Abstract
Charnley’s low-friction principle of total hip arthroplasty (THA) is recognized as the gold standard. However, complications may arise, and one of the major reasons for revising THA is dislocation. Under such a background, Pr. Gilles Bousquet invented dual-mobility cups (DMC) in the 1970s to fight against hip instability. Despite the excellent clinical results of DMC compared with conventional implants, the clinical application of DMC is limited by concerns about the dual articulations, leading to rapid wear and the subsequent osteolysis and the wear on the retaining rim of the liner due to its contact with the femoral neck causing intraprosthetic dislocation (IPD). As a result, the original design of DMC has been upgraded by using highly cross-linked polyethylene, refining the geometry of the femoral neck, etc. After the improvement, the wear rate of the contemporary DMC liners has been largely reduced compared with the first generation DMC, and the IPD incidence rate has been controlled. However, with the increasing fretting corrosion damage found at the taper-trunnion interfaces in conventional implants, the contemporary DMC may face a similar problem. This is because the additional articulation and the larger head design of DMC gain the risk of articulation wear and taper-trunnion interface corrosion. Since there are still many potential DMC engineering issues that have not been extensively researched, future studies focusing on the wear and corrosion aspects are required. The purpose of this review article is to summarize both the clinical and engineering issues for DMC with possible directions for future research.
Keywords: Dislocation, dual mobility, IPD, wear, corrosion, total hip arthroplasty
Introduction
Hip prosthesis was invented in 20th century as a replacement for natural hip joints and to treat severe hip diseases. Its clinical effectiveness has been proved by years of clinical application. The major hip prostheses on the market normally consist of an acetabular shell, an acetabular liner, a femoral head, and a femoral stem. According to the 2019 annual report from the American Joint Replacement Registry [1], the cumulative number of total hip arthroplasties (THA) in America exceeded half a million during the period 2012 to 2018 (The distribution of the procedures from 2012-2018 is shown in Table 10). It is obvious that THA is a successful surgical procedure. However, some of the patients undergoing THA need further revision surgery. The contributing factors for hip implant failure include loosening, metal related pathology, fractures, infections, etc. And one of the primary reasons for revision is dislocation [2].
Table 10.
The distribution of hip procedures in America from 2012 to 2018 [1]
| Distribution of hip procedures | The cumulative volume of THA |
|---|---|
| Primary knee | 829.0 k |
| Primary hip | 498.1 k |
| Hemiarthroplasty | 69.4 k |
| Revision knee | 58.4 k |
| Revision hip | 45.0 k |
| Hip resurfacing | 5.2 k |
According to published data, the risk of dislocation ranges from 0.2% to 7% for primary procedures, and the risk increases to 10% or higher for revision arthroplasties [3]. Moreover, the health-care cost for each dislocation revision is estimated to be US $14,000 [4]. Since dislocation has been the critical factor that affects the success of the long-term results of THA, determining how to reduce the risk of dislocation is a concern for orthopedists. There are two main approaches: surgical methods and implant designs. The former uses a direct lateral approach. The latter involves enlarging the femoral head diameter, such as the constrained liners, the larger diameter femoral heads, and the dual-mobility cups (DMC). Because the concept of DMC combines Charnley’s low friction’ principle and McKee’s the effect of big femoral head theory, it can increase the effective head size with no harm to the liner thickness or the range of motion. As a result, the DMC outperforms the constrained liners and the larger diameter femoral head design. However, DMC introduced an additional articulation surface which has the potential to cause more excessive wear than the conventional implants, so concerns about wear and corrosion still remain for dual-mobility prostheses. To the authors’ knowledge, most studies about DMC are about its short to mid-term clinical follow-up results [5-20]. There are few systematic reviews from its engineering aspects. And little is known about how the liner wears or how the taper-trunnion interfaces corrode in the DMC design [21-26]. The aim of this review is to comprehensively describe the dual-mobility prosthesis, both from the clinical and engineering aspects, and to analyze these outcomes to provide possible avenues for future studies on reducing dislocation and improving the DMC lifetime.
Background of dual-mobility cups and their current clinical use
Dual mobility bearings are comprised of two unconstrained articulations between three different components (a femoral head, a polyethylene liner, and an acetabular component) (Figure 1). The concept of dual-mobility bearings was first introduced by Gilles Bousquet in the early 1970s [27,28]. The DMC design combines the advantage of Charnley’s low-friction principle and McKee-Farrar’s theory of an increased femoral head-to-neck ratio to provide a reliable solution to dislocation complications. During the procedure, the femoral ball head is forced into the liner and captured by the liner’s retentive rim. With the retaining rim design of the polyethylene liner, the femoral head is undergoing ‘safe’ movement within the liner. Meanwhile this entire structure is mobile within the acetabular component. This construct provides a larger effective head size than the conventional implants, and that is why the dual mobility design limits the risk of dislocation.
Figure 1.

Exploded view of DMC (picture courtesy of Chunlizhengda Medical Corporation, China).
With a wide clinical range of DMC applications, a complication called intraprosthetic dissociation (IPD) has been documented. IPD, a unique complication of DMC, occurs when the femoral head is separated from the liner. Mitchell et al. [29] reported an acute IPD case and pointed out that the causes for common IPD were polyethylene wear, the obstructed movement of the outer articulation, unexpected liner-neck impingement, and acetabular loosening. So some modifications have been applied on the first generation of DMC. First, the material of the polyethylene (PE) insert is changed from ultra-high molecular weight PE to highly cross-linked PE to reduce liner wear. Second, the femoral neck is designed thinner and polished smoother than before to reduce the liner-neck impingement. Furthermore, the coating technology is altered from the alumina coating to the bilayer coating (porous titanium and hydroxyapatite) to enhance the acetabular stability [30].
Since the United States Food and Drug Administration approved DMC in 2009, it has become popular worldwide, especially for revising THA [1,31,32] (Table 2). Because of this, most global orthopedics companies have developed their own dual-mobility designs (listed in Table 3), which are usually matched with the outer diameters of the 22 mm or 28 mm femoral ball heads (Table 4).
Table 2.
