recombinant His10-KRASG12V was incubated with 150 μg of purified recombinant GST-EFR3A for 1.5 h at 4 °C in immunoprecipitation (IP) Buffer (50 mM Tris HCl, 150 mM NaCl, 1% Triton X-100 and a tablet of protease inhibitor cocktail (Roche)). 25 µl of precleared Protein G Dynabeads (ThermoFisher Scientific) bound to either a KRAS-specific antibody or IgG isotype control was added and the mixture was incubated for another 1.5 h at 4 °C, after which the beads were washed three times in the above mentioned IP Buffer at room temperature. As a negative control, IgG from the same animal species was used. Bound proteins were eluted in SDS loading buffer heated at 95 °C for 5 min.
BRAF competition assay
Recombinant BRAF protein (Abcam #ab55690) was incubated at increasing concentrations (0 µM, 0.25 µM, 0.5 µM, and 1 µM) with 150 μg purified recombinant EFR3A and 50 μg purified recombinant KRAS pre-loaded as above with GTPγS for 1.5 h at 4 °C in immunoprecipitation buffer (50 mM Tris HCl, 150 mM NaCl, 1% Triton X-100 and a tablet of protease inhibitor cocktail (Roche)). The mixture was then subjected to in vitro co-immunoprecipitation as described above.
Co-immunoprecipitation of endogenous EFR3A with ectopic KRASG12D
pcDNA3.1-FLAG-KRASG12D plasmid was transiently transfected in 1 × 107 of 293T cells as above. 48 h later, cells were washed twice with ice-cold 1X PBS and lysed in immunoprecipitation buffer (50 mM Tris HCl, 150 mM NaCl, 1% Triton X-100 and a tablet of protease inhibitor cocktail (Roche)). Cell lysates were incubated with anti-FLAG-M2 resin (Sigma #A2220) overnight at 4 °C by end-to-end rotation. The next day, the beads were washed three times in the above mentioned IP buffer at 4 °C. As a negative control, IgG from the same animal species was used. Bound proteins were eluted in SDS loading buffer by heating at 95 °C for 5 min prior to immunoblotting as described above.
Live-cell confocal microscopy
HEK-HT stably infected with lentiviruses derived from LentiCRISPR-v2.0-Puro-sgEFR3A-1 and -sgEFR3B were stably infected with lentivirus derived pCDH-GFP-KRASG12V-IRES-mCherry-CAAX. 72 h later the cells were plated on 35 mm culture plates. 24 h later cells were placed in a humidifying chamber controlled at 37 °C with 5% CO2 and live cell images collected using Andor Dragonfly spinning disk confocal microscope using 63 × 1.20 water immersion UPlan S-APO objective using an Andor iXon 897 EM-CCD camera.
Membrane fractionation
2 × 107 of the indicated derived CFPAC-1 or HEK-HT cells were grown in a 15 cm dish. Cell membrane and cytoplasmic fractions were then isolated using ProteoExtract Native Membrane Protein Extraction Kit (Millipore Sigma #444810) following the manufacturer’s protocol. Briefly, the cell monolayer was washed twice with prechilled wash buffer at room temperature. 1 ml Extraction Buffer I containing protease inhibitor cocktail was then added directly onto the cell culture. The cell culture plates were rocked gently at 4 °C for 10 min. Lysate were collected and centrifuged at 16,000 g for 15 min at 4 °C. The resulting supernatant was aliquoted as cytoplasmic/soluble fraction while the pellet was resuspended in 500 μl prechilled Extraction Buffer II containing protease inhibitor cocktail and incubated for 30 min at 4 °C with end-to-end rotation. The mixture was then centrifuged at 16,000 g for 15 min at 4 °C. This resulting supernatant fraction was aliquoted as the membrane-enriched fraction. The respective fractions were then immediately processed for downstream immunoblot analysis.
