Abstract
Objective: To analyze the expression levels of total serum interleukin (IL)-6 and tumor necrosis factor (TNF)-C in children with bronchopneumonia treated by methylprednisolone in combination with azithromycin. Methods: Eighty-three children with bronchopneumonia were randomly divided into a test group (TG) and a control group (CG). The TG was comprised of 40 children treated with methylprednisolone combined with azithromycin, whereas the CG was comprised of 43 patients who received methylprednisolone monotherapy. The post-treatment effective rates and occurrence of adverse reactions were compared between the two groups. In addition, the resolution times of symptoms such as fever, cough, moist rale, asthma, and shadow on the lung X-ray were recorded. The levels of the inflammatory factors tumor necrosis factor-C (TNF-C) and interleukin-6 (IL-6) were measured after treatment. The quality of life was evaluated and compared based on the Medical Outcome Study (MOS) 36-Item Short-Form Health Survey (SF-36). Results: The total effective rate in the TG was significantly higher than that in the CG. The expression levels of TNF-C and IL-6 in the TG were significantly lower than those in the CG. The resolution times of the clinical symptoms were significantly shorter in the TG than in the CG. The ACT (Asthma Control Test) score in the TG was significantly lower than that in the CG. The TG presented with a significantly lower incidence of adverse reactions than that the CG. Conclusion: The combined administration of methylprednisolone and antibiotics can effectively improve the levels of serum inflammatory factors and the clinical symptoms in children with bronchopneumonia.
Keywords: Pediatric bronchopneumonia, methylprednisolone, azithromycin, IL-6, TNF-C
Introduction
Bronchopneumonia is a common infectious disease affecting infants and young children [1]. Mycoplasma pneumonia is the main cause of bronchopneumonia; however, “mixed infections” caused by bacteria and viruses have been reported [2]. Bacterial pneumonia mainly causes pulmonary parenchymal damage, whereas viral pneumonia affects the interstitium [2]. The resulting ventilatory dysfunction is caused by the inflammation of the lung tissue leading to the thickening of the respiratory membrane and obstruction of the lower airway, which manifests as dyspnea, moist rales, fever, cough, wheezing, and lung pain [3].
Methylprednisolone has anti-inflammatory and anti-allergy effects that can inhibit the proliferation of the cells in the connective tissue, reduce the permeability of the capillary wall and cell membrane, lessen inflammatory exudation, and inhibit the formation and release of histamine and other toxic substances [4]. In addition, it can promote the decomposition of protein into sugar and reduce the utilization of glucose [5]. Consequently, both blood glucose and liver glycogen levels are increased, resulting in glycosuria and increased secretion of gastric juices, which leads to an increase in appetite [6]. In cases of severe toxic infection, methylprednisolone is commonly used in combination with a large number of antibacterial drugs, which act as an anti-toxin, anti-inflammatory, and anti-shock agent thereby promoting the symptoms of remission [7].
Azithromycin is a semi-synthetic 15-member macrolide antibiotic [8] used for the following bacterial infections: lower respiratory tract infections such as bronchitis and pneumonia; skin and soft tissue infections; acute otitis media; and upper respiratory tract infections such as sinusitis, pharyngitis, and tonsillitis [9]. Penicillin is commonly used for the treatment of pyogenic streptococcal pharyngitis and is used to prevent rheumatic fever, whereas azithromycin can effectively eliminate oropharyngeal streptococcus; however, there is no data on the efficacy of azithromycin for the treatment and prevention of rheumatic fever. Azithromycin can be used for minor genital infections in sexually transmitted diseases caused by Chlamydia trachomatis. It can also be used for genital infections and chancre caused by non-multidrug-resistant Gonococci sp. and Haemophilus duke (excluding combined infection with Treponema pallidum), respectively [10].
At present, most clinical treatments are based on macrolides, but some children develop refractory mycoplasma pneumonia if they use these drugs in large quantities during the course of treatment [11]. Therefore, in the present study, the efficacy of glucocorticoid methylprednisolone combined with azithromycin on bronchial pneumonia in children was explored.
