Abstract
Amiodarone-induced pulmonary toxicity (AIPT) has a variety of presentations. Amiodarone use has been rarely associated with the development of acute respiratory failure. We present a patient with a history of paroxysmal atrial fibrillation who developed acute respiratory distress syndrome despite taking a low dose of amiodarone and having no risk or precipitating factors. The diagnosis of AIPT was made after drug discontinuation and exclusion of other potential causes. The development of acute respiratory failure due to AIPT is often underdiagnosed and undertreated. Better identification of risk factors and developing appropriate diagnostic tools for risk stratification of patients receiving amiodarone is mandatory.
Keywords: Acute respiratory failure, Amiodarone, Pulmonary toxicity
Highlights
-
•
Amiodarone use is rarely associated with the development of acute respiratory failure.
-
•
The most clinical presentation of AIPT is a subacute illness presented by the nonproductive cough, progressive dyspnoea, and low-grade fever
-
•
The diagnosis of AIPT is often made after drug discontinuation and exclusion of other potential causes.
-
•
Identification of risk factors and developing appropriate diagnostic tools for risk stratification of patients receiving amiodarone is mandatory
1. Introduction
Amiodarone is a class III antiarrhythmic agent used for both supraventricular and ventricular arrhythmias [1]. The toxic effects of amiodarone on the lungs are well known, but the sudden onset of those effects is not adequately recognized and treated. Amiodarone use has been rarely associated with the development of acute respiratory distress syndrome (ARDS) [2,3]. We present a patient with a history of taking low-dose amiodarone who was admitted into the intensive care unit (ICU) due to new onset of dyspnoea and acute respiratory failure.
2. Case report
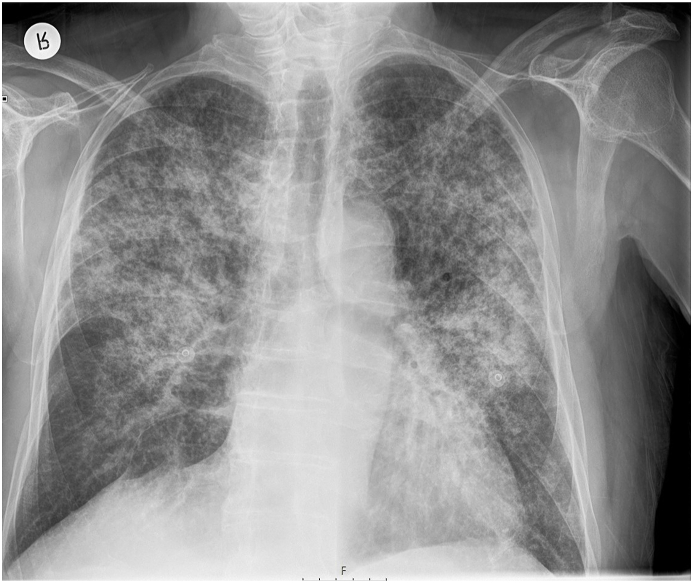
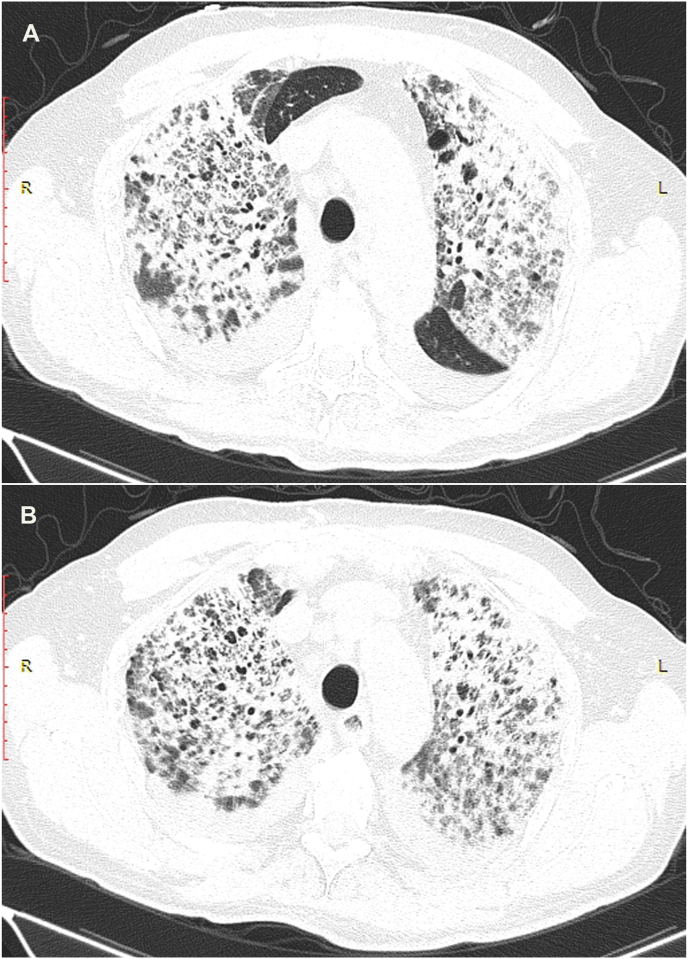
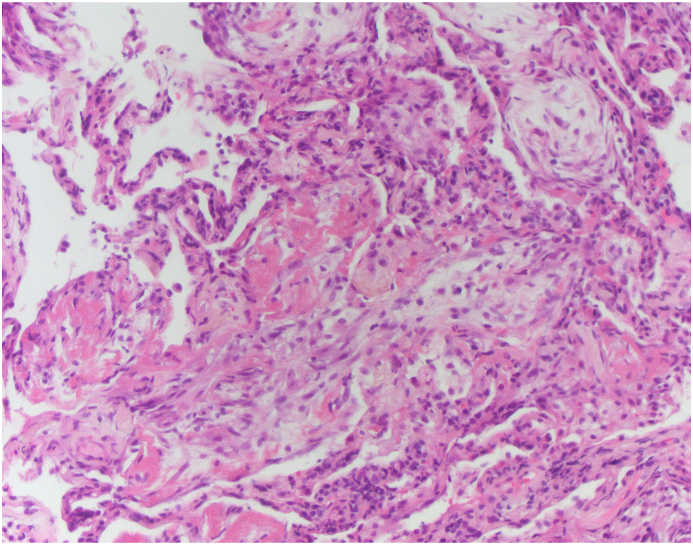
