Abstract
Body dysmorphic disorder (BDD) is characterized by preoccupations with misperceptions of one’s physical appearance. Previous neuroimaging studies in BDD have yet to examine dynamic functional connectivity (FC) patterns between brain areas, necessary to capture changes in activity in response to stimuli and task conditions. We used Leading Eigenvector Dynamics Analysis to examine whole-brain dynamic FC from fMRI data during an own-face viewing task in 29 unmedicated adults with BDD with facial concerns and 30 healthy controls. The task involved two parts: (1) unconstrained, naturalistic viewing and (2) holding visual attention in the center of the image, to reduce scanning and fixation on perceived facial flaws. An FC state consisting of bilateral medial orbitofrontal cortex regions occurred significantly less often during the visual attention condition and afterward during the unconstrained face viewing in BDD participants, compared to the first unconstrained face viewing, a pattern that differed from controls. Moreover, the probability of this state during the second unconstrained face viewing was associated with severity of obsessions and compulsions and degree of poor insight in BDD, suggesting its clinical significance. These findings have implications for understanding the pathophysiology of own-face viewing in BDD and how it is affected by modification of viewing patterns, which may have implications for novel perceptual retraining treatment designs.
Subject terms: Attention, Psychiatric disorders
Introduction
Individuals with body dysmorphic disorder (BDD) have preoccupations with misperceived appearance flaws, which they believe render them ugly and disfigured, and engage in time-consuming repetitive behaviors to check or fix their appearance. The consequences can be profound; the disorder is marked by high lifetime prevalence of suicide attempts (25%) [1] and psychiatric hospitalization (50%) [2]. Overall, 27–39% are delusional in their beliefs [3]. BDD is under-recognized, misdiagnosed, and still understudied.
Neurobiological models to explain vulnerability to BDD have been put forth (e.g., [4, 5]), but a comprehensive understanding of this condition is still emerging. Given the core phenotype of distorted perception of appearance, abnormalities of visual information processing in BDD are likely critical neurobiological substrates of the disorder [4, 6]. Specifically, deficient global/holistic and enhanced local/detailed processing may contribute to the experience of perceptual distortions [7–18]. In addition, aberrant orbitofrontal-striatal circuit activity, similar to that seen in obsessive-compulsive disorder (OCD), may play a role in the phenomenological features of obsessional preoccupations and repetitive, compulsion-like behaviors [7, 19, 20]. Selective attention biases could also potentially contribute to its psychopathological features [21]. This includes aberrant patterns of visual attention; a commonly observed phenomenological feature is excessive visual attention paid to perceived appearance defects, particularly with mirrors and photographs [22]. Studies using eye gaze tracking in BDD have found biased attention to facial areas deemed flawed, and a scanning pattern characterized by multiple fixations of brief duration [23, 24].
Such findings have led to treatment approaches [25, 26] and proposed treatments [27] that incorporate modifications of visual attention. Yet, the brain mechanisms underlying aberrant visual attention and how the neurobiological substrates of potential targets are engaged by different visual attention modification approaches, are incompletely understood. Such understanding may be critical to the development of effective new clinical treatments (National Institute for Mental Health 2020 Strategic Plan Goal 3: Strive for Prevention and Cures).
We therefore designed an experiment to test the neurobiological mechanistic effects of a strategy of modification of visual attention [28] in BDD. The experiment includes a task condition termed “modulated viewing (ModV),” which requires participants to view a centered white cross overlaid on a photo of their face, with instructions and monitoring to ensure that their gaze is on the cross. The purpose is to reduce fixation on perceived facial flaws and reduce eye scanning behaviors that typically trigger negative preoccupations about appearance in BDD. The effects of this task condition were compared with the other task condition, consisting of unconstrained “natural” viewing (NatV) of their faces.
Functional magnetic resonance imaging (fMRI) can be used to characterize neural connectivity underlying visual attention abnormalities and the effects of potential interventions. We previously conducted several functional connectivity (FC) studies in BDD [29–31]. One study tested hypotheses involving a face-processing network [29], and another tested hypotheses about visual, parietal (body processing), and striatal networks [30]. The only previous whole-brain FC analysis in BDD found that degree connectivity of the right lateral and central orbitofrontal cortex (OFC) was positively correlated with the severity of BDD symptoms during a task of viewing others’ faces [31]. All studies, however, have only investigated “static” FC, i.e., average correspondence between brain regions over the scanning periods. It remains unclear how FC changes over time in individuals with BDD.
Dynamic FC (dFC) approaches, on the other hand, can capture temporal changes in the organization of network properties. Given that cognitive processes fluctuate over timescales of milliseconds to a few seconds, dFC likely reflects the brain’s ability to shift, adapt, or anticipate changing cognitive demands or external stimuli from the environment [32, 33]. dFC changes during tasks [34, 35] and resting state [36–38] have also been demonstrated to be associated with psychiatric disorders and with reduced behavioral/cognitive performance in healthy subjects [39–42]. Understanding dynamic network organization in BDD could contribute to a more comprehensive model of neural system function/dysfunction occurring in response to symptom-relevant stimuli. Further, such an approach could reveal how neural systems react dynamically to experimental modulation, which may critically provide insights into novel therapeutic interventions for BDD.
In this study, we used Leading Eigenvector Dynamics Analysis (LEiDA) [39]. This is a method to calculate dFC at the instantaneous level (for each recorded frame) and to identify recurrent patterns of blood oxygen level dependent (BOLD) phase-locking (i.e., FC states). This method allows characterizing recurrent FC states in terms of probabilities of occurrence, duration and transition profiles for individual subjects. The dynamic properties of recurrent FC states, using LEiDA, have been associated with cognitive performance [39] and with psychedelic experiences [43] in healthy participants. In addition, LEiDA has been used to differentiate patients with remitted major depressive disorder from healthy controls [42]. These works underscore the possibility that dFC analysis could provide novel insights into the system-level disruptions underlying BDD and the effects of an experimental modulation of attention.
We employed LEiDA dFC analysis to explore the whole-brain effects of ModV within BDD and healthy controls. We further investigated if the modulation had differential effects between groups by comparing differences in probability of FC states. We hypothesized that the ModV intervention would result in changes in dFC patterns within BDD and control groups compared to NatV of their faces. We also hypothesized that during a period of NatV after ModV there would be significant effects on dFC patterns within BDD and controls compared to the first NatV (i.e., a “carry over” effect of the ModV). Finally, we hypothesized there would be significantly different dFC patterns between BDD and controls during the first NatV of faces, during ModV, and during the second NatV of faces after the ModV.
Materials and methods
Participants
The UCLA Institutional Review Board approved the study and informed written consent was obtained from all participants. Thirty-five adults with BDD aged 18–40 years and thirty-five healthy controls were enrolled. All were recruited from the community and were free from psychoactive medications for at least 8 weeks. BDD participants met DSM criteria for BDD, with face concerns. Those with concerns specifically about the region between their eyes were excluded due to the nature of ModV task. BDD participants could have comorbid depressive or anxiety disorders, since they commonly co-occur. (See Supplementary Material for exclusion criteria details).