Materials and methods
General information
Eighty-three children who presented with bronchopneumonia at our hospital from December 2017 to December 2018 were enrolled in the study. The children were randomly divided into two groups: the control group (CG) and test group (TG). The CG was comprised of 43 children, including 29 males and 14 females aged 6 months to 3 years (average age, 17.0±2.4 months); the average length of course of the disease was 11.8±2.1 weeks (range, 1-18 weeks). The TG was comprised of 40 children, including 27 males and 13 females aged 7 months to 3 years (average age, 17.3±2.6 months), and the average length of the course of the disease was 11.6±2.1 weeks (range, 1-9 weeks). Although comparable, no significant difference was observed in the general information between the two groups (Table 1).
Table 1.
General information
| Factors | The TG (n = 40) | The CG (n = 43) | F/χ2 | P |
|---|---|---|---|---|
| Gender | 3.192 | 0.9955 | ||
| Male | 27 (67.50%) | 29 (67.44%) | ||
| Female | 13 (32.50%) | 14 (32.56%) | ||
| Age | 17.3±2.6 | 17.0±2.4 | 0.5467 | 0.5861 |
| Course of disease | 11.6±2.1 | 11.8±2.1 | 0.3868 | 0.6999 |
| WBC (×109/L) | 17.68±2.45 | 16.37±2.97 | 1.844 | 0.068 |
| CRP (mg/L) | 29.38±5.62 | 28.46±6.03 | 0.863 | 0.390 |
| Pulmonary auscultation | ||||
| Rale | 17 (42.5%) | 20 (46.51%) | 1.550 | 0.213 |
| Tubular breath sound | 23 (57.5%) | 23 (53.49%) | ||
| Fever | ||||
| Yes | 22 (55.0%) | 23 (53.49%) | 0.018 | 0.894 |
| No | 18 (45.0%) | 20 (46.51%) | ||
| Cough | ||||
| Yes | 24 (60.0%) | 27 (62.79%) | 0.417 | 0.520 |
| No | 16 (40.0%) | 16 (37.21%) | ||
| Asthma | ||||
| Yes | 21 (52.5%) | 23 (53.49%) | 0.0001 | 0.976 |
| No | 19 (47.5%) | 20 (46.51%) |
WBC: white blood cell count; CRP: C-reactive protein.
Exclusion and inclusion criteria
The inclusion criteria for the study were as follows: all children met the diagnostic criteria related to bronchial pneumonia in children in Practical Pediatrics [12], they were ≤3 years old, and there was full patient compliance in the study, as well as the family members of the children signing the informed consent. The exclusion criteria included the presence of autoimmune diseases and other organ abnormalities and children with congenital pulmonary tracheal dysplasia or allergies to the drugs used in this study. This study was approved by The First Affiliated Hospital of Zhejiang Chinese Medical University.
Treatment
All the children enrolled in this study underwent conventional symptomatic treatment, including oxygen inhalation, timely administration of antipyretics, and bronchiectasis, according to the specific conditions. In the CG, methylprednisolone (1.0 mg/kg/day) was administered intravenously (Pfizer; 40 mg powder for each injection), once every 8 h. If the symptoms were alleviated after 3 days, the same dose was administered orally (Pfizer, 4 mg per tablet). After 5 days of the oral dose, the drug was stopped if the symptoms were relieved. The condition of the patient was closely monitored during the course of the treatment. If any worsening of the condition was observed, the oral dose was continued and subsequently increased, if required. Intravenous infusion was provided in severe cases. The children in the TG were given an intravenous infusion of azithromycin (10.0 mg/kg/day; 500 mg powder per injection) once daily. The dosage was changed to 5.0 mg/kg/day (250 mg per tablet) for 4 weeks after the symptoms were alleviated.
Observation indicators
The effectiveness of the clinical treatment was compared between the two groups. The evaluation criteria of the therapeutic effects were as follows: Markedly effective, wherein all the clinical signs and symptoms disappeared; Effective, where the clinical symptoms disappeared and the clinical signs showed significant improvement after treatment; and Ineffective, where the post-treatment indicators of the children were not different from those before treatment and the clinical symptoms and signs were not improved or aggravated. The resolution times of fever, cough, moist rale, asthma, pulmonary X-ray shadow, and other symptoms in the two groups were recorded, and the efficacy was evaluated by referring to previously published criteria [13]. The levels of the serum inflammatory factors TNF-C and IL-6 were measured after treatment. The quality of life of the children in the two groups were compared 3 months after treatment based on the evaluation of the SF-36 scale; the higher the score, the better the quality of life. The factors examined were physiological functions (PF), body pain (BP), general health (GH), vitality (VT), social functioning (SF), role-emotional (RE), and mental health (MH).
