Abstract
Introduction: Crohn’s disease (CD) is an inflammatory bowel disease (IBD) that affects the gastrointestinal tract and can have a major impact on the patient’s quality of life and social/professional activities. Asymptomatic patients, or those with mild symptoms, experience the active disease with subclinical manifestation. Systematic review (SR) was performed to look for evidence for the role of chemokines and adipokines as markers for CD activity. Methods: This SR was conducted by searching published studies in international and regional databases up till July, 2020. CD patients were adults with the disease in activity or remission. All adipokines and chemokines were considered for the analysis and the Rayyan QCRI system was used. Results: In total, 20 studies were included. Six addressed chemokines and eight adipokines as potential biomarkers of CD activity. CXCL8 was the most studied chemokine (8 studies) and the results were controversial, with 62.5% showing a significant association with CD activity. CXCL10 was investigated by 4 studies and 50% identified it as a potential biomarker. CCL2, CCL11, CCL26 and CXCL1 were examined by 2 articles each. CXCL8 (P=0.002/P=0.001) and CXCL1 (P<0.001) presented the lowest? P value, which qualifies them as potential markers of disease activity. All the adipokines were tested in peripheral blood but 44.4% were also tested in intestinal mucosa, while the percentage in the chemokines’ studies was 76.9% in peripheral blood, 46.1% in intestinal mucosa and 7.6% in urine sample respectively. Conclusion: The development of disease activity biomarkers for CD is becoming relevant for clinical practice. Chemokines and adipokines have the potential to signalize CD activity, but validation in larger cohorts of patients, preferable multicenter studies are still needed.
Keywords: Crohn’s disease, inflammatory bowel disease, chemokine, adipokine, inflammation
Introduction
Crohn’s disease (CD) is an inflammatory bowel disease (IBD) that can affect the entire gastrointestinal tract and its pathophysiology is associated with an exacerbated immune response. It usually impacts the patient’s quality of life, professional activities and the psychosocial environment. The diagnosis is more frequent among young individuals and the prevalence has been increasing in several developing countries. In spite of the relatively low mortality rate, CD’s high morbidity makes it a current public health problem [1].
CD is characterized by segments of transmural inflammation that can affect any part of the gastrointestinal tract, from the mouth to the anus, with a greater involvement of the terminal ileum and the proximal colon. Its endoscopic appearance is one of compound deep and serpiginous ulcers, and affects areas interspersed with normal mucosa. Non-caseous epithelioid granuloma is a specific histological marker, but it is infrequent [2]. Exacerbation and remission phases are alternated. Therefore, CD patients must be monitored regularly to avoid permanent bowel damage. It is worth mentioning that asymptomatic patients or those with mild symptoms should have the disease’s activity confirmed by complementary examinations. These cases with subclinical manifestation require intervention to change the course of the disease [3].
The diagnosis is based on clinical symptoms, the most frequent of which are fever, weight loss, anemia, abdominal pain, diarrhoea, digestive hemorrhage, extra intestinal manifestations such as arthritis, uveitis and erythema nodosum, in addition to ileocolonoscopy with biopsy and/or entero magnetic resonance imaging (MRI) [4]. Patients are monitored with clinical evaluation and complementary examinations, including endoscopy with biopsies and/or imaging and analysis of serum and fecal biomarkers. Serum C-reactive protein (CRP) and fecal calprotectin levels are markers of IBD activity, but they do not always correlate with the degree of intestinal mucosa inflammation observed during colonoscopy examination. Thus, new biomarkers are urgently needed in order to more accurately predict mucosal damage [5]. The most common tissues of which biomarkers have been evaluated are intestinal mucosa, adipose tissue, and peripheral blood of the CD patients.
The inflammatory response in CD patients is mainly based on the increasing expression of cytokines and chemokines. Cytokines are defined as a group of proteins released by the immune system that regulate the migration of interleukins and lymphocytes to the inflamed area, acting as mediators of the immune response. On the other hand, chemokines (Greek-kinos, movement) are a family of small cytokines, or signaling proteins secreted by cells. Their name is derived from their ability to induce directed chemotaxis in nearby responsive cells. They are chemotactic cytokines [6].
Cytokines are classified as chemokines according to their behavior and structural characteristics. In addition to being known for mediating chemotaxis, all chemokines are approximately 8-10 kilodaltons in mass unit and have four cysteine residues in conserved locations that are key to form their 3-dimensional shape (Figure 1). These proteins have historically been known as the SIS family of cytokines, SIG family of cytokines, SCY family of cytokines, Platelet factor-4 superfamily or intercrines. Some chemokines are considered pro-inflammatory and can be induced during an immune response to recruit cells of the immune system to a site of infection, while others are considered homeostatic and are involved in controlling the migration of cells during normal processes of tissue maintenance or development [7]. Chemokines have been classified into four main subfamilies: CXC, CC, CX3C and XC. All of these proteins exert their biological effects by interacting with G protein-linked transmembrane receptors called chemokine receptors, which are selectively found on the surfaces of their target cells [7-9].
Figure 1.
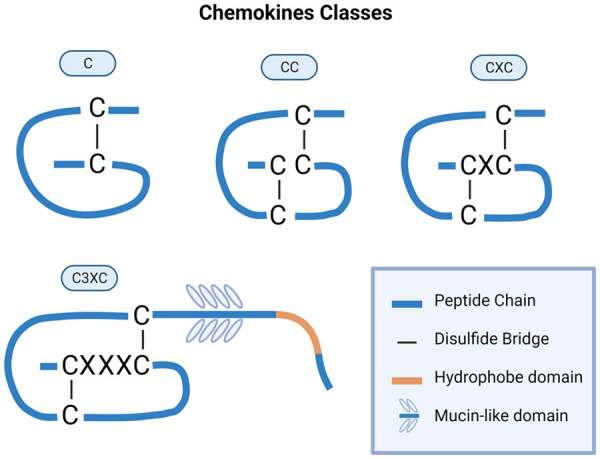
Classes of chemokines which have been defined by the arrangement of the conserved cysteine (C) residues of the mature proteins.
At the same time, the literature emphasizes the hypothetical role of mesenterial adipose tissue in the pathophysiology and CD activity [10-12]. Therefore, the traditional view of adipose tissue as a passive reservoir for energy storage is no longer valid, because recent studies have begun to regard it as an endocrine organ and have shown that it secretes relevant inflammatory factors [7,11-13]. These factors, also called adipokines, are cytokines (cell signaling proteins) secreted by adipose tissue. The first adipokine to be discovered was leptin in 1994 [13]. Since that time, hundreds of adipokines have been described [14]. They were identified and classified based on the processes being controlled. These processes include lipid homeostasis, immune function, insulin sensitivity, blood pressure control, homeostasis, appetite and energy balance [15-17]. Some studies consider the possibility that CD’s inflammatory process is due to the impaired function of the adipokine secretion [18-20].
Rodrigues et al. demonstrate that patients without clinical and endoscopic disease activity show inflammation of the mesenteric adipose tissue near the intestinal affected area observed in surgical specimens [21]. Moreover, CD patients with active disease showed decreased levels of serum adiponectin, an anti-inflammatory adipokine, when compared to those in remission and the control group, which suggests a dysregulation of an anti-inflammatory pathway to counterbalance the inflammation. There was no difference in serum and tissue leptin levels, which were similar among the groups [21].