Clinical assessments
Eligibility was determined through telephone screening followed by a clinical interview with the study physician (JDF). The Mini International Neuropsychiatric Interview and BDD Module [44, 45] were administered. The Yale-Brown Obsessive-Compulsive Scale Modified for BDD (BDD-YBOCS) [46], Montgomery-Åsberg Depression Rating Scale [47], and Hamilton Anxiety Scale [48] were used to assess BDD symptoms, depression, and anxiety, respectively. The Brown Assessment of Beliefs Scale (BABS) was used to assess insight and delusionality [49]; higher scores are indicative of poorer insight. (See Supplementary Material for assessment details).
Task paradigm
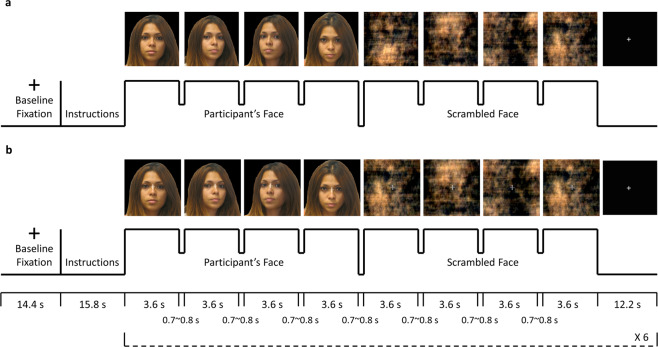
There were two sets of stimuli for NatV condition: photos of participant’s face and scrambled faces as the control task (Fig. 1a). The scrambled image was produced using phase-scrambling techniques that utilized a fast Fourier transform, so that the scrambled image had the same frequency spectrum, luminance and color as the original image, but with the phase randomly scrambled [50]. There were also two sets of stimuli for ModV condition: the same photos overlaid with a semi-transparent crosshair between the eyes, and the scrambled faces with a crosshair (Fig. 1b).
Fig. 1. fMRI task paradigm.
Four color photos of participants’ own faces at different, standardized angles were captured before the MRI session. A blocked design was used for the presentation of participant’s own face and scrambled face control stimuli for both a natural viewing and b visual modulation runs. The first four images were participant’s faces at different angles, and the next four images were scrambled faces. Each image was presented for 3.6 s, with a small gap of 0.7–0.8 s for changing the image. A fixation with duration of 12.2 s was shown after the stimuli. The presentation of participant’s face and scrambled face stimuli was repeated for six times in a single run. The stimuli for the visual modulation run (b) had a semi-transparent crosshair between the eyes of the participants’ faces and in the center of the scrambled faces. For the visual modulation run, participants were required to maintain their gaze on the crosshair. Compliance with face viewing and of viewing the crosshair was determined with live monitoring with a camera during the scan. Informed consent was obtained for publication of the volunteer’s photo in the figure.
FMRI data were acquired while participants underwent two conditions. During NatV, participants viewed unaltered photos of their face and scrambled images of their face without restrictions. During ModV, they viewed the same images while maintaining attention on the crosshair. The rationale was that fixating visual gaze on the crosshair would reduce scanning associated with piecemeal/detailed processing, and enhance holistic/global visual processing.
Participants were randomly assigned to one of the two counterbalanced groups for three fMRI runs: NatV-NatV-ModV (NNM) or NatV-ModV-NatV (NMN). They were instructed to press a button every time an image disappeared from the screen to ensure vigilance. Moreover, an eye-tracking camera attached to the fMRI goggles was used to acquire eye-tracking data. To ensure task compliance for viewing the photos and crosshairs, gaze location was continuously monitored with the camera by the experimenters, using ViewPoint software (Arrington Research) during the scan.
MRI data acquisition and preprocessing
We used a 3 T Siemens Prisma scanner for MRI data acquisition. Data preprocessing was done using fMRIPrep 1.4.0 [51]. Details of data acquisition and preprocessing, including quality control and motion correction, are available in Supplementary Material.
Dynamic FC
The anatomical automatic labeling (AAL) atlas [52] was used to parcellate the brain into 90 cortical and subcortical areas and the BOLD signals were averaged over all voxels belonging to each area. BOLD phase coherence connectivity [53–56] was used to obtain a time-resolved dFC tensor, with size N × N × T, where N = 90, the number of brain areas, and T = 42, the number of recording frames during unaltered face or scrambled face stimuli. Thus, the timepoints associated with those trials of viewing unaltered faces or scrambled faces were extracted. This was done for each of the two NatV task runs and the ModV task run. The calculation of BOLD phase coherence is available in Supplementary Material.
FC states
To identify recurrent patterns in the dFC, (i.e., FC states), LEiDA considers only the leading eigenvector V1(t) of each dFC(t) at each time t, which captures the dominant pattern of BOLD phase coherence [39]. The leading eigenvector contains 90 elements, whose sign (positive/negative) can be used to classify brain areas into two communities according to their BOLD-phase relationship [57]. All the FC patterns - represented by the V1 computed for each fMRI frame - were clustered into a reduced number of recurrent FC states, by applying a k-means clustering algorithm to all leading eigenvectors V1 across all participants. The clustering algorithm divides the samples into k clusters, with a higher k revealing more fine-grained and less frequent network configurations. The number of clusters (k) was varied over a wide range between 2 and 20 [39, 42]. We considered each cluster to represent a different FC state, whose spatial pattern is represented by the cluster centroid, a vector with 90 elements representing the BOLD phase orientation of each brain area [42]. FC states were sorted according to the probability of occurrence.
Statistical analysis
We calculated the probability of occurrence of each FC state (i.e., the fraction of epochs that it occurred throughout the scan) for each participant and condition. We focused on the probability of FC states as that measure is intuitive and it previously was found to be sensitive to task effects [35, 42]. FC state probability changes were examined for all the (k) FC states obtained in each partition into k = 2 to k = 20 clusters and statistically compared between different combinations of task sequence, time, and group using a three-way mixed ANOVA (within-subjects factor: time [first or second or third run]; between-subjects factors: group [BDD or CON] and task sequence [NNM or NMN]). To correct for multiple comparisons, the significance threshold was adjusted to 0.05/k, using a Bonferroni correction accounting for the number of independent hypotheses. As the omnibus test to encompass our hypotheses we focused on the FC states that most consistently had significant group × task × time effects across the range of k. If a significant three-way interaction effect was found, simple two-way interaction, simple-simple main effect, and simple-simple pairwise comparisons were also computed as post hoc tests. As exploratory follow-up analyses, the associations between the FC states that showed significant group × task × time interaction effects, and the symptom severity measures of BDD-YBOCS (core BDD symptom) and BABS (insight) were examined using Pearson correlation. Statistical tests were done using MATLAB and R.
