Abstract
Objective: To investigate the feasibility and effectiveness of Xialiqi capsules in rats with nonbacterial prostatitis. Methods: A total of 90 healthy male SD rats, weighing 200-220 g, were randomly divided into a blank control group (BCG, n=30), a model group (MG, n=30), and an intervention group (IG, n=30). After establishing the model of chronic nonbacterial prostatitis, IG was treated with 50 mg/kg Xialiqi capsules via gavage. The three groups received the same dose of saline via gavage for 7 consecutive days. The differences in leukocytes, phospholipid vesicle density, number of colonies, prostate mass, apparent diffusion coefficient (ADC), degree of inflammatory cell infiltration in the prostate fluid, serum tumor necrosis factor-α (TNF-α), interleukin (IL)-6, IL-8, CD3+, CD8+ levels after intervention were compared in the three groups. Results: Compared with the BCG, the number of leukocytes and colonies in the prostate fluid of the MG was elevated, and the density of lipid vesicles was decreased, and the number of leukocytes and colonies in the prostate fluid of the MG significantly decreased and the density of lipid vesicles rebounded after the intervention of Xialiqi capsules (P>0.05). Compared with the BCG, the prostate mass, ADC and the degree of inflammatory cell infiltration were elevated in the MG. There was a significant reversion of the above indices after the intervention of Xialiqi capsules (P<0.05). The serum levels of TNF-α, IL-6 and IL-8 in the MG were significantly higher than those in the IG, and the levels in the IG were higher than that in the BCG (P<0.05). The serum levels of CD3+ and CD8+ in the MG were significantly lower than those in the IG, and the levels in the IG were lower than that in the BCG (P<0.05). Conclusion: Xialiqi capsules have a good intervention effect on nonbacterial prostatitis, which can significantly alleviate the immune status and reduce the level of cytokines in the serum and tissues of rats.
Keywords: Nonbacterial prostatitis, rats, Xialiqi capsule
Introduction
Nonbacterial prostatitis is one of the most common urological diseases in males [1,2]. Typical clinical symptoms include pelvic pain and frequent urination, involving the perineum, penis, perianal region, urethra, pubic bone, and lumbosacral region. Difficulty in urination is characterized by urgency, frequency, painful urination, and nocturia [3,4]. The pathogenesis of nonbacterial prostatitis remains unclear, and its etiology is complex. Studies have shown that pathogenic infections, inflammation, abnormal neuromuscular activity of the pelvic floor, and immune disorders may all play a role in the development of the condition [5,6]. The incidence rate of nonbacterial prostatitis is very high, with survey data showing that there are over 8 million patients with chronic prostatitis worldwide each year [7]. A survey of 1000 patients with genitourinary tract disease showed that 25% of patients had prostatitis, with the number of patients with nonbacterial prostatitis was eight times higher than those with bacterial prostatitis [8].
There is no targeted treatment for nonbacterial prostatitis. In clinical practice, the aim of treatment is to improve the clinical symptoms and the quality of life, and prevent complications. The treatment concept has also shifted from the original “infection” -oriented (administration of antibiotics and anti-inflammatory drugs) to “symptoms” -oriented (improving symptoms and quality of life) [9,10]. Treatment options for nonbacterial prostatitis include Western medicine and Chinese medical modalities, of which Western medicine usually formulates an individualized and comprehensive treatment plan according to the patient’s condition using antibiotics, alpha-blockers, and non-steroidal anti-inflammatory analgesics [11-13]. Although Western medicine has achieved certain effects, it is associated with a long treatment period and more side effects. Patients are also prone to lose confidence in treatment, while Chinese medicine intervention is relatively mild and has received more attention in the treatment of prostatitis [14,15].
Xialiqi capsule is a traditional Chinese herbal formula for the treatment of prostate diseases, and its indications include mild to moderate benign prostatic hyperplasia, chronic prostatitis, etc. The formula has the characteristics of having a “quick effect and long release time” [16]. A clinical study conducted on 300 patients with type III A chronic prostatitis showed that treatment with Xialiqi capsules increased the total effective rate of treatment from 65.07% to 86.21%, with significant improvement in patients’ clinical symptoms [17]. The present study was conducted to analyze the effect of Xialiqi capsules on rats with nonbacterial prostatitis by establishing an animal model, thereby providing a new idea for the treatment of this disease.
