Abstract
The prognosis of oral squamous cell carcinoma (OSCC) largely depends on the control of lymph node metastases. We evaluate the therapeutic efficacy of G47Δ, a third-generation oncolytic herpes simplex virus type 1 (HSV-1), in mouse tongue cancer models. Intratumoral injection with G47Δ prolonged the survival in all orthotopic models investigated. In both athymic and immunocompetent models, G47Δ injected into the tongue cancer swiftly traffics to the draining cervical lymph nodes and suppresses lymph node metastases. In the immunocompetent KLN205-MUC1 model, in which the metastatic cascade that tongue cancer patients commonly experience is reproduced, intratumoral G47Δ injection even immediately prior to a tumor resection prolonged survival. Cervical lymph nodes 18 h after G47Δ treatment showed the presence of G47Δ infection and an increase in CD69-positive cells, indicating an immediate activation of T cells. Furthermore, G47Δ injected directly into enlarged metastatic lymph nodes significantly prolonged the survival at an advanced stage. Whereas intratumorally injected oncolytic HSV-1 does not readily circulate in the blood stream, G47Δ is shown to traffic in the lymphatics swiftly. The use of G47Δ can lead to entirely new treatment strategies for tongue cancer and other OSCC at all clinical stages.
Keywords: G47Δ, oncolytic virus therapy, lymph node metastasis, tongue cancer, antitumor immunity, oral squamous cell carcinoma, herpes simplex virus type 1, viral trafficking, neoadjuvant therapy, orthotopic tumor model
Graphical abstract
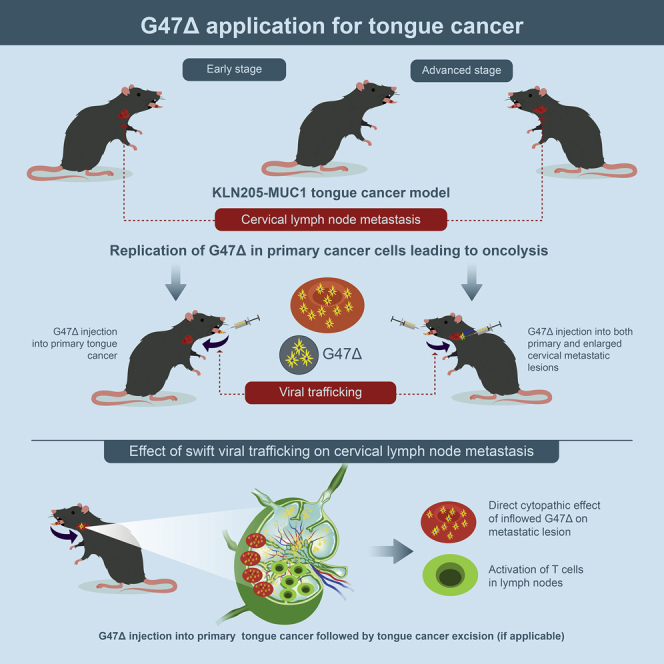
Although intratumorally injected oncolytic HSV-1 doesn’t readily circulate in the blood stream, in orthotopic tongue cancer models, G47Δ traffics in the lymphatics swiftly, activates T cells in the cervical lymph nodes, and suppresses metastases. G47Δ is efficacious even when injected immediately before tumor resection or directly to metastatic lymph nodes.
Introduction
Oral squamous cell carcinoma (OSCC), the sixth most common cancer worldwide, is treated by surgical resection with or without radiation and chemotherapy.1 OSCC exhibits a poor prognosis because it frequently metastasizes to cervical lymph nodes and ultimately spreads to distant organs, most frequently to the lung.2, 3, 4, 5, 6 A radical resection of oral and maxillofacial lesions can lead to severe impairment of oral functions and physical appearance, which impairs the quality of life (QOL) of patients. Even with a complete resection of primary lesions, micrometastasis often results in lymph node metastasis, especially in tongue cancer patients.5, 6, 7 Therefore, a successful control of lymph node metastases is essential for improving the survival rate of patients with OSCC.6
Oncolytic virus therapy is a promising therapeutic approach for intractable cancer.8 Talimogene laherparepvec (T-Vec), a double-mutated oncolytic herpes simplex virus type 1 (HSV-1) armed with granulocyte-macrophage colony stimulating factor (GM-CSF), has been approved as a drug for melanoma in the United States and Europe.9, 10, 11, 12 A variety of oncolytic viruses such as HSV-1,13, 14, 15 adenovirus,16, 17, 18 and Newcastle disease virus have been tested in patients with head and neck cancer.19 Some preclinical studies have reported the lymphatic spread of oncolytic viruses in immunodeficient animal models.20, 21, 22, 23 An important question, however, is whether cervical lymph node metastases that occur as a natural course of tongue cancer can be controlled with an oncolytic virus under immunocompetent conditions.
G47Δ is a third-generation oncolytic HSV-1 with triple mutations; deletions in both copies of the γ34.5 gene, a lacZ insertion inactivating the ICP6 gene, and a deletion of the α47 gene and the overlapping US11 promoter.24 The γ34.5 gene counteracts the host-cell-induced shutdown of protein synthesis mediated by protein kinase R upon viral infection, and because such protein synthesis shutdown is usually disabled in cancer cells, the γ34.5 mutation permits viral replication within cancer cells but not in normal cells.25 ICP6 encodes the large subunit of ribonucleotide reductase that is required for virus DNA replication,26 and therefore the ICP6 inactivation permits viral replication only in dividing cells. The α47 gene functions to antagonize host cell’s transporter associated with antigen presentation,27 and therefore the deletion of the gene precludes the downregulation of major histocompatibility complex (MHC) class I expression, causing enhancement of antitumor immune responses.24 The deletion also results in immediate early expression of the neighbor US11 gene, which results in enhanced viral replication in cancer cells.28 In this study, we evaluate the efficacy of G47Δ in mouse models of orthotopic tongue cancer. We show that, when injected into the primary tongue tumors, G47Δ not only inhibits the growth of primary lesions, but also drains into the cervical lymph nodes almost instantly and suppresses the extent of lymph node metastases.
Results
Human OSCC and mouse SCC cell lines are susceptible to G47Δ in vitro
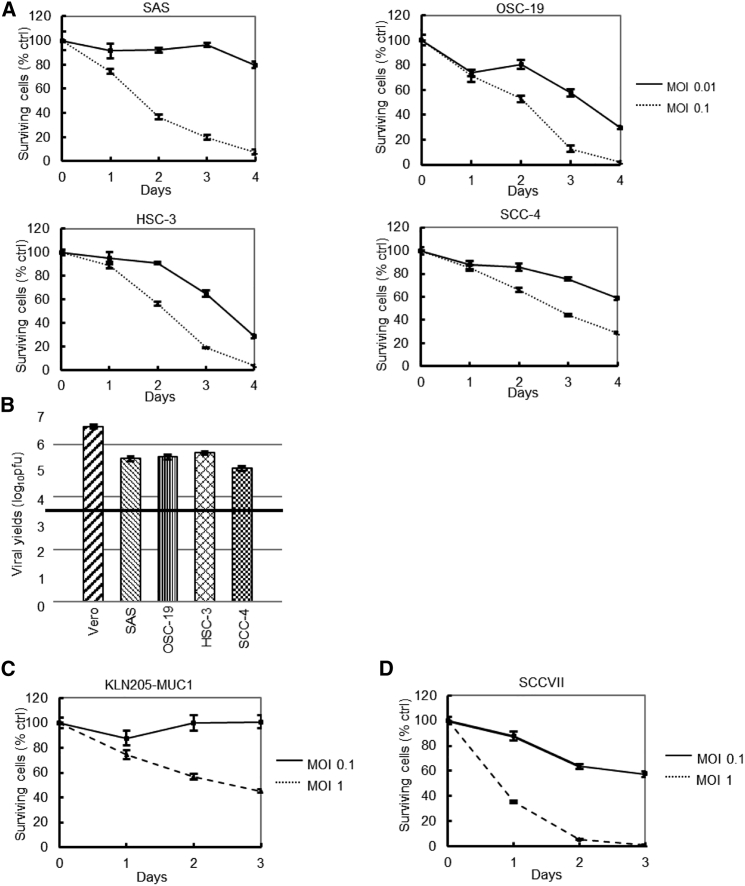
All human OSCC lines tested were susceptible to G47Δ in vitro and supported efficient viral replication (Figures 1A and 1B). Mouse squamous cell carcinoma (SCC) cell lines (KLN205-MUC1 and SCCVII cells) were also tested in vitro, revealing that KLN205-MUC1 cells were relatively resistant to G47Δ, whereas SCCVII cells were moderately susceptible to G47Δ (Figures 1C and 1D).