Spatial distribution and clustering of KRAS by transmission electron microscopy
Univariate K-Function Analysis for analyzing the distribution of one species on the plasma membrane. Basal plasma membrane sheets of parental and EFR3A/Bknockout HEK-HT and MDCK cells were prepared, fixed with 4% paraformaldehyde, 0.1% glutaraldehyde, and labeled with 4.5 nm gold directly conjugated to anti-GFP antibody11,23,69. Images were obtained with a transmission electron microscope at 100,000x magnification and intact 1μm2 areas were identified with ImageJ. Univariate K function analysis was performed and bootstrap tests were used to examine for statistical differences between replicated point patterns. Bootstrap tests were used to examine for statistical differences between replicated point patterns70,71 and statistical significance was evaluated against 1000 bootstrap samples. Lmax: Each L(r)-r curve is a mean univariate K-function of 30 replicates of each treatment group. Curves above the 99% confidence interval (=1) indicate significant clustering. Lmax is used as a summary statistic to visualize the extent of clustering and is equal to the maximum observed value of the mean L(r)-r function of each group. Bivariate K-Function Analysis to quantify the extent of co-clustering between two different species on the plasma membrane. 293T cells were seeded onto fibronectin-coated cover slips. 24 h later the cells were transiently co-transfected with mGFP- and mCherry- or mRFP-tagged proteins of interest. After 48 h, plasma membrane sheets from these cells were attached to EM grids, fixed with 4% paraformaldehyde, 0.1% glutaraldehyde, and labeled with 6 nm gold directly conjugated to anti-GFP antibody and 2 nm gold conjugated to anti-RFP antibody11,23. Images were obtained with a transmission electron microscope at 100,000x magnification and intact 1 μm2 areas were identified with ImageJ. Bivariate K function analysis was performed and bootstrap tests were used to examine for statistical differences between replicated point patterns70,71 and statistical significance was evaluated against 1000 bootstrap samples. LBI: Each Lbiv(r)-r curve is a mean bivariate K-function of 30 replicates of each treatment group. Curves above the 95% confidence interval (=1) indicate significant co-clustering. LBI is used as a summary statistic to visualize the extent of co-clustering and is equal to the integral of the mean Lbiv(r)-r function of each group. LBI values above the 95% confidence interval (=100) indicate significant co-clustering.
Drug treatment and cell viability assay
The RASG12C inhibitor sotorasib (AMG510) was purchased from Medchemexpress. The PI4KA inhibitor C7 was purchased from Ximbio. Both drugs were dissolved in DMSO. 20,000 cells were seeded in triplicate in 96 well plates. After 24 h, media was exchanged with fresh media containing increasing concentration (0, 5, 10, and 20 nM) of the indicated drugs. DMSO was used for vehicle-treated samples. After 72 h of incubation, viability was measured using Cell Titer Glo assay (Promega Corporation) following manufacturer’s protocol.
Bioinformatic and statistical analysis
The 3-dimensional RAS CRISPR enrichment scatter plot was generated using Graphing Calculator 3D. cBioportal PanCan TCGA dataset was used to identify genomic alterations in RAS-mutant cancers. cBioportal (cbioportal.org) pancreatic adenocarcinoma dataset was used to generate survival plots72,73. EFR3A gene expression profiles in normal and tumor samples were analyzed in GEPIA portal (gepia.cancer-pku.cn) using the PAAD (Pancreatic Adenocarcinoma), LUAD (Lung Adenocarcinoma), COAD (Colorectal Adenocarcinoma), BLCA (Bladder Cancer) and HNSC (Head and Neck Squamous Cell Carcinoma) dataset. The same dataset was analyzed for KRAS mutations status and compared to EFR3A expression levels. Survival plots depicting correlation between EFR3A, EFR3B, FAM126A, PI4KA, and TTC7A expression and pancreatic cancer patient samples survival outcome was determined using XENA TCGA pancreatic adenocarcinoma dataset (xenabrowser.net). Two-way t-tests were performed using GraphPad Prism (GraphPad Software), and P values are indicated in the respective graphs. Asterisks are also shown in the respective graphs, *p < 0.05, **p < 0.01, ***p < 0.001 and ****p < 0.0001. Bioinformatic statistics are indicated in the respective figure legend descriptions. All cellular experiments were repeated at least three times. The in vivo experiments contained 5 mice for each treatment group and the sample size was chosen to reflect a difference in means of 20% with a power of 90%. Synergy plots and scores were calculated using Synergy Finder (synergyfinder.fimm.fi).