Materials and methods
Animals and environment
Ninety, adult, male SD rats of SPF grade, weighing 200-220 g, with an average weight of (210.11±3.29) g, were purchased from Nanjing Junke Biological Engineering Co. The laboratory temperature was controlled at (25±2)°C, the relative humidity was controlled at 55%-68%, and the experimental rats were kept in separate cages with 5 rats in each cage. All rats were fed and watered freely during the experiment.
Methods
Establishment of model
Ninety SD rats were randomly grouped and divided into a blank control group (BCG), a model group (MG) and an intervention group (IG) (n=30 in each group). Models of nonbacterial prostatitis were established according to the literature. Except for the BCG, all rats received ketamine for anesthesia and surgical castration under aseptic conditions. Estradiol benzoate was injected subcutaneously at a dose of 0.25 mg/kg on the dorsal side from the 2nd day for 30 d [18]. The MG and IG exhibited pathological characteristics of chronic prostatitis after successful modeling. Each procedure was approved by the Animal Care and Use Committee of Longhua Hospital Shanghai University of Traditional Chinese Medicine.
Experimental treatment
The IG was treated with 200 mg/kg of Xialiqi capsule via gavage daily from the 30th day of feeding, once per day. The MG and the BCG were treated with the same dose of saline via gavage for 7 d. The rats were executed 1 h after the last dose.
Specimen collection and treatment
Two hours after the final administration of the drug, the blood was collected from the rats by eyeball removal while the prostate tissue was dissected under aseptic conditions. (1) Prostate fluid. Five μL of prostate fluid was aspirated, diluted in saline and incubated at 37°C for 24 h. The number of bacterial colonies was recorded, and 10 μL of prostate fluid was aspirated to count the number of leukocytes and phospholipid vesicles density under microscope. (2) The prostatic fluid was collected and weighed to calculate the prostate coefficient. Meanwhile, specimen sections were prepared using prostate tissue, and the inflammatory cell infiltration was evaluated after H&E staining. (3) The prostate tissue was collected to assess the severity of inflammation (grades 0-3, grade 0 refers to no inflammation, grade 3 to severe inflammatory infiltration) and cell morphology. (4) The tumor necrosis factor-α (TNF-α), interleukin (IL)-6, IL-8, CD3+, CD8+ levels in serum as well as tissue homogenates of rats in each group were determined using ELISA kits of TNF-α (Item No. RX302058R), IL-6 (Item No. RX302856R), IL-8 (Item No. RX302854R), CD3+ (Item No. RX303007R) and CD8+ (Item No. RX303002R) purchased from Quanzhou Ruixin Biotechnology Co., Ltd.
Statistical methods
SPSS 22.0 was used to analyze the data collected from the study. The measurement data were expressed as mean ± standard deviation (mean ± SD). The t-test was used for the difference between groups for data meeting normal distribution or with even variance while approximate t-test was applied for data with uneven variance. Chi-square test was performed for the difference in count data between groups, and F-test was used for comparison between multiple groups. P<0.05 indicated significant difference. Graphpad Prism 8.3 was used for plotting figures [19].
Results
Morphological changes of cells under light microscope
Under a light microscope, there was no significant abnormal change in prostate cells in the BCG. The prostate tissue of rats in the MG showed an enlarged glandular lumen, increased discharge in the lumen, and severe changes in vasodilation and congestion. The papillary hyperplasia of acinar epithelial cells was more prominent, and a large number of lymphocytes and plasma cells appeared in the interstitium. The IG also had an enlarged glandular lumen, but glandular epithelial cells only slightly proliferated. The secretion in the cavity in the IG was significantly reduced compared with the MG, and there were very few inflammatory cells scattered in the interstitium.
Post-intervention changes in the prostate
The results showed that compared with the BCG, the number of leukocytes and colonies in the prostate fluid was significantly higher and the density of phospholipid vesicles was significantly lower in the MG, while the number of leukocytes and colonies in the IG was significantly lower and the density of phospholipid vesicles was significantly higher than that in the MG (P<0.05) (Figures 1, 2 and 3). Compared with the BCG, the MG showed a significant increase in the prostate mass, prostate coefficient and degree of inflammatory cell infiltration (P<0.05). The IG showed a decrease in these indices compared with the MG (P<0.05) (Figure 4). The mean inflammatory score was (1.02±0.32) for the BCG, (2.21±0.43) for the MG and (1.56±0.23) for the IG, and there were significant differences among the three groups of rats in terms of distribution of inflammatory grading and the inflammatory score (P<0.05) (Table 1).