Figure 1.
In vitro evaluation of G47Δ activity in human OSCC and mouse SCC cell lines
(A) In vitro cytotoxicity assays for human OSCC cells. Four human OSCC cell lines were infected with G47Δ (MOI = 0.01 or 0.1). The number of surviving cells was counted daily, and the results are expressed as a percentage of mock-infected controls. All human OSCC lines tested were susceptible to G47Δ. The results are presented as the mean ± standard deviation (SD). (B) In vitro viral replication in OSCC cells. The black line indicates the initial G47Δ titer used for this assay. G47Δ showed high virus yields in all human OSCC cells tested. (C and D) In vitro cytotoxicity assays in mouse SCC cells. Two mouse SCC cell lines were infected with G47Δ (MOI = 0.1 or 1). G47Δ was susceptible to KLN205-MUC1 at a MOI of 1.0 and to SCCVII at a MOI of 0.1. Error bars, ± SD.
G47Δ is efficacious in human OSCC and mouse SCC subcutaneous tumor models
Intratumoral injections with G47Δ significantly inhibited the growth of subcutaneous SAS tumors in athymic mice in a dose-dependent manner (Figure 2A). In a separate experiment, subcutaneous SAS tumors, inoculated with G47Δ (1 × 106 pfu) or mock on days 0 and 3, were excised on day 15 for immunohistochemical analysis using Ki-67 as a proliferation marker. The rate of Ki-67 positivity of tumor cells drastically decreased in the areas with high HSV-1 positivity, presumably reflecting the areas with abundant G47Δ replication (Figure S1). Intratumoral injections with G47Δ (5 × 106 pfu) significantly inhibited the growth of subcutaneous KLN205-MUC1 tumors in DBA/2 mice. The growth of subcutaneous SCCVII tumors in C3H/He mice was significantly inhibited at lower doses (2 × 105 and 1 × 106 pfu; Figures 2B and 2C).
Figure 2.
In vivo evaluation of G47Δ efficacy for human OSCC and mouse SCC subcutaneous tumor models
(A) Efficacy of G47Δ in subcutaneous SAS tumors in athymic mice. Established subcutaneous SAS tumors were inoculated with G47Δ (2 × 105 or 1 × 106 pfu) or mock when tumors reached approximately 8 mm in diameter. Intratumoral injections with G47Δ significantly inhibited the growth of subcutaneous SAS tumors in a dose-dependent manner. (B and C) Efficacy of G47Δ in KLN205-MUC1 and SCCVII subcutaneous tumors in DBA/2 mice and C3H/He mice, respectively. Established KLN205-MUC1 (B) or SCCVII (C) subcutaneous tumors approximately 5 mm in diameter were inoculated with G47Δ at doses indicated on days 0 and 3. Intratumoral injections with G47Δ (5 × 106 pfu) significantly inhibited the growth of subcutaneous KLN205-MUC1 tumors in DBA/2 mice. Intratumoral injections with G47Δ significantly inhibited the growth of subcutaneous SCCVII tumors at both doses used (2 × 105 and 1 × 106 pfu) in C3H/He mice. Arrows indicate the timings of G47Δ injection. Tumor volume (mm3) = length × width × height (mm). The results are presented as the mean ± standard error of the mean (SEM) . ∗p < 0.05; ∗∗∗p < 0.001 (Tukey-Kramer method).
G47Δ is efficacious in orthotopic tongue cancer models of athymic mice
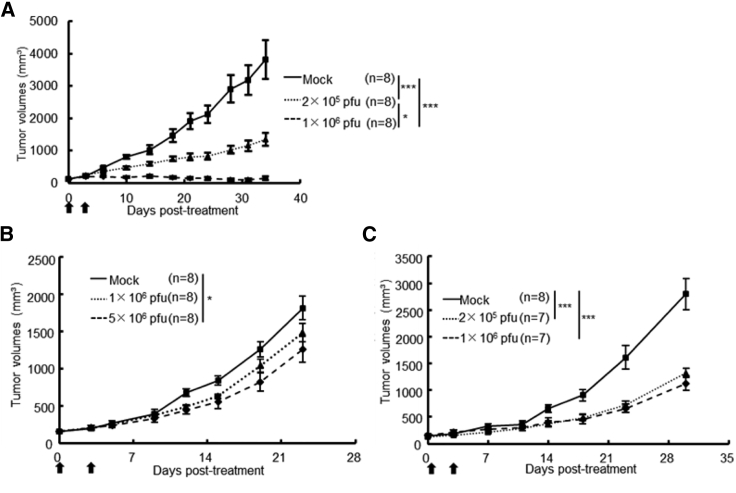
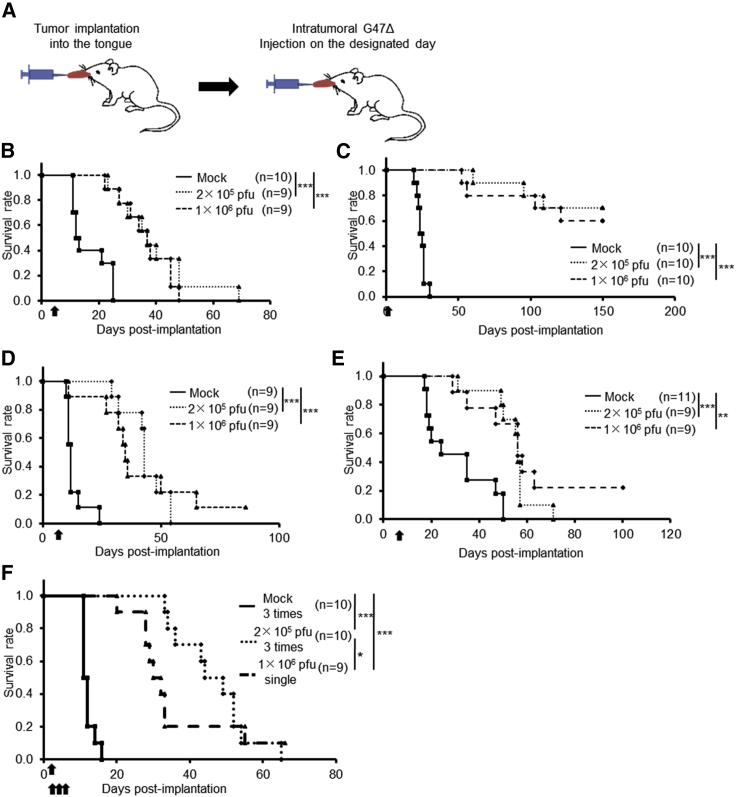
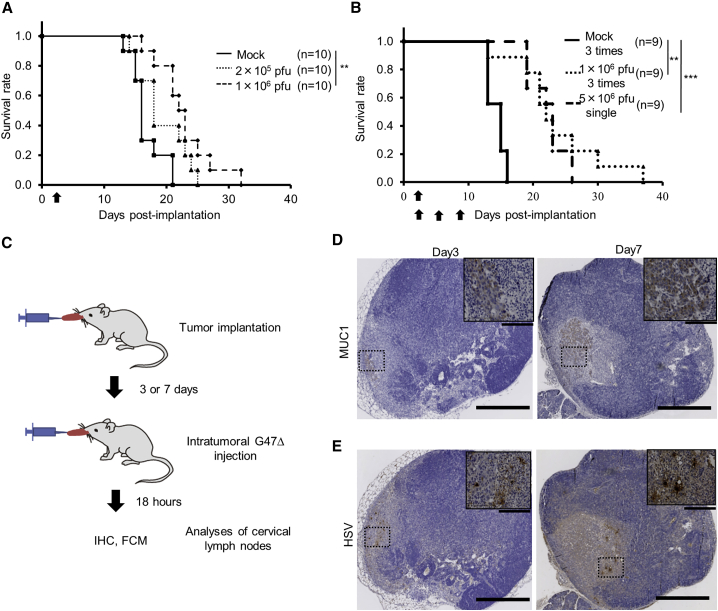
Next, we examined the efficacy of G47Δ in two orthotopic tongue cancer models in athymic mice (SAS-GFP and HSC-3; Figure 3A). The susceptibility of SAS-GFP cells to G47Δ was comparable to that of SAS cells (data not shown). Intratumoral injection with G47Δ 3 days after tumor implantation significantly prolonged the survival compared with control in both SAS-GFP and HSC-3 models (Figures 3B and 3C). In both models, there was no significant difference in efficacy between the doses used (2 × 105 pfu and 1 × 106 pfu). G47Δ was significantly efficacious in both orthotopic tongue cancer models even when it was injected at delayed time points; 5 days for SAS or 7 days for HSC-3 after tumor implantation (Figures 3D and 3E). The immunostaining for green fluorescent protein (GFP) and hematoxylin and eosin (H&E) staining of tumor samples obtained 3 and 5 days after tumor implantation of SAS-GFP cells (1 × 106) show that the tumor is well established at day 3 and grows rapidly to occupy a large portion of the tongue by day 5 (Figure S2). In the SAS-GFP human orthotopic tongue cancer model, 3-time injections with a low dose of G47Δ (2 × 105 pfu) exhibited a significantly higher efficacy than a single injection with a high dose (1 × 106 pfu; Figure 3F).