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Supplementary information
Acknowledgements
This work was supported by the National Cancer Institute (K99CA248495 to H.A., R01CA123031 and P01CA203657 to C.M.C.), the Cancer Prevention and Research Institute of Texas (RP200047 to J.F.H.), the Andrew Sowell-Wade Huggins Fellowship/Professorship in Cancer Research (W.E.K. and J.F.H.), and the NIH Duke Clinical and Translational Science Award (UL1TR002553 through a subaward to P.Z.). We thank members of the Counter laboratory and Dr. Aaron Hobbs for helpful discussions and the Shared Resources of the Duke Cancer Institute for technical support (supported by P30CA014236 from the National Cancer Institute).
Source data
Author contributions
H.A., W.E.K., and S.K. performed experiments. H.A., W.E.K., S.K., P.Z., J.F.H., and C.M.C. contributed to the conception of the study, experimental design, data interpretation, and writing the manuscript.
Data availability
All data have been analyzed using publicly available datasets that can be downloaded at cbioportal database cbioportal.org/datasets as Pancreatic adenocarcinoma [ICGC, Nature 2012; QCMG, Nature 2016; TCGA, Firehose Legacy; TCGA, PanCancer Atlas and for all other cancers, TCGA PanCancer Atlas, datasets for XENA are available at XENA database xenabrowser.net/datapages/ as TCGA Lung Adenocarcinoma, TCGA Bladder Cancer, TCGA Pancreatic Cancer, TCGA Colon Cancer, TCGA Head and Neck Cancer, TCGA Target GTEx) and datasets utilized in GEPIA analysis at gepia.cancer-pku.cn are available as TCGA Pancreatic Cancer dataset at the XENA database XENAbrowser.net/datapages). Source data are provided with this paper. All the other data are available within the article and its supplementary information. Source data are provided with this paper.
Competing interests
The authors declare no competing interests.
Footnotes
Peer review informationNature Communications thanks John Burke, Shiva Malek and Man-Tzu Wang for their contribution to the peer review of this work. Peer reviewer reports are available.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
John F. Hancock, Email: john.f.hancock@uth.tmc.edu
Christopher M. Counter, Email: count004@mc.duke.edu
Supplementary information
The online version contains supplementary material available at 10.1038/s41467-021-25523-5.
References
- 1.Prior IA, Hood FE, Hartley JL. The frequency of Ras mutations in cancer. Cancer Res. 2020;80:2969–2974. doi: 10.1158/0008-5472.CAN-19-3682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li S, Balmain A, Counter CM. A model for RAS mutation patterns in cancers: finding the sweet spot. Nat. Rev. Cancer. 2018;18:767–777. doi: 10.1038/s41568-018-0076-6. [DOI] [PubMed] [Google Scholar]
- 3.Pylayeva-Gupta Y, Grabocka E, Bar-Sagi D. RAS oncogenes: weaving a tumorigenic web. Nat. Rev. Cancer. 2011;11:761–774. doi: 10.1038/nrc3106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Canon J, et al. The clinical KRAS(G12C) inhibitor AMG 510 drives anti-tumour immunity. Nature. 2019;575:217–223. doi: 10.1038/s41586-019-1694-1. [DOI] [PubMed] [Google Scholar]
- 5.Hallin J, et al. The KRASG12C inhibitor MRTX849 provides insight toward therapeutic susceptibility of KRAS-mutant cancers in mouse models and patients. Cancer Discov. 2020;10:54–71. doi: 10.1158/2159-8290.CD-19-1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dueling KRASG12C inhibitors achieve responses. Cancer Discov. 10, 10 (2020). [DOI] [PubMed]