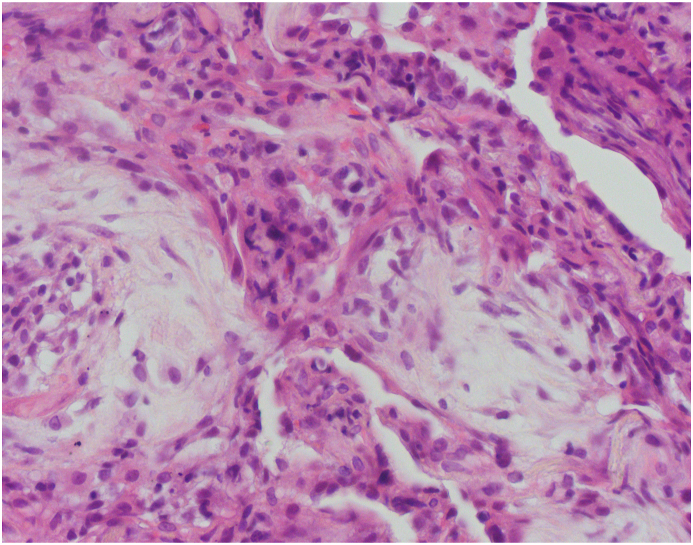
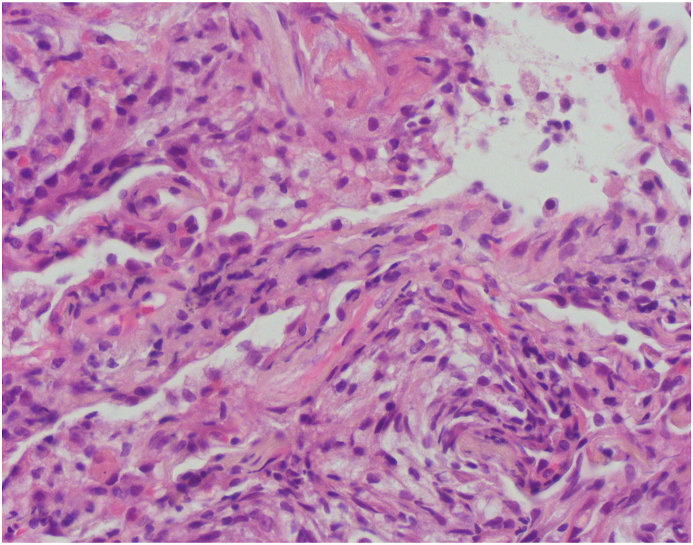
A 70-year old man with a history of paroxysmal atrial fibrillation, diabetes mellitus, and arterial hypertension presented to the Emergency Department with the symptoms of dyspnoea, cough, and fever lasting for five days. The patient had symptomatic paroxysmal atrial fibrillation and was treated with amiodarone 200 mg daily for the previous five years. His vital signs at the arrival were as follows: blood pressure 160/110 mmHg, respiratory rate 34/min with decreased oxygen saturation (89%). Electrocardiogram (ECG) showed atrial fibrillation with a fast ventricular response rate of 110/min and no signs of ischemia. Physical examination revealed diminished pulmonary sounds with right side basal crackles. Laboratory workup revealed increased levels of white blood cell count (WBC) 13,100/ml with 83% neutrophils, creatinine (180mg/dl), C-reactive protein (CRP) 296.4 mg/dl and slite elevation of gamma-glutamyl transferase (GGT) 80 mg/dl and lactate dehydrogenase (LDH) 315 mg/dl. Chest X-ray showed diffuse bilateral perihilar nonhomogeneous infiltrates (Fig. 1). Due to the acute clinical deterioration, he was immediately admitted into the intensive care unit (ICU) and treated with high-flow oxygen. Empirical antibiotic therapy with amoxicillin-clavulanic acid and levofloxacin was started. Considering the potential toxicity, the therapy with amiodarone was discontinued. A repeated chest X-ray study one week later did not show any resolution of pulmonary infiltrates. Blood and urine cultures were taken and found to be sterile. Further investigation included a high-resolution computed tomography (HRCT) scan of the chest that showed diffuse interlobar septal thickening with the extensive ground-glass opacities in the upper lungs and consolidation in the anterior segment of the right upper lobe (Figure 2. (A and B)). The differential diagnosis considered at the time included