The role of biomarkers for CD activity is to reduce the time spent to detect disease activity and the need for invasive procedures to access disease activity (such as colonoscopy). In addition, it also enables clinicians to anticipate changes in the therapeutic strategy, and thereby avoid serious complications such as perforation, intestinal occlusion, fistulas and abscesses. The ability to predict the behavior and course of the disease, as well as to identify patients at risk of rapid progression towards complications, can avert the use of invasive resources and reduce the economic impact of this disease.
There is much less research on the impact of chemokine’s and adipokine’s role in the inflammatory process of Crohn’s disease than on inflammatory factors such as cytokines or other secreted proteins. Chemokines and adipokines can be secreted by several types of cells, and they may play a relevant role in the maintenance of the inflammation in CD, either chemoattracting immune cells to the affected intestinal area (chemokines), or displaying pro- and anti-inflammatory properties which can be secreted by adipose cells from the affected mesenteric adipose tissue in CD (adipokines). Given the above, we performed a systematic review (SR) looking evidence at the literature for the role of chemokines and adipokines as markers for CD activity, with a view towards future application in clinical practice.
Method
Study design
The SR dealt with the comparison of all the empirical evidence that fits the specified eligibility criteria, to answer a specific question: “Chemokines and adipokines as activity markers of Crohn’s disease”. This analysis went on to follow some fundamental stages: definition of the research question, the databases, the search interval, the details of the search elements, the descriptors, extensive and ordered search in the databases, the inclusion and exclusion criteria, the collection of essential data, the choice of articles, assessment of eligibility and the application of exclusion criteria [22,23]. The selected articles were read and those with inconsistencies were removed from the SR. Afterwards, the analysis of results and the discussion of the study with the evidence were performed. This SR is in line with the recommendation “Preferred Reporting Items for Systematic Reviews and Meta-Analyses” (PRISMA), described in a review in 2015 [24].
Research strategy, screening and data extraction
To choose the study and export the data, the Rayyan QCRI system was used. Rayyan is software developed by the Qatar Computing Research Institute (QCRI), a tool to assist in archiving, organizing and selecting articles [25].
Publications from the years 2002 to 2020 were included in the study. The final search in the selected databases was carried out on July 20, 2020, with 344 articles identified in English, Spanish and Portuguese languages.
The selection of articles published in the Rayyan system was carried out by two reviewers, autonomously and independently, and a third reviewer was responsible for analysing and deciding on the inclusion or exclusion of the article, especially in relation to those who presented a contradictory and conflicting decision.
The search for articles was carried out in the databases PUBMED, PUBMED PM C, BVS-BIREME, SCOPUS, WEB OF SCIENCE, EMBASE, COCHRANE, EBSCOHOST, PROQUEST and ENDNOTE WEB, which are shown in Table 1.
Table 1.
Databases and the descriptors used for the systematic review
| DATABASE | Vocabulary of subjects | Discriptors and free terms used in the search strategy | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| 1 | 2 | 3 | 4 | 5 | ||
| PUBMED | Mesh | “Crohn’s Disease” | Biomarkers | Chemokines | Adipokines | Inflammation |
| Medical Subject Headings | ||||||
| PUBMED PMC | Mesh | “Crohn’s Disease” | Biomarkers | Chemokines | Adipokines | Inflammation |
| Medical Subject Headings | ||||||
| BVS/BIREME | DeCS | “Crohn’s Disease” | Biomarkers | Chemokines | Adipokines | Inflammation |
| “Enfermedad de Crohn” | Biomarcadores | Quimiocinas | Adipoquinas | Inflamación | ||
| “Doença de Crohn” | Biomarcadores | Quimiocinas | Adipocinas | Inflamação | ||
| EBSCOHORT | Mesh | “Crohn’s Disease” | Biomarkers | Chemokines | Adipokines | Inflammation |
| SCOPUS | Mesh | “Crohn’s Disease” | Biomarkers | Chemokines | Adipokines | Inflammation |
| WEB OF SCIENCE | Mesh | “Crohn’s Disease” | Biomarkers | Chemokines | Adipokines | Inflammation |
| EMBASE | Emtree | “Crohn’s Disease” | Biomarkers | Chemokines | Adipokines | Inflammation |
Inclusion criteria
The keywords “Crohn’s disease”, “chemokines”, “adipokines”, “biomarkers” and “inflammation” were chosen after reading material related to the researched topic. To expand and guide the search, an association of the descriptors was carried out by means of Boolean operators “OR” and “AND”. The Boolean operators “OR” and “AND” were used to add or restrict the search, as follows: “Crohn Disease AND Biomarkers AND (Chemokines OR Adipokines) AND Inflammation”.
Furthermore, this type of review is defined by the type of population (participants), types of interventions (and comparisons) and types of implications. The acronym PICO (population, interventions, comparators and outcome) has been widely used in this routine. In this research, the English acronym “PICO” was used as the study design. Thus, Table 2 summarizes the main aspects of interest for the SR.
Table 2.
Population, interventions, comparators and outcome parameters used for the systematic review
| Population | Crohn’s disease patients |
|---|---|
| Interventions | Studies demonstrate the population of Crohn’s disease patient with a disease activity inferred by the expression of chemokines and/or adipokines |
| Comparators | Chemokines and/or adipokines with disease activity |
| Outcome | Studies demonstrate the population of Crohn’s disease patient with a disease activity inferred by the expression of chemokines and/or adipokines |
Reviews, SRs and meta-analyses that did not show original data were excluded from this review. Moreover, we considered only studies with human samples. Therefore, animal studies were excluded.
Results
Literature search and study characteristics
The thorough search process carried out in nine databases until July 28th, 2020, resulted in the immediate retrieval of 495 potentially relevant references in the period from 2002 to 2020. The articles’ collection was made possible by the library staff of the School of Medical Sciences (SMS) of the University of Campinas (Unicamp), who provided support in the search and identification of the references. After reviewing the articles, 475 were excluded for being duplicates, for not meeting the eligibility criteria, and were excluded according to our research design. Finally, this SR included twenty studies. Figure 2 shows the flowchart of the references’ selection.
Figure 2.
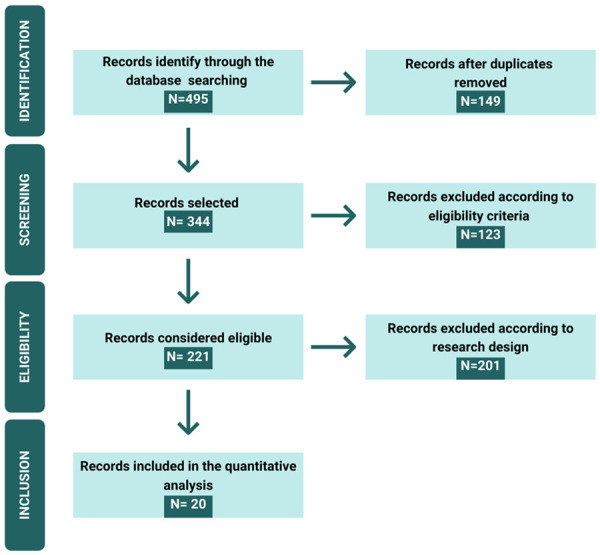
Design study flowchart. Total of references obtained in the databases and the final number of studies included in the systematic review.
