Abstract
Objective: To explore the incidence rate of aspirin resistance (AR) in patients with coronary heart disease (CHD) in the plateau, and analyze its correlation with clinical influencing factors and serological indicators. Methods: In this retrospective study, 90 patients with CHD who had lived in the plateau for a long time (>10 years) and received treatment were selected as the subjects. Patients were divided into the AR group (11-dehydrothromboxane B2 (11-DH-TXB2) >1500 pg/mg) and aspirin sensitivity group (AS group, 11-DH-TXB2 ≤1500 pg/mg) according to the content of 11-DH-TXB2 in the urine. The differences in gender, body weight, blood pressure and heart rate between the two groups were compared, and the correlation of these indexes with the incidence rate of AR was analyzed. Moreover, serum indicators were detected. Multiple variable binary logistic regression was used to detect the independent risk factors for AR. Results: The incidence rate of AR in the enrolled patients with CHD was 27.78% (25/90). The body mass index (BMI) in the AR group was significantly higher than that in the AS group (P<0.05). Patients in the AR group had significantly higher C-reactive protein (CRP) and total bilirubin levels and lower mean corpuscular volume and mean corpuscular hemoglobin compared with the AS group (all P<0.05). Binary logistic regression showed that BMI and CRP were independent factors for AR. Conclusion: AR occurs in patients with CHD who take aspirin in the plateau. Patients with high BMI or CRP level have an increased risk of AR. In addition, BMI and CRP are independent factors for AR, and bilirubin can be a predictive factor for AR.
Keywords: Plateau Tibetan, aspirin resistance, serum indicators
Introduction
Coronary heart disease (CHD), also called coronary atherosclerotic heart disease, refers to the accumulation of atherosclerotic lipid-like substances in the endarterium due to abnormal lipid metabolism, which destroys the integrity of the endarterium. With the aging of population and widespread unhealthy life style and dietary habits, the incidence rate of cardiovascular disease is increasing year by year, which brings heavy burden to the society and the family [1,2].
Cardiovascular and cerebrovascular diseases are mainly caused by thrombosis-induced arterial occlusion, and antiplatelet therapy effectively reduces the occurrence of cardiovascular and cerebrovascular events [3,4]. Aspirin has been widely applied in clinical practice due to its proved effect and cheap price. Aspirin can decrease the mortality of patients with atherosclerosis-induced cardiovascular and cerebrovascular events by 25% [5]. Recent studies have found that 8-45% of people taking aspirin have aspirin resistance (AR), that is, some patients still suffer from malignant cardiovascular events after regular administration of aspirin. Relevant Chinese studies have also proved that the effect of aspirin varies generally between individuals [6,7].
According to incomplete statistics, the number of people living in the plateau in China has been more than 10 million and is increasing year by year [8]. Unique geographical environment and climate exist in the plateau, such as rarefied air, low oxygen pressure, dry climate, low relative humidity and high ultraviolet radiation intensity. These unique climatic features bring about pathophysiological changes in local residents who are different from people living in the plain, making the incidence rates of cardiovascular and cerebrovascular diseases in the plateau significantly higher than those in the plain area [9].
Blood test is a common detection method for CHD. Red cell volume distribution width (RDW) has been reported to be useful for the differentiation of CHD. High-sensitivity C-reactive protein (hs-CRP) plays a certain role in determining the severity of CHD. Changes in serum indicators can serve as important bases for the auxiliary diagnosis of CHD [10]. However, it is still controversial whether or not there are more sensitive and authoritative indicators for reference and auxiliary diagnosis. A further investigation on differences in the sensitivity, tolerance and resistance of antiplatelet drug in patients with CHD in the plateau compared to those living in the plain and its correlation with clinical influencing factors and serological indicators contributes to the accurate guidance of antiplatelet therapy in patients in the plateau. It is also conducive to further prevention and treatment of high altitude-related diseases and cardiovascular and cerebrovascular diseases caused by hypercoagulability. Additionally, it is of great clinical value and social significance for the safe, reasonable, efficient and accurate treatment of frequently-occurring and common diseases in the plateau.
