Abstract
Sample particle size is an important parameter in the solid–liquid extraction system of natural products for obtaining their bioactive compounds. This study evaluates the effect of sample particle size on the phytochemical composition and antioxidant activity of brown macroalgae Sargassum cristaefolium. The crude ethanol extract was extracted from dried powders of S.cristeafolium with various particle sizes (> 4000 µm, > 250 µm, > 125 µm, > 45 µm, and < 45 µm). The ethanolic extracts of S.cristaefolium were analysed for Total Phenolic Content (TPC), Total Flavonoid Content (TFC), phenolic compound concentration and antioxidant activities. The extract yield and phytochemical composition were more abundant in smaller particle sizes. Furthermore, the TPC (14.19 ± 2.08 mg GAE/g extract to 43.27 ± 2.56 mg GAE/g extract) and TFC (9.6 ± 1.8 mg QE/g extract to 70.27 ± 3.59 mg QE/g extract) values also significantly increased as particle sizes decreased. In addition, phenolic compounds epicatechin (EC), epicatechin gallate (ECG), epigallocatechin (EGC), and Epigallocatechin gallate (EGCG) concentration were frequently increased in samples of smaller particle sizes based on two-way ANOVA and Tukey’s multiple comparison analysis. These results correlate with the significantly stronger antioxidant activity in samples with smaller particle sizes. The smallest particle size (< 45 µm) demonstrated the strongest antioxidant activity based on DPPH, ABTS, hydroxyl assay and FRAP. In addition, ramp function graph evaluates the desired particle size for maximum phytochemical composition and antioxidant activity is 44 µm. In conclusion, current results show the importance of particle size reduction of macroalgae samples to increase the effectivity of its biological activity.
Subject terms: Biochemistry, Biological techniques, Chemical biology, Drug discovery, Medical research
Introduction
Seaweeds or also referred to as macroalgae are currently being explored for sources of novel and sustainable compounds for both pharmaceutical and nutraceutical purposes1. Biologically active substances known as “bioactive compounds” have been well reported in various natural resources, including seaweeds2–4. Bioactive compounds such as carotenoids, polyphenols, tocotrienols, sulfated polysaccharides are of high interest5. Due to this vast variety of bioactive constituents, seaweeds have been proven to demonstrate various biological activities, especially antioxidant activity by inhibiting reactive oxygen radical-mediated oxidative stress.
Marine macroalgae belong to three major classes or phyla: Chlorophyceae (green algae), Rhodophyceae (red algae), and Phaeophyceae (brown algae). The most extensively studied macroalgae are those of the Phaeophyceae family6. Brown macroalgae are well known to be rich in polyphenol compounds which potentially contribute to its antioxidant capacity7. The brown seaweed Sargassum species have shown strong antioxidant activity8. The brown macroalgae Sargassum cristaefolium is one of the most abundant Sargassum species in the coastal area of Lombok, Indonesia.
Several parameters have been reported to influence the amount and composition of the potential bioactive compounds in extracts9. Furthermore, these parameters also affect the biological activity of the extract. Some of them include the extraction solvent, temperature, extraction time, storage conditions, and also sample particle size10. The phytochemical compositions of extracts have been reported to be influenced by the particle size of the extracted sample. Previous reports have demonstrated that reduction of sample particle size has a significant effect on the amount of bioactive constituents obtained11. However, this is the first report to evaluate the effect of particle size on the phytochemical composition and antioxidant activity of macroalgae. Due to the increasing demand to obtain bioactive compounds from macroalgae, there is a need to understand every aspect during the extraction process of the macroalgae biomass especially the particle size as it is the very initial step. Thus, this paper aims to evaluate the impact of particle size on the extraction yield, phenol content, and antioxidant activity of brown macroalgae S.cristaefolium.
Materials and methods
Chemicals and standard solutions
All chemicals were of analytical grade. Ethanol were from Merck (Darmstadt, Germany) and l-ascorbic acid by Sigma-Aldrich (Steinhein, Germany). Water used for all experiments and solutions was obtained from Milli-Q water purification system (Millipore) (Bedford, MA, USA). Folin-Ciocalteu’s phenol reagent was purchased from Merck (Darmstadt, Germany). DPPH and ABTS reagents were purchased from Tokyo Chemical Industry Co. Ltd (TCI). Standards of catechin (C), epicatechin (EC), epicatechin gallate (ECG), epigallocatechin (EGC), Quercetin (QUE), Procatechuic acid (PCA), and Epigallocatechin gallate (EGCG) were from Sigma-Aldrich (St. Louis, MO, USA).
Macroalgae sample collection and preparation
Samples of Sargassum cristaefolium were harvested offshore from Lendang luar beach, West Lombok, Indonesia (8.459692, 116.029639) in February 2021. The macroalgae samples were identified based on the algae online database12. The samples were washed with seawater and cleaned from sand debris before transported to the Laboratorium. Approximately 200 g of fresh S.cristaefolium samples were left to dry at room temperature (24 °C) regulated with an air conditioner for 4 days until the dry biomass reaches constant weight (± 50 g dry weight). The Fungicide tablets 1% concentration (Rely + On, Virkom) were also applied to avoid contamination from fungal growth. Dried samples were then stored in ziplock bags with silica gels until further use.