The chosen references included in the SR are shown in the following Tables 3, 4.
Table 3.
Scientific articles about chemokines as biomarker of CD activity included in the systematic review
| CHEMOKINE | ORIGINAL NAME | RECEPTOR | GENE | ARTICLE | SAMPLES | YEAR | CRITERIA FOR DISEASE ACTIVITY | IS IT A POTENTIAL BIOMARKER? |
|---|---|---|---|---|---|---|---|---|
| CCL2 | MCP-1 | CCR2 | Scya2 | Bourgonje et al./Christophi et al. | Peripheral Blood/Intestinal Mucosa | 2018/2012 | Calprotectin level/Histologic | NO/YES (p<0.05) |
| CCL3 | MIP-1α | CCR1/CCR5 | Scya3 | Bourgonje et al. | Peripheral Blood | 2018 | Calprotectin level | NO |
| CCL4 | MIP-1β | CCR5 | Scya4 | Bourgonje et al. | Peripheral Blood | 2018 | Calprotectin level | NO |
| CCL11 | Eotaxin-1 | CCR3 | Scya11 | Bourgonje et al./Bourgonje et al. | Peripheral Blood/Peripheral Blood | 2018/2019 | Calprotectin level/Endoscopic Index | NO/NO |
| CCL17 | TARC | CCR4 | Scya17 | Bourgonje et al. | Peripheral Blood | 2018 | Calprotectin level | NO |
| CCL20 | LARC/Exodus-1 | CCR6 | Scya20 | Christophi et al. | Peripheral Blood | 2012 | Histologic | YES (p<0.05) |
| CCL22 | MDC | CCR4 | Scya22 | Bourgonje et al. | Peripheral Blood | 2018 | Calprotectin level | NO |
| CCL26 | Eotaxin-3 | CCR3 | Scya26 | Bourgonje et al./Bourgonje et al. | Peripheral Blood | 2018/2019 | Calprotectin level/Endoscopic Index | NO/NO |
| CXCL1 | GROα | CXCR2 | Scyb1 | Hong et al./Leal et al. | Intestinal Mucosa/Intestinal Mucosa | 2017/2014 | Endoscopic Index/Endoscopic Index | YES (p<0.001)/YES (p<0.05) |
| CXCL3 | GRO3, MIP-2β | CXCR2 | Scyb2 | Leal et al. | Intestinal Mucosa | 2014 | Endoscopic Index | YES (p<0.05) |
| CXCL8 | IL-8 | CXCR1 | Scyb8 | Arsenescu et al./Boland et al. | Intestinal Mucosa/Intestinal Mucosa | 2008/2015 | Endoscopic Index/Endoscopic Index | YES (p=0.002)/YES (p<0.05) |
| CXCR2 | Bourgonje et al./Brand et al. | Peripheral Blood/Intestinal Mucosa | 2019/2006 | Endoscopic Index/Endoscopic Index | YES (p<0.05)/YES (p<0.05) | |||
| Bruno et al./Christophi et al. | Intestinal Mucosa/Intestinal Mucosa | 2015/2012 | Endoscopic Index/Histologic | NO/NO | ||||
| Taha et al./Yarur et al. | Urine/Peripheral Blood | 2002/2016 | Clinical Index/Histologic | YES (p=0.001)/NO | ||||
| CXCL10 | IP-10 | CXCR3 | Scyb10 | Boland et al./Bourgonje et al. | Intestinal Mucosa/Peripheral Blood | 2015/2018 | Endoscopic Index/Calprotectin level | YES (p<0.05)/NO |
| Christophi et al./Grip et al. | Intestinal Mucosa/Peripheral Blood | 2012/2009 | Histologic/Calprotectin Level | YES (p<0.05)/NO | ||||
| CX3CL1 | Fractalkine | CX3CR1 | Scyd1 | Brand et al. | Intestinal Mucosa | 2006 | Endoscopic Index | YES (p=0.02) |
Table 4.
Scientific articles about adipokines as biomarker of CD activity included in the systematic review
| ADIPOKINE | ARTICLE | SAMPLES | YEAR | CRITERIA FOR DISEASE ACTIVITY | IS IT A POTENTIAL BIOMARKER? |
|---|---|---|---|---|---|
| ADIPONECTIN | Karahman et al./Karmiris et al./Karmiris et al./ | Peripheral Blood/Peripheral Blood/Peripheral Blood/ | 2017/2007/2006 | Clinical Index/Clinical Index/Clinical Index/ | NO/NO/NO |
| Rodrigues et al./Theocharidou et al. | Mesenteric Biopsies/Peripheral Blood | 2012/2016 | Endoscopic Index/Clinical Index | YES (p<0.01)/NO | |
| LEPTIN | Karahman et al./Karmiris et al./Karmiris et al. | Peripheral Blood/Peripheral Blood/Peripheral Blood/ | 2017/2007/2006 | Clinical Index/Clinical Index/Clinical Index/ | NO/NO/NO |
| Rodrigues et al./Trejo-Vazquez et al. | Mesenteric Biopsies/Peripheral Blood | 2012/2017 | Endoscopic Index/Endoscopic Index | NO/YES (p<0.001) | |
| OMENTIN-1 | Yin et al. | Peripheral Blood | 2014 | Clinical Index | YES (p=0.012) |
| PAI-1 | Trejo-Vazquez et al. | Peripheral Blood | 2017 | Endoscopic Index | NO |
| RESISTIN | Karmiris et al./Karmiris et al./Konrad et al. | Peripheral Blood/Peripheral Blood/Peripheral Blood/ | 2007/2006/2007 | Clinical Index/Clinical Index/Clinical Index/ | YES (p=0.004)/NO/YES (p=0.02) |
| Theocharidou et al./Trejo-Vazquez et al. | Peripheral Blood/Peripheral Blood | 2016/2017 | Clinical Index/Endoscopic Index | YES (p=0.014)/NO | |
| VISFATIN | Trejo-Vazquez et al. | Peripheral Blood | 2007 | Endoscopic Index | NO |
| GHRELIN | Karmiris et al./Trejo-Vazquez et al. | Peripheral Blood/Peripheral Blood | 2006/2017 | Clinical Index/Endoscopic Index | NO/NO |
| IL-6 | Boland et al./Bourgonje et al. | Intestinal Mucosa/Peripheral Blood | 2015/2017 | Endoscopic Index/Calprotectin Level | YES (p<0.05)/YES (p<0.01) |
| Bourgonje et al./Christophi et al. | Peripheral Blood/Intestinal Mucosa | 2019/2012 | Endoscopic Index/Histologic Endoscopic | YES (p<0.05)/YES (p<0.05) | |
| Leal et al./Yarur et al. | Intestinal Mucosa/Peripheral Blood | 2014/2016 | Index/Histologic | YES (p<0.05)/YES (p=0.004) | |
| TNF-α | Boland et al./Bourgonje et al. | Intestinal Mucosa/Peripheral Blood | 2015/2019 | Endoscopic Index/Endoscopic Index | YES (p<0.05)/YES (p<0.05) |
| Christophi et al./Yarur et al. | Intestinal Mucosa/Peripheral Blood | 2012/2016 | Histologic/Histologic | YES (p<0.05)/YES (p<0.002) |
We evaluated several aspects of each study including the type of chemokine/adipokine investigated, the subjects, origin of the samples (peripheral blood, intestinal biopsies, urine), year of publication, and criteria for assessing disease activity and judging whether the chemokine/adipokine evaluation showed significant results (Tables 1 and 2). Figure 3 illustrates the main variables obtained from each study included in the SR.