Materials and methods
Materials
In this retrospective study, 90 patients with CHD who had lived in the plateau for a long time (>10 years) and received treatment in our hospital from December 2018 to November 2019 were selected as the subjects. There were 25 patients of the Tibetan nationality and 65 patients of the Han nationality. Patients were divided into the AR group and aspirin sensitivity (AS) group according to the content of 11-dehydrothromboxane B2 (11-DH-TXB2) in the urine [11]. Patients agreed to participate in the study through communication and signed the informed consent. The study was approved by the ethics committee of the hospital (Approval number: TR-2018-004).
Inclusion and exclusion criteria
Inclusion criteria: Patients with CHD had lived in the plateau for a long time (>10 years) and met the diagnostic criteria for CHD in Nomenclature and Diagnosis of Ischemic Heart Disease [12]; Patients took aspirin (100 mg/d) orally for more than 30 d; Patients did not take nonsteroidal anti-inflammatory drugs or drugs that might affect platelet aggregation for at least 1 week; Patients did not receive interventional therapy.
Exclusion criteria: Patients were allergic to aspirin; Patients were infused with blood platelets or whole blood recently; Patients had various hemorrhagic diseases or hematological system diseases; Patients had acute myocardial infarction, pulmonary embolism, or other severe hepatic or renal insufficiency.
Methods
Study protocol
General data of patients were collected, including gender, age, body weight, blood pressure, heart rate, and medical history (hypertension and diabetes). Patients took aspirin (Guangdong Jiuming Pharmaceutical Co., Ltd., China, GYZZ: H44021139) on time according to the doctor’s advice. Blood was drawn before medication and after 7-day medication to detect platelet aggregation, and thromboxane B2 (TXB2) level in the urine was measured. Blood tests were performed in all patients, including blood routine, hepatic and renal function, indicators for four infectious diseases, blood lipid, blood glucose, homocysteine, biochemistry and hs-CRP.
Detection methods
A hand-held pulse oximeter (YK-80A, Xuzhou Yonker Electronic Technology Co., Ltd., China) was used to measure the oxygen saturation (SpO2) of patients without oxygen inhalation. The measurement on the same fingertip was repeated thrice, and the mean value was obtained. A benchtop mercurial sphygmomanometer was used to measure the systolic blood pressure and diastolic blood pressure. The measurement was repeated thrice, and the mean value was obtained.
Enzyme linked immunosorbent assay (ELISA) was used to detect the content of TXB2 in the urine with the ELISA kit (Wuhan BOSK Biological Engineering Co., Ltd., China). The urine of patients was taken and diluted to a concentration of 10 mg/L. Then the diluted urine was detected strictly according to the instruction of the ELISA kit. After reaction termination, a microplate reader (Multiskan Spectrum, Thermo Fisher Scientific, USA) was used to read the results at 405 nm. The threshold value was determined based on the following. The content of 11-DH-TXB2 was more than the standard level of 1500 pg/mg, indicating the absence of aspirin effect. The content of 11-DH-TXB2 was less than or equal to 1500 pg/mg, indicating the presence of aspirin effect.
Elbow venous blood was collected into the 3.8% sodium citrate anticoagulant tube and heparin anticoagulant tube. Blood indicators were detected with an automatic hematology analyzer (XN-2000, Sysmex Corporation, Japan) and an automatic biochemical analyzer (AU5831, Beckman, USA).
Statistical analysis
All data in this study were analyzed by the SPSS 21.0 statistical software. The measurement data with normal distribution were expressed as mean ± standard deviation (x̅ ± sd), and comparison between groups was performed by independent-samples t test. The measurement data with skewed distribution were expressed as M (P25, P75), and a difference between groups was compared by Mann-Whitney U test. The enumeration data were expressed as percentage (%) and analyzed by chi-square test. Continuous variables were analyzed by the Pearson correlation coefficient. Furthermore, multiple variable binary logistic regression was used to detect the independent risk factors. P<0.05 indicated a significant difference.
Results
TXB2 content and aspirin resistance analysis
There were 25 patients with AR (11-DH-TXB2 >1500 pg/mg), accounting for 27.78% of the total patients, with the mean TXB2 content of 2724.85±1519.72 pg/mg. There were 65 patients with AS (11-DH-TXB2 ≤1500 pg/mg), accounting for 72.22% of the total patients, with the mean TXB2 content of 640.73±376.37 pg/mg (Figure 1).
Figure 1.