Figure 3.
In vivo evaluation of G47Δ efficacy for human orthotopic tongue cancer models in athymic mice
(A). The experimental schedule is shown. (B and C) After 3 days of tumor implantation (B, SAS-GFP; C, HSC-3), G47Δ was intratumorally inoculated at a dose of 2 × 105 or 1 × 106 pfu, respectively. In both models, a single intratumoral injection with G47Δ significantly prolonged the survival at both doses. (D and E), After 5 (D, SAS-GFP) or 7 (E, HSC-3) days of tumor implantation, G47Δ was intratumorally inoculated at a dose of 2 × 105 or 1 × 106 pfu, respectively. In both models, a single intratumoral injection with G47Δ at a delayed timing significantly prolonged the survival. ∗∗p < 0.01; ∗∗∗p < 0.001 (log-rank test). (F) G47Δ at a low dose (2 × 105 pfu) or mock was inoculated intratumorally three times, 3, 6, and 9 days after tumor implantation (SAS-GFP), or G47Δ at a high dose (1 × 106 pfu) once 3 days after tumor implantation, and the survival was observed. Three-time injections with a low dose of G47Δ exhibited a significantly higher efficacy than a single injection with a high dose. ∗p < 0.05; ∗∗∗p < 0.001 (generalized Wilcoxon test). Arrows indicate the timings of G47Δ injection.
G47Δ injected into the primary tongue tumors traffics to the cervical lymph node
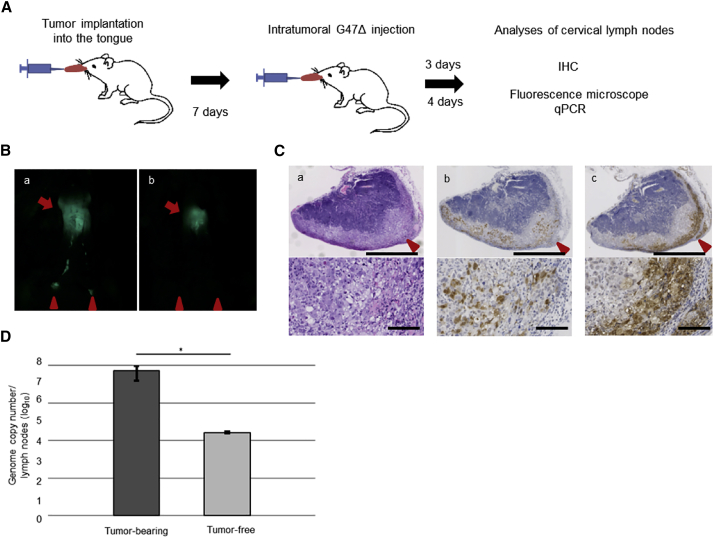
Implanted SAS and HSC-3 cells are known to cause regional lymph node metastases in orthotopic tongue cancer models.22,29 Therefore we utilized these models to investigate the effect of G47Δ injected into the primary lesion 7 days after tumor implantation on the lymph node metastases (Figure 4A). Fluorescence microscopy 4 days after G47Δ inoculation into SAS-GFP tongue tumors revealed that, whereas all mock-treated mice developed bilateral lymph node metastases depicted by GFP signals, 5 of 9 G47Δ-treated mice did not exhibit any GFP signal in the lymph nodes of either side (Figure 4B). Immunohistochemical analysis 3 days after G47Δ inoculation revealed the presence of EGFP+ tumor cells in the lymph node cortex and abundant HSV positivity, presumably representing G47Δ-infected cancer cells, distributed mainly in the subcapsular area (Figure 4C). Quantitative PCR showed significantly higher copy numbers of G47Δ DNA, but not necessarily infectious G47Δ, in the cervical lymph nodes of tumor-bearing mice than in those without tumors (p = 0.02, Figure 4D). HSV-1 infection in the metastatic cervical lymph nodes was also confirmed in the HSC-3 tongue cancer model treated with an intratumoral G47Δ injection (Figure S3). These results indicate that G47Δ injected into primary tongue tumor traffics to the cervical lymph nodes and exhibits antitumor effects locally in the regional metastatic cervical lymph nodes. G47Δ, when injected into the tongue of normal HSV-1-sensitive A/J mice, has shown to be safe, causing no side effects, in preclinical safety studies (data not shown).
Figure 4.
In vivo evaluation of G47Δ in metastatic cervical lymph nodes after injections into the primary tongue tumors in athymic mice
(A) SAS-GFP cells (1 × 106) were implanted in the tongues of athymic mice. After 7 days, G47Δ was inoculated intratumorally at a dose of 1 × 106 pfu. Cervical lymph node metastasis was analyzed by immunohistochemistry 3 days after virus inoculation or assessed using fluorescence microscopy and quantitative PCR 4 days after virus inoculation. The experimental schedule is shown. (B) Representative fluorescence micrographs of the cervical regions 4 days after virus inoculation. Arrows indicate primary tongue tumors, and arrowheads indicate metastases to the cervical lymph nodes. GFP+ tumors in the cervical lymph nodes were detected in control mice (a), but not in the G47Δ-treated mice (b). (C) Immunohistochemistry of the cervical lymph nodes 4 days after virus inoculation; H&E staining (a), EGFP staining (b), and HSV-1 staining (c). HSV-1 positivity was mainly detected in the subcapsular area. Scale bars; 500 μm (upper), 100 μm (lower). (D) Quantitative real-time PCR analysis of G47Δ DNA in the cervical lymph nodes 4 days after virus inoculation. Significantly higher copy numbers of G47Δ DNA were detected in the cervical lymph nodes of tumor-bearing mice than in those without tumors. n = 4 in each group. Error bars, ± SEM. ∗p < 0.05 (Mann-Whitney U test).