- 7.McCormick F. Sticking it to KRAS: covalent inhibitors enter the clinic. Cancer Cell. 2020;37:3–4. doi: 10.1016/j.ccell.2019.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Amodio V, et al. EGFR blockade reverts resistance to KRASG12C inhibition in colorectal cancer. Cancer Discov. 2020;10:1129–1139. doi: 10.1158/2159-8290.CD-20-0187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xue JY, et al. Rapid non-uniform adaptation to conformation-specific KRAS(G12C) inhibition. Nature. 2020;577:421–425. doi: 10.1038/s41586-019-1884-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ahearn IM, Haigis K, Bar-Sagi D, Philips MR. Regulating the regulator: post-translational modification of RAS. Nat. Rev. Mol. Cell Biol. 2011;13:39–51. doi: 10.1038/nrm3255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhou Y, Hancock JF. Ras nanoclusters: Versatile lipid-based signaling platforms. Biochim. Biophys. Acta. 2015;1853:841–849. doi: 10.1016/j.bbamcr.2014.09.008. [DOI] [PubMed] [Google Scholar]
- 12.Herrero A, Matallanas D, Kolch W. The spatiotemporal regulation of RAS signalling. Biochem. Soc. Trans. 2016;44:1517–1522. doi: 10.1042/BST20160127. [DOI] [PubMed] [Google Scholar]
- 13.Hancock JF, Magee AI, Childs JE, Marshall CJ. All ras proteins are polyisoprenylated but only some are palmitoylated. Cell. 1989;57:1167–1177. doi: 10.1016/0092-8674(89)90054-8. [DOI] [PubMed] [Google Scholar]
- 14.Laude AJ, Prior IA. Palmitoylation and localisation of RAS isoforms are modulated by the hypervariable linker domain. J. Cell Sci. 2008;121:421–427. doi: 10.1242/jcs.020107. [DOI] [PubMed] [Google Scholar]
- 15.Plowman SJ, Hancock JF. Ras signaling from plasma membrane and endomembrane microdomains. Biochim. Biophys. Acta. 2005;1746:274–283. doi: 10.1016/j.bbamcr.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 16.Apolloni A, Prior IA, Lindsay M, Parton RG, Hancock JF. H-ras but not K-ras traffics to the plasma membrane through the exocytic pathway. Mol. Cell. Biol. 2000;20:2475–2487. doi: 10.1128/MCB.20.7.2475-2487.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rocks O, et al. An acylation cycle regulates localization and activity of palmitoylated Ras isoforms. Science. 2005;307:1746–1752. doi: 10.1126/science.1105654. [DOI] [PubMed] [Google Scholar]
- 18.Hancock JF, Parton RG. Ras plasma membrane signalling platforms. Biochem. J. 2005;389:1–11. doi: 10.1042/BJ20050231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tian T, et al. Plasma membrane nanoswitches generate high-fidelity Ras signal transduction. Nat. Cell Biol. 2007;9:905–914. doi: 10.1038/ncb1615. [DOI] [PubMed] [Google Scholar]
- 20.Kholodenko BN, Hancock JF, Kolch W. Signalling ballet in space and time. Nat. Rev. Mol. Cell Biol. 2010;11:414–426. doi: 10.1038/nrm2901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harding AS, Hancock JF. Using plasma membrane nanoclusters to build better signaling circuits. Trends Cell Biol. 2008;18:364–371. doi: 10.1016/j.tcb.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nan X, et al. Single-molecule superresolution imaging allows quantitative analysis of RAF multimer formation and signaling. Proc. Natl Acad. Sci. USA. 2013;110:18519–18524. doi: 10.1073/pnas.1318188110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhou Y, et al. Lipid-sorting specificity encoded in K-Ras membrane anchor regulates signal output. Cell. 2017;168:239–251. doi: 10.1016/j.cell.2016.11.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kattan WE, et al. Targeting plasma membrane phosphatidylserine content to inhibit oncogenic KRAS function. Life Sci. Alliance. 2019;2:e201900431. doi: 10.26508/lsa.201900431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Balla T. Phosphoinositides: tiny lipids with giant impact on cell regulation. Physiol. Rev. 2013;93:1019–1137. doi: 10.1152/physrev.00028.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Balla A, Balla T. Phosphatidylinositol 4-kinases: old enzymes with emerging functions. Trends Cell Biol. 2006;16:351–361. doi: 10.1016/j.tcb.