Abstract
Background: Previous studies assessing the impact of omega-3 polyunsaturated fatty acids (ω-3 PUFA) have shown conflicting results in regard to the cardiovascular mortality. It is likely that higher dose of ω-3 PUFA would have a greater effect on the major adverse cardiovascular events (MACEs). Therefore, we performed a dose-response meta-analysis to explore the potential protective effect of ω-3 PUFA, with the increase of daily intake and extension of the intervention period, on patients with cardiovascular disease risks. Outcomes included major adverse cardiovascular events, cardiovascular and all-cause mortality. Methods: A systematic literature search of PubMed, Embase and the Cochrane Library from inception to September 31, 2019 was conducted to identify the randomized controlled trails (RCTs) of ω-3 PUFA supplementation, which reported cardiovascular events or deaths and recruited no less than 500 participants. We evaluated the effect of ω-3 PUFA through the pooled relative risks (RR) and 95% confidence intervals (95% CI), and further carried out subgroup analysis and dose-response meta-analysis. Results: Fourteen trials including 87718 individuals were reviewed. By conventional statistical significance, there was no apparent difference between the two groups on major adverse cardiovascular effects (RR 0.94, 95% CI 0.84-1.04) and all-cause mortality (RR 0.96, 95% CI 0.91-1.00), but there was an effect on the cardiovascular mortality (RR 0.93, 95% CI 0.88-0.99). However, with the dose increased and intervention period prolonged (daily dose × intervention period > 8 grams/day × years), subgroup analyses showed a more obvious reduction of MACEs (RR 0.79, 95% CI 0.65-0.95) and all-cause mortality (RR 0.93, 95% CI 0.85-1.03). Furthermore, the dose-response meta-analysis presented a 13.05% reduction of MACEs and 8.99% reduction of all-cause mortality with 10 grams/day × years increments. Conclusions: Updated with the newly published RCTs, this meta-analysis indicated that large dose and long period of interventions with ω-3 PUFA supplementation produce a close association with MACEs and cardiovascular or all-cause mortality. A dose-response beneficial effect was preliminarily established.
Keywords: Omega-3, polyunsaturated fatty acids, cardiovascular events, mortality, dose-response, meta-analysis
Introduction
Therapeutic value of the fish oil, which is rich in omega-3 polyunsaturated fatty acids (ω-3 PUFA), has been paid attention for many years. It is recommended that the daily diet should include a higher proportion of marine-sourced ω-3 PUFA, eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), to postpone cardiovascular diseases [1]. Potential mechanisms for the benefits have been discussed, including reducing platelet adherence, enhancing endothelium-dependent vasodilatation [2,3], lowing cholesterol [4] and reducing inflammation [5-7].
However, randomized controlled trials have revealed contradictory findings with both positive [8,9] and negative findings [10,11]. A recently published trail by Bhatt, which involved 8179 participants and a median of 4.9 years follow-up, reported that patients who received 4 g/d ω-3 PUFA experienced a 30% reduction in total primary endpoints events compared with the control group [12]. JELIS study, which is an open-label trail conducted in Japan, recruited 18,645 patients with a total cholesterol of 6.5 mmol/L or greater and demonstrated a 19% relative reduction of major coronary events after a 1800 mg of EPA daily on the basis of statin therapy [9]. On the contrary, the ORIGIN trial argued that there was no significant difference between the two groups on cardiovascular death (RR 0.98; 95% CI 0.87-1.10) or major cardiovascular events (RR 1.01; 95% CI 0.93-1.10) [10]. 1 g/d for 1-year ω-3 PUFA supplementation was detected to show no benefit on sudden cardiac death (1.5% in omega group and 1.5% in control group) but a small increase in the major cardiovascular and cerebrovascular events (10.4% in omega group and 8.8% in control group). These inconsistencies are reflected in recent meta-analyses [13,14]. By looking into the results reported, we propose that larger doses of ω-3 PUFA intake and a long period of intervention tend to benefit the participants more from suffering major cardiovascular events and deaths.
Considering the inconsistency of the prior studies and the tendency of a more obvious cardiovascular protective effect of a large dose of ω-3 PUFA and longer intervention, we performed an updated meta-analysis and subsequently further detected the dose-response relationship along with daily dose increase and treatment duration extension.
Methods
Literature search and study selection
We conducted comprehensive literature searches using PubMed, Embase and the Cochrane Library which covered studies from inception to September 31, 2019. Bibliographies of reviews and meta-analyses on this topic were browsed to supplement the electronic search. The searching and reporting process followed the Preferred Reporting Items for Systematic Reviews and Meta-analyses guidelines [15]. The search was designed to identify clinical trials exploring ω-3 PUFA supplementation and cardiovascular outcomes. The detailed search strategy is described in Table 1.
Table 1.
Search strategy
| No. | Search query |
|---|---|
| 1. | (omega 3 fatty acids OR n-3 fatty acids OR n-3 polyunsaturated fatty acid OR n-3 PUFA OR n-3 oils OR omega-3 FA) |
| 2. | (eicosapentaenoic acid OR EPA OR docosahaexaenoic acids OR DHA) |
| 3. | (marine OR fish oil OR fatty fish OR fish) |
| 4. | #1 OR #2 OR #3 |
| 5. | (cardiovascular events OR cardiovascular disease OR coronary heart disease OR acute myocardial infarction OR unstable angina OR revascularization OR MACE OR stroke) |
| 6. | (mortality OR death) |
| 7. | #5 OR #6 |
| 8. | #4 AND #7 |
Trials to be included should meet the following criteria: (1) randomized controlled trials conducted in humans which recruited vascular outcomes. The detailed search stratthe recruited patients were males or females aged ed olled trials conducted in humans which recruiteach study reported at least one of the following outcomes: major adverse cardiovascular events (including nonfatal myocardial infarction, coronary heart disease death, coronary revascularization, unstable angina), cardiovascular or all-cause mortality. (4) each study reported the daily dose of ω-3 PUFA and the length of the intervention period. (5) studies further included into the dose-response meta-analysis were required to report the RRs and 95% CIs in principle. The study selection process is illustrated in Figure 1. Two investigators independently evaluated the eligibility of the retrieved trials through screening titles/abstracts and further full-text reading. Discrepancies were resolved by the discussion or consultation with a third reviewer.
Figure 1.
Risk of bias of included trails summary.
Data extraction and quality evaluation
The reported results were extracted from each selected article using a pre-designed data form. The following information was extracted: the last name of the first author, publication year, country or region where the trial was conducted, number of the participants, age and sex distribution of the population, the daily dose of EPA and DHA supplementation, the intervention length, cardiovascular outcomes and enumeration data of experiment group and control group (RR and 95% CI were also recorded).