Dual mobility’s yearly number of implants by country
| Country | Organization | DMC yearly implanted | Percentage on THA |
|---|---|---|---|
| USA | American Joint replacement Registry (2019) [1] | 3,746 (Average primary THA yearly implanted number, 2012-2018) | 7.6% (Primary) |
| 16.3% (Revision) | |||
| Sweden | The Swedish Hip Arthroplasty Registry (2017) [31] | 294 (Primary and Revision) | 39% (Cemented) |
| 16% (Uncemented) | |||
| Australia | Australian Joint Replacement Registry (2016) [32] | 2640 (Primary) | 0.8% (Primary) |
| 64 (Revision) | 0.54% (Revision) |
Table 3.
Dual-mobility implants by manufacturer
| Manufacturer | Product |
|---|---|
| Aston Medical | Tregor® Medial Cup® |
| Zimmer GmbH | Stafit™ Cup |
| Amplitude | Saturne™ Cup |
| Wright Medical France | Collégia™ Cup |
| Smith & Nephew Orthopaedics AG | POLARCUP® |
| Stryker Orthopaedics | Anatomic Dual-Mobility X3® |
| Modular Dual-Mobility X3® | |
| Biomet Orthopedics | Active Articulation™ E1 |
| Serf Dedienne santé | Novae® Series Cups |
Table 4.
Major parameters of the different dual-mobility products
| Product | Poly Insert Outer Diameter (mm) | Poly Insert Inner Diameter (mm) |
|---|---|---|
| Active Articulation™ E1 | 38-60/2 mm per size | 28 |
| Pinnacle® Bi-polar head | 48-76/2 mm per size | 28, 32, 36, 40, 44 |
| POLARCUP® | 43-67/2 mm per size | 22, 28 |
| Novae® Series Cups | 41-69/2 mm per size | 22, 28 |
| Anatomic Dual-Mobility X3® | 36-58/2 mm per size | 22, 28 |
| Modular Dual-Mobility X3® |
Literature search strategy
In this study, all the relevant papers were searched in the PubMed database on 28th February 2020. The search strings and number of articles are summarized in Table 1. The exclusion criteria are: conference proceedings with summary data only, articles not written in English, duplicate publications, articles with unclear results, and articles not related to the main topic of this study. After screening, 96 articles were selected for this review. As displayed in Table 11, the number of articles rose from 2010 to 2020, which indicates the increasing popularity of dual mobility bearings, particularly since 2015.
Table 1.
The search strings and number of articles
| Research focus | Key Words | Number of selected articles |
|---|---|---|
| Clinic | ‘Bousquet’ or ‘dual-mobility’ or ‘DM cup’ and ‘survival rate’ | 57 |
| Wear | ‘Bousquet’ or ‘dual-mobility’ or ‘DM cup’ and ‘wear’ | 37 |
| Fretting and corrosion | ‘DM cup’ or ‘dual-mobility’ and ‘corrosion’ and ‘fretting’ and ‘Taper’ | 2 |
Table 11.
The number of selected articles by year
| Year | Number of articles |
|---|---|
| 2010 | 8 |
| 2011 | 10 |
| 2012 | 18 |
| 2013 | 6 |
| 2014 | 8 |
| 2015 | 10 |
| 2016 | 22 |
| 2017 | 25 |
| 2018 | 18 |
| 2019 | 15 |
| 2020 | 2 |
Clinical manifestations
Summary of the clinical results
DMC has a rich experience in clinical application, especially in its birthplace France. Prudhon et al. [10] reported a 95% survivorship of the contemporary DMC at ten years follow-up with no dislocations found. Similarly, Bousquet et al. [16] demonstrated that the survivorship at 12 years follow-up was 95.4% of 135 THAs with DMC. Martz et al. [33] reviewed 40 cases of DMC in young patients, and the survival rate was 100% without any long-term dislocations at ten years follow-up. A summary of the DMC clinical outcomes is provided in Tables 5 and 6 and Figure 2. The clinical results implicate that dual-mobility bearings have a much lower dislocation rate and equivalent survivorship rate than the conventional implants.
Table 5.
Summary of the DMC survival rates
| Researcher | Test Subjects | Survival rate | Researcher | Test Subjects | Survival rate |
|---|---|---|---|---|---|
| Alain [5] | 437 hips | 100% at 4 years | Alain [14] | 321 patients | 97.51% for all cause revision at 5 years |
| Massimo [6] | 33 hips | 97% at 5 years | Boyer [15] | 240 hips | 74% at 22 years |
| Aron [7] | 40 hips | 96% at 5 years | Bousquet [16] | 135 hips | 95.4% at 12 years |
| Hean [8] | 66 hips | 98% at 5 years | Schneider [17] | 96 patients | 95.6% at 8 years |
| Heumen [9] | 50 hips | Survival rate after 4.6 years was 100% based on dislocation | Vermersch [18] | 100 patients | 100% at minimum 5 years |
| Langlais [34] | 85 cups | 94.6% at 5 years | Philippot [19] | 438 hips | 96.3 at 15 years |
| Prudhon [10] | 105 hips | 95% at 10 years | Philippot [35] | 106 hips | 94.6% at 10 years |
| Simian [11] | 74 hips | 90% at 5 years | Philippot [36] | 384 hips | 95.9% at 18 years |
| Massin [12] | 2601hips | 95% at 10 years | Steven [37] | 453 hips | 99.6% at mean 3.3 years |
| Michael [13] | 32 patients | 78% at 2 years and 60% at 5 years | Leclercq [38] | 109 hips | 99% at 10 years |
Table 6.
Summary of the DMC dislocation rates
| Researcher | Test Subjects | Dislocation rate | Researcher | Test Subjects | Dislocation rate |
|---|---|---|---|---|---|
| Boyer [15] | 240 hips | 0% | Leclercq [38] | 200 hips | 0% |
| Bousquet [16] | 135 hips | 0% | Tarasevicius [39] | 42 hips | 0% |
| Alain [5] | 437 hips | 1.10% | Michael [13] | 10 hips | 0% |
| Philippeau [38] | 71 cups | 9.80% | Langlais [34] | 85 hips | 1.10% |
| Wegrzyn [40] | 994 hips | Dislocation rate: 1.5% | Wackenheim [41] | 59 hips | 1.70% |
| IPD rate: 0.2% | |||||
| Philippot [35] | 106 hips | 0% | Guyen [42] | 54 hips | 1.80% |
| Philippot [36] | 384 hips | 0% | Hailer [43] | 228 hips | 2% |
| Philippot [19] | 438 hips | 0% | Philippot [44] | 163 hips | 3.70% |
| Terrier [45] | 167 hips | 0% | Mertl [46] | 145 revision hips | Dislocation rate: 4.8% |
| IPD rate: 1.4% | |||||
| Hamadouche [47] | 51 hips | 2% | Alain [14] | 321 patients | 0% |
Figure 2.