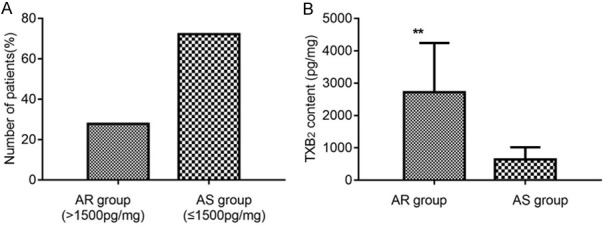
TXB2 content and proportion of patients with aspirin resistance. A: Number of patients with aspirin resistance; B: TXB2 content. Compared with AS group, **P<0.01. TXB2: thromboxane B2; AR: aspirin resistance; AS: aspirin sensitivity.
In all patients, there were 65 Han patients and 25 Tibetan patients, and their incidence rates of AR were 26.15% (17/65) and 32.00% (8/25), respectively (P>0.05). The Tibetan patients had a similar incidence rate of AR as the Han patients (Table 1).
Table 1.
Comparison of the incidence rate of aspirin resistance between Han and Tibetan patients
| Patients | n | Aspirin resistance (n, %) | χ2 | P |
|---|---|---|---|---|
| Han | 65 | 17 (26.15) | 0.308 | 0.579 |
| Tibetan | 25 | 8 (32.00) |
Comparison of clinical data
We found no significant differences in age, height and blood pressure between AR and AS groups (all P>0.05, Figure 2).
Figure 2.
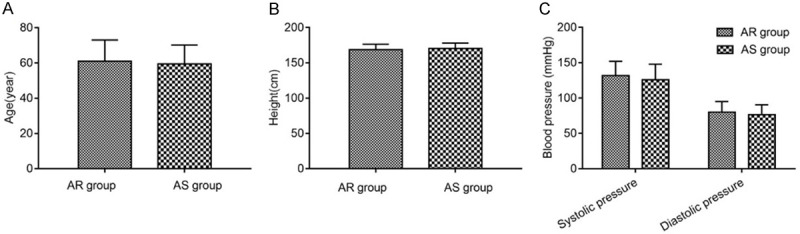
Comparison of clinical data (normal distribution). A: Age; B: Height; C: Blood pressure. AR: aspirin resistance; AS: aspirin sensitivity.
We found no significant differences in arterial oxygen saturation and heart rate (both P>0.05) but a significant difference in body mass index (BMI) between AR and AS groups (P<0.01, Figure 3). Pearson correlation analysis showed a positive correlation of the incidence rate of AR with BMI (P=0.087), suggesting that obese patients should take precautions against AR.
Figure 3.

Comparison of clinical data (skewed distribution). A: BMI index; B: Arterial oxygen saturation; C: Heart rate. Compared with the AR group, **P<0.01. BMI: body mass index; AR: aspirin resistance; AS: aspirin sensitivity.
Analysis of serum indicators
No significant differences were identified in white blood cell count, neutrophils, neutrophil percentage, red blood cells, hemoglobin, hematocrit, mean corpuscular hemoglobin concentration, red blood cell distribution width-standard deviation, red blood cell distribution width-coefficient of variation, platelets, platelet distribution width, mean platelet volume, platelet-large cell ratio, thrombocytocrit, glutamic-pyruvic transaminase (ALT), glutamic-oxalacetic transaminase (AST), AST/ALT, glutamyl transpeptidase, blood urea nitrogen, creatinine, uric acid, urine glucose, β2 microglobulin, total cholesterol, triglyceride, high density lipoprotein cholesterol, low density lipoprotein cholesterol, apolipoprotein A1, apolipoprotein B, apolipoprotein A1/apolipoprotein B, or glycated hemoglobin between the two groups (all P>0.05). The comparison of serum indicators that conformed to normal distribution is shown in Table 2. Greatly significant differences were found in mean corpuscular volume (MCV), total bilirubin (TBIL), and C-reactive protein (CRP) between AR group and AS group (all P<0.01), and a significant difference was found in mean corpuscular hemoglobin (MCH; P<0.05). The comparison of serum indicators that conformed to skewed distribution is shown in Table 3 and Figure 4. Pearson correlation analysis for the incidence rate of AR with MCV, TBIL and CRP showed a positive correlation of the incidence rate of AR with CRP (P=0.091), suggesting that patients with high CRP level might have a high probability of AR.
Table 2.