Correlation analysis
The patients were divided into good and poor groups according to the prognosis results, and the serum expression levels of IL-6 and TNF-C in the two groups were retested and analyzed by Pearson’s correlation coefficient.
Statistical analysis
Data were analyzed using SPSS version 20.0 (Shanghai Yuchuang Network Technology Co., Ltd.) and expressed as mean ± standard deviation. The counting data were compared using the χ2 test. GraphPad Prism 6 (Hangzhou Emerald Biotech Co., Ltd.) was used to plot the experimental figures. P<0.05 indicated that the difference was statistically significant.
Results
Treatment efficiency
The total effective rate in the TG was 80%, which was significantly higher (P<0.05) than that in the CG (58.14%; Table 2).
Table 2.
Comparison of effective rates between the two groups [n (%)]
| Groups | Number of cases | Markedly effective | Effective | Ineffective | Total effective rate |
|---|---|---|---|---|---|
| The CG | 43 | 10 (23.26) | 15 (34.88) | 18 (41.86) | 25 (58.14) |
| The TG | 40 | 19 (47.50) | 13 (32.50) | 8 (20.00) | 32 (80.00) |
| χ2 | 1.726 | 0.005 | 4.594 | 4.603 | |
| P | 0.189 | 0.944 | 0.032 | <0.05 |
TNF-C and IL-6 expression levels
No significant differences in the serum levels of TNF-C and IL-6 were observed between the two groups before treatment. On the other hand, the expression levels of the cytokines dropped significantly (P<0.05), particularly in the TG when compared with the CG (Figure 1).
Figure 1.

Comparison of TNF-C and IL-6 levels between the two groups before and after treatment. A: Comparison of TNF-C levels between the two groups before and after treatment. B: Comparison of IL-6 levels between the two groups before and after treatment. *P<0.05.
Resolution of symptoms
The resolution times of the symptoms represented by fever, cough, asthma, a shadow on lung X-ray, and moist rale, and the oxygen inhalation and hospitalization times in the TG were significantly shorter than those in the CG (P<0.01). No significant difference in ACT score was observed between the two groups before treatment, whereas after 4 weeks of treatment, the ACT score in the TG was significantly lower than that in the CG (P<0.05; Tables 3 and 4).
Table 3.
Comparison of symptoms resolution time (number) between the two groups of children with bronchopneumonia (x̅±SD)
| Groups | Number of cases | Fever (number) | Cough (number) | Asthma (number) | Pulmonary X-ray shadow (number) | Pulmonary rale (number) |
|---|---|---|---|---|---|---|
| The TG | 40 | 2.1±0.5 | 4.2±0.3 | 3.7±0.6 | 5.7±0.4 | 4.12±1.23 |
| The CG | 43 | 2.6±0.4 | 5.5±0.3 | 5.2±0.5 | 6.1±0.5 | 6.87±1.69 |
| t | 6.068 | 17.12 | 14.91 | 4.818 | 10.13 | |
| P | <0.001 | <0.001 | <0.001 | <0.001 | <0.05 |
Table 4.
Comparison of duration of oxygen inhalation, hospitalization status, and ACT score between the two groups
| Groups | n | Oxygen inhalation time (h) | Length of hospitalization (d) | ACT score | ||
|---|---|---|---|---|---|---|
|
| ||||||
| Before treatment | 1 week after treatment | 4 weeks after treatment | ||||
| The TG | 40 | 31.57±10.02 | 14.66±2.42 | 4.89±2.6 | 1.65±0.5 | 1.32±0.5 |
| The CG | 43 | 37.61±9.16 | 16.05±2.74 | 4.76±2.4 | 1.70±0.5 | 1.66±0.7 |
| T | - | 2.455 | 2.102 | - | - | 2.168 |
| P | - | 0.017 | 0.042 | 0.842 | 0.703 | 0.034 |
Comparison of adverse reactions between the two groups
According to statistics, the total incidence of adverse reactions in the TG was significantly lower than that in the CG (P<0.05; Table 5).