Macroalgae sample partition and extraction
Dried macroalgae samples were grounded with a food-grade grain miller to decrease the particle size. The ground samples were sieved on a Sieve shaker (Retsch As 200, USA) to separate the samples into various sizes; < 45 µm, > 45 µm, > 125 µm, > 250 µm, and > 4000 µm. All the dried samples with different particle sizes were prepared before subjected to ethanol extraction. The extraction process was conducted via cold maceration with the sample to solvent ratio of 1:10. The samples were stirred vigorously in ethanol solvent for 30 min and then subjected to centrifugation at 8000 rpm for 10 min (Tomy MX-307, Tomy Seiko Co., Ltd, Japan). The supernatant was collected and filtered with a filter cloth. This step was repeated 3 times, the final filtrate was then evaporated with a rotary evaporator at 45 °C and 50 rpm (Rotavapor R-215, Switzerland). Obtained extract pastes were then stored at 4 °C until further use.
Determination of total phenolic content
The Total Phenolic Contents (TPC) of the S.cristaefolium different particle sizes were determined by Folin-Ciocalteu colorimetric method as described by Ainsworth and Gillespie with some minor modifications13. Gallic acid (GAE) solution was used as standard and prepared by dissolving 10 mg in 10 mL of ethanol (1 mg/mL). Various concentrations of GAE (10–500 µg/mL) were prepared from the stock solution. Approximately 100 µL of the sample (1 mg/mL) was combined and mixed with 0.75 mL of the Folin-Ciocalteu reagent (diluted tenfold in dH2O before use). The liquid mixture was incubated at room temperature for 5 min. The mixture was then added about 750 µL sodium carbonate (Na2CO3), the mixture was mixed gently with pipetting. After 90 min, the absorbance of the mixture was measured at 725 nm with a UV–Vis spectrophotometer (Multiskan-Go, Thermo Scientific). The TPC value of samples was revealed as Gallic acid equivalents in milligrams per 100 g of the extract.
Determination of total flavonoid content
The total flavonoid content was measured by a colorimetric assay. About 100 µL of the sample was added to 4 mL of dH2O. Then followed by the addition of 300 µL of 5% sodium nitrite. After 5 min, 300 µL of 10% aluminium chloride was added. The mixture was incubated for an additional 6 min before the addition of 2 mL 1 M sodium hydroxide. Immediately, the mixture was diluted by the addition of 3.3 mL dH2O and vortexed. The absorbance was determined at 510 nm versus a blank. Quercetin was used as the standard for the calibration curve. The total flavonoid content of the sample was expressed as mg quercetin equivalents per gram of sample (mg/g).
High resolution mass spectromety analysis
For spectrometry analysis, a Q Exactive™ High-Resolution Accurate Mass LC–MS/MS (Thermo Scientific™) attached to a Thermo Scientific™ VanquishTM Flex UHPLC system was used. Using a flow rate of 0.3 mL/min and a 5 μL injection volume, the HPLC method was (0.1 percent formic acid in H2O MS grade as solvent A and 0.1 percent formic acid in Acetonitrile MS grade as solvent B), a gradient of 5 percent to 90 percent B in 16 min, an isocratic of 90 percent B for 4 min, and an additional 5 min 90 percent to 5 percent B. The separation was carried out on a 2.6 m Accucore™ Phenyl Hexyl 100 × 2 mm column, with an MS acquisition range of 150 to 1800 m/z. We used a sheath gas flow rate of 15, an auxiliary gas flow rate of 5, a spray voltage of 3.6 kV, a capillary temperature of 320 °C, an auxiliary gas heater temperature of 30 °C, and an S-lens RF level of 50. The resolution was set to 70,000 for the entire MS, with an AGC target of 3e6 and a maximum IT of 250 ms. Additionally, the resolution for dd-MS2 was set to 17,500, with an AGC target of 1e5 and a maximum IT of 60 ms. Furthermore, the loop count was set to 5, and the (N) CE/steeped nce was 18, 35, 53, with TopN and isolation window set to 5 and 1.0 mz, respectively. For dd setting, the minimum AGC target was 9e3, with an intensity of 1.3e5 and a charge exclusion of 4–8, > 8. The exclude isotope must be enabled, and the dynamic exclusion time must be set to 10 s. Caffeine was used as a calibrant in the study.
High performance liquid chromatography (HPLC) analysis
The concentrations of phenolic compounds were analyzed using high-performance chromatography (HPLC) (LC-20AB, SPD- M20A photodiode array detector (PDA), Shimazu, Kyoto Japan) equipped with an InfinityLab Poroshell 120 EC-C18 chromatography column, 150 mm length, 4.6 mm width, and particle size 2.7 μm at column oven temperature 26 °C. The binary gradient method was used in HPLC analysis incorporated 2% acetic acid dissolved in water (A) and a mixture of concentrated acetic acid, water, and acetonitrile (1:9:40 v/v/v) (B). The total runtime of the analysis was 93 min referring to the method described elsewhere14 as follows: (a) initially 0–25 min, 10–30% B; (b) 25–50 min, 30–40% B; (c) 50–75 min, 40–90% B; (d) 75–93 min, 10% B. An amount of 20 μL of the samples was injected onto the column, and three wavelengths 280, 360 and 520 nm were chosen for analysis in this investigation using HPLC–DAD. For quantitative purposes, a calibration curve was constructed by analysis of known concentrations of different standard compounds.