Results
Sample characteristics
Thirty-five participants with BDD and thirty-five controls were eligible and scanned. Among these, we excluded one BDD as she fell asleep during the experiment and one control as a wrong task paradigm was presented, four BDD and four controls due to excessive motion artifacts, and one BDD due to fMRIPrep errors. Twenty-nine BDD and thirty controls were finally included in the LEiDA and subsequent analyses (Table 1). A significantly positive correlation was found between the BABS and BDD-YBOCS scores in all BDD participants (ρ = 0.377, p = 0.044).
Table 1.
Sample characteristics.
| BDD (n = 29) | CON (n = 30) | Between-group statistics | |||
|---|---|---|---|---|---|
| χ2 | t | p value | |||
| Sex (Male/Female) | 4/25 | 8/22 | 1.51 | 0.22 | |
| Age (Years) | 25.5 ± 7.27 | 23.2 ± 6.82 | 1.26 | 0.21 | |
| Education (Years) | 14.6 ± 1.99 | 13.5 ± 1.72 | 2.18 | 0.03 | |
| Symptoms severity | |||||
| HAMA | 10.1 ± 6.77 | 2.5 ± 2.27 | 5.83 | <0.001 | |
| MADRS | 12 ± 8.18 | 1.1 ± 1.35 | 7.20 | <0.001 | |
| BDD-YBOCS | 26.8 ± 4.45 | NA | – | ||
| BABS | 14.9 ± 4.64 | NA | – | ||
| Psychiatric comorbidities | |||||
| Major depressive episode | 3 | ||||
| Persistent depressive disorder (dysthymia) | 4 | ||||
| Panic disorder with agoraphobia | 2 | ||||
| Agoraphobia without history of panic disorder | 1 | ||||
| Social phobia | 3 | ||||
| PTSD | 2 | ||||
| Generalized anxiety disorder | 8 | ||||
| No DSM comorbid disorder | 15 | ||||
BDD body dysmorphic disorder, CON control, HAMA Hamilton Anxiety Scale, MADRS Montgomery-Asberg Depression Rating Scale, BDD-YBOCS Yale-Brown Obsessive-Compulsive Scale Modified for BDD, BABS Brown Assessment of Beliefs Scale (insight measure), PTSD post-traumatic stress disorder, χ2 chi-square test, t independent-samples t-test.
Detection of relevant FC states during unaltered face stimuli
For each of the 19 clustering solutions considered (k = 2 to k = 20) the k FC states were compared in terms of probability during unaltered face stimuli, and the p values obtained from the three-way interaction are shown in Fig. S1. For k ≥ 5, the clustering consistently returned one or more FC states that showed significant three-way interaction during presentation of unaltered face stimuli below the p = 0.05 threshold (red dashed line in Fig. S1). Five FC states survived the correction for multiple comparisons (p < 0.05/k, green dashed line in Fig. S1) for k = 6, 7, 8, 11, and 13. In particular, FC state #2 consistently passed the significance threshold for all k ≥ 5 (highlighted with square boxes in Fig. S1, for k ≥ 5). To verify the spatial similarity of FC state #2 across the different clustering solutions between k = 5 and k = 20, FC state #2 from each partition model is represented in Fig. S2. FC state #2, consistently significant over the range of k, reveals the segregation of a functional subsystem consisting of bilateral areas in the OFC (i.e., rectus, olfactory and medial orbital part of superior frontal gyrus). The consistency of the significant three-way interaction in terms of probability for a range of partition models, reinforces the relevance of this FC state for investigating the neurophysiological basis of BDD. For the subsequent analysis, the partition model with k = 8 was selected, since it showed the lowest p value (F[2,110] = 5.86, p = 0.0038), significant after correction for the number of states in that partition, associated with the three-way interaction. The FC state #2 obtained for k = 8 is shown in Fig. 2a, b.
Fig. 2. FC state demonstrating a significant (corrected) interaction effect between group, task, and run that was consistent across multiple k-means clustering partitions.
Shown is the FC state for k = 8, the partition with the most significant interaction effect in probability of occurrence during presentation of unaltered face stimuli. a The dominant FC state is represented in cortical space, where functionally connected brain areas (represented as spheres) are colored alike. The yellow/red colored spheres represent areas in the OFC, which are positively correlated between each other, but negatively correlated with the rest of the brain areas (cyan/blue colored spheres). The FC state is also represented by the N × N phase synchronization matrix between the N = 90 brain areas, where positive values indicate pairs of areas aligned in phase, whereas negative values indicate anti-phase alignment between brain areas. b Contribution of different brain areas to the dominant FC state. Red bars represent areas in the OFC (i.e., rectus, olfactory, and medial orbital part of superior frontal gyrus), and blue bars represent the rest of the brain areas. The magnitude of values indexes the “strength” with which brain areas belong to the FC state. c Probabilities of occurrence of this state across groups, task sequences, and time. CONNNM, CONNMN, BDDNNM, and BDDNMN represent the two counterbalanced orders for each group, with probabilities for the first, second, and third runs indicated in the bar plots. For example, the healthy controls randomized to CONNNM received natural viewing (N), natural viewing (N), and then modulated viewing (M) as the first, second, and third runs.
Apart from the OFC network (i.e., FC state #2), FC state #11 for k = 11 and FC state #12 for k = 13 were also significant after correction for multiple comparisons (Fig. S1); these two FC states represent the same network, consisting of bilateral areas in the somatomotor, dorsal and ventral attention, default and visual networks (Fig. S3). However, this state did not appear consistently across the same range of partition models as did the OFC network, neither below the uncorrected 0.05 nor corrected 0.05/k significance thresholds. Thus, we focused further analyses on the OFC network.
Significance of two-way interactions and main effects in FC state probability (from three-way mixed ANOVA results) are shown in Figs. S4 and S5 as a reference.
Effects of group, task, and time
Since a significant three-way interaction between group, task, and time was detected for the FC state #2 with k = 8, post hoc tests were conducted. For the simple two-way interactions and simple-simple main effects, a Bonferroni adjustment was applied leading to statistical significance being accepted at p < 0.025. There were significant simple two-way interactions between task and time for BDD, F(2, 54) = 4.32, p = 0.018, and for CON, F(2, 56) = 4.40, p = 0.017. There was a significant simple-simple main effect of time on the probability for BDDNMN, F(2, 28) = 7.73, p = 0.002, but not for BDDNNM, F(2, 26) = 0.20, p = 0.817. There was a trend simple-simple main effect of time on the probability for CONNMN, F(1.4, 18.2) = 3.88, p = 0.053, but not for CONNNM, F(2, 30) = 1.55, p = 0.229. All simple-simple pairwise comparisons were computed between different runs, with a Bonferroni adjustment. The probability was significantly different between first and second runs (first NatV > ModV, p = 0.025), and between first and third runs (first NatV > second NatV, p = 0.031) for BDDNMN. The probability was also significantly different between second and third runs (ModV > third NatV, p = 0.018) for CONNMN (Fig. 2c).