Comparison of serum indicators (normal distribution, x̅ ± sd)
| Indicators | AR group (n=25) | AS group (n=65) | t | P |
|---|---|---|---|---|
| WBC (×109/L) | 8.23±3.68 | 7.32±2.41 | 1.138 | 0.264 |
| NEU% (%) | 70.6±8.3 | 67.6±12.9 | 1.290 | 0.201 |
| HGB (g/L) | 147±19 | 147±21 | -0.034 | 0.973 |
| MCHC (g/L) | 328±11 | 331±10 | -1.161 | 0.249 |
| RDW-SD (fL) | 44.7±5.3 | 45.8±4.8 | -0.969 | 0.335 |
| PLT (×109/L) | 199±55 | 178±47 | 1.820 | 0.072 |
| PDW (%) | 15.9±4.5 | 14.5±2.6 | 1.506 | 0.142 |
| MPV (fL) | 11.6±1.7 | 11.5±1.1 | 0.262 | 0.795 |
| P-LCR (%) | 37.7±12.5 | 37.9±8.8 | -0.074 | 0.941 |
| PCT | 0.21±0.05 | 0.20±0.05 | 0.931 | 0.354 |
| LDL-C (mmol/L) | 2.05±0.85 | 2.34±1.01 | 1.308 | 0.194 |
| Apo-A1 (g/L) | 1.10±0.20 | 1.13±0.17 | -0.869 | 0.387 |
| UA (umol/L) | 380±108 | 363±112 | 0.629 | 0.531 |
| GLU (mmol/L) | 7.11±1.98 | 6.91±1.83 | 0.454 | 0.651 |
| TCh (mmol/L) | 3.86±1.16 | 3.58±0.94 | -1.051 | 0.296 |
Note: WBC: white blood cell count; NEU%: neutrophil percentage; HGB: hemoglobin; MCHC: mean corpuscular hemoglobin concentration; RDW-SD: red blood cell distribution width-standard deviation; PLT: platelet; PDW: platelet distribution width; MPV: mean platelet volume; P-LCR: platelet-large cell ratio; PCT: thrombocytocrit; LDL-C: low density lipoprotein cholesterol; apo-A1: apolipoprotein A1; UA: uric acid; GLU: urine glucose; TCh: total cholesterol; AR: aspirin resistance; AS: aspirin sensitivity.
Table 3.
Comparison of serum indicators (skewed distribution, M (P25, P75))
| Indicators | AR group (n=25) | AS group (n=65) | Z | P |
|---|---|---|---|---|
| NEU (×109/L) | 5.70 (3.72, 8.01) | 4.56 (3.46, 6.17) | -1.495 | 0.135 |
| RBC (×1012/L) | 4.99 (4.56, 5.45) | 4.75 (4.20, 5.32) | -1.757 | 0.079 |
| HCT (%) | 45.6 (40.7, 48.8) | 46.2 (40.8, 49.3) | -0.135 | 0.893 |
| MCV (fL) | 91.4 (84.3, 94.9) | 94.3 (91.1, 97.8) | -2.955 | 0.003 |
| MCH (pg) | 30.2 (28.9, 31.5) | 31.3 (29.6, 32.3) | -2.055 | 0.040 |
| RDW-CV (%) | 13.7 (12.6, 15.3) | 13.3 (12.7, 14.2) | -1.024 | 0.306 |
| HBA1C (%) | 5.58 (5.52, 6.73) | 6.20 (5.62, 6.90) | -1.261 | 0.207 |
| ALT (U/L) | 49 (27, 70) | 31 (20, 59) | -1.617 | 0.106 |
| AST (U/L) | 41 (27, 83) | 33 (22, 96) | -0.320 | 0.749 |
| AST/ALT | 1.1 (0.8, 1.8) | 1.4 (0.8, 2.7) | -1.637 | 0.102 |
| TBIL (umol/L) | 28.8 (18.1, 36.3) | 15.9 (10.6, 24.4) | -4.144 | 0.000 |
| DBIL (umol/L) | 5.5 (4.0, 7.4) | 3.6 (2.3, 6.2) | -2.811 | 0.005 |
| IBIL (umol/L) | 22.6 (14.4, 29.2) | 10.8 (7.7, 17.6) | -4.234 | 0.000 |
| GGT (IU/L) | 33 (22, 50) | 37 (20, 51) | -0.063 | 0.950 |
| BUN (mmol/L) | 6.91 (6.08, 8.51) | 6.46 (4.91, 9.28) | -0.446 | 0.656 |
| CREA (umol/L) | 73 (57, 89) | 73 (56, 89) | -0.266 | 0.790 |
| β2-MG (ug/mL) | 2.52 (1.91, 3.41) | 2.05 (1.63, 2.74) | -1.703 | 0.089 |
| TG (mmol/L) | 1.38 (0.96, 1.85) | 1.38 (0.83, 2.11) | -0.216 | 0.829 |