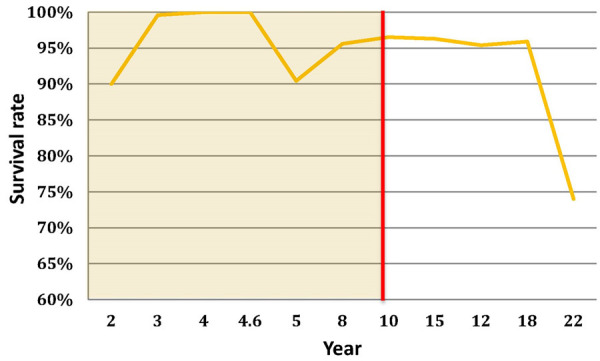
Survival rate versus implantation year for DMC (summarized from the literature from Oct 1985 to June 2014).
Because of DMC’s excellent short to mid-term clinical stability, it has been typically used for revision procedures and also as a good option for ‘risky’ primary arthroplasty [37]. Nicholas et al. [48] reviewed twenty-nine limited revisions by converting the failed monoblock metal-on-metal (MoM) THA to DMC without acetabular component extraction. The radiographic outcomes of limited revisions show no aseptic loosening or osteolysis, with lower complication rates than the complete revisions (the control group). Similarly, Moreta et al. [49] reported 10 THA revisions with DMC cemented into an existing well-fixed acetabular shell. At a mean follow-up of 3.5 years, the Harris Scores improved from 49.3 to 71.3. Among all the 10 revisions, 1 case was found with a postoperative recurrent dislocation, 2 cases with late polyethylene wear, but no cases of IPD or aseptic loosening. Jason et al. [50] reviewed seventy-one surgeries converting hip resurfacing arthroplasty to total hip arthroplasty, among which 27 cases used DMC and retained the existing acetabular component and 44 used conventional bearings for complete revision. The results showed that a higher complication rate was detected in the ‘complete revision group’ than in the ‘retained acetabular component group’, and there was no significant difference in the pre-conversion and post-conversion metal ion levels between the two groups. Overall, in the knotty revision cases, combining the existing well-fixed acetabular component with the DMC demonstrated good results compared with the standard revision THA.
On the other hand, the literature reported that IPD appeared to be an exclusive DMC complication. From the radiographs, if there is an eccentric position of the femoral head lying against the outer articulation of the shell, then IPD is diagnosed. Jean et al. [51] found that the incidence rate of IPD is almost 5%. Clinical observations and an analysis of the cause of IPD were performed and recorded in the literature. Christian et al. [52] found that the blocked movement of the outer articulation and cup loosening might be the cause of IPD. Ivan et al. [53] reviewed 19 THAs from 16 articles. They found the previous attempted closed reduction could be the possible iatrogenic etiology for early IPD. The late IPD was related to polyethylene wear and the subsequent failure of the capture mechanism. Similarly, Neri et al. [54] studied 93 retrieved DM liners, and they found IPD was highly related to the wear of liner, particularly the wear from the outer surface of the liner’s retaining rim.
Since polythene wear and the design of femoral neck and retaining rim are the acknowledged causes if IPD, optimizations have been done on the first generation DMC. Blakeney and Epinette et al. [14-55] found that, due to the excellent clinical results of contemporary DMC with highly cross-linked PE, it seemed that the wear problem had been overcome. Darrith et al. [30] reviewed 10,783 THAs using DMC and no IPD was found with contemporary DMC. However, Andrew et al. [56] reported a case of early IPD in contemporary DMC. The main cause should be the ceased movement of the outer articulation with the succeeding impingement between the femoral neck and the retentive rim. And Koper et al. [57] observed an IPD and a higher level of metal ions from the contemporary DMC follow-up, which indicates that the wear or corrosion of the metal components is the root cause of IPD in contemporary DMC. The contemporary DMC does reduce the polyethylene wear and the incidence rate of IPD but does not eliminate them. Concerns about wear and subsequent IPD still remain. And that is why some experts suggest caution should be exercised with younger and active patients when they are using DMC [27]. More research about the wear of DMC and the further optimization of its design are needed.
Retrieved studies on the in-vivo uses
As DMC has extra articulation between the liner and the acetabular component, and the liner’s wear is found highly related to IPD, some researchers predicted that wear would be higher in DMC compared with conventional implants. The most straightforward way to verify this hypothesis is through in-vivo evaluations. Boyer et al. [21,23], Imbert [22] and Adam [24] have analyzed retrieved DM liners to study the DMC clinical wear. The wear volumes of the liner’s two articulations can be directly measured using a coordinate measuring machine (CMM) or a light surface scanner. They all found the wear rate of the internal surface (Convex) was larger than the external surface (Concave), which means the motion of the inner articulation is dominant.
The median annual wear rate for traditional metal-on-polyethylene bearings is between 39 and 98 mm3/year, and Boyer et al.’s [23] research found that the median annual wear rate of DMC was 38 mm3/year. Deckard et al. [25] used Martell methodology to analyze the head penetration of 34 DM bearings. The outcomes show that the penetration of DMC is larger than conventional bearings at the early stage, but after 2 years the penetration rate of DMC is close to the conventional ones. These findings from in-vivo tests prove that the long-term wear rate of DMC is similar with conventional bearings.
Additionally, Leonard et al. [26] studied 129 retrieved DM liners using a lever-out test. The lever-out force of the liners from the same and mixed manufacturers shows no difference. This finding could support the use of mixed manufacturers of DM liners and femoral heads in revision surgeries, which doesn’t increase the risk of IPD. The results of the in-vivo tests are summarized in Table 7.
Table 7.