G47Δ immediately traffics to the cervical lymph nodes and activates T cells
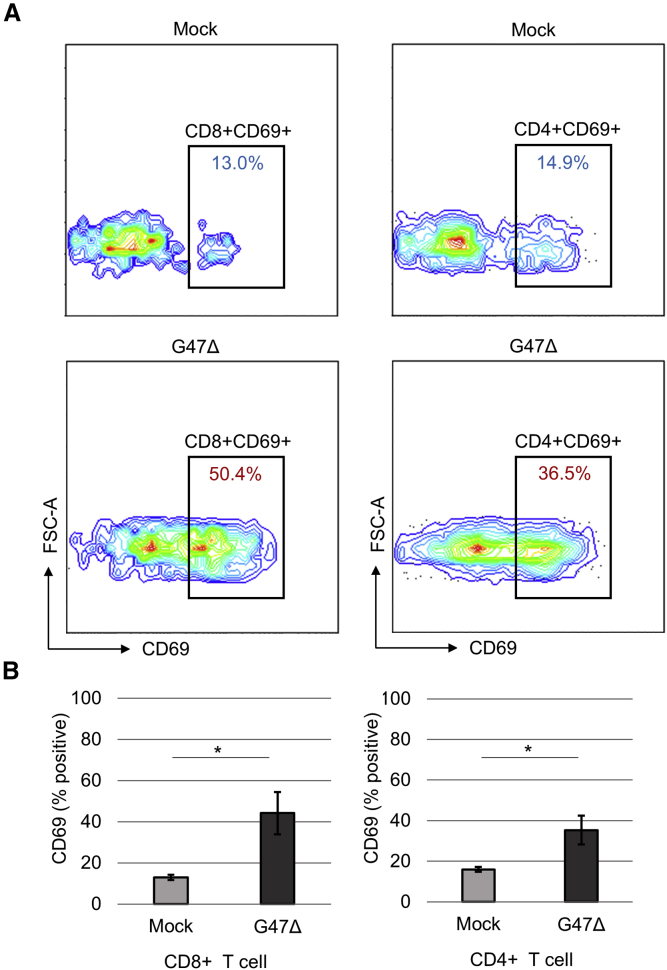
To investigate the efficacy of G47Δ under immunocompetent conditions, we generated tongue tumors by injecting KLN205-MUC1 and SCCVII cells in syngeneic DBA/2 and C3H/He mice, respectively. An intratumoral injection with G47Δ into the tongue tumors on day 3 prolonged the survival compared with control in both models (Figure 5A; Figure S4). In the KLN205-MUC1 tongue cancer model, 3-time injections with a low dose of G47Δ (1 × 106 pfu) exhibited an efficacy equivalent to a single injection with a high dose (5 × 106 pfu; Figure 5B). Immunostaining for MUC1 revealed that cervical lymph node metastases existed at day 3 of tumor implantation with the metastatic volume increasing at day 7 (Figures 5C and 5D). HSV-1 staining of metastatic cervical lymph nodes 18 h after a G47Δ injection into the tongue tumor showed G47Δ-infected tumor cells within the lymph nodes (Figure 5E). Quantitative PCR showed a high copy number of G47Δ DNA existing in the cervical lymph node as early as 10 min after G47Δ injection into the tongue tumor that gradually decreased with time in the following days (Figure S5). Flow cytometric analysis of lymphocytes in the cervical lymph nodes 18 h after G47Δ injection into the tongue tumor revealed a significantly higher proportion of CD69-positive cells among both CD8- and CD4-positive cells in G47Δ-treated animals compared with mock-treated ones (Figure 6), indicating that the G47Δ treatment causes immediate activation of T cells in the regional lymph nodes.30
Figure 5.
In vivo evaluation of G47Δ efficacy in an orthotopic tongue cancer model using immunocompetent mice
(A) KLN205-MUC1 cells (2 × 105) were implanted into the tongue of DBA/2 mice. G47Δ was intratumorally inoculated at a dose of 2 × 105 pfu or 1 × 106 pfu on day 3, and the survival was monitored. Intratumoral G47Δ injection at a dose of 1 × 106 pfu significantly prolonged the survival compared with control. ∗∗p < 0.01 (log-rank test). (B) G47Δ at a low dose (1 × 106 pfu) or mock was inoculated intratumorally three times, 3, 6, and 9 days after tumor implantation, or G47Δ at a high dose (5 × 106 pfu) once 3 days after tumor implantation, and the survival was observed. Three-time injections with a low dose of G47Δ exhibited an efficacy equivalent to a single injection with a high dose. ∗∗p < 0.01; ∗∗∗p < 0.001 (generalized Wilcoxon test). Arrows indicate the timings of G47Δ injection. (C) KLN205-MUC1 cells were implanted into the tongues of DBA/2 mice, and after 3 or 7 days, G47Δ was intratumorally inoculated at a dose of 1 × 106 pfu. The cervical lymph nodes were excised 18 h after virus inoculation and analyzed by immunohistochemistry or flow cytometry. (D and E) Immunohistochemistry of cervical lymph nodes 18 h after virus inoculation; MUC1 staining (D), HSV-1 staining (E). Metastatic MUC1-positive tumor cells and G47Δ-infected tumor cells can be observed. Insets depict enlarged images of areas indicated. Scale bars, 100 μm in high power fields, 500 μm in low power fields.
Figure 6.
Flow cytometric analysis of the cervical lymph nodes
KLN205-MUC1 cells (2 × 105) were implanted in the tongues of DBA/2 mice. After 7 days, G47Δ was intratumorally inoculated into the tongue tumors at a dose of 1 × 106 pfu. After 18 h, the cervical lymph nodes were removed and lymphocytes were analyzed by a flow cytometer. (A) Representative plots of CD69-positive cells among CD8- or CD4-positive T cells. (B) The proportions of CD69-positive lymphocytes among CD8-positive cells (left) and CD4-positive cells (right). A significantly higher proportion of CD69-positive cells among both CD8- and CD4-positive cells was detected in the G47Δ-treated animals compared with mock-treated ones. n = 4 per group. Error bars, ± SEM. ∗p < 0.05 (Student’s t test).
Intratumoral injection with G47Δ immediately prior to tumor resection prolongs survival
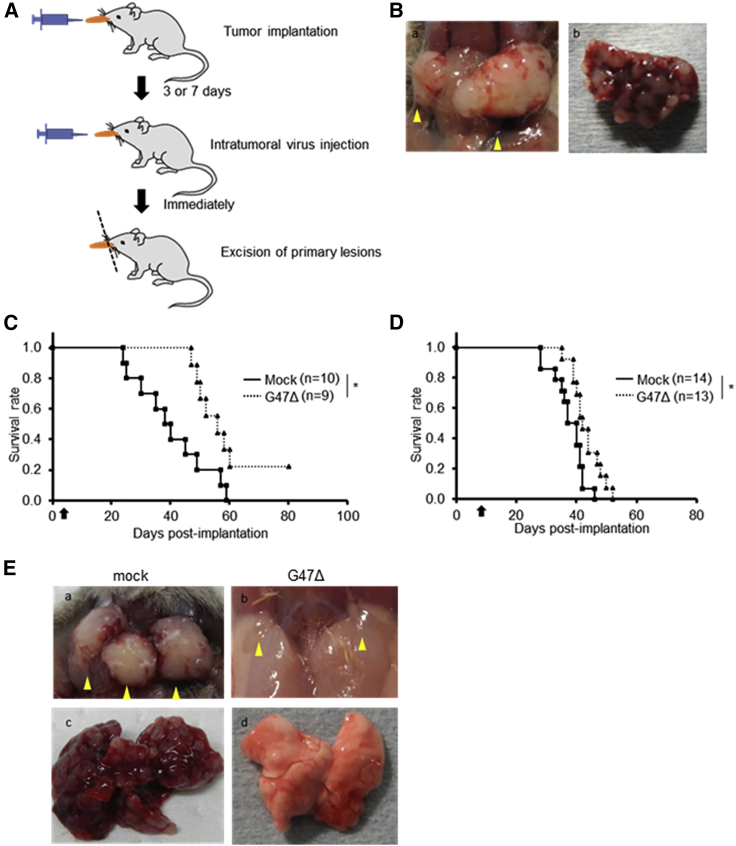
To evaluate the efficacy of G47Δ injected immediately prior to tumor resection, we injected G47Δ intratumorally 3 or 7 days after tumor implantation in the orthotopic KLN205-MUC1 model immediately after which the tongue was resected (Figure 7A). Without G47Δ treatment, the mice became moribund at around 35 days after tumor implantation due to large cervical tumors and multiple lung metastases, mimicking the clinical course of tongue cancer patients (Figure 7B). The G47Δ treatment, at both early (day 3) and late (day 7) time points, significantly prolonged the survival of tumor-implanted mice compared with control (median survival 56.0 versus 38.0 days, p = 0.01; 42.0 versus 37.0 days, p = 0.01, respectively; Figures 7C and 7D). The suppression of metastases by G47Δ treatment was also observed macroscopically in a separate experiment (Figure 7E). The results indicate that G47Δ is efficacious even when injected immediately prior to tongue tumor resection.
Figure 7.
In vivo evaluation of the efficacy of G47Δ injected into primary tumors immediately prior to tumor resection
(A) The experimental design is shown. KLN205-MUC1 cells (2 × 105) were implanted into the tongues of DBA/2 mice. After 3 or 7 days, G47Δ was intratumorally inoculated at a dose of 1 × 106 pfu, followed by immediate resection of the tongues. (B) Representative images of advanced-stage cervical lymph node (a) and lung (b) metastases 35 days after tumor implantation without treatment. (C and D) Intratumoral G47Δ injection on day 3 (C) or day 7 (D) of tumor implantation followed by immediate tumor resection significantly prolonged the survival. Arrows indicate the timings of G47Δ injection. ∗p < 0.05 (log-rank test). (E) Representative images of the cervical lymph nodes (upper: a, b) and the lung (lower; c, d) of mice 35 days after tumor implantation and treated with mock (left: a, c) or G47Δ (right: b, d) on day 3. Arrowheads indicate cervical lymph nodes.