cryptogenic organizing pneumonia (COP) but based on the imaging features, there was also a suspicion of amiodarone-induced pulmonary toxicity (AIPT). HRCT also revealed bilateral pleural effusions with paratracheal and subcarinal enlargement of lymph nodes. Transthoracic echocardiography demonstrated hypertrophic interventricular septum with normal left ventricular systolic function and no valvular abnormalities. Mitral inflow showed a pseudonormal left ventricular filling pattern consistent with elevated LV filling pressures and grade II diastolic dysfunction. Right-sided thoracocentesis was done and bacterial cultures of the pleural fluid were negative. Legionella antigen was negative. Due to the worsening of respiratory function noninvasive ventilation (NIV) was applied. After clinical stabilization, he was transferred to the Department for further investigation and treatment. After that, bronchoscopy with aspiration and transbronchial biopsy (TBB) was performed to confirm a definitive diagnosis. Bacterial cultures of the bronchial aspiration fluid were negative. Transbronchial lung tissue biopsy showed morphological findings suggestive of amiodarone exposure and indicative of its toxicity. Two small samples of lung tissue demonstrated organizing pneumonia and diffuse interstitial pneumonitis with the widening of alveolar septae infiltrated with chronic inflammatory cells and mild to moderate interstitial fibrosis. The histologic finding of hyaline membranes as seen in diffuse alveolar damage was indicative of adult respiratory distress syndrome. Reactive hyperplasia of type II pneumocytes, some of them with lipid vacuoles, were present. The intra-alveolar foamy macrophages with cytoplasmic vacuolization were characteristic of amiodarone lung toxicity (Fig. 3, Fig. 4, Fig. 5.) One week after patient's clinical condition improved and he was discharged from the hospital. A follow-up chest X-ray six weeks later showed no previously detected pulmonary infiltrates.