Summary of the in-vivo wear studies (All the values listed below are the mean values)
| Researcher | Test subjects | Results | Testing technique |
|---|---|---|---|
| Geringer [58] | 12 explants of DMC | Convex: 36.7±29.1 mm3/year | In-vivo test (CMM) |
| Concave: 22.1±39.2 mm3/year | |||
| Total wear rate: 53.9±50.3 mm3/year | |||
| Imbert [22] | 8 explants of DMC | Convex: 29.72 mm3/year | In-vivo test (CMM) |
| Concave: 15.96 mm3/year | |||
| Boyer [23] | 35 explants of DMC | Convex: 25 mm3/year | In-vivo test (Light Surface Scanner) |
| Concave: 12 mm3/year | |||
| Adam [24] | 40 retrieved DM liners | convex: 28.9 mm3/year | In-vivo test (Surface analysis) |
| concave: 25.5 mm3/year | |||
| total wear rate: 54.3 mm3 year | |||
| Deckard [25] | 34 implants of DMC | Mean volumetric wear: | Martell methodology at regular postoperative intervals |
| 783 mm3/year at 1 year | |||
| 555 mm3/year at 2 years | |||
| 104 mm3/year at 5 years | |||
| Leonard [26] | 129 retrieved DM liners | The lever-out force for same manufacturer: 272.6±68.7 N and mixed manufacturer: 299.2±89.0 N | Lever-out test |
In-vitro studies
The kinematic studies of DMC
Since DMC has additional articulation, so how the inner and outer articulations move becomes critical to understand the kinematics of DMC. Rowe et al. [59] investigated how the motion was distributed between the two articulations from both the cadaveric and in-vivo studies. They found the lubricant condition largely influenced the motion of DMC, and all the motion occurred at the inner articulation when the two articulations were well lubricated in cadaveric tests. However in the human body, they found the preponderance of outer articulation motion in most patients. This may be because more synovial fluid is reserved in the outer articulation and the femoral neck and liner impingement. Some other kinematic studies were performed on a computer. Febry et al. [60] found the inner bearing was the main articulation of DMC, and the eccentric design can improve the stability of DMC. Gao et al. [61] found that the motion of DMC is highly dependent on the friction coefficient ratios and the initial clearances/interferences between the liner and the acetabular shell. The results show the threshold value of the friction coefficient ratio between the inner and the outer articulations is 1.43, and the larger radial clearance promotes the motion of outer articulation. Overall, among the factors that affect the motion distribution of DMC, the radial clearance and lubricant conditions are the most important factors. Because the contact area and sliding distance at the outer articulation are larger than the inner articulation, some researchers believe that if the outer articulation is dominant, then the wear of DMC could be higher than the conventional bearings [61]. Because the polyethylene wear of DMC may be related to its motion, the studies on DMC wear could help us figure out which articulation is predominant.
The studies on DMC wear
Contemporary DMC shows excellent wear performance compared with the first generation DMC from experimental studies. Loving et al. [62] used contemporary DMC (ADM of Stryker) to investigate its wear performance. They found the condition of the inner articulation and the polyethylene material to be the critical factors that determine the DMC wear. And the contemporary DMC with highly cross-linked PE can reduce the maximum wear of 75% compared with conventional bearing of UHMWPE. Likewise, Netter et al. [63] tested the wear performance of highly cross-linked PE DM liners under some extreme situations. They found contemporary DMC with highly cross-linked PE showed a good wear tolerance for third-body particles and micro-separation.
As stated in section 4.2, the DMC wear rate is similar to conventional bearings. It can also be corroborated by laboratory data. In the hip simulator test, Gaudin et al. [64] compared the wear of UHMWPE liners from conventional bearings and DM bearings. The results demonstrate that the wear of DMC is slightly smaller than conventional bearings. Similarly, Saikko et al. [65] analyzed the wear of DMC and conventional bearings at different acetabular shell positions. The wear rates of both designs are not correlated with the abduction angle, and there is no significant difference in the data between the two designs. The summarized in-vitro results are listed in Table 8. According to the results of the wear studies, the wear rate of DMC is similar to conventional bearings, which implies that the inner articulation dominates in the motion distribution of DMC.
Table 8.
Summary of the in-vitro wear studies
| Researcher | Test subjects | Results | Testing technique |
|---|---|---|---|
| Gaudin [64] | Standard bearings with UHMWPE vs. DM bearings with UHMWPE | Wear volume | In vitro test (Multiple Hip Simulator Test) |
| DM liner: 97.34 mm3 at 5 million cycles | |||
| Conventional liner: 99.57 mm3 at 5 million cycles | |||
| Saikko [65] | DM bearings (Stafit) vs. Conventional bearings (Allofit Alpha) | Shell position at the abduction angle of 45° | In vitro test (Multiple Hip Simulator Test) |
| DM: 21.49±3.21 mg/million cycles | |||
| Conventional: 19.63±1.59 mg/million cycles | |||
| Shell position at the abduction angle of 60° | |||
| DM: 17.81±4.64 mg/million cycles | |||
| Conventional: 20.79±1.29 mg/million cycles |
DMC fretting and corrosion studies
DMC has been used commercially for many years but little is known about its fretting and corrosion at the taper-trunnion interfaces. With increasing findings of fretting corrosion at the taper-trunnion interface for conventional total hip implants, the same problem may also occur for the DMC. The occurrence of corrosion damage between the taper-trunnion interfaces may release metal ions, leading to osteolysis. Though there are only a few reported adverse events of corrosion failure in terms of DMC, concerns about corrosion still remain. This is because theoretically the larger diameter head design could lead to larger forces on the femoral trunnion, accelerating the corrosion [66]. Recently, Lombardo et al. [67] studied 18 retrieved DMC. The results demonstrate that DMC may have the same fretting and corrosion problems with conventional bearings. The fretting and corrosion scores are highly related, and the corrosion score is moderately correlated with the presence of embedding on the PE liner. On the other hand, Tarity et al. [68] investigated the fretting and corrosion between the interfaces of the acetabular shells and the metal inserts. The results indicated the fretting and corrosion damage observed was more severe in the metal-on-metal THAs than that in the DMC, which may indicate that the taper engagement mechanism of DMC can prevent significant fretting and corrosion damage between the shell-insert interfaces.