Figure 1.

Comparison of number of colonies of rats in the three groups after intervention. Compared with the blank control group (B), the number of colonies in the prostatic fluid in the model group (A) was significantly higher, while the number of colonies in the intervention group (C) was significantly lower than that in the model group (P<0.05).
Figure 2.

Comparison of prostate of rats in the three groups. Compared with the blank control group (B), the prostate mass in the model group was significantly increased (A), and compared with the model group, the prostate mass in the intervention group was significantly decreased (C).
Figure 3.
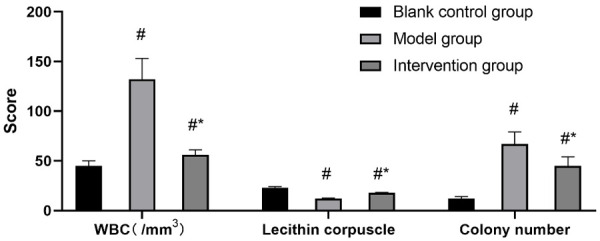
Effect of Xialiqi capsule on prostatic fluid of rats with nonbacterial prostatitis. #P<0.05 compared with the blank control group, and *P<0.05 compared with the model group.
Figure 4.

The effect of Xialiqi capsule on prostate indices in rats with nonbacterial prostatitis. #P<0.05 compared with the blank control group, and *P<0.05 compared with the model group.
Table 1.
Histopathological grade distribution and inflammatory grade score of the prostate
| Group | n | Inflammation grade | Inflammation grade score | |||
|---|---|---|---|---|---|---|
|
| ||||||
| 0 | 1 | 2 | 3 | |||
| Blank control group | 30 | 24 | 6 | 0 | 0 | 1.02±0.32 |
| Model group | 30 | 0 | 3 | 6 | 21 | 2.21±0.43 |
| Intervention group | 30 | 4 | 14 | 8 | 4 | 1.56±0.23 |
| F | - | 5.403 | 6.776 | |||
| P | - | 0.001 | <0.001 | |||
Differences in serum inflammatory and immune factors after intervention
The levels of the TNF-α, IL-6 and IL-8 were lowest in the BCG followed by the IG and the MG (P<0.05) (Figure 5). The BCG had the highest levels of CD3+ (Figure 6A) and CD8+ (Figure 6B), followed by the IG and the MG (P<0.05) (Figure 6).
Figure 5.

Differences in serum levels of inflammatory factors after intervention. A: TNF-α; B: IL-6; C: IL-8. #P<0.05 compared with the blank control group, and *P<0.05 compared with the model group.
Figure 6.

Differences in immune factor levels in serum after intervention. A: CD3+; B: CD8+. #P<0.05 compared with the blank control group, and *P<0.05 compared with the model group.
Discussion
The typical manifestations of nonbacterial prostatitis [20] include urinary frequency, urgency, painful urination, and nocturia. Some patients may also experience urethral discomfort or burning urination, vague pain in the perineum and lower abdomen, as well as soreness and pain in the lumbosacral, suprapubic, and groin regions. Nonbacterial prostatitis is characterized by slow onset, recurrent attacks, diverse symptoms, and lingering difficulty, which can have a serious impact on patients’ physical and mental health as well as their quality of life [21].
The results showed that compared with the BCG, the rats in the MG and the IG showed greater changes in prostate tissue, prostate fluid, and serum inflammatory factors and serum immune factors, namely, hyperplasia of prostate tissue, a significant increase in the number of leukocytes in prostate fluid, an increase in serum levels of inflammatory factors and altered levels of immune factors. These all indicate that the pathological characteristics of nonbacterial prostatitis are closely related to the inflammatory process. A study has been conducted on 100 patients with nonbacterial prostatitis, which showed that patients with nonbacterial prostatitis had significantly elevated levels of leukocytes in the prostate, well above normal levels, as well as high levels of serum inflammation-related factors in patients, which are all similar to the results of the present study [22]. We believe that the etiology of nonbacterial prostatitis is complex and difficult to treat. Its pathogenesis is closely associated with immune stress, as evidenced by the results of this study. The clinical effect of traditional Western medicine is not ideal, and the role of Chinese medicine is gaining importance [23,24].