Fig. 1.
Diffuse bilateral perihilar inhomogenous infiltrates on chest X-ray.
Fig. 2.
(A and B) High-resolution computed tomography scan of the chest showing diffuse interlobar septal thickening with ground-glass opacities.
Fig. 3.
Lung tissue showing organizing pneumonia, interstitial thickening and hyaline membranes (H&E stain, 100X).
Fig. 4.
The alveolar septae are thickened and infiltrated by mononuclear cell infiltration, lined by hyperplastic pneumocytes type II. Organizing pneumonia is also seen. (H&E stain, 400X).
Fig. 5.
Small aggregates of lipid-laden macrophages in alveolar spaces. Also note aforementioned morphologic changes (H&E stain, 400X).
3. Discussion
Amiodarone is a widely used antiarrhythmic agent. Acute and chronic pulmonary drug toxicity related to amiodarone is still noticed [4]. AIPT has a variety of presentations ranging from mild to very severe and includes organizing pneumonia, interstitial pneumonitis, and acute respiratory distress syndrome [5]. Amiodarone toxicity is related to several different mechanisms such as a cytotoxic effect of type II pneumocytes, an immune-mediated mechanism in genetically predisposed patients, and the activation of the angiotensin enzyme system [[6], [7], [8]]. The incidence of AIPT ranges from 0.5% and up to 17% [2]. Overall mortality rates of AIPT vary between 1% and 33% depending on the respiratory situation [9]. Risk factors associated with AIPT include a high cumulative dose (400 mg/day), duration of therapy more than two months, older age, preexisting lung disease, major surgery, and diagnostic procedure such as pulmonary angiography [10]. According to the current data it is recommended to apply 200 mg/d as a maintenance dose to keep the probability for AIPT as low as possible but in our case, acute pulmonary toxicity developed despite taking only a daily low dose of amiodarone [11]. Sweidan et al. reported a case of amiodarone induced-pulmonary toxicity in a 56- year-old male with coronary artery disease and chronic obstructive pulmonary disease after coronary artery bypass graft CABG surgery. The patient received amiodarone in a daily dose of 400 mg [12]. Abuzaid et al. also described a case of acute amiodarone pulmonary toxicity in a 57-year old patient who was treated with 400 mg daily of amiodarone for recurrent episodes of atrial fibrillation [13]. Although our patient did not have any risk or precipitating factors and despite the that he was taking a daily low dose of amiodarone (200 mg/day), he developed AIPT with signs of acute respiratory failure. All this outlines that risk factors for developing AIPT are still underrecognized and need to be investigated. Typical clinical presentation of AIPT is a subacute illness presented by the nonproductive cough, progressive dyspnoea, and low-grade fever, malaise with weight loss [14]. Our patient presented with the acute onset of respiratory symptoms which at first did not point at the amiodarone as a primary cause of respiratory failure. Nevertheless, he did not undergo any major surgery or diagnostic procedure that would explain such an acute clinical deterioration. Laboratory tests are not specific for the diagnosis of AIPT. They may show increased erythrocyte sedimentation rate and lactate dehydrogenase, leukocytosis, and rarely eosinophilia. Increased levels of BNP, which were high in our patient, do not exclude amiodarone lung toxicity, since these clinical entities may co-exist [15]. Our patient had a history of arterial hypertension, atrial fibrillation with a fast ventricular response, and signs of diastolic dysfunction confirmed on transthoracic echocardiography. Keeping that in mind, it is hard to distinguish to which extent fluid overload contributed to this condition since it is known that all of these conditions can lead to volume overload. Right-sided pleural effusion seen in our patient was the manifestation of acute decompensated heart failure precipitated with an ARDS. However, Hawatmeh, et al. reported a case of an amiodarone-induced pleural effusion without associated lung parenchymal involvement in a 73-year-old male with a history of coronary artery disease [16]. Uong et al. also reported a case of an amiodarone-induced bilateral pleural effusion without associated pneumonitis in a 70-year old female patient with a history of atrial fibrillation [17]. Our patient laboratory tests showed leukocytosis and increased levels of lactate dehydrogenase with no signs of eosinophilia. On chest X-ray images, the extent of the AIPT is very often underestimated. CT images of lungs are more relevant in the detection of early infiltrations [18]. Radiographic hallmarks of AIPT are interstitial infiltrates which can be localized or diffuse. Alveolar ground-glass opacities can also be found in AIPT, as was the case with our patient [10]. We also considered other medications associated with organizing pneumonia. Our patient was taking bisoprolol, amlodipine, rivaroxaban and amiodarone. All of these drugs, except amiodarone are not associated with the development of organizing pneumonia.