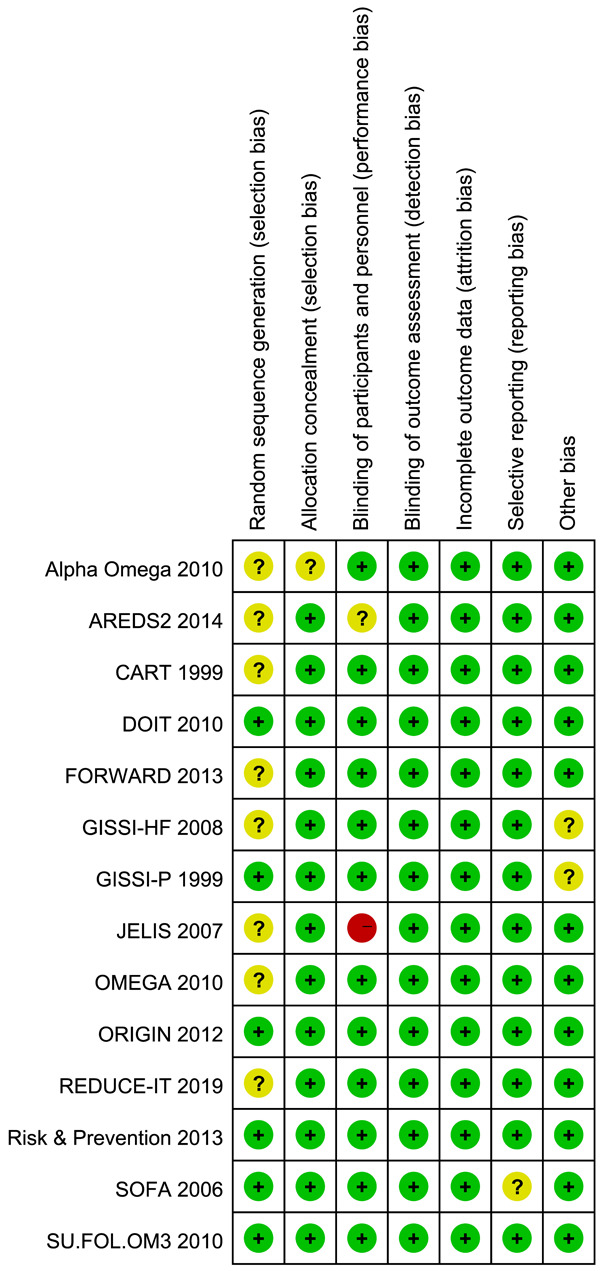
The quality of the included studies was evaluated by the Cochrane Collaboration Risk of Bias Tool which assessed the trials from six aspects. Each aspect was ranked “high risk”, “unclear risk” or “low risk”. The overall rating chart summarizing the quality is shown in Figure 1 (Risk of bias summary) and Figure 2 (Risk of bias graph).
Figure 2.
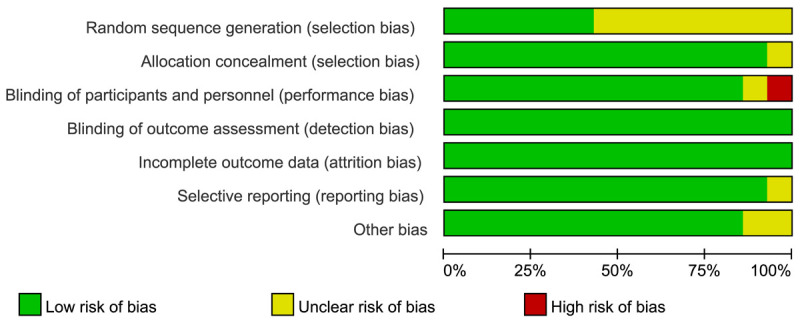
Risk of bias of included trails graph.
Statistical analysis
The number of the individuals with an event and total participants of each group was collected to calculate the pooled RRs with 95% CIs using fixed or random effects model. We assessed publication bias by inspecting funnel plots and measuring through Begg’s or Egger’s test [16]. Heterogeneity among studies was evaluated using the Cochran’s Q test and quantified using the I2 static. The I2 statistic was considered to reflect low likelihood (0-25%), moderate likelihood (26-75%), and high likelihood (76-100%) of differences. In our meta-analysis, we consider an I2 value more than 50% notable, as was a P value of less than or equal to 0.1 for heterogeneity. The sensitivity analyses were done by excluding individual studies. We also performed subgroup analysis to detect the heterogeneity and the effect of dose on MACEs. These data analyses were performed using Review Manager 5.3 and the dose-response relationship was detected using Stata SE 12.0.
Results
Basic characteristics of included trials
Figure 3 shows the selection process of the literature search. We finally included 14 studies ranging in size from 500 to 18645 participants and the main characters of the included 14 trials are listed in Table 2 [9-12,17-26]. The 14 trails comprising a total number of 87718 individuals randomly assigned 43918 people to the treatment group and 43800 to the control group. Male took up a larger proportion of each study except for one trial carried out in Japan, which occupied 68.6% of the total participants [9]. The mean age of the participants was 63 years and the follow-up period varied from 0.5 to 6.2 years.
Figure 3.
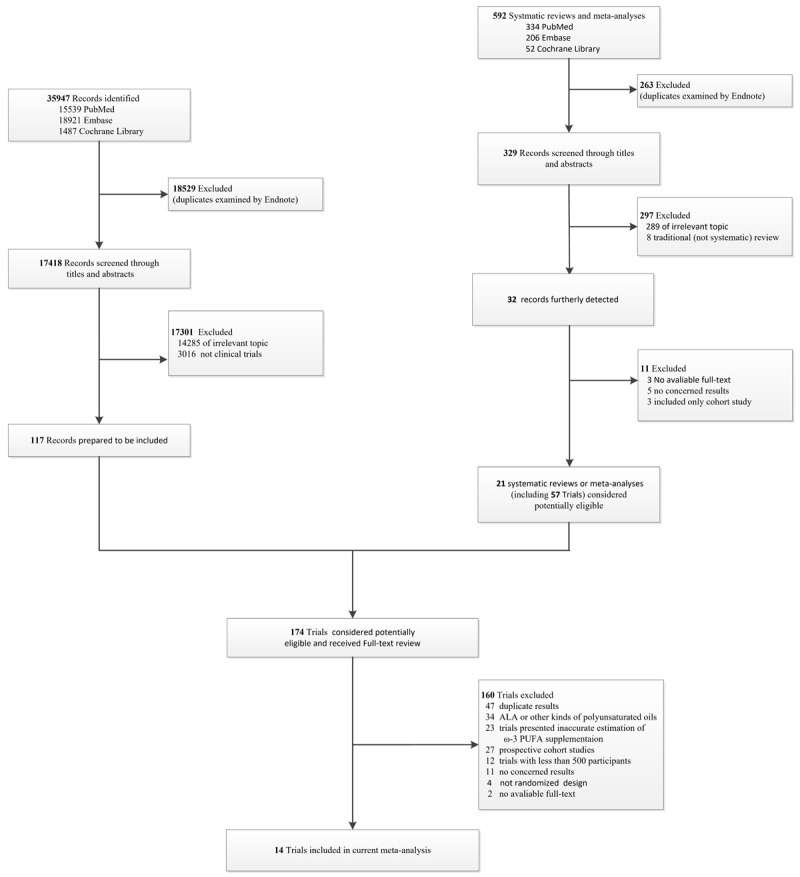
Flow chart of literature search and study selection. PUFA = polyunsaturated fatty acid; ALA = α-Linolenic acid.