DPPH radical scavenging assay
Radical scavenging activity against DPPH radical was measured as described by Mansour et al.15, with minor modifications. A sample of 100 µL with various concentrations (10–4000 µg/mL) was mixed with 100 µL solution of DPPH in a 96-well plate. A mixture of the sample with solvent without DPPH radical was added for blank. The plate was allowed to stand in the dark (RT) for 20 min. The absorbance was measured at 520 nm. The sample radical scavenging activity was measured with the equation below:
| 1 |
ABTS radical scavenging assay
The scavenging activity of extracts was measured against ABTS radical cation according to the method of Nenandis et al.16, with minor modifications. The stock solutions prepared included 7 mM ABTS aqueous solution and a 2.4 mM potassium persulfate solution. The working solution was prepared by mixing the two stock solutions in equal quantities and allowing them to react for 16 h at room temperature in the dark. The solution was then diluted by mixing 250 µL ABTS with 12 mL ethanol to obtain an absorbance of around 0.700 units at 734 nm using a spectrophotometer. Fresh ABTS solution was prepared for each assay. A volume of 1 mL sample extracts of various concentrations (10–4000 µg/mL) was mixed with 1 mL ABTS solution and the absorbance was measured at 734 nm after 7 min of incubation at room temperature. The ABTS scavenging activity of the extracts was calculated with the same equation above (1).
Hydroxial anti radical scavenging assay
The hydroxyl radical scavenging ability of the extracts was evaluated based on a Fenton-type reaction17. A volume of 1 mL sample in various concentrations (10–4000 µg/mL) was mixed with 1 mL of 9 mM ferric sulfate, 1 mL of 9 mM ethanolic salicylic acid, and 1 mL of 9 mM hydrogen peroxide. The mixture was incubated for 30 min at 37 °C. The absorbance was measured at 510 nm. Ascorbic acid was used as the positive control. The activity was calculated as Eq. (1).
Ferric reducing antioxidant power assay (FRAPS)
The Ferrous Reducing Antioxidant Power Assay (FRAPS) of the extracts were evaluated by the method described by Mansoori et al.18 with minor modifications. The Fe2+ can be monitored by measuring the formation of Perl’s Prussian blue at 700 nm. A volume of 0.25 mL samples/standard at different concentrations (10–4000 µg/mL) were mixed with 0.625 mL potassium buffer (0.2 M) and 0.625 mL of 1% potassium ferricyanide [K3Fe(CN)6] solution were added into the test tubes. The reaction mixtures were incubated for 20 min at 50 °C to complete the reaction. To this mixture, a volume of 0.625 mL 10% trichloroacetic acid (TCA) solution was added. The total mixture was centrifuged at 3000 rpm for 10 min. The supernatant (1.8 mL) was collected and mixed with 1.8 mL dH2O and 0.36 mL 0.1% Ferric chloride (FeCl3) solution. The absorbance of the solution was recorded at 700 nm using a spectrophotometer. Increased absorbance of the reaction mixture indicates increased reducing capacity.
Statistical analysis
All assays were carried out in triplicates. Data were presented as mean ± SD to evaluate significant relationships between experimental parameters. The statistical ANOVA analysis with Tukey’s multiple comparisons. The IC50 value and graphs were generated with Graphpad Prism software (v9.1.10). Optimal particle size was predicted using Response Surface Method in Design Expert 13.
Results and discussion
Effect of particle size on extract yield
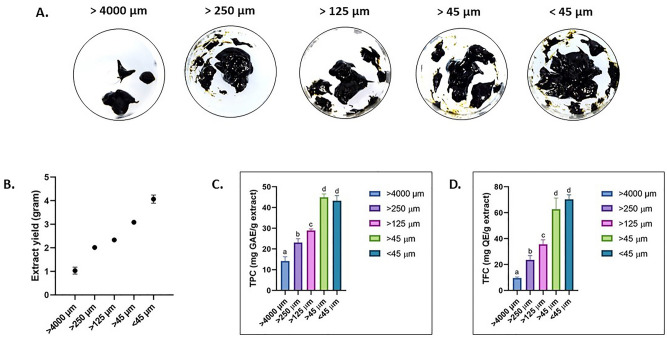
Prior to extraction, the samples were separated into various sizes (< 45 µm, > 45 µm, > 125 µm, > 250 µm, and > 4000 µm) (Fig. 1). The fraction of < 45 µm showed the maximum extract yield with approximately 4% of algal powder dry weight (Fig. 2A). A Previous study reported that extraction of green tea leaves from smaller particle sizes produced higher extract yield (> 1000 µm)19,20. In the case of macroalgae samples, a study on red macroalgae Laminaria spp. shown that smaller particle sizes produced higher extract yield21. Overall, the total extract yield increased as the sample particle size decreases (Fig. 2B). By reducing the particle size of the algal powder, the contact surface area of the sample and the ethanol solvent used for extraction is increased22. Thus resulting in a significantly higher amount of extract yield obtained.
Figure 1.

Brown macroalgae S.cristaefolium and their corresponding thallus grounded to different particle sizes.
Figure 2.
Effect of particle size on S.cristaefolium extract (A) total yield, (B) yield increase, (C) TPC-Total Phenolic Content, and (D) TFC-Total Flavonoid Content. Different letters indicating significant difference (p < 0.05).
Effect of particle size on total phenolic content (TPC) and total flavonoid content (TFC)
The phytochemical composition of S.cristaefolium ethanol extracts shown higher contents in samples of smaller particle sizes (Table 1). The extract from samples with smaller particle sizes produced significantly higher Total Phenolic Content (TPC) and Total Flavonoid Content (TFC) (Fig. 2C,D). However, no significant differences were observed between particle sizes of > 45 µm and < 45 µm. Numerous reports have demonstrated the importance of sample particle size in the solid–liquid extraction system23. However, in some studies reducing particle size does not always contribute to higher bioactive compounds retrieved. In tea, the reduction in particle size led to a higher decrease in almost all phenolic compound catechins measured24. In addition, in some cases, the granulometric characteristics do not influence the amount of bioactive compounds extracted25. For example, the total phenolic content and antioxidant activity of green propolis were increased regardless of the particle size. But in the case of macroalgae or seaweed, reduction of particle size is necessary due to its rigid biomass texture. The size reduction of a biological sample before extraction maximizes the surface area, which in turn enhances the transfer of bioactive compounds from the biological material to the solvent26. The content of TPC and TFC from several Sargassum species had been demonstrated in other studies27. However, due to different parameters and sample preparation, the results are sometimes incomparable. Based on our results, we suggest that smaller particle sizes in macroalgae dried sample is beneficial for the improvement of the TPC and TFC content which has significant effects on its antioxidant activity.