Table 5.
Comparison of adverse reactions after treatment between the two groups [n (%)]
| Groups | Number of cases | Vomiting | Abdominal pain | Rash | Diarrhea | Nausea | Total |
|---|---|---|---|---|---|---|---|
| The TG | 40 | 1 (1.71) | 1 (1.71) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 2 (3.58) |
| The CG | 43 | 2 (3.23) | 2 (3.23) | 1 (1.61) | 1 (1.62) | 2 (3.23) | 9 (14.52) |
| χ2 | 0.277 | 0.277 | 0.943 | 0.943 | 1.903 | 4.409 | |
| P | 0.599 | 0.599 | 0.331 | 0.331 | 0.168 | 0.037 |
Comparison of quality of life between the two groups after 3 months of treatment
PF, BP, GH, and other dimensions concerning the patients’ quality of life after 3 months of treatment were compared between the TG and CG. The PF, BP, GH, and VT scores in the TG were significantly higher (P<0.05) than those in the CG. Alternately, no significant differences in SF, RE, and MH were noted between the two groups (Table 6).
Table 6.
Comparison of quality of life between the two groups 3 months after treatment (x±s)
| Categories | The TG (n = 40) | The CG (n = 43) | t | p |
|---|---|---|---|---|
| PF | 81.36±10.96 | 69.29±11.25 | 5.947 | <0.001 |
| BP | 88.05±10.49 | 69.23±12.54 | 8.885 | <0.001 |
| GH | 83.66±9.81 | 71.07±12.14 | 6.223 | <0.001 |
| VT | 83.14±10.26 | 73.79±13.79 | 4.191 | <0.001 |
| SF | 63.21±10.67 | 63.54±11.37 | 0.164 | 0.8703 |
| RE | 72.63±12.09 | 69.98±12.04 | 0.232 | 1.202 |
| MH | 83.62±10.48 | 81.18±10.46 | 1.276 | 0.2045 |
Correlation analysis
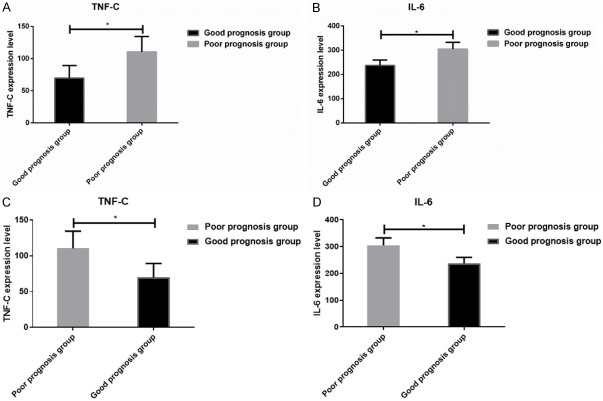
The results showed that IL-6 level was negatively correlated (r = 0.6947; P<0.05) with TNF-C level between the good and poor prognosis groups (Figures 2 and 3).
Figure 2.

Correlation analysis of IL-6 and TNF-C expression. IL-6 was negatively correlated with TNF-C level (r = 0.6947; P<0.05).
Figure 3.
Comparison of TNF-C and IL-6 levels between the good prognosis group and the poor prognosis group. *P<0.05, the poor prognosis group compare with the good prognosis group.
