Abstract
Rutaecarpine is reported as a potent inducer of CYP1A2 enzyme in rats. There are natural herbal supplements containing rutaecarpine that are designed to enhance the CYP1A2-dependent removal of caffeine from blood so that people can have coffee later in the day without causing sleep interference. This study aimed to determine the minimum amount of time needed from oral rutaecarpine administration until the observed effect of rutaecarpine on caffeine pharmacokinetics (PK) in 15 male Sprague-Dawley rats. PK parameters for caffeine and its metabolites in the control and rutaecarpine groups were calculated using WinNonlin®. Results showed that orally administered rutaecarpine at 100 mg/kg dose as early as 3 h before oral caffeine administration significantly decreased the oral systemic exposure and mean residence time of caffeine and its metabolites due to decreased caffeine bioavailability (by up to 75%) and increased clearance. The systemic exposure of caffeine and its metabolites were also decreased when caffeine was given intravenously, though this effect was less pronounced than when caffeine was given orally. Although plasma level of rutaecarpine was undetectable (less than 10 ng/mL), rutaecarpine still induced hepatic CYP1A2 activity. Results from 7-methoxyresorufin O-demethylation activity, which is specific to CYP1A2, showed that 3 h after one rutaecarpine oral dose, CYP1A2 activity in rat liver tissue was increased by 3- fold. This finding suggested that rutaecarpine effectively induced CYP1A2 activity in the liver.
Keywords: Rutaecarpine, Caffeine, Time-dependent induction, CYP1A2, Rat
Abbreviations: AHR, aryl hydrocarbon receptor; GI, gastrointestinal tract; CYP, cytochrome P450; BCA, Bicinchoninic acid; intravenous, IV; pharmacokinetics, PK; MROD, 7-methoxyresorufin O-demethylation; ANOVA, Analysis of variance
Highlights
-
•
Rutaecarpine significantly decreases caffeine exposure as early as 3 h.
-
•
Rutaecarpine markedly decreases caffeine metabolites exposure as early as 3 h.
-
•
Rutaecarpine decreases the oral bioavailability of caffeine by up to 75% in rats; .
1. Introduction
Caffeine is one of the most researched food components, with the vast majority of dietary contributions coming from beverage consumption. 85% of the U.S. population consumes at least one caffeinated beverage per day [1]. Caffeine has varying impacts on the body such as increases in the basal metabolic rate, acts as a stimulant for heart and central nervous system, and relax smooth-muscle [2,3]. However, many people are sensitive to the effects of caffeine. Therefore, they either avoid drinking caffeinated beverages altogether or avoid them close to bedtime to prevent them from interfering with their sleep. Caffeine is mainly metabolized by the liver after fast and complete absorption from the gastrointestinal tract (GI, mainly small intestine). It is extensively metabolized via cytochrome P450 1A2 (CYP1A2) N-demethylation, and has three primary metabolites: paraxanthine (1, 7-dimethylxanthine), theobromine (3, 7-dimethylxanthine), and theophylline (1, 3-dimethylxatnhine) [3,4].
The formation of paraxanthine, theobromine and theophylline in humans account for 83.9 ± 5.4%, 12.2 ± 4.1 and 3.7 ± 1.3% of caffeine metabolism, respectively [3,5,6]. In rats, the formation of paraxanthine is less while the formation of theophylline and theobromine is of the same order [7]. Each of the primary demethylation products is further metabolized to form a variety of xanthines, uric acids, and uracils [8]. Rutaecarpine, a pentacyclic indolopyridoquinazolinone alkaloid originally isolated from the unripe fruit of Evodia rutaecarpa [9], has been reported to reduce the systemic exposure of caffeine, as well as a potent inducer of cytochrome P450 (CYP) (CYP1A2 and CYP2E1) enzymes in rats [10]. It is also known to have vasodilation, anti-thrombosis, analgesic, and antianoxic effects [11]. CYP1A2 plays a critical role in drug metabolism because of its wide tissue distribution and high expression in vivo [12]. Induction of CYP1A2 enzymes is expected to alter the pharmacokinetics (PK) of drugs that are substrates of CYP1A2, such as caffeine. There are natural herbal supplements (such as Ruta Cleanse and Ruta Sleep) on the market, with rutaecarpine as the active ingredient, which are designed to speed up the removal of caffeine from the body. The recommendation is to take two capsules (equivalent to 100 mg rutaecarpine), as needed, to reduce caffeine level. Rutaecarpine has been shown to speed up caffeine elimination in rats, after once a day oral dosing for 3 days [11]. However, there is no scientific data to show how soon a single oral dose of rutaecarpine can promote caffeine elimination in vivo.
The present study aimed to determine the minimum amount of time needed from the time of oral rutaecarpine administration, to observe the effect of rutaecarpine induction on caffeine elimination. The in vivo PK of caffeine along with its metabolites (paraxanthine, theophylline and theobromine) in rats was evaluated when caffeine was administered either orally (20 mg/kg) or via intravenous (IV) bolus injection (15 mg/kg) after various oral rutaecarpine pretreatment (zero (oral group only), 3, 6, and 12 h rutaecarpine pretreatment). These doses were chosen based on a previously published study in rats. In the published paper by Noh. et al., 20 mg/kg caffeine and 80 mg/kg rutaecarpine were administered orally [11]. Therefore, for IV caffeine dosing, 15 mg/kg caffeine was selected to account for the incomplete bioavailability of caffeine. We would like to maximize the rutaecarpine dose to see what was the minimum time needed to observe the induction effect by rutaecarpine, but due to rutaecarpine's limited solubility, 100 mg/kg was used in this study.