Table 1.
Phytochemical composition and antioxidant activity of different sample particle size of S.cristaefolium ethanol extract.
| > 4000 µm | > 250 µm | > 125 µm | > 45 µm | < 45 µm | Ascorbic acid | |
|---|---|---|---|---|---|---|
| Yield (%) | 1.03 ± 0.15a | 2.05 ± 0.4b | 2.33 ± 0.7c | 3.08 ± 0.08d | 4.07 ± 0.18e | |
| Phytochemical composition | ||||||
| Total Phenolic content (mg GAE/g extract) | 14.19 ± 2.08a | 23.14 ± 1.86b | 28.92 ± 1.78c | 44.95 ± 2.62d | 43.27 ± 2.56d | |
| Total Flavonoid content (mg QE/g extract) | 9.6 ± 1.8a | 23.67 ± 3.25b | 35.67 ± 3.41c | 62.73 ± 8.61d | 70.27 ± 3.59d | |
| Free radical scavenging activity IC50 (µg/mL) | ||||||
| DPPH radical scavenging activity (IC50) | 803.1 ± 1.89a | 822.4 ± 1.78a | 438.1 ± 1.32b | 254.9 ± 2.5c | 202.7 ± 1.22c | 37.94 ± 2.89d |
| ABTS radical scavenging activity (IC50) | 758.7 ± 1.48a | 772.4 ± 3.78a | 321.5 ± 2.13b | 170.1 ± 3.26c | 151.5 ± 2.12c | 24.53 ± 2.54d |
| Hydroxyl radical scavenging activity (IC50) | 2163 ± 2.96a | 1840 ± 2.24b | 1310 ± 3.32c | 1068 ± 2.51d | 930.8 ± 4.14d | 33.11 ± 1.61e |
| Ion reducing activity | ||||||
| Ferric reducing power (Abs at 700 nm) | 0.574 ± 0.013a | 0.602 ± 0.01a | 0.759 ± 0.029b | 0.944 ± 0.015c | 0.993 ± 0.04c | 1.523 ± 0.06d |
Different letters indicating significant difference between columns (p < 0.05).
Effect of particle size on metabolite profle (HR-ESI–MS analysis)
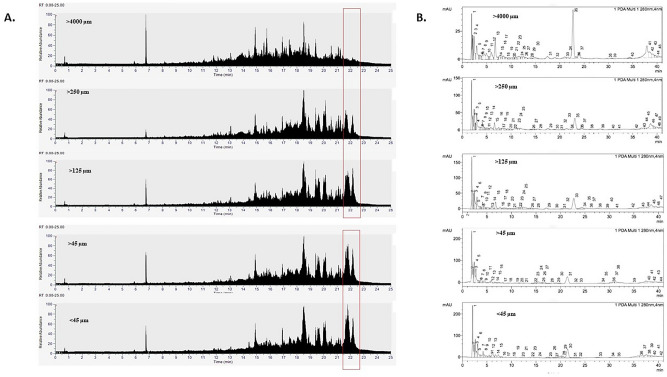
Particle size is one parameter that has a significant effect on the extraction of bioactive compounds from natural products28,29. Our results show that there is increased metabolite composition based on reduction of sample particle size (Fig. 3A). Interestingly in samples with particle size larger than 4000 µm, a significant reduction of non-polar metabolites was observed in the 22 min range. Previous research also increase in bioactive compound composition in smaller particle size of various plant leaves30. The reduction of particle size not only increases the diffusivity of bioactive compounds, but also helps to rupture the cell walls of the sample31,32. The reduction of sample particle size important in macroalgae samples, as the cell walls in macroalgae are extremely thick33.
Figure 3.
Mass Spectrometry analysis of S. cristaefolium extracts with varying particle sizes. (A) Comparative HR-ESI–MS analysis showing different metabolite compositions. The red box indicates changes in phytochemical composition. (B) HPLC analysis of phenolic compounds showing increased concentration in samples with smaller particles. Known standards are listed as follows, 1. EGC-Epigallocatechin; 2. PCA-Protocatechuic Acid; 3. C-Catechin hydrate; 4. ECG-Epicatechin gallate; 5. EGCG-Epigallocatechin gallate; 6. QUE-Quercetin.
Effect of particle size on phenolic profile (HPLC analysis)
In order to evaluate the effect of particle size on the phenolic profile of S.cristaefolium extract, the HPLC analysis was performed (Fig. 3B). This HPLC analysis revealed the presence of some major phenolic compounds such as EGC-Epigallocatechin; PCA-Protocatechuic Acid; C-Catechin hydrate; ECG-Epicatechin gallate; EGCG-Epigallocatechin gallate; QUE-Quercetin (Table 2). Presence of phenolic compounds have been well reported in seaweeds. Tabled results showed that epicatechin, which was the most frequently appearing phenolic compound in S.cristaefolium extract. These group of polyphenols are also proven to be abundant in other seaweeds such as Undaria pinnatifida and the red seaweed Palmaria palmata34. Based on our results the phenolic compound EGC, ECG, EGCG were significantly higher in samples with smaller particle sizes. Other similar studies of HPLC analysis of phenolic compounds in algal samples showed that EGC could range from 0.13 to 3.8 mg/g or from 0.25 to 0.76 mg/g35,36. The immense difference of these values could caused by many factors, not only the species and origin but also extraction process.