| HDL-C (mmol/L) | 0.92 (0.76, 1.04) | 0.93 (0.75, 1.10) | -0.257 | 0.797 |
| Apo-B (g/L) | 0.75 (0.68, 0.93) | 0.78 (0.63, 1.04) | -0.514 | 0.608 |
| Apo-A1/apo-B | 1.4 (1.2, 1.6) | 1.4 (1.1, 1.7) | -0.081 | 0.935 |
| CRP (mg/dL) | 1.405 (0.632, 3.521) | 0.400 (0.143, 1.061) | -3.608 | 0.000 |
Note: NEU: neutrophils; RBC: red blood cells; HCT: hematocrit; MCV: mean corpuscular volume; MCH: mean corpuscular hemoglobin; RDW-CV: red blood cell distribution width-coefficient of variation; HBA1C: glycated hemoglobin; ALT: glutamic-pyruvic transaminase; AST: glutamic-oxalacetic transaminase; TBIL: total bilirubin; DBIL: direct bilirubin; IBIL: indirect bilirubin; GGT: glutamyl transpeptidase; BUN: blood urea nitrogen; CREA: creatinine; β2-MG: β2 microglobulin; TG: triglyceride; HDL-C: high density lipoprotein cholesterol; apo-B: apolipoprotein B; Apo-A1/apo-B: apolipoprotein A1/apolipoprotein B; CRP: C-reactive protein; AR: aspirin resistance; AS: aspirin sensitivity.
Figure 4.

Comparison of serum indicators with skewed distribution. A: MCV; B: MCH; C: TBIL; D: CRP. Compared with AS group, *P<0.05, **P<0.01, ***P<0.001. MCV: mean corpuscular volume; MCH: mean corpuscular hemoglobin; TBIL: total bilirubin; CRP: C-reactive protein; AR: aspirin resistance; AS: aspirin sensitivity.
Results of binary logistic regression
According to the differences in serological indicators in patients with AR, logistic regression on the correlation of BMI, CRP, MCV and MCH with AR showed that BMI and CRP were risk factors for AR (P<0.05, odds ratio >1, Table 4).
Table 4.
Regression analysis on the influencing factors for aspirin resistance
| Factors | β | Standard error | Wals | P | Odds ratio (95% confidence interval) |
|---|---|---|---|---|---|
| BMI | 0.294 | 0.104 | 7.901 | 0.005 | 1.1341 (1.093, 1.646) |
| CRP | 0.909 | 0.325 | 7.812 | 0.005 | 2.281 (1.312, 4.693) |
| MCV | -0.210 | 0.084 | 6.239 | 0.012 | 0.810 (0.687, 0.956) |
| MCH | 0.043 | 0.179 | 0.059 | 0.809 | 1.044 (0.735, 1.484) |
Note: BMI: body mass index; CRP: C-reactive protein; MCV: mean corpuscular volume; MCH: mean corpuscular hemoglobin.
Discussion
Aspirin is used for the prevention and treatment of cardiovascular and cerebrovascular diseases, and has brought good effect since its first application in clinical practice more than 100 years ago. Recent studies have unveiled that the antiplatelet aggregation effects of aspirin are discrepant among patients, and patients with poor aspirin reactivity have an increasing probability of the recurrence of cardiovascular and cerebrovascular events [13-15]. In this study, we explored aspirin sensitivity, tolerance and resistance in patients with CHD in the plateau, providing more clinical and experimental data for the prevention and treatment of cardiovascular and cerebrovascular diseases caused by hypercoagulability.