2. Materials and methods
2.1. Reagents and animals
Rutaecarpine (>98%), caffeine (>99%), cremophor EL (polyethoxylated castor oil), methoxyresorufin, resorufin (95%), β-nicotinamide adenine dinucleotide phosphate (β-NADPH) and corn oil were obtained from Sigma-Aldrich (USA). Paraxanthine was from Fisher Scientific. Theophylline and theobromine were purchased from Enzo Life Sciences. Caffeine-d9 (internal standard) was purchased from CDN Isotopes (Quebec, Canada). Pierce™ Bicinchoninic acid (BCA) Protein Assay Kit was from Thermo Scientific. Tissue miser homogenizer was from Fisher Scientific, and Teflon glass hand homogenizer was obtained from Thermo Scientific. 96 well UV and fluorescence microplates (with transparent bottom) and analytical grade methanol were obtained from Spectrum Chemicals (Gardena, CA). Tristar LB 941 microplate spectrophotometer was from Berthold Technologies (Oak Ridge, TN). Heparin (10,000 U), 1 mL Norm-Ject syringes, infusion plugs, normal saline, 50% dextrose and isoflurane were obtained from Patterson Veterinary (CA). VIP 3000 isoflurane Matrx anesthesia equipment was purchased from Ebay. The rat harness was from SAI Infusion (Lake Villa, IL).
Male Sprague-Dawley rats (250–300 g) were obtained from Charles River. Upon arrival, animals were caged individually in strictly controlled conditions of 23 ± 3 °C and 50 ± 10% relative humidity and were acclimated before experiments. A 12-h light-dark cycle was maintained with free access to rodent cubes and tap water. Rats were sacrificed using CO2 gas asphyxiation followed by a physical method to ensure death. All animal protocols were approved by the Animal Care Committee of University of the Pacific and complied with the NIH Guidelines on Care for Animal Use in research. The animal model was chosen based on a previously published study in rats [11].
2.2. Rat liver and intestinal samples preparation
Animals (previously used for oral PK dosing studies first, after a 2-week washout period, used for IV PK dosing studies, then after one week washout period after IV studies were used for this tissue collection study) were randomly divided into four groups (n =3/group): a) control, b) 3 h, c) 6 h, d) 12 h treatment groups. Animals in groups a and b were sacrificed 3 h after 100 mg/kg oral rutaecarpine dose while animals in groups c and d were sacrificed 6, and 12 h, respectively, after 100 mg/kg oral rutaecarpine dose. Livers and intestines were perfused with cold phosphate buffered saline and harvested. Collected livers and intestines were placed on a dry ice-ethanol bath to freeze them instantly before storage in −80 °C.
2.3. Microsome preparation
All samples were prepared on ice at all times. Liver and intestine tissues were thawed on ice and approximately 1 g of each tissue was finely cut, followed by homogenization in tissue homogenizer with homogenization buffer (85.3 g of sucrose, 11 g of potassium chloride, 2 mL of 0.5 M EDTA pH 8, reconstituted with 0.1 M phosphate buffer pH 7.4 to make 1 L solution). Homogenization was performed until tissues were completely suspended in the buffer. The homogenate was then transferred into ice-cold centrifuge tubes and centrifuged at 4 °C for 20 min at 9000 g. The post-mitochondrial supernatant fraction was carefully collected without disturbing the pellet and transferred to ice-cold ultra-centrifuge tubes and centrifuged at 105,000 g for 65 min at 4 °C to yield microsomal pellets, which were re-suspended in 1:1 (w/v) re-suspension buffer (0.1 M phosphate buffer of pH 7.4, 0.25 M sucrose). The resulting samples were transferred to Eppendorf tubes (pre-chilled on ice) and stored in −80 °C until protein quantitation and 7-methoxyresorufin O-demethylation (MROD) assay.
2.4. BCA assay for protein quantitation
A standard calibration curve for bovine serum albumin with seven concentrations, ranging from 0 to 2 mg/mL, was prepared in duplicate. All serial dilutions were made with 0.1 M phosphate buffer. Liver and intestine microsome samples were diluted 20X with 0.1 M phosphate buffer to a final volume of 40 μL. 10 μL of standards and 10 μL microsome samples were added individually into each well of 96-well plate. With a multi-channel pipetter, 190 μL of Pierce reagent A and B mixture (50:1, respectively) was added to each well (containing samples or standards). The plate was then placed on a shaker for about 5 min and incubated at 37 °C for 30 min, after which the reactions were analyzed at 562 nm using Tristar LB 941 microplate spectrophotometer. The concentrations of microsomal samples were diluted to 10 and 5 mg/mL for the liver and intestine, respectively.
2.5. MROD assay for CYP1A2 activity
The activity of CYP1A2 is measured as a rate of the O-dealkylation of 7-methoxyresorufin into resorufin in the presence of NADPH [13,14]. Resorufin can be detected using the fluorimetric assay [15]. MROD activity was measured in the rat livers and intestinal microsomes. Two sets of master mix, one with NADPH and another without NADPH, were prepared (in 10% excess) and 75 μL of this mixture was added to each microcentrifuge. The reaction master mixture consisted of 10 μM methoxyresorufin, 5 mM NADPH, and 0.1 M phosphate buffer of pH 7.4. This master mix was allowed to equilibrate in the water bath at 37 °C for a few minutes before 25 μL of 10 mg/mL of rat liver (or 5 mg/mL of intestinal) microsome was added to each microfuge and mixed to start the reaction and incubated for 60 min. Ice cold methanol (200 μL) was then added to terminate the reaction. The mixture was vortexed and centrifuged at 16,000 g for 10 min. 200 μL of the supernatant was collected and transferred to 96 well plates which were then analyzed by a fluorescence detector. The formation of resorufin was monitored fluorometrically at an excitation wavelength of 544 nm and an emission wavelength of 590 nm. For calibration, resorufin concentrations ranging from 0 to 5 μM were prepared in 0.1 M phosphate buffer to calculate the amount of resorufin in each well.
2.6. Oral and IV PK study
Rats were randomly divided into two sets: oral and IV. Each set was divided into four groups (n =3/group): a) control, b) 3-h, c) 6-h, d) 12-h treatment groups. The oral set had an extra group (zero-hour) of rats where the rats received 100 mg/kg of rutaecarpine orally, followed immediately with 20 mg/kg of caffeine orally. The group name represented the time lapse between rutaecarpine and caffeine dose. Rats were pretreated with vehicle (control group) or 100 mg/kg of rutaecarpine orally (administered via oral gavage) for either 0, 3, 6, or 12 h before 20 mg/kg of caffeine oral dose (for oral study). For IV caffeine study, 15 mg/kg caffeine was administered IV via cannulated jugular vein 3, 6, or 12 h after 100 mg/kg oral rutaecarpine dose. Caffeine was formulated in saline (12 mg/mL) and rutaecarpine was a suspension (24 mg/mL) in vehicle containing ethanol, cremophor EL and water (20:20:60). The control group was pretreated with vehicle alone. Blood samples (∼100 μL) were collected via jugular vein immediately before and 0.083, 0.5, 1, 2, 4, 6, 7, 8, 10 and 12 h after oral/IV dose of caffeine, followed by centrifugation at 8,000 g for 5 min at 4 °C to obtain plasma, which were stored at −80 °C until analysis.