Bronchoalveolar lavage (BAL) may reveal an inclusion body in alveolar macrophages and type II pneumocytes of accumulated phospholipids [19]. To exclude other potential causes of acute respiratory failure we performed bronchoscopy with transbronchial biopsy (TBB) to confirm a diagnosis but also to rule out malignancy or atypical fungal, mycobacterial, and viral infections. Pathology remains the gold standard for diagnosing AIPT. The histopathologic findings suggesting AIPT include lipid-laden macrophages in airspaces, nonspecific interstitial pneumonitis, type II pneumocyte hyperplasia, interstitial edema, and fibrosis which was also found in our patient [11,20,21]. The intra-alveolar foamy macrophages with cytoplasmic vacuolization which were found in our patient may be associated with amiodarone toxicity. However, these histological findings can also correlate with chronic exposure to amiodarone and they are not specific for amiodarone toxicity [15].
Lung biopsy, alveolar cytogram, and foamy macrophages in BAL can help in the formulation of the diagnosis. To make a definitive diagnosis of AIPT requires the exclusion of other diagnostic possibilities, especially congestive heart failure, accompanied by an appropriate combination of symptoms or findings [22].
The first line of therapy is drug cessation. Systemic corticosteroids had been widely used, but there are currently no randomized controlled studies to confirm their benefit in terms of AIPT [10,23]. We did not administer corticosteroids because the patient clinical condition dramatically improved after one week. Additionally, we wanted to exclude some other conditions, particularly malignancy before corticosteroid administration. In our opinion, his clinical improvement was due to a combination of intensive care management, which included oxygen therapy, noninvasive ventilation, antibiotic therapy, diuretic and beta-blockers administration, pleurocentesis, and drug discontinuation. Intensive care measures, rather than amiodarone discontinuation led to clinical improvement because amiodarone is a lipophilic structure with a half-life of 25–100 days [24]. The aim of this case report was to emphasize that we should think about AIPT even in patients with no obvious risk or precipitating factors and taking only a low-dose of amiodarone. We also want to point that risk factors for developing AIPT are still underappreciated and better risk stratification of patients taking amiodarone to avoid AIPT is mandatory. Further studies are needed to investigate potential new risk factors in order to identify patients at high risk for AIPT.
4. Conclusion
To conclude, amiodarone pulmonary toxicity is a rare clinical condition, and diagnosis can be very challenging. It is usually made by exclusion of other potential causes. The combination of imaging and histological findings in our patient could be associated with amidarone pulmonary toxicity. Better identification of risk factors and developing appropriate diagnostic tools for risk stratification of patients receiving amiodarone is an emergent necessity.
CrediT author statement
Mijo Meter: Conceptualization, Validation, Writing - Original Draft preparation.
Ivana Kuzmić Prusac: Visualization.
Duška Glavaš: Writing - Reviewing & Editing.
Diana Meter: Writing - Reviewing & Editing, Supervision.