We also examined the simple two-way interaction between group and time on the probabilities, with statistical significance being accepted at p < 0.025 for task after a Bonferroni adjustment. The simple two-way interaction between group and time was significant for the NMN order (F(2, 54) = 5.92, p = 0.005), but not for the NNM order. The simple-simple main effect of group was only significant in the participants with the NMN order during the first run (F(1, 27) = 4.48, p = 0.044). The pairwise comparisons show that there was a significant difference between BDD participants and healthy controls with the NMN order during the first run (BDD > CON, p = 0.044) (Fig. 2c).
Time-evolving patterns of FC states
To better demonstrate the variation of FC states over time, we present the FC states with the highest frequency of occurrence in the BDDNMN group and an example transition of FC states from a BDD participant during face stimuli in Fig. 3. FC states were sorted according to the probability of occurrence, and therefore the FC state #1 was predominant as it most frequently occurred. From the group-level results, the FC state #2 was revealed to less frequently occur during the second and third runs (ModV and NatV, respectively) compared to the first run (NatV). These patterns were in line with the results from the pairwise comparisons between different runs.
Fig. 3. FC states with the highest frequency of occurrence in the BDDNMN group and an example sequence of FC states from a BDD participant during face stimuli.
There were six blocks of face stimuli in each run, and each block of face stimuli consisted of seven timepoints, constituting a total of 42 timepoints during face stimuli in each run. At each timepoint, the corresponding FC state is represented by its corresponding number and color, as determined in the bottom panel. From group-level results (top), the FC states were selected based on the highest frequency of occurrence among the participants from BDDNMN group. In other words, the selected FC state was the most frequent functional network across participants at that particular timepoint. The sequence of FC states is represented at the individual level for a representative patient (middle).
Clinical symptom correlations
There were significantly positive correlations between the probability of FC state #2, consisting of OFC regions, during the second natural viewing and BDD-YBOCS scores (r = 0.45, p = 0.02, uncorrected) and BABS scores (r = 0.39, p = 0.04, uncorrected) in BDD (Fig. 4). Thus, the higher the probability of occurrence of this OFC state during the second natural viewing, the worse the obsessions and compulsions and poorer insight/delusionality. There was a negative correlation between the BABS score and the probability of this FC state during visual modulation in BDD, albeit nonsignificant (r = −0.29, p = 0.13, uncorrected).
Fig. 4. Associations between functional connectivity states and symptom severity.
Correlations between the probability of occurrence of the relevant state (shown in Fig. 2) and BABS scores and BDD-YBOCS scores in BDD participants during first natural viewing run, second natural viewing run, and visual attention modulation run.
Discussion
This is the first examination of whole-brain dFC in individuals with BDD. The goals were to understand the FC states involved when viewing one’s face, the primary area of appearance concern for most with BDD, and how they change under different viewing conditions. Specifically, we investigated (1) how dFC is affected by constraining visual attention, (2) if there is a subsequent “carry over” effect when the viewing is once again unconstrained, and (3) whether effects differ in individuals with BDD compared with controls. We found a significant effect on the probability of occurrence of a dFC state involving OFC regions, which was reduced after ModV. In BDD, the probability of occurrence of this state during NatV was associated with severity of obsessions and compulsions and the degree of poor insight, suggesting potential clinical significance. These results shed light on dynamic brain states in those with BDD when symptoms are triggered and the effects on these states as a result of changing viewing patterns, which may have implications for novel perceptual retraining treatment designs.
Although understanding of the neural phenotypes associated with BDD and BDD symptoms is still limited, previous functional neuroimaging studies using face viewing tasks have uncovered the OFC subregions as demonstrating abnormal activity, or connectivity associated with symptom severity. An early study found hyperactivity in BDD compared with controls when viewing one’s face, in frontostriatal regions including the left OFC (albeit more lateral than in the dFC network found in this study) [7]. A previous study examining static FC during an others’ face viewing task found a positive correlation between degree connectivity in right lateral and right central OFC and BDD symptom severity (BDD-YBOCS scores) [31]. Analogously, a previous study in a related disorder, OCD, found an association between right lateral OFC degree connectivity and OCD symptom severity (Yale-Brown Obsessive-Compulsive Scale scores), and greater degree connectivity in unmedicated OCD than the healthy controls in right central OFC [19]. In OCD, OFC hyperactivity during resting state and symptom provocation is a well-replicated finding (see meta-analyses [58–60]). BDD is categorized in the DSM-5 as an obsessive-compulsive and related disorder [61], a conceptualization supported by findings from these previous studies in BDD. The current study’s face viewing task is highly likely to provoke BDD symptoms given that all participants had face concerns. We speculate that this may have provoked obsessive appearance-related thoughts and urges to engage in compulsive behaviors. This is supported by the significant correlation between probability in the OFC network and obsessive and compulsive symptom severity from the BDD-YBOCS. It could also account for the trend for higher probability of engagement of the OFC network in the BDD than the controls in the first NatV.
Beyond the links to experiences of obsessive thoughts and urges to do compulsive behaviors, multiple OFC subregions in this dFC state may reflect a broader involvement of this network related to the task and stimuli. The medial OFC has a role in monitoring incentive salience of stimuli [62], as well as emotional and reward or punishment associations to be learned from visual or other sensory stimuli [63]. The OFC is continuously engaged in monitoring and evaluating the valence of stimuli over multiple timescales; in particular, the medial OFC has a central role in brain networks involved in fast processing (around 130–170 ms) of salient affective stimuli (e.g., gun-like hairdryers, infant faces and vocalizations) for top-down facilitation [64–66]. Aberrant processing in these brain networks could have significant deleterious effects. In fact, a meta-analysis of fMRI tasks using emotionally valenced vs. neutral stimuli in the related disorder, OCD, showed significant medial bilateral OFC activation [58]. Own-face viewing is typically a highly emotionally arousing experience for those with BDD, which may be reflected by higher probability of engagement of the medial OFC network.
In general, the OFC is part of an extended face-processing network [67, 68]. It is functionally connected to the fusiform gyrus [69] and structurally connected to the occipital lobe and fusiform gyrus via the inferior fronto-occipital fasciculus (IFOF; specifically the IFOF-II [70]). The role of the OFC within this extended face-processing network may be in incentive salience and reinforcement processing; this is supported by findings that the medial OFC has been found to be involved in aesthetic judgments in general [71, 72], face attractiveness [73], as well as sexual preference of faces [74]. The orbitofrontal network with differential activity by group, task, and run observed in this study spans posterior to anterior medial regions, and, along this gradient, may be involved in a range of simpler to more complex reinforcement processing [62, 75]. In this study, this might correspond to visually-specific elements of the face stimuli, represented in posterior portions of this OFC network, while more abstract concepts, such as the meaning of their facial appearance, or their judgements of it, may be represented in anterior subregions.