2.7. LC-MS/MS analysis
Due to the high sensitivity of LC-MS/MS, high concentration samples were diluted by either 2 or 4-folds. To 40 μL of plasma sample/standard, 80 μL of methanol containing 0.1% formic acid and 80 ng/mL caffeine d9 (internal standard for caffeine) and 100 ng/mL nitro-rutaecarpine (internal standard for rutaecarpine) was added to precipitate the proteins. The mixture was vortexed and centrifuged at 16,000g for 20 min at 4 °C. 90 μL of resulting supernatant was collected, transferred to HPLC vials with inserts and analyzed. Samples were injected at a flow rate of 0.5 mL/min into Phenomenex Kinetex column (4.6 × 250 mm, 5 μ). The assembly consisted of Agilent 1100 system (Agilent Technologies, Palo Alto, CA, USA) with the API 3000 LC-MS/MS System, a triple quad mass spectrometer equipped with electronspray ionization source. The column temperature was maintained at 20 °C. The mobile phase for caffeine and its metabolites consisted of HPLC grade methanol and 0.1% formic acid in distilled water (1:3 v/v). Isocratic program was used for the HPLC separation at a flow rate of 0.5 mL/min and the injection volume was 20 μL. Quantification was performed using multiple reaction monitoring. The mass transitions used for caffeine, paraxanthine, theophylline, theobromine and caffeine d-9 were positive ion mode with m/z 195.1 → 138.0, 180.1 → 124.0, 180.9 → 123.9, 181.1 → 138.0 and 203.9 → 144.0, respectively; declustering potential of 41, 41, 46, 36 and 41 V, respectively; entrance potential, all at 10 V; collision energy of 27, 27, 29, 27 and 35 V, respectively; and collision exit potential of 10, 8, 22, 10 and 12 V, respectively. Retention time of theobromine was 8–9 min, paraxanthine was 11–12 min, theophylline was 13–14 min, caffeine d9 was 18–19 min, and caffeine was 19–20 min. The total run time was 21 min. The same samples were re-run with Phenomenex Synergi 4 μ fusion column (2.5 × 250 mm) for the measurement of rutaecarpine concentration. The mobile phase for rutaecarpine consisted of HPLC grade methanol and 0.1% formic acid in distilled water (4:1 v/v). The mass transitions used for rutaecarpine and nitro-rutaecarpine were positive ion mode with m/z 287.8 → 273.2 and 333.3 → 287.1, respectively; declustering potential of 81 and 36 V, respectively; entrance potential of 10 V; collision energy of 47 and 43 V, respectively; and collision exit potential of 16 and14 V, respectively. The calibration curve for caffeine, paraxanthine, theophylline, theobromine and rutaecarpine in rat plasma were derived from the regression of the peak area ratios relative to that of caffeine d9 (nitro-rutaecarpine for rutaecarpine), with 1/x as the weighing factor. The respective quality control samples were analyzed along with each batch of plasma samples. The lower limit of quantitation and detection of caffeine, paraxanthine, theophylline and theobromine were 50 ng/mL while the lower limit of quantitation and detection of rutaecarpine was 10 ng/mL. However, plasma concentrations of rutaecarpine at all time points were below the lower limit of quantitation of 10 ng/mL, therefore no PK parameters of rutaecarpine were reported here.
2.8. PK data analysis
PK parameters were calculated using WinNonlin® Professional software, version 2.1 (Pharsight, Mountain View, CA). Non-compartmental analysis for IV bolus input (Model 201) and extravascular input (Model 200) were employed to estimate the PK parameters of caffeine, paraxanthine, theophylline and theobromine. Bioavailability (F) value is calculated by plugin the corresponding average AUC values from the same hour group and using the equation below:
2.9. Statistical analysis
Analysis of variance (ANOVA), posthoc Dunnett's test (compares control group with every treatment group) and Tukey's test (compares means of each group with every other group) was performed on all the data sets. Natural log-transformed Cmax and AUCinf were used for the analysis. Data were analyzed using GraphPad Prism Version 6.01 (GraphPad Software Inc., San Diego, CA, USA). The acceptance level of statistical significance was P < 0.05.
3. Results
3.1. Time-dependent induction of the CYP1A2 activity by rutaecarpine
MROD assay showed that CYP1A2 activity was increased up to 12 h after a single oral dose of rutaecarpine in rats (Fig. 1). In the liver, CYP1A2 activity increased with increasing time between the oral administration of 100 mg/kg rutaecarpine and the harvest of liver tissues. The highest CYP1A2 activity (about 8-fold compared to control) was found in the liver of 12 h rutaecarpine pre-treatment group. The data also showed that as early as 3 h after rutaecarpine administration, CYP1A2 activity in the liver tissue was increased by almost 3-fold compared to controls. Results for rat intestinal MROD assay were not included as there was no detectable fluorescence, indicating the absence of the CYP1A2 enzyme in the intestine.
Fig. 1.
MROD assay to estimate CYP1A2 enzyme activity in rat liver microsomes for control, 3, 6, and 12 h groups. Each bar represents the mean ± S.D. (n = 3). **p < 0.0001.