Figure 3.
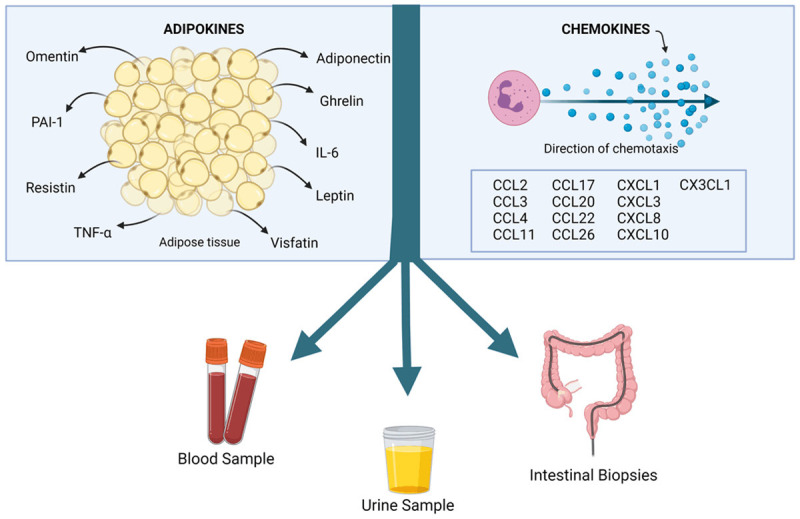
Illustrated chart of the systematic reviews’ aims, and the samples which most of the selected references worked with to correlate the chemokines and adipokine levels with Crohn’s disease activity.
Chemokines and adipokines as significant markers for Crohn’s disease activity
Among the twenty selected articles, six (30%) addressed only chemokines [2,26-30] and eight (40%) only adipokines [21,31-37] as potential biomarkers for CD activity. Six references mentioned both types of cytokines [38-43]. The samples were obtained from peripheral blood (60%), and intestinal mucosa (40%); one study evaluated urine samples (5%). This latter one analyzed peripheral blood and intestinal mucosa simultaneously. The assessment of CD activity was heterogeneous, with endoscopic index (40%), clinical index (40%), calprotectin level (10%), and histologic parameters (10%) as the main criteria.
Among the adipokines, all were tested in peripheral blood, and 44.4% were also tested in the intestinal mucosa. Adiponectin, leptin, IL-6, and TNF-α were tested in more than one type of sample, like peripheral blood and intestinal mucosa. While the percentage among the chemokine’s studies was 76.9% in peripheral blood, 46.1% intestinal mucosa, and 7.6% in urine sample. CCL2, CXCL8, and CXCL10 were tested in peripheral blood and intestinal mucosa in different studies.
CXCL8 was the most studied chemokine (eight studies), and the results were controversial, with 62.5% (five studies) showing a significant association with CD activity (P=0.002; P<0.05; P<0.05; P<0.05; P=0.001 respectively) [2,27,28,30,42]. Four researchers group explored CXCL10 and 50% identified it as a potential biomarker (P<0.05) [40,42]. CCL11 and CCL26 were considered by two articles each and they did not show significant results [38,40]. CCL2 was explored in two studies, one of them positively correlated with disease activity (P<0.05) [40]. CXCL1 was included in two studies as well, with significant results in both (P=0.001; P<0.05) [26,41]. Only three references studied CX3CL1, CCL20 and CXCL3 separately, with positive correlation with the adopted inflammation criteria (P=0.02; P<0.05; P<0.05, respectively) [27,33,34]. CCL3, CCL4, CCL17, and CCL22 were explored in one article each as well, but without significant results [38].
There were more studies that addressed adipokines than chemokines. IL-6 was studied in six studies. All of them showed it as a potential inflammation biomarker in CD (P<0.05; P<0.01; P<0.05; P<0.05; P<0.05; P=0.004, respectively) [38-43]. Five studies explored adiponectin levels, while there was only one with a significant result (P<0.01), when comparing active CD with healthy controls [21,31-34]. Resistin and leptin were investigated in another five works. Resistin presented 60% (three) of the studies which demonstrated significative P-value (P=0.04; P=0.02; P=0.014, respectively) [31,33,36], while leptin presented only one with a significant result (P<0.01) [35]. All of four references on TNF-α showed it as a potential activity biomarker for CD (P<0.05; P<0.05; P<0.05; P<0.002) [39,40,42,43]. One study explored omentin and confirmed a p of significance equal to 0.012 [37]. Ghrelin was evaluated in two studies, PAI-1 and visfatin in one; all of them revealed no significant results [31,35]. Figure 4 shows the potential biomarkers for active Crohn’s disease.
Figure 4.
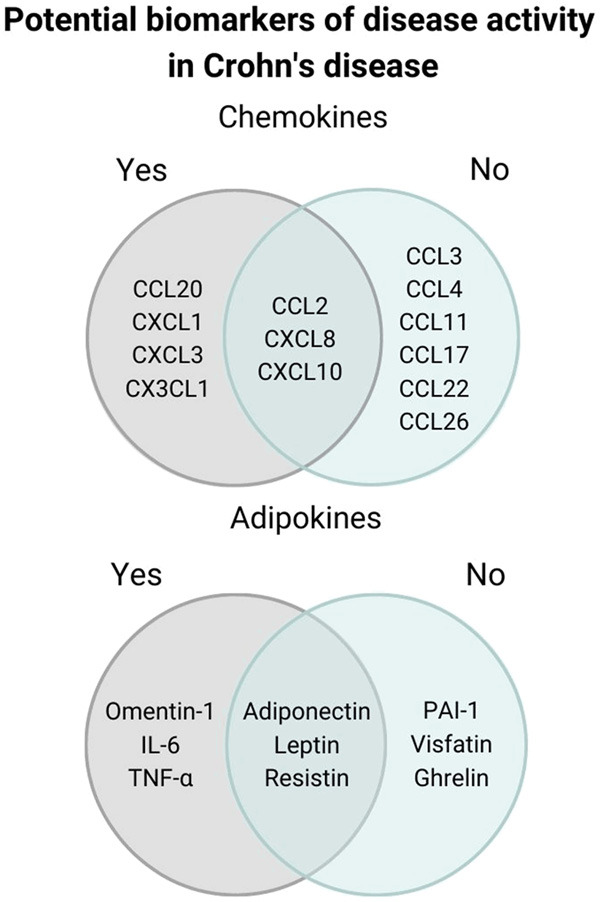
Chemokines and adipokines significantly modulated in Crohn’s disease according to the studies included in the systematic review. Potential biomarkers of disease activity are show in gray in the Venn diagram.
Discussion
This SR aims to explore what can be found in the literature about chemokines and adipokines as CD activity markers. The analysis began with an examination of convergence and divergence in the results. All studies dealt with a population of CD patients with and without activity of the disease defined by clinical index, endoscopic, radiological aspects, or calprotectin level. Therefore, the following topics were based on the studies which pointed towards chemokines and adipokines as possible markers of CD activity.