In this study, no significant difference was revealed in the prevalence of AR between the Tibetan patients and the Han patients, indicating that AR in patients with CHD was not correlated with the nationality [16]. The prevalence of AR was 27.8%, which was generally consistent with the results of previous studies. Previous studies have shown that AR may be related to gender, age, body weight, smoking history, hypertension or hyperglycemia [17-20]. The results in this study showed that the BMI was higher in the AR group than in the AS group, demonstrating that BMI might be a factor influencing AR. However, no significant differences were found in the effect of age, gender and hypertension, which might be related to the small sample size and small age span in this study. Platelet aggregation rate at baseline and after treatment in obese people is higher than that in people with normal weight, which may be related to the higher sensitivity of obesity to arachidonic acid-induced aggregative response [21,22]. Westerbacka et al. found that obesity was an independent risk factor for the occurrence of malignant cardiovascular events in patients with cardiovascular disease [23]. Inflammatory states associated with obesity, especially metabolic endotoxemia, may promote a number of mechanisms to increase platelet reactivity and platelet renewal and decrease the bioavailability of aspirin. All of these lead to adverse aspirin response and may become risk factors for reducing the pharmacodynamic response of aspirin, triggering AR.
The results of serum indicator comparison between the two groups showed significant differences in MCV, TBIL, direct bilirubin, indirect bilirubin and CRP. Binary logistic regression indicated that MCV and MCH had little effect on AR, while the relation of bilirubin and CRP with AR was worthy of attention. TBIL included direct bilirubin and indirect bilirubin. Abnormal increase of serum bilirubin level in patients with chronic heart failure was reported to be the change in serum bilirubin level caused by the blocked discharge of liver-excreted bile due to the congestive damage of hepatic cells [24]. Therefore, the occurrence of AR may cause cardiac insufficiency in patients, and then result in changes in serum bilirubin level. In this study, bilirubin can be used as a predictive factor for AR. It is also necessary to exclude cardiac insufficiency induced by other causes by combining with clinical symptoms, brain natriuretic peptide, N-terminal pro brain natriuretic peptide, electrocardiogram, heart color ultrasound, and abdominal ultrasonography.
CRP is an inflammatory indicator, which is widely used in clinical practice. In this study, the CRP level in the AR group was higher than that in the AS group, indicating that CRP had a relation with AR. Enugopal et al. found that in cultured aortic endothelial cells, the increase of CRP level decreased the mRNA level of endothelial nitric oxide synthase [25]. Endothelial nitric oxide synthase produced NO by catalytic action to regulate the cardiovascular system, which could play a role in vasodilation and regulation of coronary artery spasm, confirming the association between CRP and the cardiovascular system [26]. Therefore, patients with CHD with high CRP level have an increased risk of AR, so CRP can be used as an independent factor for predicting AR in clinical practice.
The unique climate in the plateau, such as rarefied air, low oxygen pressure, dry climate, and low relative humidity, led to higher incidence rates of malignant cardiovascular and cerebrovascular events in local residents than those in the plain area. The results also showed that patients with CHD in the plateau had a high probability of AR (27.78%), which reduced the medication effect. At present, there are many studies on the influencing factors for AR, but with different research directions [7,15]. In this study, significant differences were found in BMI, CRP and bilirubin, which brought us some inspirations. First, for patients with recently increased BMI, the dosage of aspirin should be further confirmed. Second, the level of CRP might provide predictive value for AR. A lot of clinical studies in future are required to determine the linear relationship between CRP and AR. Third, bilirubin is an indicator to predict cardiac insufficiency, and the clinical end-stage manifestation of AR is cardiac insufficiency, so bilirubin can theoretically be used as a predictive factor for AR. Moreover, the effect of liver disease, biliary system and other heart diseases on cardiac function should be ruled out. In addition, there were some shortcomings and limitations in this study. For example, the small sample size might bias the results. The study period and follow-up duration were short. Hence, further studies with larger sample sizes and longer follow-up periods are needed.
In conclusion, AR occurs in Tibetan patients with CHD who take aspirin in the plateau, which shows no difference in Han patients. Patients with high BMI or CRP level have an increased risk for AR, and bilirubin can be a predictive factor for AR.
Acknowledgements
This work was supported by the Natural Science Foundation of Qinghai Province Science and Technology Department (2018-ZJ-904), Qinghai Province Science and Technology Department Science and Technology Achievement Transformation Project (2018-SF-114) and Qinghai Provincial Health Commission Fund Project (2020-wjzdx-36).
Disclosure of conflict of interest
None.
