Abstract
Objective: To investigate the short-term efficacy of drug-eluting bead transarterial chemoembolization (DEB-TACE) loaded with epirubicin and raltitrexed in the treatment of intermediate and advanced primary hepatocellular carcinoma (PHC). Methods: One hundred patients with intermediate or advanced PHC were randomly divided into a control group (the CG, n=50) and an observation group (the OG, n=50). The CG was treated with conventional TACE (cTACE), and the OG was treated with DEB-TACE loaded with epirubicin and raltitrexed. The overall efficiency, the liver function indices, the tumor markers, the macrophage migration inhibitory factor (MIF) levels , the lesion diameters, the Child-Pugh scores, the adverse reactions, the median times to disease progression, the 1-year and 2-year recurrence rates, and the survival rates were compared between the two groups. Results: At 6 months after the surgery, the overall response rate in the OG (82.00%) was higher than it was in the CG (62.00%) (P<0.05). The serum alanine aminotransferase, total bilirubin, and aspartate aminotransferase levels were elevated in both groups after the intervention, but they were lower in the OG than they were in the CG (P<0.05). The serum alpha-fetoprotein, carcinoembryonic antigen, and MIF levels, and the lesion diameters were lower in both groups at one month after the intervention, and they were lower in the OG than they were in the CG (P<0.05). The incidence of abnormal blood test results in the OG was lower than it was in the CG (P<0.05). The OG also exhibited a longer median time to disease progression, lower 1-year and 2-year recurrence rates, and higher 1- and 2-year survival rates than the CG (P<0.05). Conclusion: DEB-TACE loaded with epirubicin and raltitrexed improves the short-term outcomes, reduces the tumor load, decreases the incidence of adverse events, and improves the survival rate in patients with intermediate and advanced PHC.
Keywords: Primary hepatocellular carcinoma, DEB-TACE, epirubicin, raltitrexed, hepatic artery embolization chemotherapy
Introduction
Primary hepatocellular carcinoma (PHC) is a common malignant tumor. The 2018 Global Cancer Statistics report showed that the PHC incidence (4.70%) and mortality (8.20%) rates ranked 6th and 4th among new cancer cases worldwide. PHC is difficult to treat and has a poor prognosis, with a 5-year survival rate from 5% to 15% [1]. PHC has an insidious onset and is not easily detected, and once it progresses to the intermediate and advanced stages, it cannot be cured by surgical resection of the tumor lesions [2]. Conventional transarterial chemoembolization (cTACE) is a less invasive palliative treatment, which injects chemotherapeutic drugs and embolizes blood vessels to the tumor using a shaped microcatheter for the superselective catheterization of the arteries, causing atrophy and necrosis of tumor tissue cells. cTACE is commonly used in the treatment of mid- and late-stage PHC. Although it can achieve certain therapeutic effects, it can also induce complications [3]. The macrophage migration inhibitory factor (MIF) is an important cytokine in the regulation of the immune system. It can regulate the biological activity of a variety of tumor-related factors, directly affect the division of normal cells and induce the transformation of oncogenes, and promote the occurrence and development of tumors. Liao et al. [4] showed that serum MIF is closely related to the occurrence and development of PHC and is an important biomarker for the auxiliary diagnosis of PHC.
Drug-eluting bead transarterial chemoembolization (DEB-TACE) is a novel drug delivery embolization system that allows high-dose chemotherapy drugs to be injected into the tumor donor artery through drug-loaded beads, and it prolongs the duration of the drug release to reduce the adverse effects caused by the rapid entry of chemotherapy drugs into the circulatio, reducing chemotherapy toxicity [5,6]. Several studies [5,7,8] have shown that DEB-TACE shows a better response for PHC than cTACE and improves the 1- and 2-year survival rates. Epirubicin and raltitrexed are commonly used in the chemotherapeutic treatment of PHC. However, the effects of DEB-TACE double-loaded with epirubicin and raltitrexed to treat intermediate and advanced PHC have been rarely reported. In this study, we compared the short-term efficacy of epiruboin and raltitrexed administrated using different methods in the treatment of PHC, and explored the clinical value of DEB-TACE.