In this study, we found that the IG treated with Xialiqi capsules had reduced prostate weight, decreased number of leukocytes in prostate fluid, higher density of phospholipid vesicles, and improved inflammatory status, and the levels of inflammatory factors such as TNF-α, IL-6 and IL-8 were also decreased significantly, but the levels of CD3+ and CD8+ appeared to increase significantly, which suggested that Xialiqi capsules effectively mitigated the inflammatory status of the prostate in patients with nonbacterial prostatitis and improved their inflammatory lesions. A randomized trial of 300 patients with type III A chronic prostatitis showed that compared with the control group treated with single antibacterial drugs, the NIH-Chronic Prostatitis Symptom Index (NIH-CPSI) decreased from (12.08±2.04) to (6.04±3.69) in the treatment group treated with Xialiqi capsules, significantly lower than of the control group (7.56±4.05) after intervention, and the researchers of the trial believe that Xialiqi capsule has the efficacy of invigorating the spleen, benefiting the kidneys, and promoting water circulation and dispersion of nodules, and has a better intervention effect on chronic prostatitis [25]. Another controlled study of 75 patients with chronic prostatitis showed that the total effective rate of Xialiqi capsule + levofloxacin capsule reached 83.33%, significantly higher than 68.33% in patients treated with antibacterial drugs alone, and the scholars consider that Xialiqi capsule can clear heat and detoxify toxins, and can exhibit better intervention effect in combination with antibacterial drugs [26]. In this study, the authors concluded that chronic prostatitis belongs to the category of “gonorrhea”, “fatigue strangury” and “white turbidity” in Chinese medicine. Traditional Chinese medicine believes that chronic prostatitis is caused by deficiency in origin and excess in superficiality. The formula of Xialiqi capsules includes Astragalus, Ligustri Lucidi Fructus, Talc, Prunella vulgaris, Amber, Cinnamon, Amur Cork-tree Bark, Semen Litchi, etc. Among them, Talc is diuretic and drenching, clearing heat and permeating dampness; Amur Cork-tree Bark clears heat and dampness; Amber activates blood circulation and disperses blood stasis. The combination of herbs can invigorate the spleen and kidney, relieve hydration, and improve many symptoms of nonbacterial prostatitis [27].
In conclusion, Xialiqi capsule has a good intervention effect on nonbacterial prostatitis, which can significantly alleviate the immune status of the rats and reduce the levels of cytokines in the serum and tissues of rats. The mechanism may be related to the inhibition of the infiltration of CD3+ and other T lymphocytes in the prostate tissue. The shortcoming of this study is that only a small number of representative inflammatory cytokines was selected, and the effect of drugs on pain level was not analyzed, which will be improved in the further research.
Acknowledgements
This work was supported by the Shanghai Municipal Health Commission special subject of Chinese traditional medicine research: study on the mechanism of the prescriptions of “Qi Ling” regulating tumor microenvironment and inhibiting CRPC cell proliferation and invasion through IL6/STAT3 [Subject number: 2020JQ002]; and the Shanghai Science and Technology Commission Shanghai Natural Science Foundation Project: study on mechanism about prescriptions of “Qi Ling” inhibiting androgen-independent transformation of prostate cancer cells by AR signaling pathway based on TRIM66/HP1γ complex [Subject number: 19ZR1458200]; and the National TCM clinical research base dragon medicine scholars (nursery plan) of Longhua Hospital Shanghai University of Traditional Chinese Medicine: Effect of Qi Ling prescriptions on regulation of JAK-STAT Signaling Pathway Against Prostate Cancer by TRIM66 [Subject number: LYTD-56] and The third batch of young Chinese name training program of LongHua Hospital Shanghai University of Traditional Chinese Medicine (Chen Lei) [Subject number: RC-2017-01-14].
Disclosure of conflict of interest
None.