3.2. Time effect of rutaecarpine treatment on caffeine PK
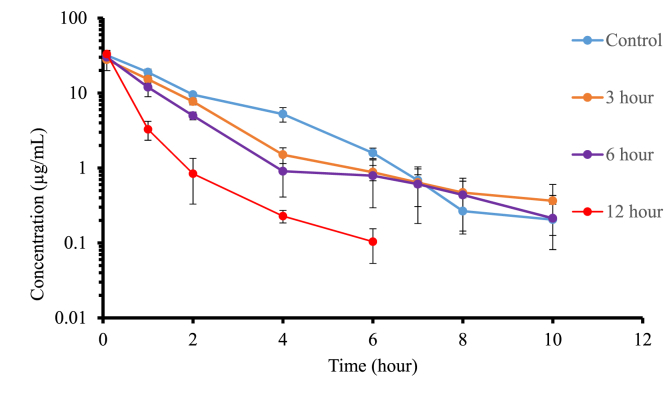
Fig. 2 (a), Fig. 2 (b) showed the concentration vs. time profile of IV (15 mg/kg) and oral caffeine (20 mg/kg) following pretreatment of rats with 100 mg/kg oral rutaecarpine. For the IV study, the concentration of caffeine was still detectable even 10 h after caffeine administration in the control, 3, and 6 h-groups, while in the 12-h group it was undetectable past 6 h (less than 50 ng/mL) (Fig. 2 (a), Table 1). Cmax of caffeine in the 3, 6, and 12-h groups was similar to control, which were 88%, 98%, and 122% of control, respectively.
Fig. 2 (a).
Logarithmic plot of time course of mean plasma concentrations of caffeine following 15 mg/kg caffeine administered intravenously in the presence and absence of 100 mg/kg oral rutaecarpine. Each value represents the mean ± S.D. (n = 3).
Fig. 2 (b).
Logarithmic plot of time course of mean plasma concentrations of caffeine following 20 mg/kg caffeine administered orally in the presence and absence of 100 mg/kg rutaecarpine. Each value represents the mean ± S.D. (n = 3).
Table 1.
Pharmacokinetic parameters of caffeine following IV administration of 15 mg/kg caffeine in the presence and absence of 100 mg/kg rutaecarpine in rats. Each value represents the mean ± S.D. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.
| Caffeine (IV) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Parameter | AUCINF (μg.h/mL) | Clearance (mL/h) | MRTinf (h) | T 1/2 (h) | Vss (mL) | |||||
| Control | 64.3 ± 2.3 | % of control | 56.1 ± 2.1 | % of control | 1.85 ± 0.16 | % of control | 1.28 ± 0.12 | % of control | 103.90 ± 11.49 | % of control |
| 3 h | 48.7 ± 5.5 | 76% | 74.6 ± 8.5 | 133% | 1.88 ± 0.28 | 102% | 2.91 ± 1.53 | 227% | 140.17 ± 23.84 | 135% |
| 6 h | 40.9 ± 4.1*** | 64% | 88.7 ± 8.9* | 158% | 1.70 ± 0.59 | 92% | 3.20 ± 2.06 | 250% | 149.10 ± 49.47 | 144% |
| 12 h | 23.5 ± 3.4**** | 37% | 155.7 ± 23.9*** | 278% | 0.52 ± 0.06** | 28% | 1.64 ± 1.08 | 128% | 79.55 ± 8.75 | 77% |
For the oral study, in the control and zero hour groups, the concentration of caffeine was still detectable even 8 h after administration, while for 3, 6, and 12-h groups, the concentration of caffeine was undetectable past 4 h (less than 50 ng/mL) (Fig. 2 (b), Table 2). Pretreated groups of 3, 6, and 12 h showed a rapid decline in caffeine plasma concentration between 2 and 4 h after caffeine administration. Similarly, for the zero-hour group, a rapid decline in caffeine plasma concentration occurred after 5 h. As shown in Table 1, Table 2, there was a more pronounced decrease (compared to control) in the systemic exposure of caffeine for caffeine administered orally versus IV, due to a decrease in clearance and a decrease in oral bioavailability for the oral group. There was a statistically significant decrease in t1/2 following oral administration, while unexpectedly, no statistically significant change was observed in the t1/2 of caffeine administered intravenously. IV study showed that clearance was increased (about 2-fold in the 12-h group) and combining the IV data with the oral data for the 12-h group showed that oral bioavailability was decreased (about 2-fold in the 12-h group) with rutaecarpine pretreatment.
Table 2.
Pharmacokinetic parameters of caffeine following oral administration of 20 mg/kg caffeine in the presence and absence of 100 mg/kg rutaecarpine in rats. Each value represents the mean ± S.D. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.
| Caffeine (oral) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Parameter | Cmax (μg/mL) | T 1/2 (h) | MRTinf (h) | AUCinf (μg.h/mL) | Tmax (h) | Cl/F (mL/h) | F (%) | ||||||
| Control | 19.5 ± 2.0 | % of control | 1.12 ± 0.15 | % of control | 2.34 ± 0.29 | % of control | 72.4 ± 10.0 | % of control | 0.8 ± 0.3 | % of control | 70.0 ± 10.0 | % of control | 81.1 ± 11.4 |
| Zero hour | 13.4 ± 3.1 | 69% | 0.62 ± 0.07*** | 55% | 2.63 ± 0.13 | 112% | 57.2 ± 10.2 | 79% | 2.0 ± 0.0**** | 250% | 89.3 ± 16.4 | 128% | 64.11 ± 11.77 |
| 3 h | 13.1 ± 0.6 | 67% | 0.61 ± 0.11*** | 54% | 1.27 ± 0.12**** | 54% | 26.9 ± 3.1**** | 37% | 0.5 ± 0.5 | 62.5% | 187.7 ± 22.5 | 268% | 39.8 ± 6.4** |
| 6 h | 10.5 ± 1.0** | 54% | 0.59 ± 0.16*** | 53% | 1.13 ± 0.23**** | 48% | 18.7 ± 5.6**** | 26% | 0.7 ± 0.3 | 87.5% | 287.4 ± 100.6 | 411% | 32.8 ± 10.2*** |
| 12 h | 12.9 ± 5.1* | 66% | 0.35 ± 0.13**** | 31% | 0.73 ± 0.17**** | 31% | 12.5 ± 4.1**** | 17% | 0.5 ± 0.0 | 62.5% | 434.8 ± 163.9* | 621% | 38.3 ± 13.6*** |
3.3. Time effect of rutaecarpine treatment on caffeine metabolites PK
As caffeine and its metabolites (paraxanthine, theophylline and theobromine) are substrates for CYP1A2, the impact of a time-dependent induction of CYP1A2 enzyme by rutaecarpine on their systemic exposure are unknown. This depends on the production and the elimination of the metabolites. In both the control and zero hour groups from the oral set, paraxanthine and theophylline were still detectable between 10 and 12 h after caffeine dose while theobromine was still detectable between 8 and 12 h after administration of caffeine. Meanwhile all the treatment groups showed rapid decline in paraxanthine plasma concentrations between 2 and 6 h after administration of caffeine, while they showed rapid decline in theophylline and theobromine plasma concentrations between 4 and 8 h after administration of caffeine (Fig. 3 (a), Fig. 3(b), Fig. 4 (a), Fig. 4 (b), Fig. 5 (a), Fig. 5 (b)). The caffeine metabolites showed as formation rate limited after IV administration as the metabolites concentration time profiles parallel caffeine profiles (control group) except for the 3-h group which could be due to large variability within the group (Fig. 3 (a), 4 (a), 5 (a)).