The summarized major parameters which affect the fretting and corrosion of the conventional bearings are listed in Table 9. Previous studies about fretting and corrosion for conventional bearings may give us an idea to investigate the fretting and corrosion of DMC. Bhalekar et al. [69] did a large amount of work on fretting and corrosion studies of ‘metal-on-polyethylene’ and ‘ceramic-on-ceramic’ THAs. They tested the contemporary ‘metal-on-polyethylene’ and ‘ceramic-on-ceramic’ prosthesis on hip simulators and used a CMM system to measure the material loss in the taper-trunnion junction. The results demonstrate that the metal ions released for both ‘metal-on-polyethylene’ (MoP) and ‘ceramic-on-ceramic’ (CoC) prostheses are mainly from taper-trunnion interfaces. Additionally, in ‘MoP’ THAs, the wear of the ball head (CoCrMo) and the femoral stem (Ti6Al4V) in the taper-trunnion junction is similar. The reason may be that the oxide layer is destroyed and electrochemical corrosion occurs. In the case of ‘CoC’, they found the wear of the articulation bearing was similar to the wear of the taper-trunnion junction. Thus the wear in the taper-trunnion junction cannot be neglected for ‘CoC’ THAs. Other studies about the explants show that the wear of the taper-trunnion interfaces mainly appears at the proximal-superior end and the distal-inferior end of the trunnion. This means the material loss at the taper-trunnion junction is correlated with the ‘toggling’ movement [70]. A demonstration of the ‘toggling’ movement is shown in Figure 3.
Table 9.
The effect of the major fretting and corrosion performance parameters on the conventional bearings
| Parameters | Fretting | Corrosion |
|---|---|---|
| Materials of articulation | Depends on the materials | Depends on the materials |
| Load & torque | Positive correlation | Positive correlation |
| Femoral head diameter | Positive correlation | Positive correlation |
| Assembly compression force | Negative correlation | Negative correlation |
| Implantation time | Positive correlation | Positive correlation |
Figure 3.

A demonstration of the main wear area and toggling motion.
Summary
In summary, fretting and corrosion damage between the acetabular shell-metal insert interfaces seems non-critical for DMC. But compared with the conventional bearings, DMC may face even worse corrosion damage from the taper-trunnion interfaces. This is because its larger diameter head design could increase the rotation torque that contributes to the corrosion. More research on the fretting and corrosion of DMC are needed in order to understand its mechanisms.
Efforts have been made to improve the lifetime of THA over the years. Charnley’s low-friction principle has achieved excellent clinical results and has been regarded as the gold standard. However dislocation was found as one of the major reasons for revising THA. Pr. Gilles Bousquet invented DMC in 1970s to deal with dislocation. Compared with other approaches, DMC seems to have innate advantage to solve the instability. This is because it combines Charnley’s ‘low friction’ principle and McKee’s ‘the effect of big femoral head’ theory. The advantage of reducing dislocation in DMC has been corroborated by the excellent clinical outcomes. With the increasing use of DMC, a unique complication called IPD appeared. Because IPD limits the clinical application of DMC, some modifications on first-generation DMC have been applied. After the improvement, it demonstrated a favorable rate of wear and a low incidence rate of IPD compared with the first-generation DMC. The literature also reports that the wear rate of contemporary DMC is similar with the conventional hip implants. It is likely that the inner articulation bearing is predominant in most cases. However, the kinematics of DMC are highly related to the condition of inner articulation, lubrication, and radial clearance etc., and the dominance of the articulation bearings in the movement of the human body is not changeless, and it might vary with the affecting factors. On the other hand, the design of DMC may have some disadvantages which could lead to potential engineering issues. For example, the additional bearing of DMC has the potential to cause the excessive wear comparative to the single bearing prosthesis. And the larger diameter head design of DMC may produce larger forces on the femoral trunnion, which can promote fretting and corrosion. Future studies may consider evaluating the metal ion levels in patients to trace the DMC corrosion and wear damage. And future studies should also focus on computational and experimental approaches. For example, using finite element analysis to simulate the movement of DMC can predict its dynamic forces, slide distances, and wear depths. With the application of the hip simulator, a long-term DMC wear study can be simulated. After the in-vitro simulation, CMM allowed the measuring the volumetric wear from the pre-test and post-test of the taper-trunnion interfaces. Analyzing these computational and laboratorial data can help us explore the wear and fretting corrosion mechanisms of DMC. If the concerns on the wear and corrosion of DMC can be eliminated, the widespread use of DMC can be expected.
Acknowledgements
This work was supported by the National Natural Science Foundation of China [51775460, 51905456].
Disclosure of conflict of interest
None.
References
- 1.Levine BR, Springer BD, Golladay GJ. Highlights of the 2019 American joint replacement registry annual report. Arthroplast Today. 2020;6:998–1000. doi: 10.1016/j.artd.2020.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bozic KJ, Kurtz SM, Lau E, Ong K, Vail TP, Berry DJ. The epidemiology of revision total hip arthroplasty in the United States. Joint Surg. 2009;91:128–133. doi: 10.2106/JBJS.H.00155. [DOI] [PubMed] [Google Scholar]
- 3.Patel PD, Potts A, Froimson MI. The dislocating hip arthroplasty: prevention and treatment. J Arthroplasty. 2007;22:86–90. doi: 10.1016/j.arth.2006.12.111. [DOI] [PubMed] [Google Scholar]