References
- 1.Holt JD, Garrett WA, McCurry TK, Teichman JM. common questions about chronic prostatitis. Am Fam Physician. 2016;93:290–296. [PubMed] [Google Scholar]
- 2.Park MJ, Park HJ, Cheon WH, Park JH, Shin BC, Park NC. Herbal phytotherapy in chronic nonbacterial prostatitis. World J Mens Health. 2019 doi: 10.5534/wjmh.190091. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 3.Polackwich AS, Shoskes DA. Chronic prostatitis/chronic pelvic pain syndrome: a review of evaluation and therapy. Prostate Cancer Prostatic Dis. 2016;19:132–138. doi: 10.1038/pcan.2016.8. [DOI] [PubMed] [Google Scholar]
- 4.Sugimoto M, Zhang X, Ueda N, Tsunemori H, Taoka R, Hayashida Y, Hirama H, Miyauchi Y, Matsuoka Y, Naito H, Osaki Y, Kekehi Y. A phosphodiesterase 5 inhibitor, tadalafil, suppresses stromal predominance and inflammation in a rat model of nonbacterial prostatitis. BMC Urol. 2019;19:99. doi: 10.1186/s12894-019-0525-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang L, Liu Y, Chen XG, Zhang Y, Chen J, Hao ZY, Fan S, Zhang LG, Du HX, Liang CZ. MicroRNA expression profile in chronic nonbacterial prostatitis revealed by next-generation small RNA sequencing. Asian J Androl. 2019;21:351–359. doi: 10.4103/aja.aja_97_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhu YX, Xu NG. Clinical treatment of chronic nonbacterial prostatitis of kidney-yang deficiency type by acupuncture of Sanhuang points. Zhen Ci Yan Jiu. 2019;44:443–445. doi: 10.13702/j.1000-0607.180693. [DOI] [PubMed] [Google Scholar]
- 7.Liu F, Liu L, Wang Z, Chen L, Yu J, Xu X. The role of ethanol in the pathogenesis of non-bacterial prostatitis. Mol Med Rep. 2019;19:3848–3854. doi: 10.3892/mmr.2019.9991. [DOI] [PubMed] [Google Scholar]
- 8.Smothers A, Young S, Constantine L. Management of chronic nonbacterial prostatitis and chronic pelvic pain syndrome in the adult male patient with comorbid conditions. J Christ Nurs. 2020;37:E21–E26. doi: 10.1097/CNJ.0000000000000745. [DOI] [PubMed] [Google Scholar]
- 9.Xiong Y, Zhou L, Qiu X, Miao C. Anti-inflammatory and anti-hyperplastic effect of Bazhengsan in a male rat model of chronic nonbacterial prostatitis. J Pharmacol Sci. 2019;139:201–208. doi: 10.1016/j.jphs.2019.01.007. [DOI] [PubMed] [Google Scholar]
- 10.Liu X, Ran X, Riaz M, Kuang H, Dou D, Cai D. Mechanism investigation of tagetes patula L. against chronic nonbacterial prostatitis by metabolomics and network pharmacology. Molecules. 2019;24:2266. doi: 10.3390/molecules24122266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kang SW, Park JH, Seok H, Park HJ, Chung JH, Kim CJ, Kim YO, Han YR, Hong D, Kim YS, Kim SK. The effects of korea red ginseng on inflammatory cytokines and apoptosis in rat model with chronic nonbacterial prostatitis. Biomed Res Int. 2019;2019:2462561. doi: 10.1155/2019/2462561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yan Z, Huang C, Huang G, Wu Y, Wang J, Yi J, Mao W, Wang W. The effect of Jiedu Huoxue decoction on rat model of experimental nonbacterial prostatitis via regulation of miRNAs. Pharm Biol. 2020;58:745–759. doi: 10.1080/13880209.2020.1797124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang X, Chen Q, Ma M, Xie W, Gong B, Huang Y, Li Y, Liu S, Hu J, Liang S, Chen J, Liu F, Sun T. Expression and regulation of brain natriuretic peptide and natriuretic peptide receptor A (NPR-A) in L6-S1 dorsal root ganglia in a rat model of chronic nonbacterial prostatitis. Med Sci Monit. 2019;25:9042–9047. doi: 10.12659/MSM.915619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tantawy SA, Elgohary HM, Kamel DM. Trans-perineal pumpkin seed oil phonophoresis as an adjunctive treatment for chronic nonbacterial prostatitis. Res Rep Urol. 2018;10:95–101. doi: 10.2147/RRU.S167896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yamaguchi H, Kurita M, Okamoto K, Kotera T, Oka M. Voiding behavior and chronic pelvic pain in two types of rat nonbacterial prostatitis models: attenuation of chronic pelvic pain by repeated administration of tadalafil. Prostate. 2019;79:446–453. doi: 10.1002/pros.23750. [DOI] [PubMed] [Google Scholar]