During ModV, this OFC network’s probability decreased in BDD group. We speculate that this could signify reduced likelihood of engagement of aberrant orbitofrontal-striatal systems while visually attending only to the center of the image; this visual attention modification prevents visual “search” behaviors including facial scanning for imperfections or defects and/or honing in on specific areas of dysmorphic concern, common in BDD [23, 24, 76]. In healthy controls, there was no significant change in the probability of occurrence of this network during ModV. If this brain state is linked to pathological engagement of an OFC network, little change in controls during ModV could simply reflect the overall low likelihood that this brain state would be triggered no matter how they viewed their faces. Alternatively, ModV may have different effects in BDD than in controls; the same brain state could theoretically be utilized for different processes in different cohorts (e.g., obsessional thinking and/or high emotional arousal might be limited just to those with BDD) and thereby be affected differently by the same intervention.
In both groups, this OFC network decreased in probability in the second NatV compared with the first (although only significant in BDD). This suggests a similar but greater magnitude effect in BDD. Evidence that this was due to the intervening ModV and not just exposure to a second NatV comes from the observation that participants randomized to the “control” condition of two NatVs without an intervening ModV did not show decreased probability in the second NatV. The positive correlation between probability of occurrence of this OFC network and worse obsessions and compulsions suggest the relevance of this state to core BDD symptom dimensions. Why this significant, positive association emerged for the second and not the first NatV is unclear. During the first task there may have been general orienting to the scanner environment and/or the task itself; perhaps only after a longer duration or greater number of face stimuli did this OFC state begin to increase its probability.
In BDD, higher probability of this OFC network was also associated with worse insight. The direction of this relationship shifted during the intervening ModV component of the task (although at trend level). The mechanisms underlying possible reversal of the relationship between the OFC network and degree of insight are unclear. Yet, empirically, those with worse insight had the greatest reduction in the probability of this potentially pathological brain state during the intervention. Insight varies across patients with BDD, although in the majority it is poor or absent [44, 61]. Insight is a highly consequential clinical variable, as those with low insight are less likely to seek and engage in treatment [3, 44, 77, 78]. Although previous studies have not investigated links between OFC function and insight in BDD, in schizophrenia OFC cortical thickness [79], larger OFC volume [80], and activation in orbitofrontal-subcortical regions [81] have been found to be associated with misattribution of symptoms, an element of impaired insight. Moreover, the OFC has a role in self-monitoring such that impairments in function may lead to reduced self-insight [82].
These results have implications for understanding effects of this visual modulation intervention in the interest of development as a perceptual retraining method. A potentially pathological OFC brain state, engaged specifically when viewing one’s face and associated with obsessions/compulsions and poor insight, is less likely to occur when visual attention is constrained to reduce focus on appearance imperfections and visual scanning. Further, this effect may persist when free viewing is subsequently allowed. This may have relevance for design of future perceptual modulation treatment strategies, as it provides an early signal that such an intervention could have a “carry over” effect after it is practiced. How often or long such an exercise might need to occur, remains to be investigated.
Given the exploratory nature of this study, neither this task nor whole-brain dFC has ever been investigated in BDD, our hypotheses were relatively general. Nevertheless, it was somewhat surprising that no significant effects emerged in networks involving visual systems, given previous functional neuroimaging studies [7, 10, 29, 30]. The correction for multiple comparisons that we employed across the wide range of k values, necessary due to the lack of prior knowledge about the number of dynamic k states to investigate, may have permitted only the strongest brain state with the highest magnitude effects to survive, which, in this case, were effects in an OFC network.
This study has several limitations. Although this is the largest BDD neuroimaging study to date, the modest sample size is a limitation. The AAL atlas used for parcellation, which has yielded meaningful results in previous dFC analyses using LEiDA [39, 42], is coarse and anatomically based. Finer-grained and functionally based parcellations might reveal more precise dynamic brain states. Further, we were not able to assess participants’ thoughts or emotional experiences while viewing their faces naturalistically or with constrained visual attention, to more directly link brain states to potentially pathological cognitions, emotions, or perceptual experiences. However, this is challenging in such experiments since asking about experiences during the task can alter these experiences. Moreover, asking after the task may have limited reliability due to difficulties with recall, especially as there were three distinct parts of the task.
In conclusion, this study provides evidence that visual attention modulation could reduce the probability of a potentially pathological OFC brain state in BDD, associated with obsessions/compulsions and poor insight. Further, this may have persistent effects when freely viewing their face afterward. This provides new knowledge of the dynamics of brain networks engaged while viewing one’s appearance, and preliminarily supports the possibility that visual attention modulation may be effective in reducing a potentially pathological brain state in BDD.
Funding and disclosure
This study was supported by the National Institute of Mental Health (R21MH110865 to JDF, R21MH110865-01A1S1 to JDF, R01MH121520 to JDF) and the Nathan Cummings Foundation (JDF). JC is funded by the Portuguese Foundation for Science and Technology (FCT), projects UIDB/50026/2020, UIDP/50026/2020, and CEECIND/03325/2017. MLK is supported by the ERC Consolidator Grant: CAREGIVING (n. 615539), Center for Music in the Brain, funded by the Danish National Research Foundation (DNRF117), and Centre for Eudaimonia and Human Flourishing funded by the Pettit and Carlsberg Foundations. The authors declare no competing interests.
Supplementary information
Effects of visual attention modulation on dynamic functional connectivity during own-face viewing in body dysmorphic disorder
Author contributions
WW was responsible for data analysis and paper writing. JC and MLK contributed to the development of LEiDA algorithm. RR and RL were responsible for experiments and data acquisition. JDF was responsible for patients’ assessment, experimental design, and paper writing. All authors read and approved the submitted manuscript.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41386-021-01039-w.