Fig. 3 (a).
Logarithmic plot of time course of mean plasma concentrations of paraxanthine following 15 mg/kg caffeine administered intravenously in the presence and absence of 100 mg/kg rutaecarpine. Each value represents the mean ± S.D. (n = 3).
Fig. 3(b).
Logarithmic plot of time course of mean plasma concentrations of paraxanthine following 20 mg/kg caffeine administered orally in the presence and absence of 100 mg/kg rutaecarpine. Each value represents the mean ± S.D. (n = 3).
Fig. 4 (a).
Logarithmic plot of time course of mean plasma concentrations of theophylline following 15 mg/kg caffeine administered intravenously in the presence and absence of 100 mg/kg rutaecarpine. Each value represents the mean ± S.D. (n = 3).
Fig. 4 (b).
Logarithmic plot of time course of mean plasma concentrations of theophylline following 20 mg/kg caffeine administered orally in the presence and absence of 100 mg/kg rutaecarpine. Each value represents the mean ± S.D. (n = 3).
Fig. 5 (a).
Logarithmic plot of time course of mean plasma concentrations of theobromine following 15 mg/kg caffeine administered intravenously in the presence and absence of 100 mg/kg rutaecarpine. Each value represents the mean ± S.D. (n = 3).
Fig. 5 (b).
Logarithmic plot of time course of mean plasma concentrations of theobromine following 20 mg/kg caffeine administered orally in the presence and absence of 100 mg/kg oral rutaecarpine. Each value represents the mean ± S.D. (n = 3).
4. Discussion
Rutaecarpine has been used in combination with other drugs in the treatment of various disorders related to the GI tract and found to produce herb-drug interactions. The basis of these herb-drug interactions is not completely understood [16]. As caffeine and its metabolites (paraxanthine, theophylline and theobromine) are substrates of CYP1A2, we hypothesized and confirmed that a time-dependent induction of CYP1A2 enzyme by rutaecarpine would reduce their systemic exposure. For the orally administered caffeine, the decrease in the systemic exposure of caffeine levels correlated with increased CYP1A2 activity seen in the MROD assay, largest for the 12-h treatment groups and smallest for the 3-h treatment groups (Figs. 1 and 2 (b)). For caffeine administered intravenously, the in vivo effects also correlate directly with changes in the CYP1A2 activity as measured in MROD assay, where the largest decrease in the systemic exposure of caffeine and its metabolites were observed in the 12 h rutaecarpine treatment group (Figs. 2 (a), 4 (a), 5 (a) and 6 (a)).
For all treatment groups (except the zero-hour group) in the oral arm, rutaecarpine significantly decreased the mean residence time for caffeine (Table 2) and its metabolites (Table 4, Table 6, Table 8). Similarly, for the IV arm of the study, rutaecarpine also significantly decreased the mean residence time for all treatment groups (Table 1, Table 3, Table 5, Table 7). These results indicated rutaecarpine administration at least 3 h before caffeine administration helped decrease the residence time of caffeine and its metabolites in the blood. Interestingly, rutaecarpine achieved this effect without achieving a detectable plasma level (less than 10 ng/mL). Rutaecarpine was shown to increase the systemic clearance (CL) of caffeine (12-h group, 278% of control, Table 1) and reduce its oral bioavailability (F) in rats (12-h group, 47% of control, Table 2). It is known that rutaecarpine induces CYP1A2 via aryl hydrocarbon receptor (AHR) [17], thus faster caffeine elimination that we observed was likely mediated by AHR. Upon rutaecarpine binding to AHR, the AHR signaling pathway is activated, which induces the cyp1a2 gene transcription, a downstream gene in the AHR signaling pathway. This induction of gene transcription would increase the intrinsic clearance value for caffeine, which leads to higher total CL for caffeine in rats as caffeine is a low hepatic extraction ratio drug and hepatic metabolism is a dominant elimination pathway for caffeine in rats. However, the mechanism by which rutaecarpine decreased the oral bioavailability of caffeine is not well understood. This decrease in caffeine oral bioavailability was unlikely due to direct rutaecarpine-caffeine interactions at the GI tract as the data from the zero-hour group (where rutaecarpine and caffeine are administered orally less than 5 min apart) showed no change in plasma concentration of caffeine up to 6 h after administration (Fig. 2 (a), Fig. 2 (b)). MROD assay with small intestine tissue samples showed no CYP1A2 activity, as expected since CYP1A2 expression was not reported in rat small intestines. Contribution of CYP1A1 to caffeine metabolism is rather insignificant, according to the literature [18]. However, rutaecarpine likely induces CYP1A1 through activation of AHR; therefore, we cannot rule out the possibility that decreased fraction escaping gut-wall elimination might be due to the induced CYP1A1 activity. Further experiment will be needed to rule out this possibility.
Table 4.