Table 2.
The basic characteristics of the included studies
| Study Name | Author | Publication year | Country | Sample Size | Mean Age (SD) | Male, No (%) | Intervention length, y | Intervention Type | Dose of EPA/DHA (mg/d) | Control |
|---|---|---|---|---|---|---|---|---|---|---|
| DOIT | Einvik et al | 2010 | Norway | 563 | 70 (3) | 563 (100) | 3 | Fish oil | 1150/800 | Corn oil Background formula |
| AREDS-2 | Bonds et al | 2014 | United States | 4203 | 74 (NA) | 1816 (43.2) | 4.5 | NA | 650/350 | with partial Lutein+zeaxanthin* |
| SU.FOL..OM3 | Galan et al | 2010 | France | 2501 | 61 (NA) | 1987 (79.4) | 4.7 | Fish oil | 400/200 | Gelatin |
| JELIS | Yokoyama et al | 2007 | Japan | 18645 | 61 (8) | 5859 (31.4) | 4.6 | Ethyl esters | 1800/NA | No supplement |
| EPA+DHA | ||||||||||
| Alpha Omega | Kromhout et al | 2010 | The Netherlands | 4837 | 69 (6) | 3783 (78.2) | 3.3 | enriched | 226/150 | Oleic-acid margarine |
| Margarine | ||||||||||
| OMEGA | Rauch et al | 2010 | Germany | 3818 | 64 (NA) | 2841 (74.4) | 1 | Ethyl esters | 460/380 | Olive oil |
| Risk & Prevention | Roncaglioni et al | 2013 | Italy | 12505 | 64 (NA) | 7687 (61.5) | 5 | Ethyl esters | 500/500 | Olive oil |
| GISSI-HF | Tavazzi et al | 2008 | Italy | 6975 | 67 (11) | 5459 (78.3) | 3.9 | Ethyl esters | 850/950 | No supplement |
| ORIGIN | Bosch et al | 2012 | 40 countries | 12536 | 64 (8) | 8150(65.0) | 6.2 | Ethyl esters | 465/375 | Olive oil |
| GISSI-P | Valagussa et al | 1999 | Italy | 11334 | 59 (11) | 9658 (85.2) | 3.5 | Ethyl esters | 850/1700 | No supplement |
| REDUCE-IT | Bhatt et al | 2019 | United States | 8179 | 64 (NA) | 5822 (71.2) | 4.9 | Icosapent ethyl | 4000/0 | NA |
| SOFA | Brouwer et al | 2006 | 8 countries | 546 | 61 (NA) | 459 (84.1) | 1 | Fish oil | 464/335 | high-oleic-acid sunflower oil |
| FORWARD | Macchia et al | 2013 | Argentina | 586 | 66(11.3) | 321(54.8) | 1 | Ethyl esters | 866 (850-882) | Olive oil |
| CART | Johansen et al | 1999 | Norway | 500 | 60 (NA) | 390 (78) | 0.5 | Ethyl esters | 2790/2250 | corn oil |
NA: not available;
It is 2 × 2 factorial-designed RCT supplemented ω-3 PUFA (350-mg DHA + 650-mg EPA), macular xanthophylls (10-mg lutein + 2-mg zeaxanthin) with background therapy of ascorbic acid (500 mg), vitamin E (dl-alpha tocopherol acetate, 400 IU), beta carotene (15 mg), and zinc (80-mg zinc oxide) with copper (2-mg cupric oxide);
Experiment group received 1 g n-3 PUFA (provided by SPA and Sigma-Tau, Italy), which provide 850 to 882 mg eicosapentaenoic acid/docosahexaenoic acid ethyl esters.
Effect of ω-3 PUFA supplementation on MACEs
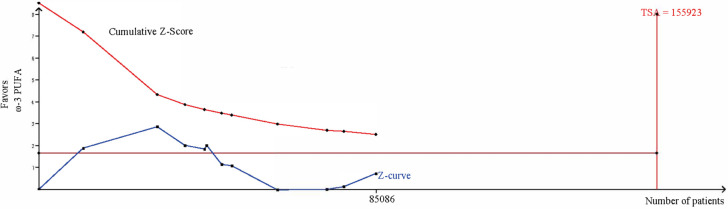
In terms of the primary composite cardiovascular outcome, the overall pooled results of the included 11 trials that reported MACEs showed nonsignificant benefit of ω-3 PUFA supplementation (RR, 0.94; 95% CI, 0.84-1.04; Random effects) (Figure 4). Notable heterogeneity, beforehand, has been detected (I2 = 91%, P < 0.00001). No apparent publication bias was observed except one study through visual examination of funnel plots (Figure 5). We conducted sensitivity analysis by excluding individual study and found that REDUCE-IT trail accounted for the majority of the heterogeneity, which turned moderate (I2 = 32%, P = 0.15) after the removal of that trial. And they remained statistically non-significant (RR, 0.97; 95% CI, 0.92-1.02) by pooling the remaining 10 trials. Considering the negative results, we carried out a trail sequence analysis, which revealed that current studies were not sufficient to draw the negative conclusion (Figure 6).
Figure 4.
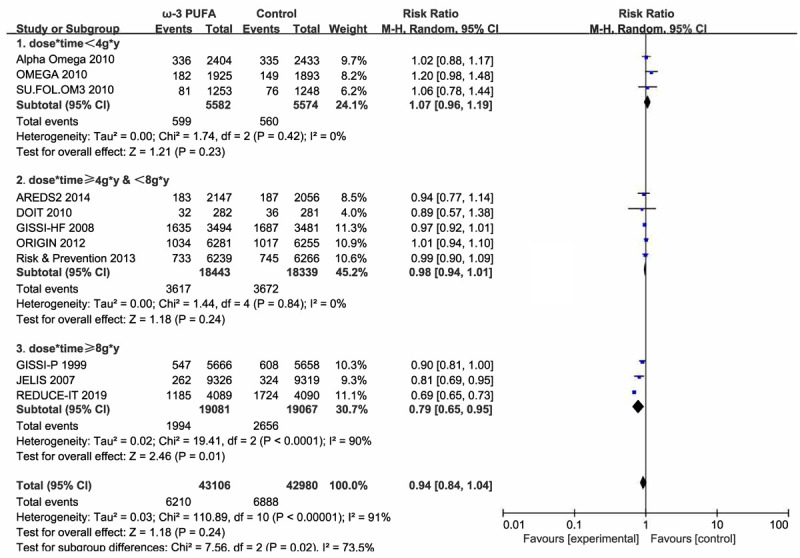
Forest plot of comparison: omega-3 PUFA vs placebo on MACEs. Stratified by dose*time.
Figure 5.