Table 2.
Amounts (mg/g sample) of selected phenolic compounds (EGC epigallocatechin; PCA protocatechuic acid; C catechin hydrate; ECG epicatechin gallate; EGCG epigallocatechin gallate; QUE quercetin) in S.cristaefolium extracts.
| Phenolic compound | Concentration (mg/g) | ||||
|---|---|---|---|---|---|
| > 4000 µm | > 250 µm | > 125 µm | > 45 µm | < 45 µm | |
| EGC | 3.30 ± 0.26a | 11.48 ± 0.4b | 13.79 ± 0.68c | 19.73 ± 0.76d | 20.04 ± 0.5e |
| PCA | 0.010 ± 0.003a | 0.014 ± 0.004a | 0.018 ± 0.004a | 0.020 ± 0.005a | 0.023 ± 0.002a |
| C | 0.069 ± 0.003a | 0.074 ± 0.006a | 0.082 ± 0.006a | 0.087 ± 0.005a | 0.091 ± 0.004a |
| ECG | 4.70 ± 0.17a | 12.01 ± 0.48b | 13. 99 ± 0.58c | 20.67 ± 0.89d | 24.97 ± 0.78e |
| EGCG | 4.13 ± 0.08a | 6.10 ± 0.57b | 7.33 ± 0.71c | 9.25 ± 0.50d | 10.67 ± 0.21e |
| QUE | 0.51 ± 0.04a | 0.57 ± 0.03a | 0.61 ± 0.09a | 0.84 ± 0.11a | 1.20 ± 0.08a |
Different letters indicating significant difference between columns (p < 0.05).
Effect of particle size in antioxidant activity
The role of particle size has been previously reported to significantly affect the antioxidant activity of the extract. This has been observed in several natural products including wheat, banana, and blackberries37–39. As previously described in our results, the phytochemical composition are significantly affected by sample particle size. A significant correlation has been reported between the antioxidant activity of natural products due to their phenolic constituents40–42.
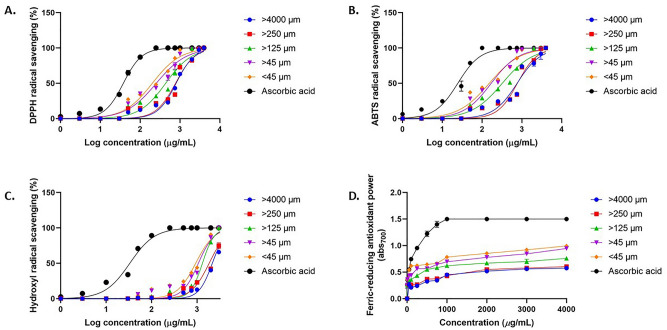
The antioxidant activity of macroalgae has been well reported in previous studies2,38,43,44. The most important and studied bioactive compounds from marine macroalgae are polyphenols, polysaccharides, carotenoids, and polyunstaturated fatty acids45,46. The phenolic compounds are the main contributors to the antioxidant activity, and this is shown that S.cristaefolium extract with higher TPC value and phenolic compound concentrations show stronger antioxidant activity. The extract from the smaller particle size samples produced stronger antioxidant activity based on DPPH, ABTS, hydroxyl assay, including Ferric Reducing Antioxidant Power (FRAP) (Fig. 4). Brown macroalgae Sargassum wightii has shown strong inhibition against free-radical DPPH and ABTS with IC50 around 320 to 800 µg/mL47. Another study shown presence of major antioxidant components such as Sargahydroquinoic acid (SHQA), sargachromanol (SCM) and sargaquinoic acid (SQA) in Sargassum serratifolium. These components demonstrate strong antioxidant capacities with IC50 values below 100 µg/mL48. Among all antioxidant assay, S.cristaefolium extract show strongest activity against ABTS radical. The ABTS assay is considered more sensitive in identifying antioxidant activity because of the faster reaction kinetics, and its response to antioxidants is higher compared to other radicals49. Overall, the extract with smaller particle size has more potential to react with free radicals and terminate them into non-reactive stable form.
Figure 4.
Effect of particle size on S.cristaefolium extract antioxidant activity based on (A) DPPH, (B) ABTS, (C) hydroxyl, and (D) ferric reducing assay. Experiments are done in triplicates, values are expressed as means ± SEM.