The chemokines were considered by their acronym (CCL, CXCL, CX3CL) or their “original names”. Some articles conducted studies based on the chemokine receptors. We standardized the chemokine description by using acronyms below.
CCL20
CCL20 is a small cytokine belonging to the CC chemokine family. It is strongly chemotactic for lymphocytes and weakly attracts neutrophils. Christophi et al. analyzed human intestinal mucosa and confirmed through RT-PCR that there is an increase of CCL20 levels 10 times more significant, and a higher CCL20/CCR6 ratio in CD patients with active disease compared to those in remission, suggesting a possible marker for CD activity [40].
CXCL10
It is an 8.7 kDa protein, encoded by the CXCL10 gene and is secreted by several cell types in response to IFN-γ. Boland et al., using biopsies from ileum and colon segments, showed increased mRNA CXCL10 expression in CD patients with active disease when compared to those in remission, suggesting this chemokine as a potential biomarker [42]. This result was supported by another study, which found increased levels of CXCL10 in CD inflamed colonic biopsies [42]. However, other two studies did not find a relation between CXCL10 level and fecal calprotectin in active CD patients [29,39].
CXCL3
Chemokine (C-X-C motif) ligand 3 was studied by Leal et al., who analyzed potential biomarkers for anti-TNF-α therapy response in the intestinal mucosa. They showed an upregulation of CXCL1 and CXCL3 chemokines, besides IL1B, S100A8, S100A9, SLC64A14, FCG3B (CD16b) and IL17A transcriptional levels in CD patients with active disease, regardless of the anti-TNF therapy use, suggesting these transcripts as markers of inflammation in CD [41].
CCL3, CCL4, CCL17, CCL22 and CCL26
Another group of chemokines was depicted in a serum cytokine study (IL-6, CCL2, CCL3, CCL4, CCL11, CCL17, CCL22, CCL26, SAA, IL-17A, TNF-β and others), and a correlation with CRP and fecal calprotectin was performed. Only IL-6, IFN-γ, IL17A, and TNF-β showed a significant association with these stablished serum biomarkers of disease activity [38,39].
CXCL1
CXCL1 is a small peptide belonging to the CXC chemokine family that acts as a relevant chemoattractant for several immune cells. Hong et al. and Leal at al. noticed an increased CXCL1 level in CD inflamed intestinal mucosa compared to CD in remission. Interestingly, intestinal mucosa of CD in remission still presented higher CXCL1 levels compared to healthy controls without CD. Moreover, CXCL1 was associated better with disease activity than CRP [26,41].
CXCL8 and CCL11
CXCL8 is the main mediator of the initial innate immune response to intracellular microbes and higher levels were found in UC patients, but not in CD patients. The samples were obtained from visibly inflamed colonic mucosa biopsies and were compared to paired samples from non-inflamed colonic mucosa from the same individuals [2,27,28,40,42].
The endoscopic criteria are still the standard for determining CD activity, despite of the high sensibility and specificity of the calprotectin test, which is a less invasive method. Thus, in a recent study, chemokines levels were compared with endoscopy disease activity. The SAA, IL-6, IL-8, and CCL11 levels in combination showed a reliable prediction for endoscopy inflammation activity in CD and can be important for monitoring active disease [39]. CCL11, CXCL8, SAA, IL17A, and TNF-α, individually, showed better predictive performances compared to CRP, fecal calprotectin and clinical scores, and they were equivalent to endoscopic parameters of disease activity [39].
CX3CL1
There was a significant increase of C3XCL1 transcript level in inflamed lesions of CD patients when compared to the non-inflamed colonic mucosa, suggesting it as a marker of disease activity [27].
Concerning the studies which discussed the adipokines and their association or not with CD activity, there is the following.
Leptin and adiponectin
The adipokines called leptin and adiponectin were mentioned in five articles, all with human populations and blood sample analyzes. Karmiris et al., in two publications, evaluated the circulating levels of leptin, adiponectin and resistin in IBD patients, and analyzed the correlation between adipokines and treatment with infliximab. They found a possible association between adipokines and disease activity, but the changes in serum levels of the examined adipokines were not correlated with CRP or with clinical disease activity indexes (CDAI) [31]. Besides, in another study, Karmiris et al. compared serum levels of adipokines among UC, CD and healthy controls. Patients with UC and CD were divided into two groups: with disease activity and in remission [32]. Both studies showed no significant difference in the levels of the studied adipokines [31,32]. Likewise, another study concluded that serum adiponectin did not show a significant difference between the activity and in remission CD groups [33]. Rodrigues et al. confirmed lower levels of serum adiponectin in active CD patients compared to healthy controls, but without significant difference compared to those in remission, and serum leptin was similar among the groups. This article expanded the analysis of adiponectin for mesenteric adipose tissue evaluation and verified lower expression in active CD as well, suggesting a deficient anti-inflammatory mechanism in this disease [21].
Corroborating the publications already cited, Karahman et al., confirmed that CD patients with CDAI <150 and ≥150 did not show any significant difference regarding serum levels of adiponectin and leptin [34]. However, Trejo-Vasquez et al. assessed the serum level of adipokines and their association with endoscopic activity of the disease. They showed that serum leptin is decreased in CD patients compared to healthy controls, and even lower when comparing CD patients with disease activity to patients in remission. There was no significant difference in the serum concentration of other adipokines evaluated when comparing patients with or without disease activity [35].
Resistin
Resistin is a central blocker of leptin, which induces satiety. Karmiris et al. did not identify any significant association of resistin serum levels and CRP or with Crohn’s disease Activity index (CDAI) [31,32]. Indeed, Trejo-Vasquez et al. found no significant differences in the serum concentration assessed when comparing patients with or without disease activity [35]. On the other hand, Konrad et al. confirmed that serum resistin concentrations were significantly associated with elevated leukocytes, CRP and disease activity, suggesting that resistin is more sensitive to inflammatory processes than CRP [36]. These were consistent with the findings reported by Theocharidou et al., which showed significantly higher serum resistin levels in active CD compared to CD in remission [33].
Visfatin and PAI-1
Visfatin and PAI-1 were cited only in a publication by Trejo-Vasquez et al., and they did not show a significant difference in the serum concentration of the evaluated adipokines when comparing CD patients with or without disease activity [35].
Ghrelin
Ghrelin is a peptide hormone produced mainly by the epsilon cells of the stomach and pancreas and it is released in the bloodstream. Karmiris et al. and Trejo-Vasquez et al. did not find any significant difference in ghrelin serum concentration when comparing CD patients with or without disease activity [31,35].
Omentin-1
Omentin-1 is an adipocytokine of 313 amino acids, which is expressed in visceral omental tissue among others. This adipokine was studied by Yin et al., who demonstrated that its decrease in the serum can be considered an independent predictive marker of clinical disease activity for both CD and UC [37].