References
- 1.Duncan MS, Freiberg MS, Greevy RA, Kundu S, Vasan RS, Tindle HA. Association of smoking cessation with subsequent risk of cardiovascular disease. JAMA. 2019;322:642–650. doi: 10.1001/jama.2019.10298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kim H, Caulfield LE, Garcia-Larsen V, Steffen LM, Coresh J, Rebholz CM. Plant-based diets are associated with a lower risk of incident cardiovascular disease, cardiovascular disease mortality, and all-cause mortality in a general population of middle-aged adults. J Am Heart Assoc. 2019;8:e012865. doi: 10.1161/JAHA.119.012865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim BK, Hong SJ, Cho YH, Yun KH, Kim YH, Suh Y, Cho JY, Her AY, Cho S, Jeon DW, Yoo SY, Cho DK, Hong BK, Kwon H, Ahn CM, Shin DH, Nam CM, Kim JS, Ko YG, Choi D, Hong MK, Jang Y. Effect of ticagrelor monotherapy vs ticagrelor with aspirin on major bleeding and cardiovascular events in patients with acute coronary syndrome: the TICO randomized clinical trial. JAMA. 2020;323:2407–2416. doi: 10.1001/jama.2020.7580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sharma AN, Deyell JS, Sharma SN, Barseghian A. Role of and recent evidence for antiplatelet therapy in prevention of cardiovascular disease in diabetes. Curr Cardiol Rep. 2019;21:78. doi: 10.1007/s11886-019-1168-y. [DOI] [PubMed] [Google Scholar]
- 5.Barbarawi M, Kheiri B, Zayed Y, Gakhal I, Al-Abdouh A, Barbarawi O, Rashdan L, Rizk F, Bachuwa G, Alkotob ML. Aspirin efficacy in primary prevention: a meta-analysis of randomized controlled trials. High Blood Press Cardiovasc Prev. 2019;26:283–291. doi: 10.1007/s40292-019-00325-5. [DOI] [PubMed] [Google Scholar]
- 6.Patrono C. Aspirin. Platelets. In: Patrono C, editor. 4th edition. New York: Academic Press; 2019. pp. 921–936. [Google Scholar]
- 7.Wang J, Liu J, Zhou Y, Wang F, Xu K, Kong D, Bai J, Chen J, Gong X, Meng H, Li C. Association among PlA1/A2 gene polymorphism, laboratory aspirin resistance and clinical outcomes in patients with coronary artery disease: an updated meta-analysis. Sci Rep. 2019;9:13177. doi: 10.1038/s41598-019-49123-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fan W, Meng M, Lu J, Dong X, Wei H, Wang X, Zhang Q. Decoupling elasticity and driving factors of energy consumption and economic development in the qinghai-tibet plateau. Sustainability. 2020;12:1326. [Google Scholar]
- 9.Taco-Vasquez ED, Barrera F, Serrano-Duenas M, Jimenez E, Rocuts A, Riveros Perez E. Association between blood viscosity and cardiovascular risk factors in patients with arterial hypertension in a high altitude setting. Cureus. 2019;11:e3925. doi: 10.7759/cureus.3925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen D, Liang M, Jin C, Sun Y, Xu D, Lin Y. Expression of inflammatory factors and oxidative stress markers in serum of patients with coronary heart disease and correlation with coronary artery calcium score. Exp Ther Med. 2020;20:2127–2133. doi: 10.3892/etm.2020.8958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McCullough PA, Vasudevan A, Sathyamoorthy M, Schussler JM, Velasco CE, Lopez LR, Swift C, Peterson M, Bennett-Firmin J, Schiffmann R, Bottiglieri T. Urinary 11-dehydro-thromboxane b(2) and mortality in patients with stable coronary artery disease. Am J Cardiol. 2017;119:972–977. doi: 10.1016/j.amjcard.2016.12.004. [DOI] [PubMed] [Google Scholar]
- 12.Zhao LL, Qiu XJ, Wang WB, Li RM, Wang DS. NMR metabolomics and random forests models to identify potential plasma biomarkers of blood stasis syndrome with coronary heart disease patients. Front Physiol. 2019;10:1109. doi: 10.3389/fphys.2019.01109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang L, Li X, Wang D, Lv H, Si X, Li X, Sun Y, Wang D, Chen K, Kang X, Lou X, Zhang G, Ma N. Risk factors of recurrent ischemic events after acute noncardiogenic ischemic stroke. Curr Pharm Des. 2019;25:4827–4834. doi: 10.2174/1381612825666191029103756. [DOI] [PubMed] [Google Scholar]