Pharmacokinetic parameters of paraxanthine following oral administration of 20 mg/kg caffeine in the presence and absence of 100 mg/kg rutaecarpine in rats. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.
| Paraxanthine (oral caffeine) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Parameter | Cmax (μg/mL) | T 1/2 (h) | MRTinf (h) | AUCinf (μg.h/mL) | Tmax (h) | AUCm/AUCp | ||||||
| Control | 2.8 ± 0.9 | % of control | 1.59 ± 0.28 | % of control | 5.06 ± 1.07 | % of control | 19.0 ± 6.6 | % of control | 4.00 ± 0.00 | % of control | 0.68 ± 0.06 | % of control |
| Zero hour | 3.3 ± 0.1 | 118% | 0.71 ± 0.04 | 45% | 5.10 ± 0.16 | 101% | 15.2 ± 1.2 | 80% | 6.00 ± 0.00**** | 150% | 0.67 ± 0.02 | 99% |
| 3 h | 2.1 ± 0.4 | 75% | 1.11 ± 0.56 | 70% | 3.03 ± 0.38* | 60% | 8.7 ± 2.6** | 46% | 2.00 ± 0.00**** | 50% | 0.65 ± 0.07 | 95% |
| 6 h | 1.9 ± 0.1 | 68% | 0.82 ± 0.36 | 52% | 2.16 ± 0.47*** | 43% | 5.0 ± 0.7*** | 26% | 2.00 ± 0.00**** | 50% | 0.56 ± 0.04 | 82% |
| 12 h | 3.2 ± 1.0 | 114% | 0.55 ± 0.39 | 35% | 1.57 ± 0.33*** | 31% | 6.5 ± 2.1*** | 34% | 1.33 ± 0.58**** | 33% | 0.74 ± 0.10 | 109% |
Table 6.
Pharmacokinetic parameters of theophylline following oral administration of 20 mg/kg caffeine in the presence and absence of 100 mg/kg rutaecarpine. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.
| Theophylline (oral caffeine) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Parameter | Cmax (μg/mL) | T 1/2 (h) | MRTinf (h) | AUCinf (μg.h/mL) | Tmax (h) | AUCm/AUCp | ||||||
| Control | 6.6 ± 1.6 | % of control | 1.09 ± 0.10 | % of control | 4.37 ± 0.96 | % of control | 37.7 ± 6.6 | % of control | 3.33 ± 1.15 | % of control | 0.85 ± 0.06 | % of control |
| Zero hour | 8.5 ± 0.3 | 129% | 0.62 ± 0.07 | 57% | 4.77 ± 0.10 | 109% | 36.5 ± 0.5 | 97% | 6.00 ± 0.00**** | 180% | 0.89 ± 0.04 | 105% |
| 3 h | 6.4 ± 1.5 | 97% | 0.97 ± 0.15 | 89% | 2.48 ± 0.22*** | 57% | 20.7 ± 5.6*** | 55% | 2.00 ± 0.00* | 60% | 0.91 ± 0.05 | 108% |
| 6 h | 9.9 ± 0.9* | 150% | 0.73 ± 0.08** | 67% | 1.93 ± 0.22**** | 44% | 27.9 ± 2.2* | 74% | 2.00 ± 0.00* | 60% | 1.16 ± 0.15* | 137% |
| 12 h | 8.4 ± 1.6 | 127% | 0.37 ± 0.03**** | 34% | 1.21 ± 0.19**** | 28% | 15.3 ± 3.1**** | 41% | 1.00 ± 0.00**** | 30% | 1.10 ± 0.11 | 130% |
Table 8.
Pharmacokinetic parameters of theobromine following oral administration of 20 mg/kg caffeine in the presence and absence of rutaecarpine in rats. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.
| Theobromine (oral caffeine) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Parameter | Cmax (μg/mL) | T1/2 (h) | MRTinf (h) | AUCinf (μg.h/mL) | Tmax (h) | AUCm/AUCp | ||||||
| Control | 3.16 ± 0.74 | % of control | 2.66 ± 0.22 | % of control | 5.96 ± 0.88 | % of control | 23.8 ± 3.8 | % of control | 4.00 ± 0.00 | % of control | 0.74 ± 0.03 | % of control |
| Zero hour | 2.12 ± 0.03* | 67% | 1.39 ± 0.68*** | 52% | 6.35 ± 0.60 | 107% | 9.1 ± 2.7 | 38% | 6.00 ± 0.00**** | 150% | 0.54 ± 0.07 | 73% |
| 3 h | 3.07 ± 0.43 | 97% | 1.26 ± 0.18*** | 47% | 3.20 ± 0.20*** | 54% | 14.2 ± 1.9** | 60% | 2.00 ± 0.00* | 50% | 0.81 ± 0.03 | 109% |
| 6 h | 5.86 ± 1.09*** | 185% | 1.22 ± 0.19*** | 46% | 2.65 ± 0.33**** | 44% | 21.0 ± 1.6* | 88% | 2.00 ± 0.00* | 50% | 1.06 ± 0.15** | 144% |
| 12 h | 4.91 ± 0.97* | 155% | 0.86 ± 0.15**** | 32% | 1.64 ± 0.18**** | 28% | 11.3 ± 2.4**** | 47% | 1.33 ± 0.58**** | 33% | 0.98 ± 0.08* | 132% |
Table 3.
Pharmacokinetic parameters of paraxanthine after administration of 15 mg/kg caffeine intravenously in the. presence and absence of rutaecarpine in rats. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.
| Paraxanthine (IV caffeine) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Parameter | AUCINF (μg.h/mL) | Tmax (h) | MRTinf (h) | T 1/2 (h) | AUCm/AUCp | |||||
| Control | 21.571 ± 2.743 | % of control | 4.667 ± 1.15 | % of control | 5.03 ± 0.43 | % of control | 1.349 ± 0.09 | % of control | 0.74 ± 0.04 | % of control |
| 3 h | 8.31 ± 0.72**** | 39% | 2.67 ± 1.15* | 57% | 2.85 ± 0.28**** | 57% | 0.47 ± 0.10**** | 35% | 0.54 ± 0.03** | 73% |
| 6 h | 6.17 ± 0.59**** | 29% | 2.0 ± 0.00** | 43% | 2.57 ± 0.27**** | 51% | 0.82 ± 0.07** | 61% | 0.51 ± 0.04** | 69% |
| 12 h | 3.86 ± 1.30**** | 18% | 1.0 ± 0.00*** | 21% | 1.28 ± 0.212**** | 25% | 0.49 ± 0.24**** | 37% | 0.42 ± 0.13*** | 57% |
Table 5.