Validation analysis of optimum particle size
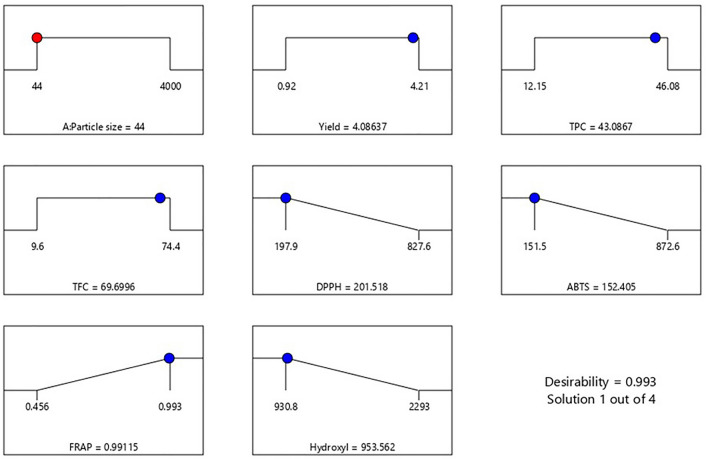
Desirability optimization of sample particle size condition was carried out for accumulative phytochemical composition and antioxidant activity. During desirability determination, the criteria proposed for selecting the optimum particle size conditions for macroalgae S.cristaefolium were for independent responses; TPC and TFC were maximized while Antioxidant activities (DPPH, ABTS, Hydroxyl anti-radical scavenging assay, FRAPS) were minimized. By applying the desirability function approach, the optimum particle size of S.cristaefolium samples was obtained. Figure 5 showed desirability ramps that were developed from optimum points via numerical optimization. A triplicate experiment was set up to validate the optimized condition. The composite desirability (0.993) is close to 1, which indicates the settings seem to achieve favourable results for all responses as a whole50. Based on predictive model, the optimum particle size is 44 µm to obtain favourable results of phytochemical composition and antioxidant activity. This prediction correlates to results showing that sample particle sizes < 45 µm showed maximum phytochemical composition and antioxidant activity.
Figure 5.
Desirability analysis. Plots ramp showing the optimal particle size that maximize TPC, TFC, and antioxidant activity.
Conclusion
The present study has shown that particle size is important in improving the phytochemical constituents and antioxidant activity of macroalgae S.cristaefolium. The constituent of bioactive compounds; phenolic and flavonoid compounds increased due to reduction of particle size. Metabolite profile and major phenolic compounds were also significantly increased compared to larger particles. Furthermore, this contributes to the increase of antioxidant activity of the extracts with smaller particle size. Particle size less than 45 µm showed the best phytochemical composition and antioxidant activity. This procedure may be extended to other applications to obtain more valuable bioactive constituents from macroalgae samples.
Acknowledgements
We acknowledge the lab members of Bioscience and Biotechnology University of Mataram for assistance in the macroalgae sample collection.
Author contributions
The funding of this research was granted to E.S.P. Hence, E.S.P. was responsible for the overall experiment design, methodology, investigation, analysis also the manuscript preparation. A.F. provided High Resolution Mass Spectrometry (HRMS) data of the extracts. F.F. and A.B.J. provided the HPLC data analysis. A.S.A. contributed in the collections of macroalgae samples. N.W.R.M., B.K.I., and H.P. helped with sample preparation and biochemical assays, A.L.S. and S.W. assisted with interpretation of the results and manuscript proofreading.
Funding
This research is funded by the grant provided by the Kementerian Pendidikan, Kebudayaan, Riset dan Teknologi scheme of Basic Research 2021.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Tanna B, Mishra A. Metabolites unravel nutraceutical potential of edible seaweeds: An emerging source of functional food. Compr. Rev. Food Sci. Food Saf. 2018;17:1613–1624. doi: 10.1111/1541-4337.12396. [DOI] [PubMed] [Google Scholar]
- 2.Olasehinde TA, Olaniran AO, Okoh AI. Macroalgae as a valuable source of naturally occurring bioactive compounds for the treatment of Alzheimer’s disease. Mar. Drugs. 2019;17:609. doi: 10.3390/md17110609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barbosa M, Valentão P, Andrade PB. Bioactive compounds from macroalgae in the new millennium: implications for neurodegenerative diseases. Mar. Drugs. 2014;12:4934–4972. doi: 10.3390/md12094934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Øverland M, Mydland LT, Skrede A. Marine macroalgae as sources of protein and bioactive compounds in feed for monogastric animals. J. Sci. Food Agric. 2019;99:13–24. doi: 10.1002/jsfa.9143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sansone C, Brunet C. Marine Algal Antioxidants. Antioxidants (Basel) 2020;9:206. doi: 10.3390/antiox9030206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chan C-X, Ho C-L, Phang S-M. Trends in seaweed research. Trends Plant Sci. 2006;11:165–166. doi: 10.1016/j.tplants.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 7.Tierney MS, et al. Enrichment of polyphenol contents and antioxidant activities of Irish brown macroalgae using food-friendly techniques based on polarity and molecular size. Food Chem. 2013;139:753–761. doi: 10.1016/j.foodchem.2013.01.019. [DOI] [PubMed] [Google Scholar]
- 8.Prasedya ES, et al. Antioxidant activity of brown macroalgae Sargassum ethanol extract from Lombok coast, Indonesia. IOP Conf. Ser. Earth Environ. Sci. 2021;712:012038. doi: 10.1088/1755-1315/712/1/012038. [DOI] [Google Scholar]
- 9.Altemimi A, Lakhssassi N, Baharlouei A, Watson DG, Lightfoot DA. Phytochemicals: Extraction, isolation, and identification of bioactive compounds from plant extracts. Plants (Basel) 2017;6:42. doi: 10.3390/plants6040042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sasidharan S, Chen Y, Saravanan D, Sundram KM, Yoga Latha L. Extraction, isolation and characterization of bioactive compounds from plants’ extracts. Afr. J. Tradit. Complement. Altern. Med. 2010;8:1–10. doi: 10.4314/ajtcam.v8i1.60483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Belscak-Cvitanović A, et al. Physical properties and bioactive constituents of powdered mixtures and drinks prepared with cocoa and various sweeteners. J. Agric. Food Chem. 2010;58:7187–7195. doi: 10.1021/jf1005484. [DOI] [PubMed] [Google Scholar]
- 12.Algaebase :: Listing the World’s Algae. https://www.algaebase.org/.