References
- 1.Phillips KA, Menard W. Suicidality in body dysmorphic disorder: a prospective study. Am J Psychiatry. 2006;163:1280–2. doi: 10.1176/ajp.2006.163.7.1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Phillips KA, McElroy SL, Keck PE, Jr, Hudson JI, Pope HG., Jr A comparison of delusional and nondelusional body dysmorphic disorder in 100 cases. Psychopharmacol Bull. 1994;30:179–86. [PubMed] [Google Scholar]
- 3.Phillips KA. Psychosis in body dysmorphic disorder. J Psychiatr Res. 2004;38:63–72. doi: 10.1016/S0022-3956(03)00098-0. [DOI] [PubMed] [Google Scholar]
- 4.Li W, Arienzo D, Feusner JD. Body dysmorphic disorder: neurobiological features and an updated model. Z Klin Psychol Psychother. 2013;42:184–91. doi: 10.1026/1616-3443/a000213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grace SA, Labuschagne I, Kaplan RA, Rossell SL. The neurobiology of body dysmorphic disorder: a systematic review and theoretical model. Neurosci Biobehav Rev. 2017;83:83–96. doi: 10.1016/j.neubiorev.2017.10.003. [DOI] [PubMed] [Google Scholar]
- 6.Beilharz F, Castle DJ, Grace S, Rossell SL. A systematic review of visual processing and associated treatments in body dysmorphic disorder. Acta Psychiatr Scand. 2017;136:16–36. doi: 10.1111/acps.12705. [DOI] [PubMed] [Google Scholar]
- 7.Feusner JD, Moody T, Hembacher E, Townsend J, McKinley M, Moller H, et al. Abnormalities of visual processing and frontostriatal systems in body dysmorphic disorder. Arch Gen Psychiatry. 2010;67:197–205. doi: 10.1001/archgenpsychiatry.2009.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Feusner JD, Townsend J, Bystritsky A, Bookheimer S. Visual information processing of faces in body dysmorphic disorder. Arch Gen Psychiatry. 2007;64:1417–25. doi: 10.1001/archpsyc.64.12.1417. [DOI] [PubMed] [Google Scholar]
- 9.Feusner JD, Hembacher E, Moller H, Moody TD. Abnormalities of object visual processing in body dysmorphic disorder. Psychol Med. 2011;41:2385–97. doi: 10.1017/S0033291711000572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li W, Lai TM, Bohon C, Loo SK, McCurdy D, Strober M, et al. Anorexia nervosa and body dysmorphic disorder are associated with abnormalities in processing visual information. Psychol Med. 2015;45:2111–22. doi: 10.1017/S0033291715000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li W, Lai TM, Loo SK, Strober M, Mohammad-Rezazadeh I, Khalsa S, et al. Aberrant early visual neural activity and brain-behavior relationships in anorexia nervosa and body dysmorphic disorder. Front Hum Neurosci. 2015;9:301. doi: 10.3389/fnhum.2015.00301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Deckersbach T, Savage CR, Phillips KA, Wilhelm S, Buhlmann U, Rauch SL, et al. Characteristics of memory dysfunction in body dysmorphic disorder. J Int Neuropsychol Soc. 2000;6:673–81. doi: 10.1017/S1355617700666055. [DOI] [PubMed] [Google Scholar]
- 13.Feusner JD, Moller H, Altstein L, Sugar C, Bookheimer S, Yoon J, et al. Inverted face processing in body dysmorphic disorder. J Psychiatr Res. 2010;44:1088–94. doi: 10.1016/j.jpsychires.2010.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jefferies K, Laws KR, Fineberg NA. Superior face recognition in body dysmorphic disorder. J Obsessive Compuls Relat Disord. 2012;1:175–9. doi: 10.1016/j.jocrd.2012.03.002. [DOI] [Google Scholar]
- 15.Stangier U, Adam-Schwebe S, Müller T, Wolter M. Discrimination of facial appearance stimuli in body dysmorphic disorder. J Abnorm Psychol. 2008;117:435–43. doi: 10.1037/0021-843X.117.2.435. [DOI] [PubMed] [Google Scholar]
- 16.Mundy EM, Sadusky A. Abnormalities in visual processing amongst students with body image concerns. Adv Cogn Psychol. 2014;10:39–48. doi: 10.5709/acp-0155-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dhir S, Ryan HS, McKay EL, Mundy ME. Parameters of visual processing abnormalities in adults with body image concerns. PLoS One. 2018;13:e0207585. doi: 10.1371/journal.pone.0207585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moody TD, Morfini F, Cheng G, Sheen CL, Kerr WT, Strober M, et al. Brain activation and connectivity in anorexia nervosa and body dysmorphic disorder when viewing bodies: relationships to clinical symptoms and perception of appearance. Brain Imaging Behav. 2020. 2020. 10.1007/s11682-020-00323-5. [DOI] [PMC free article] [PubMed]
- 19.Beucke JC, Sepulcre J, Talukdar T, Linnman C, Zschenderlein K, Endrass T, et al. Abnormally high degree connectivity of the orbitofrontal cortex in obsessive-compulsive disorder. JAMA Psychiatry. 2013;70:619–29. doi: 10.1001/jamapsychiatry.2013.173. [DOI] [PubMed] [Google Scholar]
- 20.Rotge J-Y, Guehl D, Dilharreguy B, Cuny E, Tignol J, Bioulac B, et al. Provocation of obsessive-compulsive symptoms: a quantitative voxel-based meta-analysis of functional neuroimaging studies. J Psychiatry Neurosci. 2008;33:405–12. [PMC free article] [PubMed] [Google Scholar]
- 21.Johnson S, Williamson P, Wade TD. A systematic review and meta-analysis of cognitive processing deficits associated with body dysmorphic disorder. Behav Res Ther. 2018;107:83–94. doi: 10.1016/j.brat.2018.05.013. [DOI] [PubMed] [Google Scholar]
- 22.Phillips KA. The broken mirror: understanding and treating body dysmorphic disorder. Oxford, UK: Oxford University Press; 2005.
- 23.Greenberg JL, Reuman L, Hartmann AS, Kasarskis I, Wilhelm S. Visual hot spots: an eye tracking study of attention bias in body dysmorphic disorder. J Psychiatr Res. 2014;57:125–32. doi: 10.1016/j.jpsychires.2014.06.015. [DOI] [PubMed] [Google Scholar]
- 24.Grocholewski A, Kliem S, Heinrichs N. Selective attention to imagined facial ugliness is specific to body dysmorphic disorder. Body Image. 2012;9:261–9. doi: 10.1016/j.bodyim.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 25.Johnson S, Egan SJ, Andersson G, Carlbring P, Shafran R, Wade TD. Internet-delivered cognitive behavioural therapy for perfectionism: Targeting dysmorphic concern. Body Image. 2019;30:44–55. doi: 10.1016/j.bodyim.2019.05.002. [DOI] [PubMed] [Google Scholar]
- 26.Wilhelm S, Phillips KA, Greenberg JL. Efficacy and Posttreatment effects of therapist-delivered cognitive behavioral therapy vs supportive psychotherapy for adults with body dysmorphic disorder: a randomized clinical trial. JAMA Psychiatry. 2019;76:363–73. [DOI] [PMC free article] [PubMed]
- 27.Beilharz F, Rossell SL. Treatment modifications and suggestions to address visual abnormalities in body dysmorphic disorder. J Cogn Psychother. 2017;31:272–84. doi: 10.1891/0889-8391.31.4.272. [DOI] [PubMed] [Google Scholar]
- 28.Feusner J, Deshpande R, Strober M. A translational neuroscience approach to body image disturbance and its remediation in anorexia nervosa. Int J Eat Disord. 2017;50:1014–7. doi: 10.1002/eat.22742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moody TD, Sasaki MA, Bohon C, Strober MA, Bookheimer SY, Sheen CL, et al. Functional connectivity for face processing in individuals with body dysmorphic disorder and anorexia nervosa. Psychological Med. 2015;45:3491–503. doi: 10.1017/S0033291715001397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moody TD, Morfini F, Cheng G, Sheen CL, Kerr W, Strober M, et al. Brain activation and connectivity in anorexia nervosa and body dysmorphic disorder when viewing bodies: relationships to clinical symptoms and perception of appearance. Brain Imaging Behav. 2020. 10.1007/s11682-020-00323-5. [DOI] [PMC free article] [PubMed]