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 27.Baird D, Stefan C, Audhya A, Weys S, Emr SD. Assembly of the PtdIns 4-kinase Stt4 complex at the plasma membrane requires Ypp1 and Efr3. J. Cell Biol. 2008;183:1061–1074. doi: 10.1083/jcb.200804003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nakatsu F, et al. PtdIns4P synthesis by PI4KIIIα at the plasma membrane and its impact on plasma membrane identity. J. Cell Biol. 2012;199:1003–1016. doi: 10.1083/jcb.201206095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bojjireddy N, Guzman-Hernandez ML, Reinhard NR, Jovic M, Balla T. EFR3s are palmitoylated plasma membrane proteins that control responsiveness to G-protein-coupled receptors. J. Cell Sci. 2015;128:118–128. doi: 10.1242/jcs.157495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Baskin JM, et al. The leukodystrophy protein FAM126A (hyccin) regulates PtdIns(4)P synthesis at the plasma membrane. Nat. Cell Biol. 2016;18:132–138. doi: 10.1038/ncb3271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wu X, et al. Structural insights into assembly and regulation of the plasma membrane phosphatidylinositol 4-kinase complex. Dev. Cell. 2014;28:19–29. doi: 10.1016/j.devcel.2013.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lees JA, et al. Architecture of the human PI4KIIIα lipid kinase complex. Proc. Natl Acad. Sci. USA. 2017;114:13720–13725. doi: 10.1073/pnas.1718471115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gupta AR, et al. Rare deleterious mutations of the gene EFR3A in autism spectrum disorders. Mol. Autism. 2014;5:31. doi: 10.1186/2040-2392-5-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Qian Q, et al. Brain-specific ablation of Efr3a promotes adult hippocampal neurogenesis via the brain-derived neurotrophic factor pathway. FASEB J. 2017;31:2104–2113. doi: 10.1096/fj.201601207R. [DOI] [PubMed] [Google Scholar]
- 35.García-Echeverría C. Protein and lipid kinase inhibitors as targeted anticancer agents of the Ras/Raf/MEK and PI3K/PKB pathways. Purinergic Signal. 2009;5:117–125. doi: 10.1007/s11302-008-9111-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Burke JE. Structural basis for regulation of phosphoinositide kinases and their involvement in human disease. Mol. Cell. 2018;71:653–673. doi: 10.1016/j.molcel.2018.08.005. [DOI] [PubMed] [Google Scholar]
- 37.Sapon-Cousineau V, Sapon-Cousineau S, Assouline S. PI3K inhibitors and their role as novel agents for targeted therapy in lymphoma. Curr. Treat. Options Oncol. 2020;21:51. doi: 10.1007/s11864-020-00746-8. [DOI] [PubMed] [Google Scholar]
- 38.Goldfinger LE, et al. An experimentally derived database of candidate Ras-interacting proteins. J. Proteome Res. 2007;6:1806–1811. doi: 10.1021/pr060630l. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Roux KJ, Kim DI, Raida M, Burke B. A promiscuous biotin ligase fusion protein identifies proximal and interacting proteins in mammalian cells. J. Cell Biol. 2012;196:801–810. doi: 10.1083/jcb.201112098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Trinkle-Mulcahy L. Recent advances in proximity-based labeling methods for interactome mapping. F1000Res. 2019;8:F1000 Faculty Rev-135. doi: 10.12688/f1000research.16903.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ritchie C, et al. Analysis of K-Ras interactions by biotin ligase tagging. Cancer Genomics Proteom. 2017;14:225–239. doi: 10.21873/cgp.20034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Adhikari H, Counter CM. Interrogating the protein interactomes of RAS isoforms identifies PIP5K1A as a KRAS-specific vulnerability. Nat. Commun. 2018;9:3646. doi: 10.1038/s41467-018-05692-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bigenzahn JW, et al. LZTR1 is a regulator of RAS ubiquitination and signaling. Science. 2018;362:1171–1177. doi: 10.1126/science.aap8210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kovalski JR, et al. The functional proximal proteome of oncogenic Ras includes mTORC2. Mol. Cell. 2019;73:830–844. doi: 10.1016/j.molcel.2018.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Terrell EM, et al. Distinct binding preferences between Ras and Raf family members and the impact on oncogenic Ras signaling. Mol. Cell. 2019;76:872–884. doi: 10.1016/j.molcel.2019.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hahn WC, et al. Creation of human tumour cells with defined genetic elements. Nature. 1999;400:464–468. doi: 10.1038/22780. [DOI] [PubMed] [Google Scholar]