Discussion
Pediatric bronchopneumonia mainly occurs secondary to lower respiratory tract infections. The conventional methods used to treat this condition include drug therapy and oxygen inhalation. If the disease is serious or at the terminal stage, it is often necessary to perform lung transplantation, lobectomy, or other treatments [14]. Therefore, early diagnosis and active treatment are of great importance to prevent the progression of the disease. Drug therapy often includes the use of glucocorticoids, bronchodilators, and antibacterial drugs, alone or in combination. The commonly used corticosteroids are beclomethasone dipropionate, prednisone, and methylprednisolone [15]. Although the inhalation of hormone drugs can target the airway and reduce the adverse reactions of systemic administration, the aerosol may not reach the bronchioles due to respiratory obstruction and is, therefore, not an effective method for patients with bronchopneumonia [16]. Hence, intravenous therapy and oral administration were simultaneously adopted in the present study. Wheezing was gradually improved in the children in both groups after treatment with methylprednisolone, and the levels of the serum inflammatory factors IL-6 and TNF-C were significantly decreased, indicating the effectiveness of the drug. Moreover, the incidence of adverse reactions was low in this study, and no serious adverse reactions occurred. However, considering the side effects of systemic medication on growth and development in children, it has been reported that long-term administration of methylprednisolone once every 30 days with 3 days of intravenous injection (10-30 mg/kg/day) can be used for patients undergoing organ transplantation or in those with severe pediatric bronchopneumonia [17].
The main pathogens involved in bronchopneumonia are Diplococcus pneumoniae. Azithromycin is often used as the representative of the 14- and 15-member macrolides against Haemophilus influenzae [18]. Azithromycin has both antibacterial anti-inflammatory properties [19]. As can be seen from the results of the present study, the combined use of methylprednisolone and azithromycin significantly shortened the resolution times of the clinical symptoms, such as fever, asthma, and cough, compared with the use of methylprednisolone alone. In addition, the levels of IL-6 and TNF-C in the TG group were significantly better than those in the CG after treatment; likewise, the occurrence of adverse reactions was lower in the TG. The changes in the trends of the two indicators were mutually verified. The possible causes for the occurrence of this phenomenon might be as follows: on the one hand, pediatric bronchopneumonia is generally found late because of the difficulties in diagnosis, and irreversible fibrosis is generated in the tissue during the pathogenesis before treatment [20]; thus, the effect of drugs on airway obstruction is generally limited. On the other hand, the relapse of illness, especially in children, is closely related to repeated bacterial infection. Continuous administration of antibacterial drugs can prevent this situation to a certain extent, making the patient’s condition more stable. These findings illustrate the need for long-term use of antibacterial drugs in children with bronchopneumonia [21].
The study also has limitation that the effect of drugs on airway obstruction is generally limited, and the long term prognosis of these children was not investigated. In future research, long term follow up and a larger sample size will be implemented.
In summary, the combination of prednisone and antibacterial drugs can quickly improve the serum inflammatory factors and clinical symptoms in children with bronchopneumonia. Although the current study failed to examine the long-term efficacy and adverse reactions in these patients, the results demonstrated that the combined administration of the two drugs is significantly more effective than monotherapy. Additional studies are required to confirm this finding.
Acknowledgements
This work was supported by the Special Project of Science Popularization of Hebei Science and Technology Department (20557706K).
Disclosure of conflict of interest
None.
References
- 1.Dhanapal C, Mathew J, Manavalan SMR. A hospital based study on some clinico-epidemiological aspect of bronchopneumonia among infants and young children. Editorial Advisory Board. 2009;20:85. [Google Scholar]