- 4.Sanchez-Sotelo J, Haidukewych GJ, Boberg CJ. Hospital cost of dislocation after primary total hip arthroplasty. Bone Joint Surg Am. 2006;88:290–294. doi: 10.2106/JBJS.D.02799. [DOI] [PubMed] [Google Scholar]
- 5.Epinette JA, Béracassat R, Tracol P, Pagazani G, Vandenbussche E. Are modern dual mobility cups a valuable option in reducing instability after primary hip arthroplasty, even in younger patients? J Arthroplasty. 2014;29:1323–1328. doi: 10.1016/j.arth.2013.12.011. [DOI] [PubMed] [Google Scholar]
- 6.Massin P, Besnier L. Acetabular revision of total hip arthroplasty using a press-fit dual mobility cup. Orthop Traumatol Surg Res. 2010;96:9–13. doi: 10.1016/j.rcot.2009.11.006. [DOI] [PubMed] [Google Scholar]
- 7.Grazioli A, Ek ETH, Rüdiger HA. Biomechanical concept and clinical outcome of dual mobility cups. Int Orthop. 2012;36:2411–2418. doi: 10.1007/s00264-012-1678-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Haen TX, Lonjon G, Vandenbussche E. Can cemented dual-mobility cups be used without a reinforcement device in cases of mild acetabular bone stock alteration in total hip arthroplasty? Orthop Traumatol Surg Res. 2015;101:923–927. doi: 10.1016/j.otsr.2015.09.027. [DOI] [PubMed] [Google Scholar]
- 9.van Heumen M, Heesterbeek PJ, Swierstra BA, Van Hellemondt GG, Goosen JH. Dual mobility acetabular component in revision total hip arthroplasty for persistent dislocation: no dislocations in 50 hips after 1-5 years. J Orthop Traumatol. 2014;16:15–20. doi: 10.1007/s10195-014-0318-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Prudhon JL, Ferreira A, Verdier R. Dual mobility cup: dislocation rate and survivorship at ten years of follow-up. Int Orthop. 2013;37:2345–2350. doi: 10.1007/s00264-013-2067-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Simian E, Chatellard R, Druon J, Berhouet J, Rosset P. Dual mobility cup in revision total hip arthroplasty: dislocation rate and survival after 5 years. Orthop Traumatol Surg Res. 2015;101:577–581. doi: 10.1016/j.otsr.2015.05.002. [DOI] [PubMed] [Google Scholar]
- 12.Massin P, Orain V, Philippot R, Farizon F, Fessy MH. Fixation failures of dual mobility cups: a mid-term study of 2601 hip replacements. Clin Orthop Relat Res. 2012;470:1932–1940. doi: 10.1007/s11999-011-2213-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bus MP, Szafranski A, Sellevold S, Goryn T, Jutte PC, Bramer JAM, Fiocco M, Streitbürger A, Kotrych D, van de Sande MA, Dijkstra PD. LUMiC(®) endoprosthetic reconstruction after periacetabular tumor resection: short-term results. Clin Orthop Relat Res. 2017;475:686–695. doi: 10.1007/s11999-016-4805-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Epinette JA, Harwin SF, Rowan FE, Tracol P, Mont MA, Chughtai M, Westrich GH. Early experience with dual mobility acetabular systems featuring highly cross-linked polyethylene liners for primary hip arthroplasty in patients under fifty five years of age: an international multi-centre preliminary study. Int Orthop. 2017;41:543–550. doi: 10.1007/s00264-016-3367-0. [DOI] [PubMed] [Google Scholar]
- 15.Boyer B, Philippot R, Geringer J, Farizon F. Primary total hip arthroplasty with dual mobility socket to prevent dislocation: a 22-year follow-up of 240 hips. Int Orthop. 2012;36:511–518. doi: 10.1007/s00264-011-1289-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Farizon F, de Lavison R, Azoulai JJ, Bousquet G. Results with a cementless alumina-coated cup with dual mobility. A twelve-year follow-up study. Int Orthop. 1998;22:219–224. doi: 10.1007/s002640050246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schneider L, Philippot R, Boyer B, Farizon F. Revision total hip arthroplasty using a reconstruction cage device and a cemented dual mobility cup. Orthop Traumatol Surg Res. 2011;97:807–813. doi: 10.1016/j.otsr.2011.09.010. [DOI] [PubMed] [Google Scholar]
- 18.Vermersch T, Viste A, Desmarchelier R, Fessy MH. Prospective longitudinal study of one hundred patients with total hip arthroplasty using a second-generation cementless dual-mobility cup. Int Orthop. 2015;39:2097–2101. doi: 10.1007/s00264-015-2985-2. [DOI] [PubMed] [Google Scholar]
- 19.Philippot R, Farizon F, Camilleri JP, Boyer B, Derhi G, Bonnan J, Fessy MH, Lecuire F. Survival of cementless dual mobility socket with a mean 17 years follow-up. Rev Chir Orthop Reparatrice Appar Mot. 2008;94:e23–e27. doi: 10.1016/j.rco.2007.10.013. [DOI] [PubMed] [Google Scholar]
- 20.Essig J, Javois C, Potel JF, Boussaton M. Prothèse totale de hanche par voie externe: étude prospective comparative voie mini-invasive versus standard. Rev Chir Orthop Reparatrice Appar Mot. 2005;91:76. doi: 10.1016/s0035-1040(05)84557-x. [DOI] [PubMed] [Google Scholar]
- 21.Boyer B, Neri T, Geringer J, Di Iorio A, Philippot R, Farizon F. Understanding wear in dual mobility total hip replacement: first generation explant wear patterns. Int Orthop. 2017;41:529–533. doi: 10.1007/s00264-016-3362-5. [DOI] [PubMed] [Google Scholar]
- 22.Imbert L, Geringer J, Boyer B, Farizon F. Wear analysis of hip explants, dual mobility concept: comparison of quantitative and qualitative analyses. J Engineer Tribol. 2012;226:838–853. [Google Scholar]
- 23.Boyer B, Neri T, Geringer J, Di Iorio A, Philippot R, Farizon F. Long-term wear of dual mobility total hip replacement cups: explant study. Int Orthop. 2018;42:41–47. doi: 10.1007/s00264-017-3525-z. [DOI] [PubMed] [Google Scholar]
- 24.Adam P, Farizon F, Fessy MH. Dual mobility retentive acetabular liners and wear: surface analysis of 40 retrieved polyethylene implants. Orthop Traumatol Surg Res. 2014;100:85–91. doi: 10.1016/j.otsr.2013.12.011. [DOI] [PubMed] [Google Scholar]
- 25.Deckard ER, Azzam KA, Meneghini RM. Contemporary dual mobility head penetration at five years: concern for the additional convex bearing surface? J Arthroplasty. 2018;33:S280–S284. doi: 10.1016/j.arth.2018.02.061. [DOI] [PubMed] [Google Scholar]