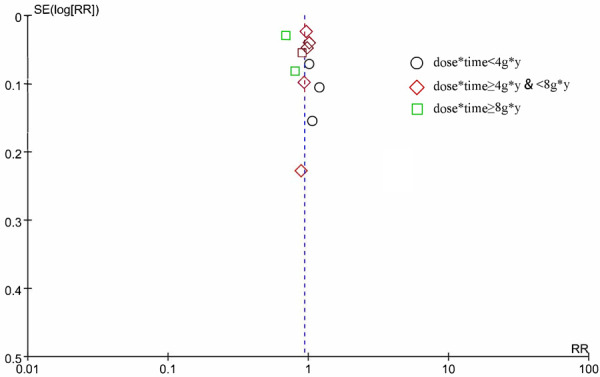
Funnel plot of 11 trials reported MACEs. Stratified by dose*time.
Figure 6.
Trail sequencing analysis of the included studies reporting MACEs.
It appears that trials with larger doses of ω-3 PUFA tend to show more significant benefits [12,20]. We conducted a subgroup analysis stratified by daily dose of ω-3 PUFA supplementation but no statistical significance was found (dose < 1 g/d: RR 1.03 (0.97-1.10); ≥1 g/d and < 2 g/d: RR 0.95 (0.88-1.01); ≥2 g*y: RR 0.80 (0.63 1.01)). However, detecting the benefit effect of longer intervention [9,12] with an intensive dose (dose*time ≥d g/d*y) revealed a distinct benefit (RR, 0.79; 95% CI, 0.65-0.95; Figure 2) while conservative therapy (dose*time < 4 g/d*y) had no benefit on MACEs (RR, 1.07; 95% CI, 0.96-1.19). We then performed a dose-response meta-analysis, and non-liner dose-response model was applied (Testparm dose2, Goodness-of-fit chi2 = 7.01, Prob > chi2 = 0.0081). Dose-response analysis revealed a 1.39% reduction of MACEs with 1 g/d*y increment of dose*time and a 13.05% reduction of 10 g/d*y increments. The dose-response plotting is shown in Figure 7.
Figure 7.
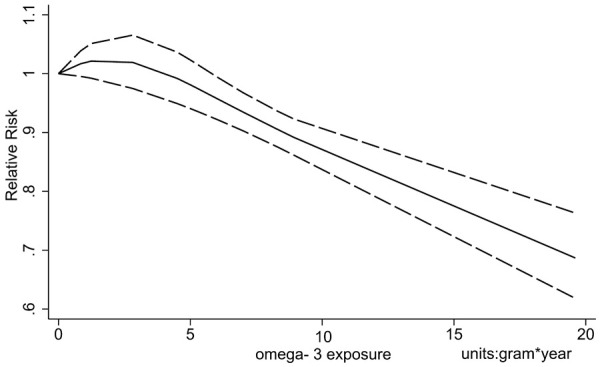
Dose-response relationship of MACEs with increment of dose*time.
Effect of ω-3 PUFA supplementation on cardiovascular and all-cause mortality
Eight trials with a total of 49872 individuals reported the incidence of cardiovascular death, with pooled results (RR 0.93, 95% CI 0.88-0.99; Fixed effects; Figure 8) indicating significant difference between the ω-3 PUFA group and the control group. Heterogeneity detection (I2 = 0) indicated that trials were consistent with this outcome.
Figure 8.
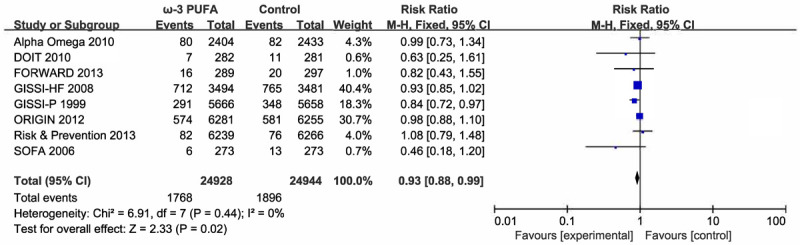
Forest plot of comparison: omega-3 PUFA vs placebo on cardiovascular mortality.
Eleven studies with a total of 62831 participants provided all-cause mortality results, which demonstrated a non-inferior effect (RR 0.96, 95% CI 0.91-1.00). Fixed-effect model was applied due to tolerable heterogeneity (I2 = 25%). Subgroup analysis was also conducted to further explore the effect and manifested a more obvious protective impact (dose*time < 4 g/d*y: RR 1.03; ≥4 g/d*y and < 8 g/d*y: RR 0.96; > 8 g/d*y: RR 0.93; Figure 9). Dose-response meta-analysis was also performed, and liner relationship was suitable for the analysis (Testparm dose2, Goodness-of-fit chi2 = 0.47, Prob > chi2 = 0.4926). Notably, of the 11 included trials reporting the all-cause mortality, 4 trials did not report the RR and 95% CI, which were then estimated through the number of the events of the experiment and control group [19,21,23,26]. The dose-response relationship demonstrated an 8.99% reduction of all-cause mortality with 10 g/d*y increment of dose*time (Figure 10).
Figure 9.
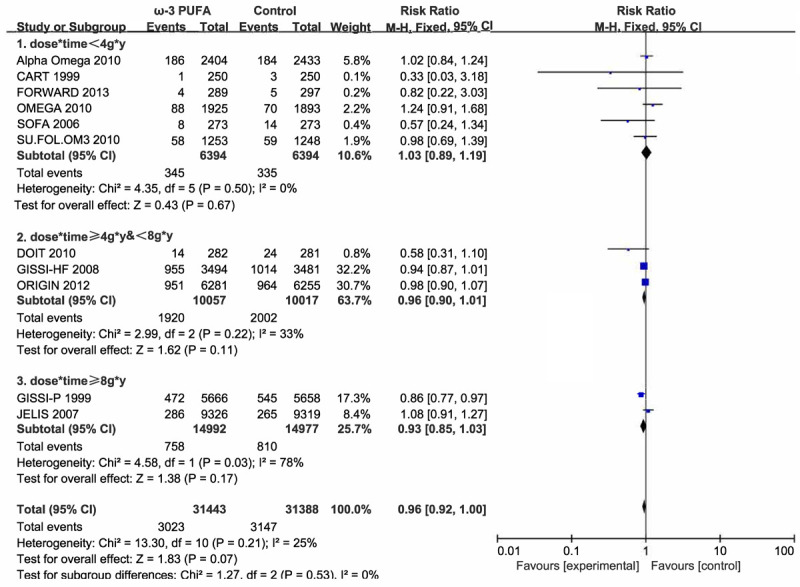
Forest plot of comparison: omega-3 PUFA vs placebo on all-cause mortality. Stratified by dose*time.
Figure 10.