Author contributions
The case report described here was carried out in collaboration with all authors. Mijo Meter contributed to the conception and interpretation of data for the case report, he performed the literature review and wrote the initial draft. Ivana Kuzmić Prusac prepared and presented histopathologic analysis. Duška Glavaš revised draft critically and contributed in analysing the data. Diana Meter revised the manuscript critically for important intellectual content and gave the final approval of the version to be published. All authors made an agreement to be accountable for all aspects of the case report in ensuring that all questions are appropriately investigated and resolved. All authors agree to the publication.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Contributor Information
Mijo Meter, Email: mijometer05@gmail.com.
Ivana Kuzmić Prusac, Email: ikuzmicp@mefst.hr.
Duška Glavaš, Email: duska.glavas@gmail.com.
Diana Meter, Email: dianabajo53@gmail.com.
References
- 1.Vassallo P., Trohman R. Prescribing amiodarone. J. Am. Med. Assoc. 2007;298(11):1312–1322. doi: 10.1001/jama.298.11.1312. [DOI] [PubMed] [Google Scholar]
- 2.Schwaiblmair M., Berghaus T., Haeckel T., Wagner T., von Scheidt W. Amiodarone-induced pulmonary toxicity: an under-recognized and severe adverse effect? Clin. Res. Cardiol. 2010;99(11):693–700. doi: 10.1007/s00392-010-0181-3. [DOI] [PubMed] [Google Scholar]
- 3.Kumar S., Bangalore S., Kumari R., Grosu H., Jean R. Amiodarone-induced acute respiratory distress syndrome masquerading as acute heart failure. J. Emerg. Med. 2012;43(5):e311–e314. doi: 10.1016/j.jemermed.2010.07.024. [DOI] [PubMed] [Google Scholar]
- 4.Dharmarajan T., Shah A., Dharmarajan L. Amiodarone-induced pulmonary toxicity: potentially fatal, recognize early during life! J. Am. Geriatr. Soc. 2008;56(7):1363–1365. doi: 10.1111/j.1532-5415.2008.01715.x. [DOI] [PubMed] [Google Scholar]
- 5.Colby R., Geyer H. Amiodarone-induced pulmonary toxicity. J. Am. Acad. Physician Assistants. 2017;30(11):23–26. doi: 10.1097/01.JAA.0000524713.17719.c8. [DOI] [PubMed] [Google Scholar]
- 6.Uhal B., Wang R., Laukka J., Zhuang J., Soledad-Conrad V., Filippatos G. Inhibition of amiodarone-induced lung fibrosis but not alveolitis by angiotensin system antagonists. Pharmacol. Toxicol. 2003;92(2):81–87. doi: 10.1034/j.1600-0773.2003.920204.x. [DOI] [PubMed] [Google Scholar]
- 7.Uhal B., Zhang H., Abdul-Hafez A., Shu R., Li X. Amiodarone induces angiotensinogen gene expression in lung alveolar epithelial cells through activation protein-1. Basic Clin. Pharmacol. Toxicol. 2007;100(1):59–66. doi: 10.1111/j.1742-7843.2007.00006.x. [DOI] [PubMed] [Google Scholar]
- 8.Nikaido A., Tada T., Nakamura K., Murakami M., Banba K., Nishii N. Clinical features of and effects of angiotensin system antagonists on amiodarone-induced pulmonary toxicity. Int. J. Cardiol. 2010;140(3):328–335. doi: 10.1016/j.ijcard.2008.11.106. [DOI] [PubMed] [Google Scholar]
- 9.Hughes M., Binning A. Intravenous amiodarone in intensive care. Intensive Care Med. 2000;26(12):1730–1739. doi: 10.1007/s001340000668. [DOI] [PubMed] [Google Scholar]
- 10.Wolkove N., Baltzan M. Amiodarone pulmonary toxicity. Can. Respir. J. J. Can. Thorac. Soc. 2009;16(2):43–48. doi: 10.1155/2009/282540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goldschlager N., Epstein A., Naccarelli G., Olshansky B., Singh B., Collard H. A practical guide for clinicians who treat patients with amiodarone: 2007. Heart Rhythm. 2007;4(9):1250–1259. doi: 10.1016/j.hrthm.2007.07.020. [DOI] [PubMed] [Google Scholar]