- 16.Park JS, Jin MH, Hong CH. Neurologic mechanisms underlying voiding dysfunction due to prostatitis in a rat model of nonbacterial prostatic inflammation. Int Neurourol J. 2018;22:90–98. doi: 10.5213/inj.1836124.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yousefi S, Ahmadi-Hamedani M, Narenji Sani R, Moslemi HR, Ghafari Khaligh S, Darvishi MM. Pentoxifylline mitigates detrimental impact of chronic nonbacterial prostatitis on sperm characteristics, reproductive hormones and histopathology in rats. Andrologia. 2018;50 doi: 10.1111/and.12932. [DOI] [PubMed] [Google Scholar]
- 18.Seong KM, Jang G, Kim DW, Kim S, Song BK. Hwanglyunhaedok pharmacopuncture versus saline pharmacopuncture on chronic nonbacterial prostatitis/chronic pelvic pain syndrome. J Acupunct Meridian Stud. 2017;10:245–251. doi: 10.1016/j.jams.2017.06.001. [DOI] [PubMed] [Google Scholar]
- 19.Paulis G. Inflammatory mechanisms and oxidative stress in prostatitis: the possible role of antioxidant therapy. Res Rep Urol. 2018;10:75–87. doi: 10.2147/RRU.S170400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Meyers J. Exercise is effective therapy for chronic nonbacterial prostatitis and chronic pelvic pain syndrome. Am Fam Physician. 2016;94:533. [PubMed] [Google Scholar]
- 21.Ma W, Hu Q, Diao L, Cai Y, Feng J. The effect of drug oil moxibustion for contents of Zinc and C-reactive protein in succus prostaticus of chronic nonbacterial prostatitis. Zhongguo Zhen Jiu. 2017;37:840–844. doi: 10.13703/j.0255-2930.2017.08.011. [DOI] [PubMed] [Google Scholar]
- 22.Borovskaya TG, Kamalova SI, Polyektova ME, Mashanova VA, Vychuzhanina AV, Kseneva SI, Plotnikov MB, Goldberg VE. Experimental analysis of the efficacy of dihydroquercetin on the model of chronic nonbacterial inflammation of the prostatic gland. Bull Exp Biol Med. 2018;164:617–619. doi: 10.1007/s10517-018-4044-7. [DOI] [PubMed] [Google Scholar]
- 23.Liu YJ, Song GH, Liu GT. Investigation of the effect of traditional Chinese medicine on pain and inflammation in chronic nonbacterial prostatitis in rats. Andrologia. 2016;48:714–722. doi: 10.1111/and.12544. [DOI] [PubMed] [Google Scholar]
- 24.Wang HJ, Tyagi P, Chen YM, Chancellor MB, Chuang YC. Low energy shock wave therapy inhibits inflammatory molecules and suppresses prostatic pain and hypersensitivity in a capsaicin induced prostatitis model in rats. Int J Mol Sci. 2019;20:4777. doi: 10.3390/ijms20194777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Almeer RS, Muhammad NAE, Othman MS, Aref AM, Elgamal B, Moneim AEA. The potential protective effect of orange peel and selenium against 17β-estradiol- induced chronic non-bacterial prostatitis in rats. Anticancer Agents Med Chem. 2020;20:1061–1071. doi: 10.2174/1871520620666200331102609. [DOI] [PubMed] [Google Scholar]
- 26.Hajighorbani M, Ahmadi-Hamedani M, Shahab E, Hayati F, Kafshdoozan K, Keramati K, Amini AH. Evaluation of the protective effect of pentoxifylline on carrageenan-induced chronic non-bacterial prostatitis in rats. Inflammopharmacology. 2017;25:343–350. doi: 10.1007/s10787-017-0335-2. [DOI] [PubMed] [Google Scholar]
- 27.Funahashi Y, Takahashi R, Mizoguchi S, Suzuki T, Takaoka E, Ni J, Wang Z, DeFranco DB, de Groat WC, Tyagi P, Yoshimura N. Bladder overactivity and afferent hyperexcitability induced by prostate-to-bladder cross-sensitization in rats with prostatic inflammation. J Physiol. 2019;597:2063–2078. doi: 10.1113/JP277452. [DOI] [PMC free article] [PubMed] [Google Scholar]