G47Δ injection into metastatic cervical lymph nodes prolongs survival at an advanced stage
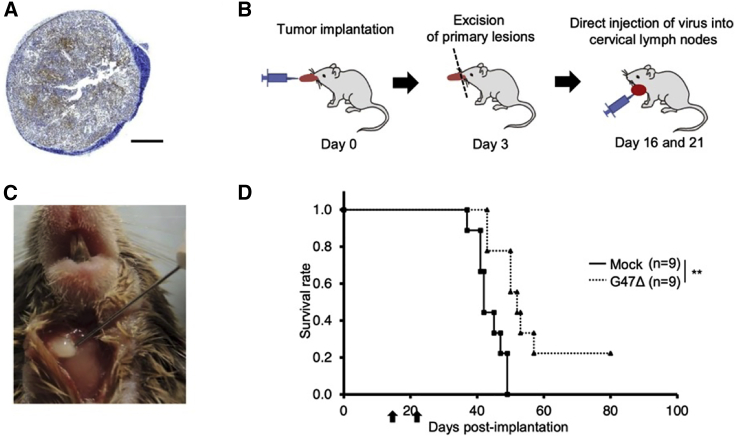
As the prognosis of patients with tongue cancer largely depends on the control of cervical metastases,31 we next evaluated the efficacy of G47Δ when injected into the metastatic cervical lymph nodes. KLN205-MUC1 cells were implanted in the tongue of DBA/2 mice, and the primary tumors were resected on day 3. After confirming the enlargement of metastatic cervical lymph nodes on day 15 (Figure 8A), G47Δ was injected into the bilateral cervical lymph nodes under direct view twice on days 16 and 21 (Figures 8B and 8C). The G47Δ treatment led to a significant prolongation of survival compared with mock-treated control (median survival 52.0 versus 42.0 days, p < 0.01; Figure 8D). Whereas all 9 mice in the control group died, 2 out of 9 mice in the G47Δ-treated group survived. The surviving mice were determined to be free of metastasis, both in the cervical lymph nodes and lungs, by macroscopic inspection at the end of the study (day 80; data not shown). G47Δ therefore may exert its efficacy even at an advanced stage by injecting into metastatic lymph nodes.
Figure 8.
In vivo evaluation of the efficacy of G47Δ injected into the metastatic cervical lymph nodes
(A) A representative image of MUC1 immunostaining of the cervical lymph node 15 days after KLN205-MUC1 cells were implanted in the tongue of DBA/2 mice. Scale bar, 500 μm. (B and C) KLN205-MUC1 cells (2 × 105) were implanted into the tongues of DBA/2 mice. After 3 days, the tongues were resected, and G47Δ (2.5 × 106 pfu) was inoculated into the bilateral cervical lymph nodes under direct view twice on days 16 and 21. (D) Two G47Δ injections into enlarged metastatic lymph nodes significantly prolonged the survival. Arrows indicate the timings of G47Δ injection. ∗∗p < 0.01 (log-rank test).
Discussion
Treatment of OSCC is problematic as the tumor cells metastasize to the cervical lymph nodes. Moreover, due its location, extensive resection cannot be performed as it could severely impair the patient QOL. We seek to overcome these obstacles by employing a new therapeutic modality, namely a third-generation oncolytic HSV-1, G47Δ. We show that, in fact, a single intratumoral injection with G47Δ significantly prolongs the survival of athymic mice bearing orthotopic tongue cancer of SAS-GFP or HSC-3. In the SAS-GFP tongue cancer model, 3-time injections with a low dose of G47Δ exhibited a significantly higher efficacy than a single injection with a dose higher than the total of three low doses, implicating that multiple doses are more efficacious than a single dose. The G47Δ treatment efficiently suppressed the extent of cervical lymph node metastases, and the presence of G47Δ in the lymph nodes was evident at 4 days after inoculation into the primary tumor.
We further generated an orthotopic tongue cancer model using KLN205-MUC1 cells in HSV-1-sensitive DBA/2 mice to investigate the efficacy of G47Δ under immunocompetent conditions. This model reproduces the metastatic cascade that tongue cancer patients commonly experience. Again, a single intratumoral injection with G47Δ prolongs the survival of immunocompetent mice bearing tongue cancer. In this model, tumor cells are already metastatic to cervical lymph nodes 3 days after tumor implantation to the tongue. Surprisingly, the presence of G47Δ in those lymph nodes was evident at 18 h by HSV-1 immunostaining and at 10 min by quantitative real-time PCR after inoculation into the primary tumor, indicating that G47Δ traffics to the regional lymph nodes almost instantly. Injection with G47Δ into the tongue cancer followed by an immediate resection effectively prolonged the survival even at a relatively late stage (7 days after tumor implantation), further supporting the finding that G47Δ swiftly traffics to the regional lymph nodes.
In contrast to the fact that intratumorally administered oncolytic HSV-1 does not readily circulate within the blood stream, we show that G47Δ can traffic within the lymphatics without delay. The phenomenon that oncolytic viruses can traffic to the regional lymph nodes after injection into primary tumors has been reported.22 However, we further elucidate that such trafficking occurs almost instantly. Therefore, at least at a very early time point, viruses are likely capable of trafficking to lymph nodes without tumor cells. One of the controversies in the field of OSCC is whether to perform prophylactic neck dissection in clinical node-negative OSCC patients. While elective neck dissection has been reported to result in higher rates of survival than therapeutic neck dissection,32 lymphatics draining the oral cavity are considered to work as a mechanical or immunologic barrier to the spread of cancer and therefore should be preserved until clinically involved.33 Our results show that G47Δ injected into the primary tongue cancer presurgically can swiftly traffic to the cervical lymph nodes and suppress the extent of metastases. Therefore, with the use of G47Δ at hand, the extent of neck dissection could be minimized. Our results further show that, in the KLN205-MUC1 model, G47Δ injected into metastatic lymph nodes after primary tumor resection prolongs the survival and can lead to a cure even at an advanced stage. The fact that cervical lymph nodes can be directly inoculated with G47Δ with efficacy after manifesting metastatic tumor growth supports the notion that prophylactic neck dissection should be avoided. Because human cells are generally more susceptible to G47Δ than murine cells, a higher efficacy may be expected in clinical settings. Whether lymphatic trafficking of oncolytic viruses is a phenomenon observed most frequently in the cervical region needs further investigation.
The triple mutations of G47Δ include a deletion of the α47 gene that causes enhanced MHC class I presentation of infected tumor cells, and intratumoral G47Δ injection has been shown to induce specific antitumor immune responses efficiently.24 In this study, flow cytometry of lymphocytes in the cervical lymph nodes 18 h after G47Δ injection into the tongue cancer showed a significant increase in CD69-positive cells among both CD8- and CD4-positive cells, indicating that G47Δ not only traffics to cervical lymph nodes swiftly but also activates T cells immediately. We also observed a higher proportion of CD69-positive cells among CD8-positive cells in the cervical lymph nodes 18 h after G47Δ was injected into the normal tongue of DBA/2 mice compared with mock (data not shown), suggesting that this early activation of T cells is at least partly due to an immune response against G47Δ. Presumably, this activation of T cells, when accompanied by a tumor cell destruction by G47Δ and a processing by antigen presenting cells in the regional lymph nodes, can facilitate the elicitation of specific antitumor immune responses.34, 35, 36, 37, 38 MHC class I expression has been reported to correlate with the prognosis of OSCC,39 and oncolytic virus therapy, especially using G47Δ, is known to turn immunologically “cold” tumors “hot.”40 It is likely that G47Δ not only suppresses cervical lymph node metastases via its swift trafficking but also acts via the stimulation of host antitumor immunity.