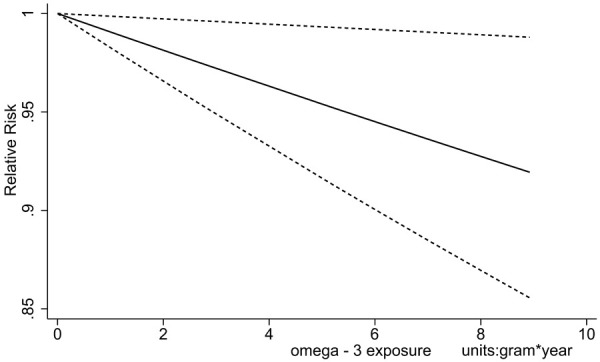
Dose-response relationship of all-cause mortality with increment of dose*time.
Quality of the evidence was evaluated though summary of findings (SoF) table conducted via GRADE profiler (version 3.6), and the SoF table is listed in Table 3.
Table 3.
SoF tables ω-3 PUFA compared to placebo for patients with coronary disease
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No. of Participants (studies) | Quality of the evidence (GRADE) | |
|---|---|---|---|---|---|
|
| |||||
| Assumed risk | Corresponding risk | ||||
| Placebo | Ω-3 PUFA | ||||
| The effect of ω-3 PUFA on MACCE clinical events | Study Population | RR 0.94 | 86086 | ⊕⊕⊕⊕ | |
| 160 per 1000 | 151 per 1000 | (0.84 to 1.04) | (11 studies) | high | |
| (135 to 167) | |||||
| Moderate | |||||
| 119 per 1000 | 112 per 1000 | ||||
| (100 to 124) | |||||
| The effect of ω-3 PUFA on cardiovascular mortality clinical events | Study Population | RR 0.93 | 49872 | ⊕⊕⊕⊕ | |
| 76 per 1000 | 71 per 1000 | (0.88 to 0.99) | (8 studies) | high | |
| (67 to 75) | |||||
| Moderate | |||||
| 56 per 1000 | 51 per 1000 | ||||
| (48 to 54) | |||||
| The effect of ω-3 PUFA on all-cause mortality clinical events | Study Population | RR 0.96 | 62831 | ⊕⊕⊕⊕ | |
| 100 per 1000 | 96 per 1000 | (0.92 to 1) | (11 studies) | high | |
| (92 to 100) | |||||
| Moderate | |||||
| 51 per 1000 | 49 per 1000 | ||||
| (47 to 51) | |||||
The basis for the assumed risk (e.g., the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI).
CI: Confidence interval; RR: Risk ratio; GRADE Working Group grades of evidence. High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate.
Discussion
Previous trials and meta-analyses have shown diverse results and drawn paradoxical conclusions. Some reviews and meta-analyses conservatively demonstrated a beneficial effect of marine-derived EPA and DHA supplementation in reducing cardiovascular events [27] and deaths [28,29] while others showing no association [30-33]. However, despite the meta-analyses not reaching conventional statistical significance, a majority of them revealed a modest reduction of MACEs and favored the ω-3 PUFA intake. Updated with new publications and relatively consistent with previous meta-analyses, we still did not detect a distinct protective effect on cardiovascular mortality (RR 0.94, 95% CI 0.84-1.04) and all-cause mortality (RR 0.96, 95% CI 0.91-1.00). However, it cannot be definitely asserted that there is no benefit of ω-3 PUFA supplementation. Subgroup analysis demonstrated a more obvious protective effect of a larger dose of ω-3 PUFA along with a longer period of supplementation. The inconsistent results of former individual studies could be partially explained by dose and time, which led us to perform the dose-response meta-analysis showing a 13.05% reduction of MACE with 10 g/d*y increments. What is more, as shown in Figure 4, we detected a slight upward trend of relative risk when the numerical data onto dose*time was relatively small, which is corresponding to the subgroup of dose*time < 4 g/d*y (RR, 1.07; 95% CI, 0.96-1.19) and putting forward the dauntless hypothesis that a small dose of omega-3 fatty acid may be of no benefit and even detrimental to health.
Obvious heterogeneity was detected in results of MACEs and the REDUCE-IT trial was responsible for the majority of it. We analyzed the trials and ascribed the potential reason to the large dose and long period of intervention, which reached up to 19.6 g/d*y and was much more than that of other trials. Besides the reduced total primary endpoint events (61 versus 89 per 1000 patient years for icosapent ethyl versus placebo, respectively; RR 0.70, 95% CI 0.62-0.78), this long-term randomized controlled double-blinded trial further reported first and subsequent primary endpoints (55.2% and 44.8% occurred respectively). However, as a study carried out in the same period, the VITAL, which is a 2 × 2 factorial designed trial and enrolled 25874 participants, demonstrated no benefit of cardiovascular events by ω-3 PUFA supplementation [34]. Partially explained by the low dosage of the supplementation, the negative results can be owing to the subjects, who are at a relatively low risk of MACEs. As is verified in the previous trails [9,17,20,35] and meta-analysis [13], patients with a high level of triglyceride and low-density cholesterol are more prone to benefit from ω-3 PUFA supplementation compared to the low-risk populations.
Potential physiological effects of ω-3 PUFA that might influence CVD risks have been discussed by Mozaffarian et al including mainly four aspects [36]. First, anti-arrhythmic effect was realized though modulating cardiac electrophysiology. Both animal experiments and human trials have endorsed that ω-3 PUFA may prevent ventricular arrhythmia [37-40] rather than atrial fibrillation [41-43]. Second, ω-3 PUFA reduces plasma triglyceride levels, and improves myocardial efficiency and left ventricular diastolic function. This effect was also verified by the OMEGA-REMODEL randomized clinical trial, which manifested that 4 g/d ω-3 PUFA supplementation lasting 6 months evidently reduced adverse left ventricular remodeling, non-infarct myocardial fibrosis and inflammation biomarkers of patients suffered from an acute myocardial infarction beyond current guideline-based standard of care [44]. Third, these hepatic effects might also lead to modest shunting of carbohydrates and/or glycerol to glucose production, which could raise plasma glucose levels but reduce hepatic steatosis and insulin resistance and not adversely affect peripheral insulin resistance or systemic metabolic dysfunction. Lastly, these changes together contribute to the established blood pressure-lowering effects of ω-3 PUFA, which have been supported by many clinical trials [45-47] and meta-analyses [48,49].
Strengths of this meta-analysis are as follows: 1. We focused on the doses of ω-3 PUFA as well as intervention period and created a new variable (dose*time) to subgroup the included trials and found significant reduction of cardiovascular events. It should be noted that no statistically significant result has been detected after stratifying the participants via daily dose merely. Dose-response meta-analysis further verified the benefit of large dose and long period of intervention. 2. Strict inclusion and exclusion criteria were followed to ensure the included trials of good quality. We also processed the risk of bias evaluation and SoF table to evaluate the reliability. 3. Trail sequencing analysis was also conducted, which indicated that the present studies were not sufficient to confidently draw a negative conclusion overall. 4. Besides the traditional process of literature search from databases, we also identified and screened the relevant reviews and meta-analyses to ensure no omissions of associated studies.