Pharmacokinetic parameters of theophylline after administration of 15 mg/kg caffeine intravenously in the presence and absence of rutaecarpine in rats. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.
| Theophylline (IV caffeine) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Parameter | AUCINF (μg.h/mL) | Tmax (h) | MRTinf (h) | T 1/2 (h) | AUCm/AUCp | |||||
| Control | 29.412 ± 1.286 | % of control | 4.000 ± 0.00 | % of control | 4.34 ± 0.29 | % of control | 0.985 ± 0.12 | % of control | 0.81 ± 0.01 | % of control |
| 3 h | 17.532 ± 1.331**** | 60% | 2.000 ± 0.00* | 50% | 2.58 ± 0.20**** | 59% | 0.653 ± 0.14* | 66% | 0.74 ± 0.03 | 91% |
| 6 h | 16.181 ± 1.082**** | 55% | 1.000 ± 0.00** | 25% | 2.11 ± 0.19**** | 49% | 0.811 ± 0.17 | 83% | 0.76 ± 0.04 | 94% |
| 12 h | 10.169 ± 3.599**** | 34% | 1.000 ± 0.00*** | 25% | 1.04 ± 0.118**** | 24% | 0.384 ± 0.02*** | 39% | 0.72 ± 0.13 | 89% |
Table 7.
Pharmacokinetic parameters of theobromine after administration of 15 mg/kg caffeine intravenously in the presence and absence of rutaecarpine in rats. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.
| Theobromine (IV caffeine) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Parameter | AUCINF (μg.h/mL) | Tmax (h) | MRTinf (h) | T 1/2 (h) | AUCm/AUCp | |||||
| Control | 17.1 ± 1.5 | % of control | 5.333 ± 1.15 | % of control | 5.13 ± 0.35 | % of control | 1.4 ± 0.18 | % of control | 0.68 ± 0.03 | % of control |
| 3 h | 6.9 ± 0.3**** | 40% | 2.7 ± 1.15* | 51% | 3.09 ± 0.23**** | 60% | 0.9 ± 0.30 | 64% | 0.50 ± 0.01** | 74% |
| 6 h | 8.7 ± 1.3**** | 51% | 2.0 ± 0.00** | 38% | 3.09 ± 0.56**** | 60% | 1.4 ± 0.57 | 100% | 0.60 ± 0.05 | 88% |
| 12 h | 4.6 ± 1.1**** | 27% | 1.0 ± 0.00*** | 19% | 1.45 ± 0.074**** | 28% | 0.7 ± 0.03 | 50% | 0.48 ± 0.09** | 71% |
Rutaecarpine is rather hydrophobic but has a high hepatic extraction ratio (CL = 63 mL/min/kg in rats) [19]. Low rutaecarpine oral bioavailability could be due to high gut and/or liver first-pass effect. However, MROD and PK data suggested that as long as rutaecarpine reached the liver, it can still induce CYP1A2 enzyme in the liver, without having to be absorbed into the systemic blood circulation. As increased first-pass effect might not be the only reason for the F changes, factors including effects of rutaecarpine on expression of other enzymes and/or transporters involved in the caffeine disposition might need to be explored further [20]. We are currently testing if the decrease in caffeine bioavailability by rutaecarpine is also mediated by the AHR pathway.
Another interesting observation to note is the terminal slope of the plasma concentration time curve of caffeine between 6 and 7-h time point increased for the zero-hour group compared to control, and the slope paralleled the control group again, between 7 and 8-h time point (Fig. 2 (b)). This effect was also seen in the IV arm of the study (Fig. 2(a)), albeit less clearly. For example, for the 12-h rutaecarpine treatment group, the plasma caffeine concentration vs. time curve appeared biphasic. The slope for the early time points was steeper compared to control groups, and the slope paralleled the control group for the later time points. An increase in the slope for the 6–7 h points in the zero-hour group could be due to an increase in the elimination of caffeine by CYP1A2 induction. However, the reason for the slope to parallel the control group for later time points for the 12-h group is still unclear, especially as MROD data showed CYP1A2 activity was highest in liver harvested from rats treated with 12 h of rutaecarpine compared with 3, and 6 h groups.
5. Conclusion
Orally administered rutaecarpine at 100 mg/kg in suspension form significantly decreased the oral systemic exposure and mean residence time of caffeine and its metabolites (paraxanthine, theophylline and theobromine), as early as 3 h before oral caffeine administration in rats. Similarly, the systemic exposure of caffeine and its metabolites was also decreased when caffeine was given intravenously, though the effect was less pronounced compared to when caffeine was given orally. Furthermore, in vitro MROD data also showed that as early as 3 h after oral rutaecarpine administration, CYP1A2 activity in the liver tissue was significantly increased (almost 3-fold compared to control rats) and the highest activity (7-fold compared to control) was found in the liver of rats in 12-h treatment group.
Funding
MP was supported by a Scholarly and Artistic Activity Grant from the University of the Pacific.
Author contributions
ER and DJ performed the experiment; ER, WK, MP and ZZ interpreted the data and conducted the analyses; ER, MP and ZZ draft the contents; MP and ZZ supervised the study. All authors provided critical review and approval of the manuscript.
Declaration of competing interest
The authors have declared that no conflicts of interest exist.
Acknowledgements
We would like to thank Linnet Biopharmaceuticals for supplying rutaecarpine for the study.
Contributor Information
Rohit Kumar Estari, Email: r_estari@u.pacific.edu.
Jin Dong, Email: j_dong5@u.pacific.edu.
William K. Chan, Email: wchan@pacific.edu.
Miki Susanto Park, Email: mpark@pacific.edu.
Zhu Zhou, Email: zzhou1@york.cuny.edu.