Presurgical inoculation of tongue cancer with G47Δ results in immediate trafficking of G47Δ to the cervical lymph nodes and suppression of lymphatic metastases. The use of G47Δ even immediately before tumor resection can therefore minimize the extent of neck dissection, thereby preserving the QOL of patients. Injections with G47Δ into metastatic lymph nodes can also be efficacious at an advanced stage. Furthermore, because G47Δ also acts via systemic immunity, immune checkpoint inhibitors may augment the efficacy of G47Δ.40,41 These results show that G47Δ can be employed in the development of entirely new treatment strategies for tongue cancer and other OSCC at various clinical stages.
Materials and methods
Cell lines and virus
Vero (African green monkey kidney) cells were purchased from the American Type Culture Collection (Rockville, MD, USA) and cultured as described previously.24,42 Human OSCC cell lines SAS, OSC-19, HSC-3, and SCC-4 were purchased from the Health Science Research Resources Bank (Osaka, Japan). A mouse SCC cell line, KLN205-MUC1, derived from DBA/2 strain was obtained from RIKEN BioResource Center (Tsukuba, Japan). A mouse SCC cell line, SCCVII, derived from C3H/He strain was a generous gift from Professor Yoshiaki Yura (Second Department of Oral and Maxillofacial Surgery, Osaka University, Osaka, Japan). The cells were cultured according to the instructions provided by the suppliers. G47Δ was grown in Vero cells, and virus titers were determined by using 6-well plates with semi-confluent Vero cells infected in serial dilutions as described previously.24,43,44 Mock-infected extract (mock) was prepared from virus buffer-infected cells, using the same procedures as those used for virus inoculum.
In vitro cytotoxicity studies
Cytopathic effects were evaluated as described previously.24,45, 46, 47 Briefly, human OSCC cells or mouse SCC cells were seeded on six-well plates at 2 × 105 cells/well and infected with mock or G47Δ at an MOI of 0.01, 0.1, or 1.0 in triplicates. The number of surviving cells was counted daily with a Coulter Counter (Beckman Coulter, Fullerton, CA, USA) and expressed as a percentage of mock-infected controls.
Viral replication studies
Four human OSCC cell lines and Vero cells (control) were seeded in 6-well plates (5 × 105 cells/well) and infected with G47Δ at a multiplicity of infection (MOI) of 0.01. The infections were performed in triplicates. After incubation at 37°C for 24 h, the number of progeny virus was titered on Vero cells.
Immunohistochemistry
Excised subcutaneous tumors and lymph nodes were formalin-fixed, paraffin-embedded, and cut into 4-μm sections using a microtome. Antibodies against HSV-1 (Dako, Glostrup, Denmark), enhanced green fluorescent protein (EGFP; Abcam, Cambridge, UK), anti-mouse Ki67 (Abcam), and mucin 1 (MUC 1; Abcam) were used for immunohistochemistry. EnVision+ System-HRP Labeled Polymer Anti-Rabbit IgG (Dako) was used as a secondary antibody. Immunostaining was visualized using DAB Substrate Kit (Vector Laboratories, Burlingame, CA, USA). Some sections were counterstained with H&E.
Generation of EGFP-expressing SAS cells and in vivo fluorescence imaging
To detect lymph node metastasis by in vivo fluorescence imaging, we generated SAS cells that stably express EGFP (SAS-GFP) using a retroviral vector pQCLIN-EGFP.48 Visualization of SAS-GFP cells in the primary lesions and metastatic cervical lymph nodes in the SAS-GFP tongue cancer model was performed by observing directly under an MVX10 stereo fluorescence microscope (Olympus, Tokyo, Japan).
Quantitative real-time PCR
G47Δ DNA copy numbers in lymph nodes were measured by quantitative real-time PCR using the TaqMan system (7500 Fast Real-Time PCR System; Applied Biosystems, Foster City, CA, USA). DNA was extracted from the cervical lymph nodes using the QIAamp DNA Mini kit (QIAGEN, Hilden, Germany). Quantitative real-time PCR was performed on an Applied Biosystems 7500 Fast Real-Time PCR system (Thermo Fisher Scientific, Waltham, MA, USA) using the following probes and primers: TaqMan probe, 5ʹ-TTCTggTgCACCTgCggATCCC-3ʹ; forward primer, 5ʹ-CTCgCCTTTACCgCATCCT-3ʹ; reverse primer, 5ʹ-TgTAAAACgACggCCAgTgA-3′. Genomic copies of G47Δ were quantified using a plasmid containing the sequence amplified by the primers mentioned above.
Animal studies
Female BALB/c nu/nu, DBA/2, and C3H/He mice (5–6 weeks old) were purchased from Japan SLC (Hamamatsu, Shizuoka, Japan). All experimental animal protocols were approved by the Committee for Ethics of Animal Experimentation and were in accordance with the Guideline for Animal Experiments at the University of Tokyo.
Subcutaneous tumor models
Subcutaneous tumors were generated by inoculating 1 × 106 cells into the left flanks of athymic or syngeneic mice using a 26G needle. Subcutaneous tumors of approximately 8 mm (SAS) or 5 mm (KLN205-MUC1 and SCCVII) in diameter were inoculated with G47Δ or mock in 20 μL of 10% glycerol/Dulbecco’s phosphate-buffered saline on days 0 and 3, and the tumor sizes were measured twice a week.46 Mice were euthanized when the tumor size reached 24 mm in maximum diameter.
Orthotopic tongue cancer models
Under general anesthesia with intraperitoneal administration of ketamine and xylazine, tongue tumors were generated by implanting 1 × 106 cells of SAS-GFP or HSC-3 into the left side of the tongue of athymic mice. Tongue tumors were inoculated with G47Δ or mock using a Hamilton syringe (Hamilton Company, Reno, NV, USA) at indicated time points. KLN205-MUC1 and SCCVII tongue tumors were generated by implanting 2 × 105 cells into the left side of the tongue of syngeneic DBA/2 mice and C3H/He mice, respectively, and G47Δ was inoculated into the tumors as described above. In SAS-GFP, HSC-3, and SCCVII tongue cancer models, implanted tumor cells grow within the tongue and metastasize to cervical lymph nodes, and animals typically die of primary tumor growth. KLN205-MUC1 tongue cancer model reproduces the metastatic cascade commonly observed in tongue cancer patients: cervical lymph node metastasis occurs without exception, followed by lung metastasis. Animals typically die of primary tumor growth, but when the primary tumor has been resected, lung metastasis usually causes death. The survival of mice inoculated with G47Δ or mock was observed every day. A mouse was euthanized when it became moribund in accordance with a protocol approved by the Institutional Animal Care and Use Committee and defined to have survived until the following day. Direct injections with G47Δ to the cervical lymph nodes were performed using a 30G needle under general anesthesia with intraperitoneal administration of ketamine and xylazine.
Flow cytometry
A single-cell suspension was prepared from the cervical lymph nodes removed from three mice. After lysis of red blood cells in 1 × RBC Lysis Buffer Solution (eBioscience, San Diego, CA, USA) and selection of live cells using the Zombie Yellow Fixable Viability Kit (BioLegend, San Diego, CA, USA), lymphocytes were incubated with mouse anti- CD16/32 antibody (Tonbo Biosciences, San Diego, CA, USA) followed by incubation with fluorescein allophycocyanin-conjugated mouse anti-CD8a (BD Biosciences, San Jose, CA, USA), phycoerythrin-Cy7-conjugated mouse anti-CD69 (BioLegend), BrilliantViolet785-conjugated anti-mouse CD3 (BioLegend), fluorescein isothiocyanate-conjugated anti-CD4 (eBioscience), or fluorophore-conjugated isotype controls (eBioscience). The cells were analyzed using CytoFLEX (V5-B5-R3 configuration, Beckman Coulter, Fullerton, CA, USA), and data were analyzed with CytExpert software (Beckman Coulter).
Statistical analysis
The Tukey-Kramer method was used to analyze the data from subcutaneous tumor models. Real-time PCR data were analyzed using Mann-Whitney U test. Kaplan-Meier analysis was used for survival studies, and significance was evaluated by log-rank test, except for comparing the survival between the single injection group and the triple injections group (Figures 3F and 5B), for which the generalized Wilcoxon test was used, with pairwise over strata. Data analysis was performed using SPSS v.22 software (IBM, New York, NY, USA). Student’s t test was used for the flow cytometric analyses.