- 31.Beucke JC, Sepulcre J, Buhlmann U, Kathmann N, Moody T, Feusner JD. Degree connectivity in body dysmorphic disorder and relationships with obsessive and compulsive symptoms. Eur Neuropsychopharmacol. 2016;26:1657–66. doi: 10.1016/j.euroneuro.2016.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hutchison RM, Womelsdorf T, Allen EA, Bandettini PA, Calhoun VD, Corbetta M, et al. Dynamic functional connectivity: promise, issues, and interpretations. Neuroimage. 2013;80:360–78. doi: 10.1016/j.neuroimage.2013.05.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thompson GJ, Magnuson ME, Merritt MD, Schwarb H, Pan W-J, McKinley A, et al. Short-time windows of correlation between large-scale functional brain networks predict vigilance intraindividually and interindividually. Hum Brain Mapp. 2013;34:3280–98. doi: 10.1002/hbm.22140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sakoğlu U, Pearlson GD, Kiehl KA, Wang YM, Michael AM, Calhoun VD. A method for evaluating dynamic functional network connectivity and task-modulation: application to schizophrenia. MAGMA. 2010;23:351–66. doi: 10.1007/s10334-010-0197-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stark EA, Cabral J, Riem MME, Van IJzendoorn MH, Stein A, Kringelbach ML. The power of smiling: the adult brain networks underlying learned infant emotionality. Cereb cortex. 2019. 10.1093/cercor/bhz219. [DOI] [PMC free article] [PubMed]
- 36.Damaraju E, Allen EA, Belger A, Ford JM, McEwen S, Mathalon DH, et al. Dynamic functional connectivity analysis reveals transient states of dysconnectivity in schizophrenia. Neuroimage Clin. 2014;5:298–308. doi: 10.1016/j.nicl.2014.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jin C, Jia H, Lanka P, Rangaprakash D, Li L, Liu T, et al. Dynamic brain connectivity is a better predictor of PTSD than static connectivity. Hum Brain Mapp. 2017;38:4479–96. doi: 10.1002/hbm.23676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kaiser RH, Whitfield-Gabrieli S, Dillon DG, Goer F, Beltzer M, Minkel J, et al. Dynamic resting-state functional connectivity in major depression. Neuropsychopharmacology. 2016;41:1822–30. doi: 10.1038/npp.2015.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cabral J, Vidaurre D, Marques P, Magalhães R, Silva Moreira P, Miguel Soares J, et al. Cognitive performance in healthy older adults relates to spontaneous switching between states of functional connectivity during rest. Sci Rep. 2017;7:5135. doi: 10.1038/s41598-017-05425-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jia H, Hu X, Deshpande G. Behavioral relevance of the dynamics of the functional brain connectome. Brain Connect. 2014;4:741–59. doi: 10.1089/brain.2014.0300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Madhyastha TM, Askren MK, Boord P, Grabowski TJ. Dynamic connectivity at rest predicts attention task performance. Brain Connectivity. 2015;5:45–59. doi: 10.1089/brain.2014.0248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Figueroa CA, Cabral J, Mocking RJT, Rapuano KM, van Hartevelt TJ, Deco G, et al. Altered ability to access a clinically relevant control network in patients remitted from major depressive disorder. Hum Brain Mapp. 2019;40:2771–86. doi: 10.1002/hbm.24559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lord L-D, Expert P, Atasoy S, Roseman L, Rapuano K, Lambiotte R, et al. Dynamical exploration of the repertoire of brain networks at rest is modulated by psilocybin. NeuroImage. 2019;199:127–42. doi: 10.1016/j.neuroimage.2019.05.060. [DOI] [PubMed] [Google Scholar]
- 44.Eisen JL, Phillips KA, Coles ME, Rasmussen SA. Insight in obsessive compulsive disorder and body dysmorphic disorder. Compr Psychiatry. 2004;45:10–15. doi: 10.1016/j.comppsych.2003.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rief W, Buhlmann U, Wilhelm S, Borkenhagen A, Brähler E. The prevalence of body dysmorphic disorder: a population-based survey. Psychol Med. 2006;36:877–85. doi: 10.1017/S0033291706007264. [DOI] [PubMed] [Google Scholar]
- 46.Phillips KA, Hollander E, Rasmussen SA, Aronowitz BR, DeCaria C, Goodman WK. A severity rating scale for body dysmorphic disorder: development, reliability, and validity of a modified version of the Yale-Brown Obsessive Compulsive Scale. Psychopharmacol Bull. 1997;33:17–22. [PubMed] [Google Scholar]
- 47.Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134:382–9. doi: 10.1192/bjp.134.4.382. [DOI] [PubMed] [Google Scholar]
- 48.Hamilton M. The assessment of anxiety states by rating. Br J Med Psychol. 1959;32:50–55. doi: 10.1111/j.2044-8341.1959.tb00467.x. [DOI] [PubMed] [Google Scholar]
- 49.Eisen JL, Phillips KA, Baer L, Beer DA, Atala KD, Rasmussen SA. The brown assessment of beliefs scale: reliability and validity. Am J Psychiatry. 1998;155:102–8. doi: 10.1176/ajp.155.1.102. [DOI] [PubMed] [Google Scholar]
- 50.Koenig-Robert R, VanRullen R. SWIFT: a novel method to track the neural correlates of recognition. Neuroimage. 2013;81:273–82. doi: 10.1016/j.neuroimage.2013.04.116. [DOI] [PubMed] [Google Scholar]
- 51.Esteban O, Markiewicz CJ, Blair RW, Moodie CA, Isik AI, Erramuzpe A, et al. fMRIPrep: a robust preprocessing pipeline for functional MRI. Nat Methods. 2019;16:111–6. doi: 10.1038/s41592-018-0235-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15:273–89. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- 53.Deco G, Cabral J, Woolrich MW, Stevner ABA, van Hartevelt TJ, Kringelbach ML. Single or multiple frequency generators in on-going brain activity: a mechanistic whole-brain model of empirical MEG data. NeuroImage. 2017;152:538–50. doi: 10.1016/j.neuroimage.2017.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Deco G, Kringelbach M. Metastability and coherence: extending the communication through coherence hypothesis using a whole-brain computational perspective. Trends Neurosci. 2016;39:432. doi: 10.1016/j.tins.2016.04.006. [DOI] [PubMed] [Google Scholar]
- 55.Glerean E, Salmi J, Lahnakoski JM, Jääskeläinen IP, Sams M. Functional magnetic resonance imaging phase synchronization as a measure of dynamic functional connectivity. Brain Connect. 2012;2:91–101. doi: 10.1089/brain.2011.0068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ponce-Alvarez A, Deco G, Hagmann P, Romani GL, Mantini D, Corbetta M. Resting-state temporal synchronization networks emerge from connectivity topology and heterogeneity. PLoS Comput Biol. 2015;11:e1004100. doi: 10.1371/journal.pcbi.1004100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Newman MEJ. Finding community structure in networks using the eigenvectors of matrices. Phys Rev E. 2006;74:036104-1–036104-19. [DOI] [PubMed]
- 58.Thorsen AL, Hagland P, Radua J, Mataix-Cols D, Kvale G, Hansen B, et al. Emotional processing in obsessive-compulsive disorder: a systematic review and meta-analysis of 25 functional neuroimaging studies. Biol Psychiatry Cogn Neurosci Neuroimaging. 2018;3:563–71. doi: 10.1016/j.bpsc.2018.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Whiteside SP, Port JD, Abramowitz JS. A meta–analysis of functional neuroimaging in obsessive–compulsive disorder. Psychiatry Res. 2004;132:69–79. doi: 10.1016/j.pscychresns.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 60.Menzies L, Chamberlain SR, Laird AR, Thelen SM, Sahakian BJ, Bullmore ET. Integrating evidence from neuroimaging and neuropsychological studies of obsessive-compulsive disorder: the orbitofronto-striatal model revisited. Neurosci Biobehav Rev. 2008;32:525–49. doi: 10.1016/j.neubiorev.2007.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.American Psychiatric Association. Diagnostic and statistical manual of mental disorders (DSM-5®). Washington D.C., USA: American Psychiatric Pub; 2013.