- 47.Deer EL, et al. Phenotype and genotype of pancreatic cancer cell lines. Pancreas. 2010;39:425–435. doi: 10.1097/MPA.0b013e3181c15963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Farnsworth CL, Feig LA. Dominant inhibitory mutations in the Mg(2+)-binding site of RasH prevent its activation by GTP. Mol. Cell. Biol. 1991;11:4822–4829. doi: 10.1128/mcb.11.10.4822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nassar N, et al. The 2.2 A crystal structure of the Ras-binding domain of the serine/threonine kinase c-Raf1 in complex with Rap1A and a GTP analogue. Nature. 1995;375:554–560. doi: 10.1038/375554a0. [DOI] [PubMed] [Google Scholar]
- 50.Chavan TS, et al. Plasma membrane regulates Ras signaling networks. Cell. Logist. 2015;5:e1136374. doi: 10.1080/21592799.2015.1136374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pivovarov AS, Calahorro F, Walker RJ. Na+/K+-pump and neurotransmitter membrane receptors. Invertebr. Neurosci. 2018;19:1. doi: 10.1007/s10158-018-0221-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Butera G, et al. Regulation of autophagy by nuclear GAPDH and its aggregates in cancer and neurodegenerative disorders. Int. J. Mol. Sci. 2019;20:2062. doi: 10.3390/ijms20092062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ahearn I, Zhou M, Philips MR. Posttranslational modifications of RAS proteins. Cold Spring Harb. Perspect. Med. 2018;8:a031484. doi: 10.1101/cshperspect.a031484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cadwallader KA, Paterson H, Macdonald SG, Hancock JF. N-terminally myristoylated Ras proteins require palmitoylation or a polybasic domain for plasma membrane localization. Mol. Cell. Biol. 1994;14:4722–4730. doi: 10.1128/mcb.14.7.4722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hayashi N, Titani K. N-myristoylated proteins, key components in intracellular signal transduction systems enabling rapid and flexible cell responses. Proc. Jpn. Acad. Ser. B: Phys. Biol. Sci. 2010;86:494–508. doi: 10.2183/pjab.86.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yeung T, et al. Membrane phosphatidylserine regulates surface charge and protein localization. Science. 2008;319:210–213. doi: 10.1126/science.1152066. [DOI] [PubMed] [Google Scholar]
- 57.Chung J, et al. PI4P/phosphatidylserine countertransport at ORP5- and ORP8-mediated ER-plasma membrane contacts. Science. 2015;349:428–432. doi: 10.1126/science.aab1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hammond GRV, Machner MP, Balla T. A novel probe for phosphatidylinositol 4-phosphate reveals multiple pools beyond the Golgi. J. Cell Biol. 2014;205:113–126. doi: 10.1083/jcb.201312072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Moser von Filseck J, et al. Phosphatidylserine transport by ORP/Osh proteins is driven by phosphatidylinositol 4-phosphate. Science. 2015;349:432–436. doi: 10.1126/science.aab1346. [DOI] [PubMed] [Google Scholar]