Acknowledgments
We thank Drs. Yasushi Ino for useful advice, Yoshiaki Yura for providing the SCCVII cell line, and Toshihiro Inubushi for his encouragement and advice. This work was supported in part by research grants from the Japan Agency of Medical Research and Development (AMED; grant numbers JP18ck0106416 and JP20lm0203140 to T.T.).
Author contributions
T.U. and M.I. contributed to investigation, methodology, data curation, data analysis, and writing. A.S. contributed to investigation. H.N. and M.K. contributed to conceptualization. H.F. and M.T. contributed to supervision. H.I. contributed to writing and editing. T.T. contributed to conceptualization, methodology, data analysis, supervision, writing, editing, and funding acquisition. All authors reviewed the manuscript.
Declaration of interests
T.T. owns the patent right for G47Δ in multiple countries including Japan.
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.omto.2021.06.008.
Supplemental information
References
- 1.Jemal A., Siegel R., Xu J., Ward E. Cancer statistics, 2010. CA Cancer J. Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Schmalbach C.E., Miller F.R. Occult primary head and neck carcinoma. Curr. Oncol. Rep. 2007;9:139–146. doi: 10.1007/s11912-007-0012-5. [DOI] [PubMed] [Google Scholar]
- 3.Ebrahimi A., Zhang W.J., Gao K., Clark J.R. Nodal yield and survival in oral squamous cancer: Defining the standard of care. Cancer. 2011;117:2917–2925. doi: 10.1002/cncr.25834. [DOI] [PubMed] [Google Scholar]
- 4.Shingaki S., Takada M., Sasai K., Bibi R., Kobayashi T., Nomura T., Saito C. Impact of lymph node metastasis on the pattern of failure and survival in oral carcinomas. Am. J. Surg. 2003;185:278–284. doi: 10.1016/s0002-9610(02)01378-8. [DOI] [PubMed] [Google Scholar]
- 5.Dias F.L., Lima R.A., Kligerman J., Farias T.P., Soares J.R., Manfro G., Sa G.M. Relevance of skip metastases for squamous cell carcinoma of the oral tongue and the floor of the mouth. Otolaryngol. Head Neck Surg. 2006;134:460–465. doi: 10.1016/j.otohns.2005.09.025. [DOI] [PubMed] [Google Scholar]
- 6.Forastiere A., Koch W., Trotti A., Sidransky D. Head and neck cancer. N. Engl. J. Med. 2001;345:1890–1900. doi: 10.1056/NEJMra001375. [DOI] [PubMed] [Google Scholar]
- 7.Werner J.A., Dünne A.A., Myers J.N. Functional anatomy of the upper aerodigestive tract’s lymphatic drainage system and its role in metastasis of squamous cell carcinoma. Head Neck. 2003;25:322–332. doi: 10.1002/hed.10257. [DOI] [PubMed] [Google Scholar]
- 8.Aghi M., Martuza R.L. Oncolytic viral therapies - the clinical experience. Oncogene. 2005;24:7802–7816. doi: 10.1038/sj.onc.1209037. [DOI] [PubMed] [Google Scholar]
- 9.Ott P.A., Hodi F.S. Talimogene Laherparepvec for the treatment of advanced melanoma. Clin. Cancer Res. 2016;22:3127–3131. doi: 10.1158/1078-0432.CCR-15-2709. [DOI] [PubMed] [Google Scholar]
- 10.Pol J., Kroemer G., Galluzzi L. First oncolytic virus approved for melanoma immunotherapy. OncoImmunology. 2015;5:e1115641. doi: 10.1080/2162402X.2015.1115641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Conry R.M., Westbrook B., McKee S., Norwood T.G. Talimogene laherparepvec: First in class oncolytic virotherapy. Hum. Vaccin. Immunother. 2018;14:839–846. doi: 10.1080/21645515.2017.1412896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rehman H., Silk A.W., Kane M.P., Kaufman H.L. Into the clinic: Talimogene laherparepvec (T-VEC), a first-in-class intratumoral oncolytic viral therapy. J. Immunother. Cancer. 2016;4:53. doi: 10.1186/s40425-016-0158-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mace A.T., Ganly I., Soutar D.S., Brown S.M. Potential for efficacy of the oncolytic Herpes simplex virus 1716 in patients with oral squamous cell carcinoma. Head Neck. 2008;30:1045–1051. doi: 10.1002/hed.20840. [DOI] [PubMed] [Google Scholar]
- 14.Hu J.C., Coffin R.S., Davis C.J., Graham N.J., Groves N., Guest P.J., Harrington K.J., James N.D., Love C.A., McNeish I. A phase I study of OncoVEXGM-CSF, a second-generation oncolytic herpes simplex virus expressing granulocyte macrophage colony-stimulating factor. Clin. Cancer Res. 2006;12:6737–6747. doi: 10.1158/1078-0432.CCR-06-0759. [DOI] [PubMed] [Google Scholar]
- 15.Harrington K.J., Hingorani M., Tanay M.A., Hickey J., Bhide S.A., Clarke P.M., Renouf L.C., Thway K., Sibtain A., McNeish I.A. Phase I/II study of oncolytic HSV GM-CSF in combination with radiotherapy and cisplatin in untreated stage III/IV squamous cell cancer of the head and neck. Clin. Cancer Res. 2010;16:4005–4015. doi: 10.1158/1078-0432.CCR-10-0196. [DOI] [PubMed] [Google Scholar]
- 16.Ganly I., Kirn D., Eckhardt G., Rodriguez G.I., Soutar D.S., Otto R., Robertson A.G., Park O., Gulley M.L., Heise C. A phase I study of Onyx-015, an E1B attenuated adenovirus, administered intratumorally to patients with recurrent head and neck cancer. Clin. Cancer Res. 2000;6:798–806. [PubMed] [Google Scholar]
- 17.Nemunaitis J., Ganly I., Khuri F., Arseneau J., Kuhn J., McCarty T., Landers S., Maples P., Romel L., Randlev B. Selective replication and oncolysis in p53 mutant tumors with ONYX-015, an E1B-55kD gene-deleted adenovirus, in patients with advanced head and neck cancer: a phase II trial. Cancer Res. 2000;60:6359–6366. [PubMed] [Google Scholar]
- 18.Rudin C.M., Cohen E.E., Papadimitrakopoulou V.A., Silverman S., Jr., Recant W., El-Naggar A.K., Stenson K., Lippman S.M., Hong W.K., Vokes E.E. An attenuated adenovirus, ONYX-015, as mouthwash therapy for premalignant oral dysplasia. J. Clin. Oncol. 2003;21:4546–4552. doi: 10.1200/JCO.2003.03.544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pecora A.L., Rizvi N., Cohen G.I., Meropol N.J., Sterman D., Marshall J.L., Goldberg S., Gross P., O’Neil J.D., Groene W.S. Phase I trial of intravenous administration of PV701, an oncolytic virus, in patients with advanced solid cancers. J. Clin. Oncol. 2002;20:2251–2266. doi: 10.1200/JCO.2002.08.042. [DOI] [PubMed] [Google Scholar]
- 20.Wong R.J., Joe J.K., Kim S.H., Shah J.P., Horsburgh B., Fong Y. Oncolytic herpesvirus effectively treats murine squamous cell carcinoma and spreads by natural lymphatics to treat sites of lymphatic metastases. Hum. Gene Ther. 2002;13:1213–1223. doi: 10.1089/104303402320138998. [DOI] [PubMed] [Google Scholar]
- 21.Kishimoto H., Kojima T., Watanabe Y., Kagawa S., Fujiwara T., Uno F., Teraishi F., Kyo S., Mizuguchi H., Hashimoto Y. In vivo imaging of lymph node metastasis with telomerase-specific replication-selective adenovirus. Nat. Med. 2006;12:1213–1219. doi: 10.1038/nm1404. [DOI] [PubMed] [Google Scholar]
- 22.Kurihara Y., Watanabe Y., Onimatsu H., Kojima T., Shirota T., Hatori M., Liu D., Kyo S., Mizuguchi H., Urata Y. Telomerase-specific virotheranostics for human head and neck cancer. Clin. Cancer Res. 2009;15:2335–2343. doi: 10.1158/1078-0432.CCR-08-2690. [DOI] [PubMed] [Google Scholar]
- 23.Tanaka Y., Araki K., Tanaka S., Miyagawa Y., Suzuki H., Kamide D., Tomifuji M., Uno K., Kimura E., Yamashita T. Sentinel lymph node-targeted therapy by oncolytic sendai virus suppresses micrometastasis of head and neck squamous cell carcinoma in an orthotopic nude mouse model. Mol. Cancer Ther. 2019;18:1430–1438. doi: 10.1158/1535-7163.MCT-18-1372. [DOI] [PubMed] [Google Scholar]