Interleukin-6
This adipokine, unlike those mentioned above, was evaluated in human biopsies of intestinal mucosa. Christophi et al. concluded that IL-6, MCP-1 (CCL2), CCR2, CCL20, and IP-10 (CXCL10) were increased in active CD. TNF-α, IL-8 and IL-17A induced by NF-KB were elevated in active IBD [40]. Likewise, Leal et al. demonstrated that IL-6 responds to anti-TNF-α and is negatively regulated regardless of the therapeutic response. IL1B, S100A8, S100A9, CXCL2, CXCL6, S100A12, SLC64A14, FCG3B (CD16b) and IL17A genes were upregulated in patients with active CD. REG1A, IL-8, S100A8, S100A9, IL1B, MMP1, MMP3 are inflammation-dependent genes that are downregulated as mucosal lesion recovery occurs [41].
Boland et al. demonstrated the IL-6 expression separating by using biopsy topography. Thus, in the ileum, IL-1β, IL-6, IL-8, TNF-α, IP-10, MMP-3 and S100-A8 were increased in active CD, while IL-6, IL-8, TNF-α, IP-10, MMP-3 and S100-A8 were increased in colonic active CD. Concerning the rectal mucosa, they identified higher levels of IL-8, TNF-α, IP-10, MMP3 and S100-A8 in active disease [42]. Yarur et al. analyzed blood samples and observed significantly lower levels of ICAM-1, IL-6 and TNF-α in patients with healed mucosa, i.e., in remission of the disease, as compared with the group with CD in activity. Reaffirming the hypothesis that the serum analysis may not reflect the inflammatory changes in the tissue [43]. However, a more recent article reported by Bourgonje et al. compared serum biomarkers with fecal calprotectin demonstrating that the increase of fecal calprotectin levels is associated to an increase of serum IFN-γ and IL-6. It also had a significant correlation with serum SAA, PCR, IL-17A and TNF-β [38].
One of the main limitations of the present review is the variety of the studies included in the analysis concerning the methods applied for the chemokine and adipokine analysis and the criteria used for the active CD definition. The different methods of cytokine quantification from distinct type of tissue samples besides the distinct inflammation criteria for analysis complicated the comparison among the studies. Most of the studies which addressed adipokines were based only on clinical criteria which may not always correlate with the presence of bowel lesions. Another limitation is the comparison between active CD patients with healthy controls but not with those in remission. For this reason, our SR is more descriptive than comparative, but it is still relevant since the data found here point towards chemokines and adipokines that should be explored in future large studies.
Moreover, an important limitation about chemokine and adipokine analysis in CD is that it has a vast role in inflammation. For example, the adipose tissue has been the major source of the adipokine TNF-α, but other cells can also produce it as well, possessing a crucial pro-inflammatory function, and helping with resistance against infections and cancer [44]. Therefore, there is a wide range of scenarios that can influence TNF-α activity and make the association with some specific aspect of CD difficult. The IL-6 adipokine, as the TNF-α, is produced by macrophages, and only 30% of the circulating IL-6 is released by adipose tissue [44]. Even the ghrelin has the stomach and pancreas as a source, besides the adipose tissue. Thus, most of the adipokines can be influenced by diverse inflammatory processes besides the CD [45]. Similar to the adipokines, the chemokines can be influenced by other diseases and by acute inflammation [46].
Conclusions
Despite the limitations and the small number of studies found in the literature that followed the criteria of this SR, some chemokines (CCL2, CCL20, CXCL1, CXCL3, CXCL8, CXCL10 and CXCL1) and adipokines (leptin, IL-6 and TNF-α) have unveiled promising results that may enable us to distinguish active versus in remission forms of CD based on objective criteria of inflammation such as endoscopic, histologic or radiological criteria. Indeed, most chemokines and adipokines with significant levels were taken from peripheral blood, which is less invasive. Given the chronic character of CD, it is helpful to detect intestinal lesions before clinical symptoms become noticeable to avoid permanent bowel damage. With this in mind, less invasive biomarkers for CD, such as the ones we highlighted in this review, may prove to become effective tools for their management and follow-up.
Acknowledgements
We thank Tristan Torriani for the English revision of our manuscript. We thank Ana Paula de Morais e Oliveira for helping to search the articles in the databases. This work was supported by National Council for Scientific and Technological Development (CNPq) [Grant number #301388/2018-0 for R.F.L.]. J.D.C.M. (author) received a Master scholarship from the Brazilian Coordination for the Improvement of Higher Education Personnel (CAPES-Coordenação de Aperfeiçoamento de Pessoal de Nível Superior-Brasil), Finance Code 001. L.B.P. (co-author) received a Post-doctoral scholarship from Funding for Education, Research and Extension Support (FAEPEX), University of Campinas [Grant number #2332/20].
Disclosure of conflict of interest
None.
Abbreviations
- CD
Crohn’s disease
- IBD
inflammatory bowel disease
- MRI
entero magnetic resonance imaging
- CRP
C-reactive protein
- SR
systematic review
- QCRI
Qatar Computing Research Institute
- SMS
School of Medical Sciences
- Unicamp
University of Campinas
- CDAI
clinical disease activity index
- PAI-1
plasminogen activator inhibitor-1
- UC
ulcerative colitis
- IL-6
interleukin 6
- MCP-1
Monocyte chemoattractant protein-1
- CCL2
C-C Motif Chemokine Ligand 2
- CCR2
C-C chemokine receptor type 2
- CCL20
C-C Motif Chemokine Ligand 20
- IP-10
Interferon gamma-induced protein 10
- CXCL10
C-X-C Motif Chemokine Ligand 10
- TNF-α
Tumour necrosis factor α
- IL-8
Interleukin 8
- IL-17A
Interleukin-17A
- NF-κB
factor nuclear kappa B
- IL1β
Interleukin 1 Beta
- S100A8
S100 Calcium Binding Protein A8
- S100A9
S100 Calcium Binding Protein A9
- CXCL2
C-X-C Motif Chemokine Ligand 2
- CXCL6
C-X-C Motif Chemokine Ligand 6
- S100A12
S100 calcium-binding protein A12
- SLC64A14
solute carrier family 6 member 14
- FCG3B
Fc Fragment of IgG Receptor IIIb
- REG1A
Regenerating Family Member 1 Alpha
- MMP1
matrix Metallopeptidase 1
- MMP3
matrix Metallopeptidase 3
- ICAM-1