- 26.Buller LT, Torres L, Baral EC, Wright TM, Ast MP. No difference in force required for intraprosthetic dislocation of mixed manufacturer vs same manufacturer dual mobility articulations. J Arthroplasty. 2020;35:597–602. doi: 10.1016/j.arth.2019.09.039. [DOI] [PubMed] [Google Scholar]
- 27.Stroh A, Naziri Q, Johnson AJ, Mont MA. Dual-mobility bearings: a review of the literature. Expert Rev Med Devices. 2012;9:23–31. doi: 10.1586/erd.11.57. [DOI] [PubMed] [Google Scholar]
- 28.Neri T, Philippot R, Klasan A, Putnis S, Leie M, Boyer B, Farizon F. Dual mobility acetabular cups for total hip arthroplasty: advantages and drawbacks. Expert Rev Med Devices. 2018;15:835–845. doi: 10.1080/17434440.2018.1538781. [DOI] [PubMed] [Google Scholar]
- 29.Klement MR, Hubbard EW, Wellman SS, Lachiewicz PF. Acute intraprosthetic dissociation of a dual-mobility hip in the United States. American J Orthop (Belle Mead NJ) 2017;46:E154–E159. [PubMed] [Google Scholar]
- 30.Darrith B, Courtney PM, Della Valle CJ. Outcomes of dual mobility components in total hip arthroplasty: a systematic review of the literature. Bone Joint J. 2018;100-B:11–19. doi: 10.1302/0301-620X.100B1.BJJ-2017-0462.R1. [DOI] [PubMed] [Google Scholar]
- 31.Miura D, Busija L, Page RS, de Steiger R, Lorimer M, Ackerman IN. Lifetime risk of primary shoulder arthroplasty from 2008-2017: a population-level analysis using national registry data. Arthritis Care Res (Hoboken) 2020 doi: 10.1002/acr.24353. [DOI] [PubMed] [Google Scholar]
- 32.Evans JT, Evans JP, Walker RW, Blom AW, Whitehouse MR, Sayers A. How long does a hip replacement last? A systematic review and meta-analysis of case series and national registry reports with more than 15 years of follow-up. Lancet. 2019;393:647–654. doi: 10.1016/S0140-6736(18)31665-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Martz P, Maczynski A, Elsair S, Labattut L, Viard B, Baulot E. Total hip arthroplasty with dual mobility cup in osteonecrosis of the femoral head in young patients: over ten years of follow-up. Int Orthop. 2017;41:605–610. doi: 10.1007/s00264-016-3344-7. [DOI] [PubMed] [Google Scholar]
- 34.Langlais FL, Ropars M, Gaucher F, Musset T, Chaix O. Dual mobility cemented cups have low dislocation rates in THA revisions. Clin Orthop Relat Res. 2008;466:389–395. doi: 10.1007/s11999-007-0047-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Philippot R, Adam P, Farizon F, Fessy MH, Bousquet G. Survival of cementless dual mobility sockets: ten-year follow-up. Rev Chir Orthop Reparatrice Appar Mot. 2006;92:326–331. doi: 10.1016/s0035-1040(06)75762-2. [DOI] [PubMed] [Google Scholar]
- 36.Philippot R, Camilleri JP, Boyer B, Adam P, Farizon F. The use of a dual-articulation acetabular cup system to prevent dislocation after primary total hip arthroplasty: analysis of 384 cases at a mean follow-up of 15 years. Int Orthop. 2009;33:927–932. doi: 10.1007/s00264-008-0589-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Harwin SF, Mistry JB, Chughtai M, Khlopas A, Gwam C, Newman JM, Higuera CA, Bonutti PM, Malkani AL, Kolisek FR, Delanois RE, Mont MA. Dual mobility acetabular cups in primary total hip arthroplasty in patients at high risk for dislocation. Surg Technol Int. 2017;30:251–258. [PubMed] [Google Scholar]
- 38.Philippeau JM, Durand JM, Carret JP, Leclercq S, Waast D, Gouin F. Dual mobility design socket use in preventing total hip replacement dislocation following tumor resection. Orthop Traumatol Surg Res. 2010;96:2–8. doi: 10.1016/j.rcot.2009.12.011. [DOI] [PubMed] [Google Scholar]
- 39.Tarasevicius S, Busevicius M, Robertsson O, Wingstrand H. Dual mobility cup reduces dislocation rate after arthroplasty for femoral neck fracture. BMC Musculoskelet Disord. 2010;11:175–175. doi: 10.1186/1471-2474-11-175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wegrzyn J, Tebaa E, Jacquel A, Carret JP, Béjui-Hugues J, Pibarot V. Can dual mobility cups prevent dislocation in all situations after revision total hip arthroplasty? J Arthroplasty. 2015;30:631–640. doi: 10.1016/j.arth.2014.10.034. [DOI] [PubMed] [Google Scholar]
- 41.Leiber-Wackenheim F, Brunschweiler B, Ehlinger M, Gabrion A, Mertl P. Treatment of recurrent THR dislocation using of a cementless dual-mobility cup: a 59 cases series with a mean 8 years’ follow-up. Orthop Traumatol Surg Res. 2011;97:8–13. doi: 10.1016/j.otsr.2010.08.003. [DOI] [PubMed] [Google Scholar]
- 42.Guyen O, Pibarot V, Vaz G, Chevillotte C, Béjui-Hugues J. Use of a dual mobility socket to manage total hip arthroplasty instability. Clin Orthop Relat Res. 2009;467:465–472. doi: 10.1007/s11999-008-0476-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hailer NP, Weiss RJ, Stark A, Kärrholm J. Dual-mobility cups for revision due to instability are associated with a low rate of re-revisions due to dislocation: 228 patients from the Swedish hip arthroplasty register. Acta Orthop. 2012;83:566–571. doi: 10.3109/17453674.2012.742395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Philippot R, Boyer B, Farizon F. Intraprosthetic dislocation: a specific complication of the dual-mobility system. Clin Orthop Relat Res. 2013;471:965–970. doi: 10.1007/s11999-012-2639-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Terrier A, Latypova A, Guillemin M, Parvex V, Guyen O. Dual mobility cups provide biomechanical advantages in situations at risk for dislocation: a finite element analysis. Int Orthop. 2017;41:551–556. doi: 10.1007/s00264-016-3368-z. [DOI] [PubMed] [Google Scholar]
- 46.Mertl P, Combes A, Leiber-Wackenheim F, Fessy MH, Girard J, Migaud H. Recurrence of dislocation following total hip arthroplasty revision using dual mobility cups was rare in 180 hips followed over 7 years. HSS J. 2012;8:251–256. doi: 10.1007/s11420-012-9301-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hamadouche M, Biau DJ, Huten D, Musset T, Gaucher F. The use of a cemented dual mobility socket to treat recurrent dislocation. Clin Orthop Relat Res. 2010;468:3248–3254. doi: 10.1007/s11999-010-1404-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Colacchio ND, Wooten CJ, Martin JR, Masonis JL, Fehring TK. Dual mobility for monoblock metal-on-metal revision-is it safe? J Arthroplasty. 2020;35:508–512. doi: 10.1016/j.arth.2019.09.028. [DOI] [PubMed] [Google Scholar]