- 60.Sohn M, et al. Lenz-Majewski mutations in PTDSS1 affect phosphatidylinositol 4-phosphate metabolism at ER-PM and ER-Golgi junctions. Proc. Natl Acad. Sci. USA. 2016;113:4314–4319. doi: 10.1073/pnas.1525719113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhang H, et al. PDGFRs are critical for PI3K/Akt activation and negatively regulated by mTOR. J. Clin. Invest. 2007;117:730–738. doi: 10.1172/JCI28984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Waring MJ, et al. Potent, selective small molecule inhibitors of type III phosphatidylinositol-4-kinase α- but not β-inhibit the phosphatidylinositol signaling cascade and cancer cell proliferation. Chem. Commun. Camb. Engl. 2014;50:5388–5390. doi: 10.1039/C3CC48391F. [DOI] [PubMed] [Google Scholar]
- 63.Bojjireddy N, et al. Pharmacological and genetic targeting of the PI4KA enzyme reveals its important role in maintaining plasma membrane phosphatidylinositol 4-phosphate and phosphatidylinositol 4,5-bisphosphate levels. J. Biol. Chem. 2014;289:6120–6132. doi: 10.1074/jbc.M113.531426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Park Y, Park JM, Kim DH, Kwon J, Kim IA. Inhibition of PI4K IIIα radiosensitizes in human tumor xenograft and immune-competent syngeneic murine tumor model. Oncotarget. 2017;8:110392–110405. doi: 10.18632/oncotarget.22778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kattan WE, Hancock JF. RAS Function in cancer cells: translating membrane biology and biochemistry into new therapeutics. Biochem. J. 2020;477:2893–2919. doi: 10.1042/BCJ20190839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ianevski A, He L, Aittokallio T, Tang J. SynergyFinder: a web application for analyzing drug combination dose-response matrix data. Bioinform. Oxf. Engl. 2017;33:2413–2415. doi: 10.1093/bioinformatics/btx162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kitagawa M, et al. Dual blockade of the lipid kinase PIP4Ks and mitotic pathways leads to cancer-selective lethality. Nat. Commun. 2017;8:2200. doi: 10.1038/s41467-017-02287-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Adhikari H, Counter CM. Using BioID to characterize the RAS interactome. Methods Mol. Biol. 2021;2262:271–280. doi: 10.1007/978-1-0716-1190-6_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Prior IA, Muncke C, Parton RG, Hancock JF. Direct visualization of Ras proteins in spatially distinct cell surface microdomains. J. Cell Biol. 2003;160:165–170. doi: 10.1083/jcb.200209091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Diggle PJ, Mateu J, Clough HE. A comparison between parametric and non-parametric approaches to the analysis of replicated spatial point patterns. Adv. Appl. Probab. 2000;32:331–343. doi: 10.1239/aap/1013540166. [DOI] [Google Scholar]
- 71.Plowman SJ, Ariotti N, Goodall A, Parton RG, Hancock JF. Electrostatic interactions positively regulate K-Ras nanocluster formation and function. Mol. Cell. Biol. 2008;28:4377–4385. doi: 10.1128/MCB.00050-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cerami E, et al. The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2012;2:401–404. doi: 10.1158/2159-8290.CD-12-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gao J, et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci. Signal. 2013;6:l1. doi: 10.1126/scisignal.2004088. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data have been analyzed using publicly available datasets that can be downloaded at cbioportal database cbioportal.org/datasets as Pancreatic adenocarcinoma [ICGC, Nature 2012; QCMG, Nature 2016; TCGA, Firehose Legacy; TCGA, PanCancer Atlas and for all other cancers, TCGA PanCancer Atlas, datasets for XENA are available at XENA database xenabrowser.net/datapages/ as TCGA Lung Adenocarcinoma, TCGA Bladder Cancer, TCGA Pancreatic Cancer, TCGA Colon Cancer, TCGA Head and Neck Cancer, TCGA Target GTEx) and datasets utilized in GEPIA analysis at gepia.cancer-pku.cn are available as TCGA Pancreatic Cancer dataset at the XENA database XENAbrowser.net/datapages). Source data are provided with this paper. All the other data are available within the article and its supplementary information. Source data are provided with this paper.