There were several limitations of this review. First, data were collected from published trials and it was restricted to obtain the individual-level data. Furthermore, the primary outcome of MACEs was limited to cardiovascular death, nonfatal myocardial infarction, coronary revascularization, unstable angina, but not include ischemic stroke. Second, we only evaluated the data from the MACEs and cardiovascular/all-cause mortality while safety data were not collected and analyzed, mainly comprising increased gastrointestinal disturbances and liver injury. Finally, the control groups were exposed to different levels of fish oil due to various dietary habits and it remained difficult to quantify the effect. They also received different kinds of control (olive oil, sunflower oil, corn oil, oleic-acid margarine or blank). Olive oil, which has been reported to have beneficial effects on lipoprotein metabolism, might have disguised the real benefit of ω-3 PUFA supplementation [50,51].
Conclusion
The meta-analysis from randomized controlled trials indicated that supplementation of ω-3 PUFA in patients with cardiovascular disease risks produced a modest protective effect and large doses with long period of intervention would enhance the salutary association with major adverse cardiovascular events. Dose-response analysis demonstrated a 13.05% reduction with 10 g/d*y increments. In the meantime, ω-3 PUFA was beneficial on the aspect of both cardiovascular and all-cause mortality.
Acknowledgements
This research was supported by the Nanjing Health Science and Technology Development Special Fund (No. YKK19164).
Disclosure of conflict of interest
None.
References
- 1.Eckel RH, Jakicic JM, Ard JD, de Jesus JM, Houston Miller N, Hubbard VS, Lee IM, Lichtenstein AH, Loria CM, Millen BE, Nonas CA, Sacks FM, Smith SC, Svetkey LP, Wadden TA, Yanovski SZ. 2013 AHA/ACC guideline on lifestyle management to reduce cardiovascular risk. J Am Coll Cardiol. 2014;63:2960–2984. doi: 10.1016/j.jacc.2013.11.003. [DOI] [PubMed] [Google Scholar]
- 2.Sun R, Wang X, Liu Y, Xia M. Dietary supplementation with fish oil alters the expression levels of proteins governing mitochondrial dynamics and prevents high-fat diet-induced endothelial dysfunction. Br J Nutr. 2014;112:145–153. doi: 10.1017/S0007114514000701. [DOI] [PubMed] [Google Scholar]
- 3.Sneddon AA, McLeod E, Wahle KW, Arthur JR. Cytokine-induced monocyte adhesion to endothelial cells involves platelet-activating factor: suppression by conjugated linoleic acid. Biochim Biophys Acta. 2006;1761:793–801. doi: 10.1016/j.bbalip.2006.05.014. [DOI] [PubMed] [Google Scholar]
- 4.Edward RR, Innes JK, Marino LV, Calder PC. Influence of different intravenous lipid emulsions on growth, development and laboratory and clinical outcomes in hospitalised paediatric patients: a systematic review. Clin Nutr. 2018;37:765–783. doi: 10.1016/j.clnu.2017.07.003. [DOI] [PubMed] [Google Scholar]
- 5.Minihane AM, Vinoy S, Russell WR, Baka A, Roche HM, Tuohy KM, Teeling JL, Blaak EE, Fenech M, Vauzour D, McArdle HJ, Kremer BH, Sterkman L, Vafeiadou K, Benedetti MM, Williams CM, Calder PC. Low-grade inflammation, diet composition and health: current research evidence and its translation. Br J Nutr. 2015;114:999–1012. doi: 10.1017/S0007114515002093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lin N, Shi JJ, Li YM, Zhang XY, Chen Y, Calder PC, Tang LJ. What is the impact of n-3 PUFAs on inflammation markers in type 2 diabetic mellitus populations? A systematic review and meta-analysis of randomized controlled trials. Lipids Health Dis. 2016;15:133. doi: 10.1186/s12944-016-0303-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Innes JK, Calder PC. The differential effects of eicosapentaenoic acid and docosahexaenoic acid on cardiometabolic risk factors: a systematic review. Int J Mol Sci. 2018;19:532–553. doi: 10.3390/ijms19020532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bhatt DL, Steg PG, Miller M, Brinton EA, Jacobson TA, Ketchum SB, Doyle RT Jr, Juliano RA, Jiao L, Granowitz C, Tardif JC, Gregson J, Pocock SJ, Ballantyne CM REDUCE-IT Investigators. Effects of icosapent ethyl on total ischemic events: from REDUCE-IT. J Am Coll Cardiol. 2019;73:2791–2802. doi: 10.1016/j.jacc.2019.02.032. [DOI] [PubMed] [Google Scholar]
- 9.Yokoyama M, Origasa H, Matsuzaki M, Matsuzawa Y, Saito Y, Ishikawa Y, Oikawa S, Sasaki J, Hishida H, Itakura H, Kita T, Kitabatake A, Nakaya N, Sakata T, Shimada K, Shirato K. Effects of eicosapentaenoic acid on major coronary events in hypercholesterolaemic patients (JELIS): a randomised open-label, blinded endpoint analysis. Lancet. 2007;369:1090–1098. doi: 10.1016/S0140-6736(07)60527-3. [DOI] [PubMed] [Google Scholar]
- 10.Bosch J, Gerstein HC, Dagenais GR, Diaz R, Dyal L, Jung H, Maggiono AP, Probstfield J, Ramachandran A, Riddle MC, Ryden LE, Yusuf S. n-3 fatty acids and cardiovascular outcomes in patients with dysglycemia. N Engl J Med. 2012;367:309–318. doi: 10.1056/NEJMoa1203859. [DOI] [PubMed] [Google Scholar]
- 11.Rauch B, Schiele R, Schneider S, Diller F, Victor N, Gohlke H, Gottwik M, Steinbeck G, Del Castillo U, Sack R, Worth H, Katus H, Spitzer W, Sabin G, Senges J OMEGA Study Group. OMEGA, a randomized, placebo-controlled trial to test the effect of highly purified omega-3 fatty acids on top of modern guideline-adjusted therapy after myocardial infarction. Circulation. 2010;122:2152–2159. doi: 10.1161/CIRCULATIONAHA.110.948562. [DOI] [PubMed] [Google Scholar]
- 12.Bhatt DL, Steg PG, Miller M, Brinton EA, Jacobson TA, Ketchum SB, Doyle RT Jr, Juliano RA, Jiao L, Granowitz C, Tardif JC, Gregson J, Pocock SJ, Ballantyne CM REDUCE-IT Investigators. Effects of icosapent ethyl on total ischemic events: from REDUCE-IT. J Am Coll Cardiol. 2019;73:2791–2802. doi: 10.1016/j.jacc.2019.02.032. [DOI] [PubMed] [Google Scholar]