Materials and methods
General information
One hundred patients with intermediate or advanced PHC admitted to our hospital from March 2016 to May 2018 were recruited as the study cohort, including 56 males and 44 females, ranging in age from 43 to 78 years old, with a mean age of (58.93±6.78) years. There were 72 single lesion patients and 28 multiple lesion patients. The patients were randomly divided into a control group (the CG, n=50) and an observation group (the OG, n=50).
Diagnostic criteria. The PHC was confirmed through CT and MRI examinations and pathological examinations and met the diagnostic criteria for PHC according to the 2017 edition of the “Diagnostic and Treatment Standard for PHC”. The PHC could not be surgically resected (single lesion diameter > 5 cm, or the diameter of 2 to 3 lesions > 3 cm and was stage B Barcelona-Clinic liver cancer (BCLC).
Inclusion criteria: (1) Patients who met the diagnostic criteria [9], (2) Patients under 80 years old, (3) Patients with an expected survival time ≥ 3 months, (4) Patients with ≤ 3 tumors and a maximum tumor diameter ≤ 10 cm, (5) A or B on the Child-Pugh staging, (6) An Eastern Collaborative Oncology Group (ECOG) score ≤ 2, (7) No bleeding tendency, normal coagulation or a coagulation dysfunction corrected with treatment, (8) A white blood cell count ≥ × 109/L, a platelet count ≥ 50 × 109/L, (9) An international normalized ratio (INR) of prothrombin time (PT) ≤ 2.3 or a PT prolonged by ≤ 3 seconds, (10) Blood creatinine < 1.5 times the upper limit.
Exclusion criteria: (1) Diffuse hepatocellular carcinoma, cholangiocarcinoma, (2) Grade III-IV portal vein tumor thrombus, (3) A history of ablative surgery, (4) A tumor volume > 2/3 of the liver volume, (5) Severe cirrhosis, ascites, (6) Vital organ lesions and dysfunction, (7) Combined uncontrolled infection, (8) Pregnant and lactating women, (9) A history of organ transplantation, and (10) Patients who could not undergo a CT/MRI examination.
Methods
In the CG, the agents were administered using cTACE. The right femoral artery was punctured by Seldinger, and the catheter was inserted into the superior mesenteric artery. Arteriography was performed to observe the shape of the portal vein and the presence of stenoses or obstructions. An abdominal arteriography was performed to observe the tumor diameter, location, and degree of staining. Epirubicin (Hisun Pfizer Pharmaceutical Co., Ltd., Art. No. 20170918) 30-50 mg/m2 + raltitrexed (Nanjing Chia Tai Tianqing Pharmaceutical Co., Ltd., Art. No. 170912) 4 mg/m2 was injected into the tumor donor artery, and 8-15 mL of iodinated oil was given to embolize the stained tumor. This study was approved by the Ethics Committee of Ji’an Central Hospital. All study participants provided written informed consent before participating in the study.
The OG was administered DEB-TACE using CalliSpheres microspheres (Suzhou Hengrui Jialisheng Biomedical Technology Co., Ltd., 20153771072), 100-300 µm or 300-500 µm in diameter. The microspheres were loaded with 1 mL of epirubicin hydrochloride (Zhejiang Haizheng Pharmaceutical Co., Ltd., H20000497, specification: 10 mg) 30-50 mg/m2 + raltitrexed (Nanjing Zhengda Tianqing Pharmaceutical Co., Ltd., H20090325, 2 mg) 4 mg/m2, respectively, for 15-30 min, and the suspension of the drug-loaded embolic microspheres mixed with a nonionic contrast agent was slowly injected (1 mL/min) into the tumor blood supply artery after the superselective catheterization. 8 Spheres blank microspheres or poppy B iodine oil (Jiangsu Hengrui Medicine Co., Ltd, H20163348, 10 ml) were used for the embolization until the end point of the embolization (the slowing or stagnation of the blood flow) was reached, if necessary.