- 12.Sweidan A., Singh N., Dang N., Lam V., Datta J. amiodarone-induced pulmonary toxicity – a frequently missed complication. Clin. Med. Insights Case Rep. 2016;9 doi: 10.4137/CCRep.S39809. CCRep.S39809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abuzaid A., Saad M., Ayan M., Kabach A., Haddad T., Smer A. Acute amiodarone pulmonary toxicity after drug holiday: a case report and review of the literature. Case Rep. Cardiol. 2015:1–3. doi: 10.1155/2015/927438. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dusman R., Stanton M., Miles W., Klein L., Zipes D., Fineberg N. Clinical features of amiodarone-induced pulmonary toxicity. Circulation. 1990;82(1):51–59. doi: 10.1161/01.cir.82.1.51. [DOI] [PubMed] [Google Scholar]
- 15.Camus P., Martin W., Rosenow E. Amiodarone pulmonary toxicity. Clin. Chest Med. 2004;25(1):65–75. doi: 10.1016/S0272-5231(03)00144-8. [DOI] [PubMed] [Google Scholar]
- 16.Hawatmeh A., Thawabi M., Jmeian A., Shaaban H., Shamoon F. Amiodarone-induced loculated pleural effusion without pulmonary parenchymal involvement: a case report and literature review. J. Nat. Sci. Biol. Med. 2017;8(1):130–133. doi: 10.4103/0976-9668.198345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Uong V., Nugent K., Alalawi R., Raj R. Amiodarone-induced loculated pleural effusion: case report and review of the literature. Pharmacotherapy. 2010 Feb;30(2):218. doi: 10.1592/phco.30.2.218. PMID: 20099996. [DOI] [PubMed] [Google Scholar]
- 18.Oyama N., Oyama N., Yokoshiki H., Kamishima T., Nambu T., Tsutsui H. Detection of amiodarone-induced pulmonary toxicity in supine and prone positions-high-resolution computed tomography study. Circ. J. 2005;69(4):466–470. doi: 10.1253/circj.69.466. [DOI] [PubMed] [Google Scholar]
- 19.Dean P., Groshart K., Porterfield J., Iansmith D., Golden E. Amiodarone-associated pulmonary toxicity: a clinical and pathologic study of eleven cases. Am. J. Clin. Pathol. 1987;87(1):7–13. doi: 10.1093/ajcp/87.1.7. [DOI] [PubMed] [Google Scholar]
- 20.Camus P., Fanton A., Bonniaud P., Camus C., Foucher P. Interstitial lung disease induced by drugs and radiation. Respiration. 2004;71(4):301–326. doi: 10.1159/000079633. [DOI] [PubMed] [Google Scholar]
- 21.Bedrossian C., Warren C., Ohar J., Bhan R. Amiodarone pulmonary toxicity: cytopathology, ultrastructure, and immunocytochemistry. Ann. Diagn. Pathol. 1997;1(1):47–56. doi: 10.1016/s1092-9134(97)80008-1. [DOI] [PubMed] [Google Scholar]
- 22.Terzo F., Ricci A., D'Ascanio M., Raffa S., Mariotta S. Amiodarone-induced Pulmonary toxicity with an excellent response to treatment: a case report. Respir. Med. Case Rep. 2019 Nov 29;29:100974. doi: 10.1016/j.rmcr.2019.100974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nacca N., Bhamidipati C.M., Yuhico L.S., Pinnamaneni S., Szombathy T. Severe amiodarone induced pulmonary toxicity. J. Thorac. Dis. 2012;4(6):667–670. doi: 10.3978/j.issn.2072-1439.2012.06.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Essrani R., Mehershahi S., Essrani R.K., Ravi S.J.K., Bhura S., Sudhakaran A. Amiodarone-induced acute liver injury. Case Rep. Gastroenterol. 2020 Feb 20;14(1):87–90. doi: 10.1159/000506184. [DOI] [PMC free article] [PubMed] [Google Scholar]