- 14.Patrono C, Baigent C. Role of aspirin in primary prevention of cardiovascular disease. Nat Rev Cardiol. 2019;16:675–686. doi: 10.1038/s41569-019-0225-y. [DOI] [PubMed] [Google Scholar]
- 15.Jing Y, Yue X, Yang S, Li S. Association of aspirin resistance with increased mortality in ischemic stroke. J Nutr Health Aging. 2019;23:266–270. doi: 10.1007/s12603-019-1168-z. [DOI] [PubMed] [Google Scholar]
- 16.Liu X, Tang BP, MM Ti, Kuang ZY, Wang J. Clinical study of aspirin resistance in uygur and han patients with coronary heart disease. J Xinjiang Med Univ. 2008;31:446–448. [Google Scholar]
- 17.Ebrahimi P, Farhadi Z, Behzadifar M, Shabaninejad H, Abolghasem Gorji H, Taheri Mirghaed M, Salemi M, Amin K, Mohammadibakhsh R, Bragazzi NL, Sohrabi R. Prevalence rate of laboratory defined aspirin resistance in cardiovascular disease patients: a systematic review and meta-analysis. Caspian J Intern Med. 2020;11:124–134. doi: 10.22088/cjim.11.2.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Paven E, Dillinger JG, Bal Dit Sollier C, Vidal-Trecan T, Berge N, Dautry R, Gautier JF, Drouet L, Riveline JP, Henry P. Determinants of aspirin resistance in patients with type 2 diabetes. Diabetes Metab. 2020;46:370–376. doi: 10.1016/j.diabet.2019.11.002. [DOI] [PubMed] [Google Scholar]
- 19.Ardeshna D, Khare S, Jagadish PS, Bhattad V, Cave B, Khouzam RN. The dilemma of aspirin resistance in obese Patients. Ann Transl Med. 2019;7:404. doi: 10.21037/atm.2019.07.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Elayeh E, Aburish E, Yousef AM. Effect of dividing low dose aspirin on platelet reactivity and the correlation with patient-related factors. Jordan J Pharm Sci. 2020;13:185–195. [Google Scholar]
- 21.Bordeaux BC, Qayyum R, Yanek LR, Vaidya D, Becker LC, Faraday N, Becker DM. Effect of obesity on platelet reactivity and response to low-dose aspirin. Prev Cardiol. 2010;13:56–62. doi: 10.1111/j.1751-7141.2009.00058.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Norgard NB. Obesity and altered aspirin pharmacology. Clin Pharmacokinet. 2018;57:663–672. doi: 10.1007/s40262-017-0611-8. [DOI] [PubMed] [Google Scholar]
- 23.Westerbacka J, Yki-Järvinen H, Turpeinen A, Rissanen A, Vehkavaara S, Syrjälä M, Lassila R. Inhibition of platelet-collagen interaction: an in vivo action of insulin abolished by insulin resistance in obesity. Arterioscler Thromb Vasc Biol. 2002;22:167–172. doi: 10.1161/hq0102.101546. [DOI] [PubMed] [Google Scholar]
- 24.Qi ZM, Zhang XY, Ding FX, Liu F. Clinical significance of changes in serum uric acid, bilirubin and high sensitivity C-reactive protein levels in patients with chronic heart failure. Chin Cardiov Res. 2013;11:519–521. [Google Scholar]
- 25.Venugopal SK, Devaraj S, Jialal I. C-reactive protein decreases prostacyclin release from human aortic endothelial cells. Circulation. 2003;108:1676–1678. doi: 10.1161/01.CIR.0000094736.10595.A1. [DOI] [PubMed] [Google Scholar]
- 26.Tajfard M, Tavakoly Sany SB, Avan A, Latiff LA, Rahimi HR, Moohebati M, Hasanzadeh M, Ghazizadeh H, Esmaeily H, Doosti H, Taghipour A, Ghayour-Mobarhan M, Ferns GA, Emamian M, Bin Abd Mutalib MS. Relationship between serum high sensitivity C-reactive protein with angiographic severity of coronary artery disease and traditional cardiovascular risk factors. J Cell Physiol. 2019;234:10289–10299. doi: 10.1002/jcp.27945. [DOI] [PubMed] [Google Scholar]