- 13.Ainsworth EA, Gillespie KM. Estimation of total phenolic content and other oxidation substrates in plant tissues using Folin-Ciocalteu reagent. Nat. Protoc. 2007;2:875–877. doi: 10.1038/nprot.2007.102. [DOI] [PubMed] [Google Scholar]
- 14.Fahrurrozi, et al. Effect of small-scale box-fermentation on catechin and epicatechin content of lampung cocoa beans varieties. Int. J. Adv. Sci. Eng. Inf. Technol. 2021;11:1029–1034. doi: 10.18517/ijaseit.11.3.14846. [DOI] [Google Scholar]
- 15.Ben Mansour R, et al. Assessment of antioxidant activity and neuroprotective capacity on PC12 cell line of Frankenia thymifolia and related phenolic LC-MS/MS identification. Evid. Based Complement. Altern. Med. 2016;2016:1–8. doi: 10.1155/2016/2843463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nenadis N, Wang L-F, Tsimidou M, Zhang H-Y. Estimation of scavenging activity of phenolic compounds using the ABTS·+ assay. J. Agric. Food Chem. 2004;52:4669–4674. doi: 10.1021/jf0400056. [DOI] [PubMed] [Google Scholar]
- 17.Chen Y-H, Tu C-J, Wu H-T. Growth-inhibitory effects of the red alga Gelidium amansii on cultured cells. Biol. Pharm. Bull. 2004;27:180–184. doi: 10.1248/bpb.27.180. [DOI] [PubMed] [Google Scholar]
- 18.Mansoori A, et al. Phytochemical characterization and assessment of crude extracts from Lantana camara L. for antioxidant and antimicrobial activity. Front. Agron. 2020;2:582268. doi: 10.3389/fagro.2020.582268. [DOI] [Google Scholar]
- 19.Vuong QV, Golding JB, Stathopoulos CE, Nguyen MH, Roach PD. Optimizing conditions for the extraction of catechins from green tea using hot water. J. Sep. Sci. 2011;34:3099–3106. doi: 10.1002/jssc.201000863. [DOI] [PubMed] [Google Scholar]
- 20.Makanjuola SA. Influence of particle size and extraction solvent on antioxidant properties of extracts of tea, ginger, and tea–ginger blend. Food Sci. Nutr. 2017;5:1179–1185. doi: 10.1002/fsn3.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Montingelli ME, Benyounis KY, Stokes J, Olabi AG. Pretreatment of macroalgal biomass for biogas production. Energy Convers. Manage. 2016;108:202–209. doi: 10.1016/j.enconman.2015.11.008. [DOI] [Google Scholar]
- 22.Crampon C, Boutin O, Badens E. Supercritical carbon dioxide extraction of molecules of interest from microalgae and seaweeds. Ind. Eng. Chem. Res. 2011;50:8941–8953. doi: 10.1021/ie102297d. [DOI] [Google Scholar]
- 23.Sheng Z, Zhao J, Muhammad I, Zhang Y. Optimization of total phenolic content from Terminalia chebula Retz. fruits using response surface methodology and evaluation of their antioxidant activities. PLoS ONE. 2018;13:e0202368. doi: 10.1371/journal.pone.0202368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hu J, Chen Y, Ni D. Effect of superfine grinding on quality and antioxidant property of fine green tea powder. Lebensm Wiss Technol. 2012;45:8–12. doi: 10.1016/j.lwt.2011.08.002. [DOI] [Google Scholar]
- 25.Augusto-Obara TR, et al. Benefits of superfine grinding method on antioxidant and antifungal characteristic of Brazilian green propolis extract. Sci. Agric. 2019;76:398–404. doi: 10.1590/1678-992x-2018-0056. [DOI] [Google Scholar]
- 26.Prakash Maran J, Manikandan S, Vigna Nivetha C, Dinesh R. Ultrasound assisted extraction of bioactive compounds from Nephelium lappaceum L. fruit peel using central composite face centered response surface design. Arab. J. Chem. 2017;10:S1145–S1157. doi: 10.1016/j.arabjc.2013.02.007. [DOI] [Google Scholar]
- 27.Baek SH, et al. The comparison of total phenolics, total antioxidant, and anti-tyrosinase activities of Korean Sargassum species. J. Food Qual. 2021;2021:e6640789. doi: 10.1155/2021/6640789. [DOI] [Google Scholar]
- 28.Lucas-González R, Fernández-López J, Pérez-Álvarez JÁ, Viuda-Martos M. Effect of particle size on phytochemical composition and antioxidant properties of two persimmon flours from Diospyros kaki Thunb vars. ‘Rojo Brillante’ and ‘Triumph’ co-products. J. Sci. Food Agric. 2018;98:504–510. doi: 10.1002/jsfa.8487. [DOI] [PubMed] [Google Scholar]
- 29.Tchabo W, et al. Impact of extraction parameters and their optimization on the nutraceuticals and antioxidant properties of aqueous extract mulberry leaf. Int. J. Food Prop. 2018;21:717–732. doi: 10.1080/10942912.2018.1446025. [DOI] [Google Scholar]
- 30.Alsaud N, Farid M. Insight into the influence of grinding on the extraction efficiency of selected bioactive compounds from various plant leaves. Appl. Sci. 2020;10:6362. doi: 10.3390/app10186362. [DOI] [Google Scholar]
- 31.Syed Jaapar SZ, Morad NA, Iwai Y, Nordin MFM. Effects of processing parameters in the sonic assisted water extraction (SAWE) of 6-gingerol. Ultrasonics Sonochem. 2017;38:62–74. doi: 10.1016/j.ultsonch.2017.02.034. [DOI] [PubMed] [Google Scholar]