- 13.Alexander DD, Miller PE, Van Elswyk ME, Kuratko CN, Bylsma LC. A meta-analysis of randomized controlled trials and prospective cohort studies of eicosapentaenoic and docosahexaenoic long-chain omega-3 fatty acids and coronary heart disease risk. Mayo Clin Proc. 2017;92:15–29. doi: 10.1016/j.mayocp.2016.10.018. [DOI] [PubMed] [Google Scholar]
- 14.Aung T, Halsey J, Kromhout D, Gerstein HC, Marchioli R, Tavazzi L, Geleijnse JM, Rauch B, Ness A, Galan P, Chew EY, Bosch J, Collins R, Lewington S, Armitage J, Clarke R Omega-3 Treatment Trialists’ Collaboration. Associations of omega-3 fatty acid supplement use with cardiovascular disease risks: meta-analysis of 10 trials involving 77917 individuals. JAMA Cardiol. 2018;3:225–234. doi: 10.1001/jamacardio.2017.5205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moher D, Liberati A, Tetzlaff J, Altman DG PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. 2009;62:1006–1012. doi: 10.1016/j.jclinepi.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 16.Rothstein H, Sutton A, Borenstein M. Publication bias in meta-analysis: prevention, assessment and adjustments. Psychometrika. 2007;72:269–271. [Google Scholar]
- 17.Einvik G, Klemsdal T, Sandvik L, Hjerkinn E. A randomized clinical trial on n-3 polyunsaturated fatty acids supplementation and all-cause mortality in elderly men at high cardiovascular risk. Eur J Cardiovasc Prev Rehabil. 2010;17:588–592. doi: 10.1097/HJR.0b013e328339cc70. [DOI] [PubMed] [Google Scholar]
- 18.Galan P, Kesse-Guyot E, Czernichow S, Briancon S, Blacher J, Hercberg S SU.FOL.OM3 Collaborative Group. Effects of B vitamins and omega 3 fatty acids on cardiovascular diseases: a randomised placebo controlled trial. BMJ. 2010;341:c6273. doi: 10.1136/bmj.c6273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tavazzi L, Maggioni AP, Marchioli R, Barlera S, Franzosi MG, Latini R, Lucci D, Nicolosi GL, Porcu M, Tognoni G Gissi-HF Investigators. Effect of n-3 polyunsaturated fatty acids in patients with chronic heart failure (the GISSI-HF trial): a randomised, double-blind, placebo-controlled trial. Lancet. 2008;372:1223–1230. doi: 10.1016/S0140-6736(08)61239-8. [DOI] [PubMed] [Google Scholar]
- 20.Dietary supplementation with n-3 polyunsaturated fatty acids and vitamin E after myocardial infarction: results of the GISSI-Prevenzione trial. Lancet. 1999;354:447–455. [PubMed] [Google Scholar]
- 21.Johansen O, Brekke M, Seljeflot I, Abdelnoor M, Arnesen H. N-3 fatty acids do not prevent restenosis after coronary angioplasty: results from the CART study. J Am Coll Cardiol. 1999;33:1619–1626. doi: 10.1016/s0735-1097(99)00054-6. [DOI] [PubMed] [Google Scholar]
- 22.Kromhout D, Giltay EJ, Geleijnse JM Alpha Omega Trial Group. N-3 fatty acids and cardiovascular events after myocardial infarction. N Engl J Med. 2010;363:2015–2026. doi: 10.1056/NEJMoa1003603. [DOI] [PubMed] [Google Scholar]
- 23.Macchia A, Grancelli H, Varini S, Nul D, Laffaye N, Mariani J, Ferrante D, Badra R, Figal J, Ramos S, Tognoni G, Doval HC GESICA Investigators. Omega-3 fatty acids for the prevention of recurrent symptomatic atrial fibrillation: results of the forward (randomized trial to assess efficacy of pufa for the maintenance of sinus rhythm in persistent atrial fibrillation) trial. J Am Coll Cardiol. 2013;61:463–468. doi: 10.1016/j.jacc.2012.11.021. [DOI] [PubMed] [Google Scholar]
- 24.Risk and Prevention Study Collaborative Group. Roncaglioni MC, Tombesi M, Avanzini F, Barlera S, Caimi V, Longoni P, Marzona I, Milani V, Silletta MG, Tognoni G, Marchioli R. N-3 fatty acids in patients with multiple cardiovascular risk factors. N Engl J Med. 2013;368:1800–1808. doi: 10.1056/NEJMoa1205409. [DOI] [PubMed] [Google Scholar]
- 25.Bonds DE, Harrington M, Worrall BB, Bertoni AG, Eaton CB, Hsia J, Robinson J, Clemons TE, Fine LJ, Chew EY. Effect of long-chain omega-3 fatty acids and lutein + zeaxanthin supplements on cardiovascular outcomes: results of the age-related eye disease study 2 (AREDS2) randomized clinical trial. JAMA Intern Med. 2014;174:763–771. doi: 10.1001/jamainternmed.2014.328. [DOI] [PubMed] [Google Scholar]
- 26.Brouwer IA, Zock PL, Camm AJ, Böcker D, Hauer RN, Wever EF, Dullemeijer C, Ronden JE, Katan MB, Lubinski A, Buschler H, Schouten EG SOFA Study Group. Effect of fish oil on ventricular tachyarrhythmia and death in patients with implantable cardioverter defibrillators. JAMA. 2006;295:2613–2619. doi: 10.1001/jama.295.22.2613. [DOI] [PubMed] [Google Scholar]
- 27.Alexander DD, Miller PE, Van Elswyk ME, Kuratko CN, Bylsma LC. A meta-analysis of randomized controlled trials and prospective cohort studies of eicosapentaenoic and docosahexaenoic long-chain omega-3 fatty acids and coronary heart disease risk. Mayo Clinic Proceedings. 2017;92:15–29. doi: 10.1016/j.mayocp.2016.10.018. [DOI] [PubMed] [Google Scholar]
- 28.Bucher HC, Hengstler P, Schindler C, Meier G. N-3 polyunsaturated fatty acids in coronary heart disease: a meta-analysis of randomized controlled trials. Am J Med. 2002;112:298–304. doi: 10.1016/s0002-9343(01)01114-7. [DOI] [PubMed] [Google Scholar]
- 29.Maki KC, Palacios OM, Bell M, Toth PP. Use of supplemental long-chain omega-3 fatty acids and risk for cardiac death: an updated meta-analysis and review of research gaps. J Clin Lipidol. 2017;11:1152–1160. e1152. doi: 10.1016/j.jacl.2017.07.010. [DOI] [PubMed] [Google Scholar]
- 30.Aung T, Halsey J, Kromhout D, Gerstein HC, Marchioli R, Tavazzi L, Geleijnse JM, Rauch B, Ness A, Galan P, Chew EY, Bosch J, Collins R, Lewington S, Armitage J, Clarke R. Associations of omega-3 fatty acid supplement use with cardiovascular disease risks meta-analysis of 10 trials involving 77 917 individuals. JAMA Cardiol. 2018;3:225–234. doi: 10.1001/jamacardio.2017.5205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen Q, Cheng LQ, Xiao TH, Zhang YX, Zhu M, Zhang R, Li K, Wang Y, Li Y. Effects of omega-3 fatty acid for sudden cardiac death prevention in patients with cardiovascular disease: a contemporary meta-analysis of randomized, controlled trials. Cardiovasc Drugs Ther. 2011;25:259–265. doi: 10.1007/s10557-011-6306-8. [DOI] [PubMed] [Google Scholar]