Intercellular Adhesion Molecule 1
- IFN-γ
Interferon-gama
- SAA
Serum Amyloid A
- TNF-β
Tumor necrosis factor β
- CCL
C-C Motif Chemokine Ligand
- CXCL
C-X-C Motif Chemokine Ligand
- CX3CL
C-X3-C motif Chemokine Ligand
- CCL25
C-C Motif Chemokine Ligand 25
- CCR9
C-C chemokine receptor type 9
- RT-PCR
Reverse Transcription Polymerase Chain Reaction
- CCR6
C-C chemokine receptor type 6
- mRNA
messenger ribonucleic acid
- CCL19
C-C Motif Chemokine Ligand 19
- CCL21
C-C Motif Chemokine Ligand 21
- CCR7
C-C Motif Chemokine Receptor 7
- ELISA
Enzyme-Linked Immunosorbent Assay
- CCL3
C-C Motif Chemokine Ligand 3
- CCL4
C-C Motif Chemokine Ligand 4
- CCL11
C-C Motif Chemokine Ligand 11
- CCL17
C-C Motif Chemokine Ligand 17
- CCL22
C-C Motif Chemokine Ligand 22
- CCL26
C-C Motif Chemokine Ligand 26
- CXCL1
C-X-C Motif Chemokine Ligand 1
- CXCL8
C-X-C motif chemokine ligand 8
References
- 1.Loftus EV Jr. Clinical epidemiology of inflammatory bowel disease: incidence, prevalence, and environmental influences. Gastroenterology. 2004;126:1504–17. doi: 10.1053/j.gastro.2004.01.063. [DOI] [PubMed] [Google Scholar]
- 2.Arsenescu R, Bruno ME, Rogier EW, Stefka AT, McMahan AE, Wright TB, Nasser MS, de Villiers WJ, Kaetzel CS. Signature biomarkers in Crohn’s disease: toward a molecular classification. Mucosal Immunol. 2008;1:399–411. doi: 10.1038/mi.2008.32. [DOI] [PubMed] [Google Scholar]
- 3.Baumgart DC, Sandborn WJ. Crohn’s disease. Lancet. 2012;380:1590–605. doi: 10.1016/S0140-6736(12)60026-9. [DOI] [PubMed] [Google Scholar]
- 4.Abraham C, Cho JH. Inflammatory bowel disease. N Engl J Med. 2009;361:2066–78. doi: 10.1056/NEJMra0804647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dragoni G, Innocenti T, Galli A. Biomarkers of inflammation in inflammatory bowel disease: how long before abandoning single-marker approaches? Dig Dis. 2020;39:190–203. doi: 10.1159/000511641. [DOI] [PubMed] [Google Scholar]
- 6.Mélik-Parsadaniantz S, Rostène W. Chemokines and neuromodulation. J Neuroimmunol. 2009;198:62–8. doi: 10.1016/j.jneuroim.2008.04.022. [DOI] [PubMed] [Google Scholar]
- 7.Fernandez EJ, Lolis E. Structure, function, and inhibition of chemokines. Annu Rev Pharmacol Toxicol. 2002;42:469–99. doi: 10.1146/annurev.pharmtox.42.091901.115838. [DOI] [PubMed] [Google Scholar]
- 8.Abbas AK, Lichtman AH, Pillai S. Cellular and molecular immunology. Elsevier; 2018. pp. 39–45. [Google Scholar]
- 9.Zlotnik A, Yoshie O. Chemokines: a new classification system and their role in immunity. Immunity. 2000;12:121–7. doi: 10.1016/s1074-7613(00)80165-x. [DOI] [PubMed] [Google Scholar]
- 10.Schäffler A, Schölmerich J, Büchler C. Mechanisms of disease: adipocytokines and visceral adipose tissue-emerging role in intestinal and mesenteric diseases. Nat Clin Pract Gastroenterol Hepatol. 2005;2:103–11. doi: 10.1038/ncpgasthep0090. [DOI] [PubMed] [Google Scholar]
- 11.Peyrin-Biroulet L, Chamaillard M, Gonzalez F, Beclin E, Decourcelle C, Antunes L, Gay J, Neut C, Colombel JF, Desreumaux P. Mesenteric fat in Crohn’s disease: a pathogenetic hallmark or an innocent bystander? Gut. 2007;56:577–83. doi: 10.1136/gut.2005.082925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zulian A, Cancello R, Micheletto G, Gentilini D, Gilardini L, Danelli P, Invitti C. Visceral adipocytes: old actors in obesity and new protagonists in Crohn’s disease? Gut. 2012;61:86–94. doi: 10.1136/gutjnl-2011-300391. [DOI] [PubMed] [Google Scholar]
- 13.Conde J, Scotece M, Gómez R, López V, Gómez-Reino JJ, Lago F, Gualillo O. Adipokines: biofactors from white adipose tissue. A complex hub among inflammation, metabolism, and immunity. Biofactors. 2011;37:413–20. doi: 10.1002/biof.185. [DOI] [PubMed] [Google Scholar]
- 14.Lehr S, Hartwig S, Sell H. Adipokines: a treasure trove for the discovery of biomarkers for metabolic disorders. Proteomics Clin Appl. 2012;6:91–101. doi: 10.1002/prca.201100052. [DOI] [PubMed] [Google Scholar]
- 15.Karmiris K, Koutroubakis IE, Kouroumalis EA. The emerging role of adipocytokines as inflammatory mediators in inflammatory bowel disease. Inflamm Bowel Dis. 2005;11:847–55. doi: 10.1097/01.mib.0000178915.54264.8f. [DOI] [PubMed] [Google Scholar]
- 16.Kershaw EE, Flier JS. Adipose tissue as an endocrine organ. J Clin Endocrinoland Metab. 2004;89:2548–56. doi: 10.1210/jc.2004-0395. [DOI] [PubMed] [Google Scholar]
- 17.Karastergiou K, Mohamed-Ali V. The autocrine and paracrine roles of adipokines. Mol Cell Endocrinol. 2010;318:69–78. doi: 10.1016/j.mce.2009.11.011. [DOI] [PubMed] [Google Scholar]
- 18.Harper JW, Zisman TL. Interaction of obesity and inflammatory bowel disease. World J Gastroenterol. 2016;22:7868–81. doi: 10.3748/wjg.v22.i35.7868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morshedzadeh N, Rahimlou M, Asadzadeh Aghdaei H, Shahrokh S, Reza Zali M, Mirmiran P. Association between adipokines levels with inflammatory bowel disease (ibd): systematic reviews. Dig Dis Sci. 2017;62:3280–86. doi: 10.1007/s10620-017-4806-5. [DOI] [PubMed] [Google Scholar]
- 20.Bilski J, Mazur-Bialy A, Wojcik D, Surmiak M, Magierowski M, Sliwowski Z, Pajdo R, Kwiecien S, Danielak A, Ptak-Belowska A, Brzozowski T. Role of obesity, mesenteric adipose tissue, and adipokines in inflammatory bowel diseases. Biomolecules. 2019;9:780. doi: 10.3390/biom9120780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rodrigues VS, Milanski M, Fagundes JJ, Torsoni AS, Ayrizono ML, Nunez CE, Dias CB, Meirelles LR, Dalal S, Coy CS, Velloso LA, Leal RF. Serum levels and mesenteric fat tissue expression of adiponectin and leptin in patients with Crohn’s disease. Clin Exp Immunol. 2012;170:358–64. doi: 10.1111/j.1365-2249.2012.04660.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Clarke M, Oxman AD. Cochrane reviewers’ handbook 4.2. In: Clarke M, Oxman AD, editors. Oxford: The Cochrane Collaboration; 2003. Issue 1. [Google Scholar]
- 23.Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA. Cochrane handbook for systematic reviews of interventions V 6.1. Cochrane. 2020. www.training.cochrane.org/handbook. [DOI] [PMC free article] [PubMed]
- 24.Galvão TF, Pansani TSA, Harrad D. Main items to report systematic reviews and meta-analyzes: the PRISMA recommendation. Epidemiology and Health Services. 2015;24:335–342. [Google Scholar]