- 49.Moreta J, Uriarte I, Foruria X, Urra I, Aguirre U, Martínez-de Los Mozos JL. Cementation of a dual-mobility cup into a well-fixed cementless shell in patients with high risk of dislocation undergoing revision total hip arthroplasty. Hip Int. 2021;31:97–102. doi: 10.1177/1120700019873617. [DOI] [PubMed] [Google Scholar]
- 50.Blevins JL, Shen TS, Morgenstern R, DeNova TA, Su EP. Conversion of hip resurfacing with retention of monoblock acetabular shell using dual-mobility components. J Arthroplasty. 2019;34:2037–2044. doi: 10.1016/j.arth.2019.04.065. [DOI] [PubMed] [Google Scholar]
- 51.Langlois J, El Hage S, Hamadouche M. Intraprosthetic dislocation: a potentially serious complication of dual mobility acetabular cups. Skeletal Radiol. 2014;43:1013–1016. doi: 10.1007/s00256-014-1824-7. [DOI] [PubMed] [Google Scholar]
- 52.Fabry C, Langlois J, Hamadouche M, Bader R. Intra-prosthetic dislocation of dual-mobility cups after total hip arthroplasty: potential causes from a clinical and biomechanical perspective. Int Orthop. 2016;40:901–906. doi: 10.1007/s00264-015-3000-7. [DOI] [PubMed] [Google Scholar]
- 53.De Martino I, D’Apolito R, Waddell BS, McLawhorn AS, Sculco PK, Sculco TP. Early intraprosthetic dislocation in dual-mobility implants: a systematic review. Arthroplast Today. 2017;3:197–202. doi: 10.1016/j.artd.2016.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Neri T, Boyer B, Geringer J, Di Iorio A, Caton JH, PhiIippot R, Farizon F. Intraprosthetic dislocation of dual mobility total hip arthroplasty: still occurring? Int Orthop. 2019;43:1097–1105. doi: 10.1007/s00264-018-4054-0. [DOI] [PubMed] [Google Scholar]
- 55.Blakeney WG, Epinette JA, Vendittoli PA. Dual mobility total hip arthroplasty: should everyone get one? EFORT Open Rev. 2019;4:541–547. doi: 10.1302/2058-5241.4.180045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Odland AN, Sierra RJ. Intraprosthetic dislocation of a contemporary dual-mobility design used during conversion THA. Orthopedics. 2014;37:e1124–e1128. doi: 10.3928/01477447-20141124-90. [DOI] [PubMed] [Google Scholar]
- 57.Koper M, Verdijk R, Bos K. Asymptomatic intraprosthetic dual mobility cup dislocation with increased metal ion levels. Arthroplasty Today. 2019;5:38–42. doi: 10.1016/j.artd.2018.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Geringer J, Boyer B, Farizon F. Understanding the dual mobility concept for total hip arthroplasty. Investigations on a multiscale analysis-highlighting the role of arthrofibrosis. Wear. 2011;271:2379–2385. [Google Scholar]
- 59.Rowe SM, Chung JY, Moon ES, Yoon TR, Seo HY, Lee JJ. Why does outer joint motion predominate in bipolar hip prosthesis? Experimental and clinical studies. Acta Orthop Scand. 2004;75:701–707. doi: 10.1080/00016470410004067. [DOI] [PubMed] [Google Scholar]
- 60.Fabry C, Woernle C, Bader R. Self-centering dual-mobility total hip systems: prediction of relative movements and realignment of different intermediate components. Proc Inst Mech Eng H. 2014;228:477–485. doi: 10.1177/0954411914531116. [DOI] [PubMed] [Google Scholar]
- 61.Gao Y, Chai W, Wang L, Wang M, Jin Z. Effect of friction and clearance on kinematics and contact mechanics of dual mobility hip implant. Proc Inst Mech Eng H. 2016;230:39–49. doi: 10.1177/0954411915617198. [DOI] [PubMed] [Google Scholar]
- 62.Loving L, Lee RK, Herrera L, Essner AP, Nevelos JE. Wear performance evaluation of a contemporary dual mobility hip bearing using multiple hip simulator testing conditions. J Arthroplasty. 2013;28:1041–1046. doi: 10.1016/j.arth.2012.09.011. [DOI] [PubMed] [Google Scholar]
- 63.Netter JD, Hermida JC, Chen PC, Nevelos JE, D’Lima DD. Effect of microseparation and third-body particles on dual-mobility crosslinked hip liner wear. J Arthroplasty. 2014;29:1849–1853. doi: 10.1016/j.arth.2014.04.010. [DOI] [PubMed] [Google Scholar]
- 64.Gaudin G, Ferreira A, Gaillard R, Prudhon JL, Caton JH, Lustig S. Equivalent wear performance of dual mobility bearing compared with standard bearing in total hip arthroplasty: in vitro study. Int Orthop. 2017;41:521–527. doi: 10.1007/s00264-016-3346-5. [DOI] [PubMed] [Google Scholar]
- 65.Saikko V, Shen M. Wear comparison between a dual mobility total hip prosthesis and a typical modular design using a hip joint simulator. Wear. 2010;268:617–621. [Google Scholar]
- 66.Cooper HJ, Della Valle CJ. Large diameter femoral heads: is bigger always better? Bone Joint J. 2014;96-B:23–26. doi: 10.1302/0301-620X.96B11.34342. [DOI] [PubMed] [Google Scholar]
- 67.Lombardo DJ, Siljander MP, Gehrke CK, Moore DD, Karadsheh MS, Baker EA. Fretting and corrosion damage of retrieved dual-mobility total hip arthroplasty systems. J Arthroplasty. 2019;34:1273–1278. doi: 10.1016/j.arth.2019.02.008. [DOI] [PubMed] [Google Scholar]
- 68.Tarity TD, Koch CN, Burket JC, Wright TM, Westrich GH. Fretting and corrosion at the backside of modular cobalt chromium acetabular inserts: a retrieval analysis. J Arthroplasty. 2017;32:1033–1039. doi: 10.1016/j.arth.2016.09.038. [DOI] [PubMed] [Google Scholar]
- 69.Bhalekar RM, Smith SL, Joyce TJ. Hip simulator testing of the taper-trunnion junction and bearing surfaces of contemporary metal-on-cross-linked-polyethylene hip prostheses. J Biomed Mater Res. 2020;108:156–166. doi: 10.1002/jbm.b.34374. [DOI] [PubMed] [Google Scholar]
- 70.Bhalekar RM, Smith SL, Joyce TJ. Wear at the taper-trunnion junction of contemporary ceramic-on-ceramic hips shown in a multistation hip simulator. J Biomed Mater Res. 2019;107:1199–1209. doi: 10.1002/jbm.b.34213. [DOI] [PubMed] [Google Scholar]