- 32.Khoueiry G, Abi Rafeh N, Sullivan E, Saiful F, Jaffery Z, Kenigsberg DN, Krishnan SC, Khanal S, Bekheit S, Kowalski M. Do omega-3 polyunsaturated fatty acids reduce risk of sudden cardiac death and ventricular arrhythmias? A meta-analysis of randomized trials. Heart Lung. 2013;42:251–256. doi: 10.1016/j.hrtlng.2013.03.006. [DOI] [PubMed] [Google Scholar]
- 33.Rizos EC, Ntzani EE, Bika E, Kostapanos MS, Elisaf MS. Association between omega-3 fatty acid supplementation and risk of major cardiovascular disease events a systematic review and meta-analysis. JAMA. 2012;308:1025–1033. doi: 10.1001/2012.jama.11374. [DOI] [PubMed] [Google Scholar]
- 34.Manson JE, Cook NR, Lee IM, Christen W, Bassuk SS, Mora S, Gibson H, Albert CM, Gordon D, Copeland T, D’Agostino D, Friedenberg G, Ridge C, Bubes V, Giovannucci EL, Willett WC, Buring JE VITAL Research Group. Marine n-3 fatty acids and prevention of cardiovascular disease and cancer. N Engl J Med. 2019;380:23–32. doi: 10.1056/NEJMoa1811403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schacky Cv, Angerer P, Kothny W, Theisen K, Mudra H. The effect of dietary ω-3 fatty acids on coronary atherosclerosis: a randomized, double-blind, placebo-controlled trial. Ann Itern Med. 1999;130:554–562. doi: 10.7326/0003-4819-130-7-199904060-00003. [DOI] [PubMed] [Google Scholar]
- 36.Mozaffarian D, Wu JH. Omega-3 fatty acids and cardiovascular disease: effects on risk factors, molecular pathways, and clinical events. J Am Coll Cardiol. 2011;58:2047–2067. doi: 10.1016/j.jacc.2011.06.063. [DOI] [PubMed] [Google Scholar]
- 37.McLennan PL. Myocardial membrane fatty acids and the antiarrhythmic actions of dietary fish oil in animal models. Lipids. 2001;36:S111–114. doi: 10.1007/s11745-001-0692-x. [DOI] [PubMed] [Google Scholar]
- 38.Billman GE, Kang JX, Leaf A. Prevention of ischemia-induced cardiac sudden death by n-3 polyunsaturated fatty acids in dogs. Lipids. 1997;32:1161–8. doi: 10.1007/s11745-997-0149-2. [DOI] [PubMed] [Google Scholar]
- 39.Weisman D, Beinart R, Erez A, Koren-Morag N, Goldenberg I, Eldar M, Glikson M, Luria D. Effect of supplemented intake of omega-3 fatty acids on arrhythmias in patients with ICD: fish oil therapy may reduce ventricular arrhythmia. J Interv Card Electrophysiol. 2017;49:255–261. doi: 10.1007/s10840-017-0267-1. [DOI] [PubMed] [Google Scholar]
- 40.McLennan PL, Abeywardena MY, Charnock JS. Dietary fish oil prevents ventricular fibrillation following coronary artery occlusion and reperfusion. Am Heart J. 1988;116:709–17. doi: 10.1016/0002-8703(88)90328-6. [DOI] [PubMed] [Google Scholar]
- 41.Li FR, Chen GC, Qin J, Wu X. DietaryFish and long-chain n-3 polyunsaturated fatty acids intake and risk of atrial fibrillation: a meta-analysis. Nutrients. 2017;9:955. doi: 10.3390/nu9090955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cheng X, Chen S, Hu Q, Yin Y, Liu Z. Fish oil increase the risk of recurrent atrial fibrillation: result from a meta-analysis. Int J Cardiol. 2013;168:4538–4541. doi: 10.1016/j.ijcard.2013.06.096. [DOI] [PubMed] [Google Scholar]
- 43.Mariani J, Doval HC, Nul D, Varini S, Grancelli H, Ferrante D, Tognoni G, Macchia A. n-3 polyunsaturated fatty acids to prevent atrial fibrillation: updated systematic review and meta-analysis of randomized controlled trials. Ann Med. 2013;2:e005–033. doi: 10.1161/JAHA.112.005033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Heydari B, Abdullah S, Pottala JV, Shah R, Abbasi S, Mandry D, Francis SA, Lumish H, Ghoshhajra BB, Hoffmann U, Appelbaum E, Feng JH, Blankstein R, Steigner M, McConnell JP, Harris W, Antman EM, Jerosch-Herold M, Kwong RY. Effect of omega-3 acid ethyl esters on left ventricular remodeling after acute myocardial infarction: the OMEGA-REMODEL randomized clinical trial. Circulation. 2016;134:378–391. doi: 10.1161/CIRCULATIONAHA.115.019949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bønaa KH, Bjerve KS, Straume B, Gram IT, Thelle D. Effect of eicosapentaenoic and docosahexaenoic acids on blood pressure in hypertension. A population-based intervention trial from the Tromsø study. Engl J Med. 1990;32:795–801. doi: 10.1056/NEJM199003223221202. [DOI] [PubMed] [Google Scholar]
- 46.Knapp HR, FitzGerald GA. The antihypertensive effects of fish oil. A controlled study of polyunsaturated fatty acid supplements in essential hypertension. N Engl J Med. 1989;320:1037–1043. doi: 10.1056/NEJM198904203201603. [DOI] [PubMed] [Google Scholar]
- 47.Passfall J, Philipp T, Woermann F, Quass P, Thiede M, Haller H. Different effects of eicosapentaenoic acid and olive oil on blood pressure, intracellular free platelet calcium, and plasma lipids in patients with essential hypertension. Clin Investigator. 1993;71:628–33. doi: 10.1007/BF00184490. [DOI] [PubMed] [Google Scholar]
- 48.Yang B, Shi MQ, Li ZH, Yang JJ, Li D. Fish, long-chain n-3 PUFA and incidence of elevated blood pressure: a meta-analysis of prospective cohort studies. Nutrients. 2016;8:1–12. doi: 10.3390/nu8010058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Miller PE, Van Elswyk M, Alexander DD. Long-chain omega-3 fatty acids eicosapentaenoic acid and docosahexaenoic acid and blood pressure: a meta-analysis of randomized controlled trials. Am J Hypertens. 2014;27:885–896. doi: 10.1093/ajh/hpu024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Herna’ez A, Farra’s M, Fito’ M. Olive oil phenolic compounds and high-density lipoprotein function. Curr Opin Lipidol. 2016;27:47–53. doi: 10.1097/MOL.0000000000000261. [DOI] [PubMed] [Google Scholar]
- 51.Perona JS, Fito M, Covas MI, Garcia M, Ruiz-Gutierrez V. Olive oil phenols modulate the triacylglycerol molecular species of human very low-density lipoprotein. A randomized, crossover, controlled trial. Metabolism. 2011;60:893–899. doi: 10.1016/j.metabol.2010.08.010. [DOI] [PubMed] [Google Scholar]