- 25.Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan-a web and mobile app for systematic reviews. Syst Rev. 2016;5:210. doi: 10.1186/s13643-016-0384-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hong SN, Joung JG, Bae JS, Lee CS, Koo JS, Park SJ, Im JP, Kim YS, Kim JW, Park WY, Kim YH. RNA-seq reveals transcriptomic differences in inflamed and noninflamed intestinal mucosa of Crohn’s disease patients compared with normal mucosa of healthy controls. Inflamm Bowel Dis. 2017;23:1098–1108. doi: 10.1097/MIB.0000000000001066. [DOI] [PubMed] [Google Scholar]
- 27.Brand S, Hofbauer K, Dambacher J, Schnitzler F, Staudinger T, Pfennig S, Seiderer J, Tillack C, Konrad A, Göke B, Ochsenkühn T, Lohse P. Increased expression of the chemokine fractalkine in Crohn’s disease and association of the fractalkine receptor T280M polymorphism with a fibrostenosing disease phenotype. Am J Gastroenterol. 2006;101:99–106. doi: 10.1111/j.1572-0241.2005.00361.x. [DOI] [PubMed] [Google Scholar]
- 28.Bruno ME, Rogier EW, Arsenescu RI, Flomenhoft DR, Kurkjian CJ, Ellis GI, Kaetzel CS. Correlation of biomarker expression in colonic mucosa with disease phenotype in Crohn’s disease and ulcerative colitis. Dig Dis Sci. 2015;60:2976–84. doi: 10.1007/s10620-015-3700-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grip O, Janciauskiene S. Atorvastatin reduces plasma levels of chemokine (CXCL10) in patients with Crohn’s disease. PLoS One. 2009;4:e5263. doi: 10.1371/journal.pone.0005263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Taha AS, Grant V, Kelly RW. Urinalysis for interleukin-8 in the non-invasive diagnosis of acute and chronic inflammatory diseases. Postgrad Med J. 2003;79:159–63. doi: 10.1136/pmj.79.929.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Karmiris K, Koutroubakis IE, Xidakis C, Polychronaki M, Voudouri T, Kouroumalis EA. Circulating levels of leptin, adiponectin, resistin, and ghrelin in inflammatory bowel disease. Inflamm Bowel Dis. 2006;12:100–5. doi: 10.1097/01.MIB.0000200345.38837.46. [DOI] [PubMed] [Google Scholar]
- 32.Karmiris K, Koutroubakis IE, Xidakis C, Polychronaki M, Kouroumalis EA. The effect of infliximab on circulating levels of leptin, adiponectin and resistin in patients with inflammatory bowel disease. Eur J Gastroenterol Hepatol. 2007;19:789–94. doi: 10.1097/MEG.0b013e3282202bca. [DOI] [PubMed] [Google Scholar]
- 33.Theocharidou E, Balaska A, Vogiatzis K, Tellis CC, Gossios TD, Athyros VG, Tselepis AD, Karagiannis A. Hypertrophic mesenteric adipose tissue may play a role in atherogenesis in inflammatory bowel diseases. Inflamm Bowel Dis. 2016;22:2206–12. doi: 10.1097/MIB.0000000000000873. [DOI] [PubMed] [Google Scholar]
- 34.Kahraman R, Calhan T, Sahin A, Ozdil K, Caliskan Z, Bireller ES, Cakmakoglu B. Are adipocytokines inflammatory or metabolic mediators in patients with inflammatory bowel disease? Ther Clin Risk Manag. 2017;13:1295–1301. doi: 10.2147/TCRM.S140618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Trejo-Vazquez F, Garza-Veloz I, Villela-Ramirez GA, Ortiz-Castro Y, Mauricio-Saucedo P, Cardenas-Vargas E, Diaz-Baez M, Cid-Baez MA, Castañeda-Miranda R, Ortiz-Rodriguez JM, Solis-Sanchez LO, Martinez-Fierro ML. Positive association between leptin serum levels and disease activity on endoscopy in inflammatory bowel disease: a case-control study. Exp Ther Med. 2018;15:3336–44. doi: 10.3892/etm.2018.5835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Konrad A, Lehrke M, Schachinger V, Seibold F, Stark R, Ochsenkühn T, Parhofer KG, Göke B, Broedl UC. Resistin is an inflammatory marker of inflammatory bowel disease in humans. Eur J Gastroenterol Hepatol. 2007;19:1070–4. doi: 10.1097/MEG.0b013e3282f16251. [DOI] [PubMed] [Google Scholar]
- 37.Yin J, Hou P, Wu Z, Nie Y. Decreased levels of serum omentin-1 in patients with inflammatory bowel disease. Med Sci Monit. 2015;21:118–22. doi: 10.12659/MSM.892081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bourgonje AR, von Martels JZH, de Vos P, Faber KN, Dijkstra G. Increased fecal calprotectin levels in Crohn’s disease correlate with elevated serum Th1- and Th17-associated cytokines. PLoS One. 2018;13:e0193202. doi: 10.1371/journal.pone.0193202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bourgonje AR, von Martels JZH, Gabriëls RY, Blokzijl T, Buist-Homan M, Heegsma J, Jansen BH, van Dullemen HM, Festen EAM, Ter Steege RWF, Visschedijk MC, Weersma RK, de Vos P, Faber KN, Dijkstra G. A combined set of four serum inflammatory biomarkers reliably predicts endoscopic disease activity in inflammatory bowel disease. Front Med (Lausanne) 2019;6:251. doi: 10.3389/fmed.2019.00251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Christophi GP, Rong R, Holtzapple PG, Massa PT, Landas SK. Immune markers and differential signaling networks in ulcerative colitis and Crohn’s disease. Inflamm Bowel Dis. 2012;18:2342–56. doi: 10.1002/ibd.22957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Leal RF, Planell N, Kajekar R, Lozano JJ, Ordás I, Dotti I, Esteller M, Masamunt MC, Parmar H, Ricart E, Panés J, Salas A. Identification of inflammatory mediators in patients with Crohn’s disease unresponsive to anti-TNFα therapy. Gut. 2015;64:233–42. doi: 10.1136/gutjnl-2013-306518. [DOI] [PubMed] [Google Scholar]
- 42.Boland BS, Boyle DL, Sandborn WJ, Firestein GS, Levesque BG, Hillman J, Zhang B, Proudfoot J, Eckmann L, Ernst PB, Rivera-Nieves J, Pola S, Copur-Dahi N, Zou G, Chang JT. Validation of gene expression biomarker analysis for biopsy-based clinical trials in Crohn’s disease. Inflamm Bowel Dis. 2015;21:323–30. doi: 10.1097/MIB.0000000000000264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yarur AJ, Quintero MA, Jain A, Czul F, Barkin JS, Abreu MT. Serum amyloid a as a surrogate marker for mucosal and histologic inflammation in patients with Crohn’s disease. Inflamm Bowel Dis. 2017;23:158–64. doi: 10.1097/MIB.0000000000000991. [DOI] [PubMed] [Google Scholar]
- 44.Aldhahi W, Hamdy O. Adipokines, inflammation, and the endothelium in diabetes. Curr Diab Rep. 2003;3:293–8. doi: 10.1007/s11892-003-0020-2. [DOI] [PubMed] [Google Scholar]
- 45.Granata R, Ghigo E. Products of the ghrelin gene, the pancreatic β-cell and the adipocyte. Endocr Rev. 2013;25:144–56. doi: 10.1159/000346306. [DOI] [PubMed] [Google Scholar]
- 46.Charo IF, Ransohoff RM. The many roles of chemokines and chemokine receptors in inflammation. N Engl J Med. 2006;354:610–21. doi: 10.1056/NEJMra052723. [DOI] [PubMed] [Google Scholar]