- 62.Kringelbach ML, Rolls ET. The functional neuroanatomy of the human orbitofrontal cortex: evidence from neuroimaging and neuropsychology. Prog Neurobiol. 2004;72:341–72. doi: 10.1016/j.pneurobio.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 63.Rolls ET, Critchley HD, Browning AS, Inoue K. Face-selective and auditory neurons in the primate orbitofrontal cortex. Exp Brain Res. 2006;170:74–87. doi: 10.1007/s00221-005-0191-y. [DOI] [PubMed] [Google Scholar]
- 64.Bar M, Kassam KS, Ghuman AS, Boshyan J, Schmid AM, Dale AM, et al. Top-down facilitation of visual recognition. Proc Natl Acad Sci USA. 2006;103:449–54. doi: 10.1073/pnas.0507062103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kringelbach ML, Lehtonen A, Squire S, Harvey AG, Craske MG, Holliday IE, et al. A specific and rapid neural signature for parental instinct. PLoS One. 2008;3:e1664. doi: 10.1371/journal.pone.0001664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Young KS, Parsons CE, Jegindoe Elmholdt E-M, Woolrich MW, van Hartevelt TJ, Stevner ABA, et al. Evidence for a caregiving instinct: rapid differentiation of infant from adult vocalizations using magnetoencephalography. Cereb Cortex. 2016;26:1309–21. doi: 10.1093/cercor/bhv306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Haxby JV, Hoffman EA, Gobbini MI. Human neural systems for face recognition and social communication. Biol Psychiatry. 2002;51:59–67. doi: 10.1016/S0006-3223(01)01330-0. [DOI] [PubMed] [Google Scholar]
- 68.Haxby JV, Hoffman EA, Gobbini MI. The distributed human neural system for face perception. Trends Cogn Sci. 2000;4:223–33. doi: 10.1016/S1364-6613(00)01482-0. [DOI] [PubMed] [Google Scholar]
- 69.Fairhall SL, Ishai A. Effective connectivity within the distributed cortical network for face perception. Cereb Cortex. 2007;17:2400–6. doi: 10.1093/cercor/bhl148. [DOI] [PubMed] [Google Scholar]
- 70.Wu Y, Sun D, Wang Y, Wang Y. Subcomponents and connectivity of the inferior fronto-occipital fasciculus revealed by diffusion spectrum imaging fiber tracking. Front Neuroanat. 2016;10:88. doi: 10.3389/fnana.2016.00088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ishizu T, Zeki S. The brain’s specialized systems for aesthetic and perceptual judgment. Eur J Neurosci. 2013;37:1413–20. doi: 10.1111/ejn.12135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jacobsen T, Schubotz RI, Höfel L, Cramon DYV. Brain correlates of aesthetic judgment of beauty. Neuroimage. 2006;29:276–85. doi: 10.1016/j.neuroimage.2005.07.010. [DOI] [PubMed] [Google Scholar]
- 73.Hahn AC, Perrett DI. Neural and behavioral responses to attractiveness in adult and infant faces. Neurosci Biobehav Rev. 2014;46:591–603. doi: 10.1016/j.neubiorev.2014.08.015. [DOI] [PubMed] [Google Scholar]
- 74.Kranz F, Ishai A. Face perception is modulated by sexual preference. Curr Biol. 2006;16:63–8. doi: 10.1016/j.cub.2005.10.070. [DOI] [PubMed] [Google Scholar]
- 75.O’Doherty J, Kringelbach ML, Rolls ET, Hornak J, Andrews C. Abstract reward and punishment representations in the human orbitofrontal cortex. Nat Neurosci. 2001;4:95–102. doi: 10.1038/82959. [DOI] [PubMed] [Google Scholar]
- 76.Grocholewski A, Heinrichs N, Lingnau A. Selektive aufmerksamkeit im gesichtsbereich bei personen mit einem kosmetisch-medizinischen behandlungswunsch. Z Klin Psychol Psychother. 2007;36:57–66. doi: 10.1026/1616-3443.36.1.57. [DOI] [Google Scholar]
- 77.Phillips KA, Menard W, Pagano ME, Fay C, Stout RL. Delusional versus nondelusional body dysmorphic disorder: Clinical features and course of illness. J Psychiatr Res. 2006;40:95–104. doi: 10.1016/j.jpsychires.2005.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mancuso SG, Knoesen NP, Castle DJ. Delusional versus nondelusional body dysmorphic disorder. Compr Psychiatry. 2010;51:177–82. doi: 10.1016/j.comppsych.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 79.Buchy L, Ad-Dab’bagh Y, Lepage C, Malla A, Joober R, Evans A, et al. Symptom attribution in first episode psychosis: a cortical thickness study. Psychiatry Res. 2012;203:6–13. doi: 10.1016/j.pscychresns.2011.09.009. [DOI] [PubMed] [Google Scholar]
- 80.Shad MU, Muddasani S, Keshavan MS. Prefrontal subregions and dimensions of insight in first-episode schizophrenia—a pilot study. Psychiatry Res. 2006;146:35–42. doi: 10.1016/j.pscychresns.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 81.Shad MU, Keshavan MS. Neurobiology of insight deficits in schizophrenia: an fMRI study. Schizophr Res. 2015;165:220–6. doi: 10.1016/j.schres.2015.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Beer JS, John OP, Scabini D, Knight RT. Orbitofrontal cortex and social behavior: integrating self-monitoring and emotion-cognition interactions. J Cogn Neurosci. 2006;18:871–9. doi: 10.1162/jocn.2006.18.6.871. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Effects of visual attention modulation on dynamic functional connectivity during own-face viewing in body dysmorphic disorder