The patients in both groups were treated once every 3 months for a total of 3 cycles, and were routinely given postoperative symptomatic support treatment such as antiemetics, hepatoprotective therapies, and energy supplementation, with a 2-year follow-up period starting after the first cycle of the intervention.
Outcome measurement
(1) At 6 months after the first intervention cycle, the efficacy was evaluated using the criteria for solid tumors (RECIST): complete remission (CR): the target lesion completely disappeared for 4 weeks; partial remission (PR): the reduction in the long diameter of lesions ≥ 30% over 4 weeks; disease progression (PD): the diameter increased ≥ 20% and the number of lesions increased; stable disease (SD): change of the target lesion between PR/PD. The overall response rate (ORR) = (CR+PR)/50 × 100%. (2) Liver function: 3 mL of peripheral venous blood was collected before and at 1 month after intervention. The alanine aminotransferase (ALT) was measured using the colorimetric method. The total bilirubin (TBiL) was measured using bilirubin oxidase. The aspartate aminotransferase (AST) was measured using the kinetic method. (3) Tumor markers and related macrophage factors: the serum was collected and the alpha-fetoprotein (AFP), carcinoembryonic antigen (CEA), and macrophage migration inhibitory factor (MIF) levels were measured using enzyme-linked immunosorbent assays. (4) Observation of the lesion diameters and the Child-Pugh grading before and at 1 month after the intervention. The Pugh-Child score is determined by scoring the five clinical measures of liver disease. A score of 1, 2, or 3 is given to each measure, with 3 being the most severe. Grade A: 5-6; Grade B: 7-9; Grade C 10-15. (5) Adverse reactions, including fever, abdominal pain, nausea and vomiting, painkillers/morphine, abnormal blood count, and malaise, were recorded. (6) During the 2-year follow-up, the median time to disease progression and the survival rate were recorded.
Statistical analysis
SPSS 25.0 software was used for the statistical analysis. The measurement data were expressed as (x̅±s) and compared using t tests. The count data were expressed as rates (%) and compared using χ2 tests. The rank data were compared using Z tests. GraphPad Prism 8 graphics software was used to draw the figures. P < 0.05 was considered statistically significant.
Results
Comparison of the baseline data
There were no significant differences between the two groups in terms of the baseline data such as gender, age, morbidity, lesion diameter, or Child-Pugh score (P > 0.05), so they were comparable, as shown in Table 1.
Table 1.
Comparison of the baseline data [x̅±s, n (%)]
| Group | n | Gender (M/F) | Age (years) | Onset of disease | Lesion diameter (cm) | Child-Pugh score | ||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| Single- | Multiple | Grade A | Grade B | |||||
| Observation group | 50 | 29/21 | 59.30±6.63 | 35 | 15 | 6.21±0.61 | 33 | 17 |
| Control group | 50 | 27/23 | 58.65±7.18 | 37 | 13 | 6.15±0.62 | 34 | 16 |
| t/χ2 | 0.162 | 0.470 | 0.198 | 0.488 | 0.045 | |||
| P | 0.687 | 0.639 | 0.656 | 0.627 | 0.832 | |||
Comparison of the efficacy at 6 months after the surgery
The ORR in the OG (82.00%) was higher than it was in the CG (62.00%) (P < 0.05), suggesting that DEB-TACE loaded with epirubicin and raltitrexed improves the short-term outcome (Table 2).
Table 2.
Comparison of the efficacy at 6 months after the intervention [n (%)]
| Group | n | CR | PR | PD | SD | ORR |
|---|---|---|---|---|---|---|
| Observation group | 50 | 19 (38.00) | 22 (44.00) | 7 (14.00) | 2 (4.00) | 41 (82.00) |
| Control group | 50 | 13 (26.00) | 18 (36.00) | 13 (26.00) | 6 (12.00) | 31 (62.00) |
| χ2 | 4.960 | |||||
| P | 0.026 |
Comparison of the liver function
The liver indicators were elevated in both groups at 1 month after the intervention, and they were lower in the OG than they were in the CG (P < 0.05), suggesting that DEB-TACE loaded with epirubicin and raltitrexed causes less damage to patients’ liver function (Figure 1).