References
- 1.Hughes J.R., McHugh P., Holtzman S. Caffeine and schizophrenia. Psychiatr. Serv. 1998;49:1415–1417. doi: 10.1176/ps.49.11.1415. [DOI] [PubMed] [Google Scholar]
- 2.Brent R.L., Christian M.S., Diener R.M. Evaluation of the reproductive and developmental risks of caffeine. Birth Defects Res B Dev Reprod Toxicol. 2011;92:152–187. doi: 10.1002/bdrb.20288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Choi E.J., Bae S.H., Pak J.B., Kwon M.J., Jang S.M., Zheng Y.F., Lee Y.S., Lee S.J., Bae S.Y. Simultaneous quantification of caffeine and its three primary metabolites in rat plasma by liquid chromatography–tandem mass spectrometry. Food Chem. 2013;141(3):2735–2742. doi: 10.1016/j.foodchem.2013.05.069. [DOI] [PubMed] [Google Scholar]
- 4.Jodynis-Liebert J., Flieger J., Matuszewska A., Juszczyk J. Serum metabolite/caffeine ratios as a test for liver function. J. Clin. Pharmacol. 2004;44:338–347. doi: 10.1177/0091270004263468. [DOI] [PubMed] [Google Scholar]
- 5.McLean C., Graham T.E. Effects of exercise and thermal stress on caffeine pharmacokinetics in men and eumenorrheic women. J Appl Physiol 1985. 2002;93:1471–1478. doi: 10.1152/japplphysiol.00762.2000. [DOI] [PubMed] [Google Scholar]
- 6.Guerreiro S., Toulorge D., Hirsch E., Marien M., Sokoloff P., Michel P.P. Paraxanthine,the primary metabolite of caffeine, provides protection against dopaminergic cell death via stimulation of ryanodine receptor channels. Mol. Pharmacol. 2008;74:980–989. doi: 10.1124/mol.108.048207. [DOI] [PubMed] [Google Scholar]
- 7.Bonati M., Latini R., Tognoni G., Young J.F., Garattini S. Interspecies comparison of in vivo caffeine pk in man, monkey, rabbit, rat and mouse. Drug Metab. Rev. 1984;15(7):1355–1383. doi: 10.3109/03602538409029964. [DOI] [PubMed] [Google Scholar]
- 8.Kalow W., Tang B.K. Use of caffeine metabolite ratios to explore CYP1A2 and xanthine oxidase activities. Clin. Pharmacol. Ther. 1991;50:508–519. doi: 10.3109/03602538409029964. [DOI] [PubMed] [Google Scholar]
- 9.Son J.K., Chang H.W., Jahng Y. Progress in studies on rutaecarpine. II.--Synthesis and structure-biological activity relationships. Molecules. 2015;20:10800–10821. doi: 10.3390/molecules200610800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jan W.C., Lin L.C., Chieh-Fu-Chen. Tsai T.H. Herb-drug interaction of Evodia rutaecarpa extract on the pharmacokinetics of theophylline in rats. J. Ethnopharmacol. 2005;102:440–445. doi: 10.1016/j.jep.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 11.Noh K., Seo Y.M., Lee S.K., Bista S.R., Kang M.J., Jahng Y., Kim E., Kang W., Jeong T.C. Effects of rutaecarpine on the metabolism and urinary excretion of caffeine in rats. Arch Pharm. Res. (Seoul) 2011;34:119–125. doi: 10.1007/s12272-011-0114-3. [DOI] [PubMed] [Google Scholar]
- 12.Shi J., Geng M.Y., Liu C.X. Comparative studies of the effects of two novel sugar drug candidates on the CYP 1A2 and CYP 2E1 enzymes in different sexed rats using a "cocktail" approach. Molecules. 2004;9:978–987. doi: 10.3390/91100978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Burke M.D., Thompson S., Weaver R.J., Wolf C.R., Mayer R.T. Cytochrome P450 specificities of alkoxyresorufin O-dealkylation in human and rat liver. Biochem. Pharmacol. 1994;48:923–936. doi: 10.1016/0006-2952(94)90363-8. [DOI] [PubMed] [Google Scholar]
- 14.Kojima M., Sekimoto M., Degawa M. A novel gender-related difference in the constitutive expression of hepatic cytochrome P4501A subfamily enzymes in Meishan pigs. Biochem. Pharmacol. 2008;75:1076–1082. doi: 10.1016/j.bcp.2007.10.030. [DOI] [PubMed] [Google Scholar]
- 15.Machala M., Soucek P., Neca J., Ulrich R., Lamka J., Szotáková B., Skálová L. Inter species comparisons of hepatic cytochrome P450 enzyme levels in male ruminants. Arch. Toxicol. 2003;77:555–560. doi: 10.1007/s00204-003-0477-4. [DOI] [PubMed] [Google Scholar]
- 16.Zhu Q.N., Zhang D., Jin T., Wu Q., Liu J., Lu Y.F. Rutaecarpine effects on expression of hepatic phase-1, phase-2 metabolism and transporter genes as a basis of herb-drug interactions. J. Ethnopharmacol. 2013;147:215–219. doi: 10.1016/j.jep.2013.03.005. [DOI] [PubMed] [Google Scholar]
- 17.Wen B., Roongta V., Liu L., Moore D.J. Metabolic activation of the indoloquinazoline alkaloids evodiamine and rutaecarpine by human liver microsomes: dehydrogenation and inactivation of cytochrome P450 3A4. Drug Metab. Dispos. 2014;42:1044–1054. doi: 10.1124/dmd.114.057414. [DOI] [PubMed] [Google Scholar]
- 18.Kot M., Daniel W.A. Relative contribution of rat cytochrome P450 isoforms to the metabolism of caffeine: the pathway and concentration dependence. Biochem. Pharmacol. 2008;75:1538–1549. doi: 10.1016/j.bcp.2007.12.017. [DOI] [PubMed] [Google Scholar]
- 19.Ko H.-C., Tsai T.-H., Chou C.-J., Hsu S.-Y., Li S.-Y., Chen C.-F. High performance chromatic determination of rutaecarpine in rat plasma: application to pharmacokinetic study. J. Chromatogr. 1994;655:27–31. doi: 10.1016/s0378-4347(94)80128-2. [DOI] [PubMed] [Google Scholar]
- 20.Seo Y.M., Noh K., Kong M.J., Lee D.H., Kang M.J., Jahng Y., Kang W., Jeong B.S., Jeong T.C. Effects of rutaecarpine on the pharmacokinetics of caffeine and its three metabolites in Rats. Biomol. Therap. 2011;19(2):243–247. doi: 10.1007/s12272-011-0114-3. [DOI] [Google Scholar]