- 2.Zhang X, Ji W, Ji Z, Ding Y, Zhu H, Yan Y, Huang Y, He Y, Ye J, Ji X. Epidemiological study on respiratory syncytial virus and its bronchopneumonia among children in Suzhou. Zhonghua Yu Fang Yi Xue Za Zhi. 2007;41:371–374. [PubMed] [Google Scholar]
- 3.Chang CC, Cheng AC, Chang AB. Over-the-counter (OTC) medications to reduce cough as an adjunct to antibiotics for acute pneumonia in children and adults. Cochrane Database Syst Rev. 2014:CD006088. doi: 10.1002/14651858.CD006088.pub2. [DOI] [PubMed] [Google Scholar]
- 4.Feinberg SM, Feinberg AR, Pruzansky J, Fisherman EW. Methylprednisolone (medrol), a potent new anti-inflammatory steroid: therapeutic results in allergic diseases. J Am Med Assoc. 1957;165:1560–1562. doi: 10.1001/jama.1957.72980300006009b. [DOI] [PubMed] [Google Scholar]
- 5.Can M, Gul S, Bektas S, Hanci V, Acikgoz S. Effects of dexmedetomidine or methylprednisolone on inflammatory responses in spinal cord injury. Acta Anaesthesiol Scand. 2009;53:1068–1072. doi: 10.1111/j.1399-6576.2009.02019.x. [DOI] [PubMed] [Google Scholar]
- 6.Jin JY, Jusko WJ. Pharmacodynamics of glucose regulation by methylprednisolone. II. Normal rats. Biopharm Drug Dispos. 2009;30:35–48. doi: 10.1002/bdd.642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Higa F, Saito A, Inadome J, Kusano N, Kitsukawa K. Influence of methylprednisolone on the intracellular antimicrobial activity of erythromycin and clindamycin against legionella pneumophila. J Antimicrob Chemother. 1993;31:901–908. doi: 10.1093/jac/31.6.901. [DOI] [PubMed] [Google Scholar]
- 8.Luke DR, Foulds G, Cohen SF, Levy B. Safety, toleration, and pharmacokinetics of intravenous azithromycin. Antimicrob Agents Chemother. 1996;40:2577–2581. doi: 10.1128/aac.40.11.2577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Girard D, Finegan S, Dunne M, Lame M. Enhanced efficacy of single-dose versus multi-dose azithromycin regimens in preclinical infection models. J Antimicrob Chemother. 2005;56:365–371. doi: 10.1093/jac/dki241. [DOI] [PubMed] [Google Scholar]
- 10.Lau CY, Qureshi AK. Azithromycin versus doxycycline for genital chlamydial infections: a meta-analysis of randomized clinical trials. Sex Transm Dis. 2002;29:497–502. doi: 10.1097/00007435-200209000-00001. [DOI] [PubMed] [Google Scholar]
- 11.Bao F, Qu J, Liu Z, Qin X, Cao B. The clinical characteristics, treatment and outcome of macrolide-resistant mycoplasma pneumoniae pneumonia in children. Zhonghua Jie He He Hu Xi Za Zhi. 2013;36:756–761. [PubMed] [Google Scholar]
- 12.Baublis J. Practical pediatrics. AMA J Dis Child. 1959;98:812–812. [Google Scholar]
- 13.Che XY. Effect of aerosol inhalation of ipratropium bromide combined with budesonide and terbutaline on cytokines in children with bronchopneumonia. Journal of Hainan Medical University. 2016;22:65–68. [Google Scholar]
- 14.Yu J. Postinfectious bronchiolitis obliterans in children: lessons from bronchiolitis obliterans after lung transplantation and hematopoietic stem cell transplantation. Korean J Pediatr. 2015;58:459. doi: 10.3345/kjp.2015.58.12.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Van Bever HP. Corticosteroids for bronchiolitis. Lancet. 1996;348:753–754. doi: 10.1016/s0140-6736(05)65640-1. [DOI] [PubMed] [Google Scholar]
- 16.Morawska L, Johnson G, Bell S. Could BFFB mode breath aerosol play a role in H5N1 transmission? 2012;7:2–9. [Google Scholar]
- 17.Keown P, Shackleton C, Ferguson B. The influence of long-term morbidity on health status and rehabilitation following paediatric organ transplantation. Eur J Pediatr. 1992;151(Suppl 1):S70–S75. doi: 10.1007/BF02125807. [DOI] [PubMed] [Google Scholar]
- 18.Belousov D, Cheberda A. Pharmacoeconomic analysis of naftidrofuryl in patients with chronic obliterating diseases of lower limb arteries. Value Health. 2016;19:A44. [Google Scholar]
- 19.Fusui Z. Clinical analysis of azithromycin and prednisone on mycoplasma pneumonia. 2012:1810–1811. [Google Scholar]
- 20.Peyrol S, Cordier J, Grimaud J. Intra-alveolar fibrosis of idiopathic bronchiolitis obliterans-organizing pneumonia. Cell-matrix patterns. Am J Pathol. 1990;137:155–170. [PMC free article] [PubMed] [Google Scholar]
- 21.Yamamoto M, Kondo A, Tamura M, Izumi T, Ina Y, Noda M. Long-term therapeutic effects of erythromycin and newquinolone antibacterial agents on diffuse panbronchiolitis. Nihon Kyobu Shikkan Gakkai Zasshi. 1990;28:1305–1313. [PubMed] [Google Scholar]