- 24.Todo T., Martuza R.L., Rabkin S.D., Johnson P.A. Oncolytic herpes simplex virus vector with enhanced MHC class I presentation and tumor cell killing. Proc. Natl. Acad. Sci. USA. 2001;98:6396–6401. doi: 10.1073/pnas.101136398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cassady K.A., Gross M., Roizman B. The second-site mutation in the herpes simplex virus recombinants lacking the gamma134.5 genes precludes shutoff of protein synthesis by blocking the phosphorylation of eIF-2alpha. J. Virol. 1998;72:7005–7011. doi: 10.1128/jvi.72.9.7005-7011.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goldstein D.J., Weller S.K. Herpes simplex virus type 1-induced ribonucleotide reductase activity is dispensable for virus growth and DNA synthesis: isolation and characterization of an ICP6 lacZ insertion mutant. J. Virol. 1988;62:196–205. doi: 10.1128/jvi.62.1.196-205.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.York I.A., Roop C., Andrews D.W., Riddell S.R., Graham F.L., Johnson D.C. A cytosolic herpes simplex virus protein inhibits antigen presentation to CD8+ T lymphocytes. Cell. 1994;77:525–535. doi: 10.1016/0092-8674(94)90215-1. [DOI] [PubMed] [Google Scholar]
- 28.Mohr I., Sternberg D., Ward S., Leib D., Mulvey M., Gluzman Y. A herpes simplex virus type 1 gamma34.5 second-site suppressor mutant that exhibits enhanced growth in cultured glioblastoma cells is severely attenuated in animals. J. Virol. 2001;75:5189–5196. doi: 10.1128/JVI.75.11.5189-5196.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Morita Y., Hata K., Nakanishi M., Nishisho T., Yura Y., Yoneda T. Cyclooxygenase-2 promotes tumor lymphangiogenesis and lymph node metastasis in oral squamous cell carcinoma. Int. J. Oncol. 2012;41:885–892. doi: 10.3892/ijo.2012.1529. [DOI] [PubMed] [Google Scholar]
- 30.Cosulich M.E., Rubartelli A., Risso A., Cozzolino F., Bargellesi A. Functional characterization of an antigen involved in an early step of T-cell activation. Proc. Natl. Acad. Sci. USA. 1987;84:4205–4209. doi: 10.1073/pnas.84.12.4205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Safi A.F., Kauke M., Grandoch A., Nickenig H.J., Drebber U., Zöller J., Kreppel M. The importance of log odds of positive lymph nodes for locoregional recurrence in oral squamous cell carcinoma. Oral Oncol. 2017;72:48–55. doi: 10.1016/j.oraloncology.2017.07.005. [DOI] [PubMed] [Google Scholar]
- 32.D’Cruz A.K., Vaish R., Kapre N., Dandekar M., Gupta S., Hawaldar R., Agarwal J.P., Pantvaidya G., Chaukar D., Deshmukh A., Head and Neck Disease Management Group Elective versus therapeutic neck dissection in node-negative oral cancer. N. Engl. J. Med. 2015;373:521–529. doi: 10.1056/NEJMoa1506007. [DOI] [PubMed] [Google Scholar]
- 33.Baker R.R., Wood S., Jr., Cong P.V., Kim S.T., Tolo V.T. Role of the cervical lymph nodes as a barrier to metastatic tumor. Am. J. Surg. 1969;118:654–659. [Google Scholar]
- 34.Granot T., Yamanashi Y., Meruelo D. Sindbis viral vectors transiently deliver tumor-associated antigens to lymph nodes and elicit diversified antitumor CD8+ T-cell immunity. Mol. Ther. 2014;22:112–122. doi: 10.1038/mt.2013.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim R., Emi M., Tanabe K., Arihiro K. Immunobiology of the sentinel lymph node and its potential role for antitumour immunity. Lancet Oncol. 2006;7:1006–1016. doi: 10.1016/S1470-2045(06)70975-5. [DOI] [PubMed] [Google Scholar]
- 36.Walker L.S., Abbas A.K. The enemy within: keeping self-reactive T cells at bay in the periphery. Nat. Rev. Immunol. 2002;2:11–19. doi: 10.1038/nri701. [DOI] [PubMed] [Google Scholar]
- 37.Thomas D.A., Massagué J. TGF-beta directly targets cytotoxic T cell functions during tumor evasion of immune surveillance. Cancer Cell. 2005;8:369–380. doi: 10.1016/j.ccr.2005.10.012. [DOI] [PubMed] [Google Scholar]
- 38.Sobol P.T., Boudreau J.E., Stephenson K., Wan Y., Lichty B.D., Mossman K.L. Adaptive antiviral immunity is a determinant of the therapeutic success of oncolytic virotherapy. Mol. Ther. 2011;19:335–344. doi: 10.1038/mt.2010.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Koike K., Dehari H., Shimizu S., Nishiyama K., Sonoda T., Ogi K., Kobayashi J., Sasaki T., Sasaya T., Tsuchihashi K. Prognostic value of HLA class I expression in patients with oral squamous cell carcinoma. Cancer Sci. 2020;111:1491–1499. doi: 10.1111/cas.14388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yamada T., Tateishi R., Iwai M., Koike K., Todo T. Neoadjuvant use of oncolytic herpes virus G47Δ enhances the antitumor efficacy of radiofrequency ablation. Mol. Ther. Oncolytics. 2020;18:535–545. doi: 10.1016/j.omto.2020.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Saha D., Martuza R.L., Rabkin S.D. Macrophage polarization contributes to glioblastoma eradication by combination immunovirotherapy and immune checkpoint blockade. Cancer Cell. 2017;32:253–267.e5. doi: 10.1016/j.ccell.2017.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Todo T., Martuza R.L., Dallman M.J., Rabkin S.D. In situ expression of soluble B7-1 in the context of oncolytic herpes simplex virus induces potent antitumor immunity. Cancer Res. 2001;61:153–161. [PubMed] [Google Scholar]
- 43.Mineta T., Rabkin S.D., Yazaki T., Hunter W.D., Martuza R.L. Attenuated multi-mutated herpes simplex virus-1 for the treatment of malignant gliomas. Nat. Med. 1995;1:938–943. doi: 10.1038/nm0995-938. [DOI] [PubMed] [Google Scholar]
- 44.Miyatake S., Martuza R.L., Rabkin S.D. Defective herpes simplex virus vectors expressing thymidine kinase for the treatment of malignant glioma. Cancer Gene Ther. 1997;4:222–228. [PubMed] [Google Scholar]
- 45.Ino Y., Saeki Y., Fukuhara H., Todo T. Triple combination of oncolytic herpes simplex virus-1 vectors armed with interleukin-12, interleukin-18, or soluble B7-1 results in enhanced antitumor efficacy. Clin. Cancer Res. 2006;12:643–652. doi: 10.1158/1078-0432.CCR-05-1494. [DOI] [PubMed] [Google Scholar]
- 46.Fukuhara H., Martuza R.L., Rabkin S.D., Ito Y., Todo T. Oncolytic herpes simplex virus vector g47delta in combination with androgen ablation for the treatment of human prostate adenocarcinoma. Clin. Cancer Res. 2005;11:7886–7890. doi: 10.1158/1078-0432.CCR-05-1090. [DOI] [PubMed] [Google Scholar]
- 47.Todo T., Rabkin S.D., Sundaresan P., Wu A., Meehan K.R., Herscowitz H.B., Martuza R.L. Systemic antitumor immunity in experimental brain tumor therapy using a multimutated, replication-competent herpes simplex virus. Hum. Gene Ther. 1999;10:2741–2755. doi: 10.1089/10430349950016483. [DOI] [PubMed] [Google Scholar]
- 48.Tanaka K., Arao T., Maegawa M., Matsumoto K., Kaneda H., Kudo K., Fujita Y., Yokote H., Yanagihara K., Yamada Y. SRPX2 is overexpressed in gastric cancer and promotes cellular migration and adhesion. Int. J. Cancer. 2009;124:1072–1080. doi: 10.1002/ijc.24065. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.