- 32.Yang B, Liu X, Gao Y. Extraction optimization of bioactive compounds (crocin, geniposide and total phenolic compounds) from Gardenia (Gardenia jasminoides Ellis) fruits with response surface methodology. Innov. Food Sci. Emerg. Technol. 2009;10:610–615. doi: 10.1016/j.ifset.2009.03.003. [DOI] [Google Scholar]
- 33.Martone PT, et al. Cellulose-rich secondary walls in wave-swept red macroalgae fortify flexible tissues. Planta. 2019;250:1867–1879. doi: 10.1007/s00425-019-03269-1. [DOI] [PubMed] [Google Scholar]
- 34.Machu L, et al. Phenolic content and antioxidant capacity in algal food products. Molecules. 2015;20:1118–1133. doi: 10.3390/molecules20011118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rodríguez-Bernaldo de Quirós A, Lage-Yusty MA, López-Hernández J. Determination of phenolic compounds in macroalgae for human consumption. Food Chem. 2010;121:634–638. doi: 10.1016/j.foodchem.2009.12.078. [DOI] [Google Scholar]
- 36.Yoshie Y, Wang W, Petillo D, Suzuki T. Distribution of catechins in Japanese seaweeds. Fish. Sci. 2000;66:998–1000. doi: 10.1046/j.1444-2906.2000.00160.x. [DOI] [Google Scholar]
- 37.Savlak N, Türker B, Yeşilkanat N. Effects of particle size distribution on some physical, chemical and functional properties of unripe banana flour. Food Chem. 2016;213:180–186. doi: 10.1016/j.foodchem.2016.06.064. [DOI] [PubMed] [Google Scholar]
- 38.Brewer LR, Kubola J, Siriamornpun S, Herald TJ, Shi Y-C. Wheat bran particle size influence on phytochemical extractability and antioxidant properties. Food Chem. 2014;152:483–490. doi: 10.1016/j.foodchem.2013.11.128. [DOI] [PubMed] [Google Scholar]
- 39.Sójka M, Kolodziejczyk K, Milala J. Polyphenolic and basic chemical composition of black chokeberry industrial by-products. Ind. Crops Prod. 2013;51:77–86. doi: 10.1016/j.indcrop.2013.08.051. [DOI] [Google Scholar]
- 40.Khorasani Esmaeili A, Mat Taha R, Mohajer S, Banisalam B. Antioxidant activity and total phenolic and flavonoid content of various solvent extracts from in vivo and in vitro grown Trifolium pratense L. (Red Clover) BioMed Res. Int. 2015;2015:e643285. doi: 10.1155/2015/643285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Piluzza G, Bullitta S. Correlations between phenolic content and antioxidant properties in twenty-four plant species of traditional ethnoveterinary use in the Mediterranean area. Pharm. Biol. 2011;49:240–247. doi: 10.3109/13880209.2010.501083. [DOI] [PubMed] [Google Scholar]
- 42.Turumtay EA, et al. Correlation between phenolic compounds and antioxidant activity of Anzer tea (Thymus praecox Opiz subsp. caucasicus var. caucasicus) Ind. Crops Prod. 2014;52:687–694. doi: 10.1016/j.indcrop.2013.11.042. [DOI] [Google Scholar]
- 43.Ismail MM, Alotaibi BS, El-Sheekh MM. Therapeutic uses of red macroalgae. Molecules. 2020;25:4411. doi: 10.3390/molecules25194411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Moubayed NMS, Al Houri HJ, Al Khulaifi MM, Al Farraj DA. Antimicrobial, antioxidant properties and chemical composition of seaweeds collected from Saudi Arabia (Red Sea and Arabian Gulf) Saudi J. Biol. Sci. 2017;24:162–169. doi: 10.1016/j.sjbs.2016.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gomez-Zavaglia A, Prieto Lage MA, Jimenez-Lopez C, Mejuto JC, Simal-Gandara J. The potential of seaweeds as a source of functional ingredients of prebiotic and antioxidant value. Antioxidants (Basel) 2019;8:406. doi: 10.3390/antiox8090406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sanjeewa KKA, Kim E-A, Son K-T, Jeon Y-J. Bioactive properties and potentials cosmeceutical applications of phlorotannins isolated from brown seaweeds: A review. J. Photochem. Photobiol. B. 2016;162:100–105. doi: 10.1016/j.jphotobiol.2016.06.027. [DOI] [PubMed] [Google Scholar]
- 47.Maneesh A, Chakraborty K, Makkar F. Pharmacological activities of brown seaweed Sargassum wightii (Family Sargassaceae) using different in vitro models. Int. J. Food Prop. 2017;20:931–945. doi: 10.1080/10942912.2016.1189434. [DOI] [Google Scholar]
- 48.Lim S, et al. Evaluation of antioxidant activities of various solvent extract from Sargassum serratifolium and its major antioxidant components. Food Chem. 2019;278:178–184. doi: 10.1016/j.foodchem.2018.11.058. [DOI] [PubMed] [Google Scholar]
- 49.Lee KJ, Oh YC, Cho WK, Ma JY. Antioxidant and anti-inflammatory activity determination of one hundred kinds of pure chemical compounds using offline and online screening HPLC assay. Evid.-Based Complement. Altern. Med. 2015;2015:e165457. doi: 10.1155/2015/165457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Amdoun R, et al. The desirability optimization methodology; a tool to predict two antagonist responses in biotechnological systems: case of biomass growth and hyoscyamine content in elicited Datura starmonium hairy roots. Iran J. Biotechnol. 2018;16:e1339. doi: 10.21859/ijb.1339. [DOI] [PMC free article] [PubMed] [Google Scholar]