Figure 1.
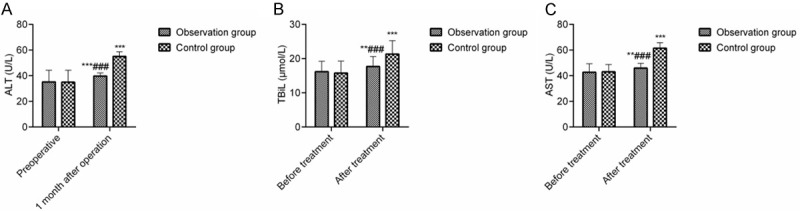
Comparison of the blood glucose and liver function levels. Note: (A) ALT; (B) TBiL; (C) AST. Compared with the preoperative levels, **P < 0.01, ***P < 0.001; compared with the one-month postoperative level, ###P < 0.001.
Comparison of the serum tumor markers and the macrophage cytokine levels
The serum AFP, CEA, and MIF levels showed no statistically significant differences between the two groups before the surgery (P > 0.05), and they were decreased in both groups at 1 month after the intervention and were lower in the OG than they were in the CG (P < 0.05), indicating that DEB-TACE loaded with epirubicin and raltitrexed improves the serum tumor markers and related macrophage cytokine levels and reduces the tumor load (Figure 2).
Figure 2.
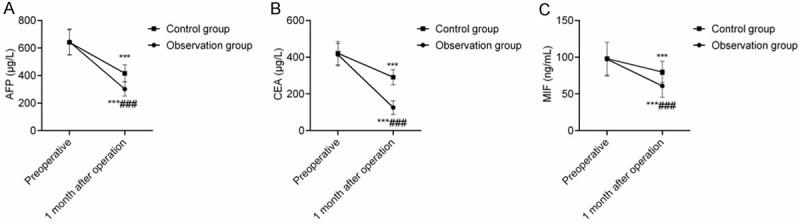
Comparison of the serum tumor markers and macrophage cytokine levels. Note: (A) AFP; (B) CEA; (C) MIF. Compared with the preoperative level, **P < 0.01, ***P < 0.001; compared with the one-month postoperative level, ###P < 0.001.
Comparison of the lesion diameters and the Child-Pugh scores
The lesion diameters were lower in both groups at 1 month after the intervention and were smaller in the OG than in the CG (P < 0.05). The difference in the Child-Pugh scores between the two groups at 1 month after intervention was not statistically significant (P > 0.05), indicating that DEB-TACE loaded with epirubicin and raltitrexed can shrink the lesion diameters (Table 3).
Table 3.
Comparison of the lesion diameters and Child-Pugh scores in the two groups [x̅±s n (%)]
| Group | n | Lesion diameter (cm) | Child-Pugh score | ||
|---|---|---|---|---|---|
|
| |||||
| Grade A | Grade B | Grade C | |||
| Observation group | 50 | 2.05±0.76* | 30 (60.00) | 20 (40.00) | 0 (0.00) |
| Control group | 50 | 3.16±0.78* | 25 (50.00) | 23 (46.00) | 2 (2.00) |
| t/χ2 | 7.207 | 2.664 | |||
| P | 0.000 | 0.264 | |||
indicates P < 0.05 compared to the preoperative level.
Comparison of the adverse reactions
The incidences of fever, pain, malaise, and nausea and vomiting showed no significant differences between the two groups (P > 0.05), and they were lower in the OG than they were in the CG (P < 0.05), indicating that DEB-TACE loaded with epirubicin and raltitrexed can reduce the occurrence of adverse reactions during chemotherapy (Table 4).
Table 4.
Comparison of the adverse reactions [n (%)]
| Group | n | Fever | Pain | Fatigue | Nausea and vomiting | Abnormal blood test results |
|---|---|---|---|---|---|---|
| Observation group | 50 | 15 (30.00) | 17 (34.00) | 8 (16.00) | 16 (32.00) | 6 (12.00) |
| Control group | 50 | 19 (38.00) | 23 (46.00) | 15 (30.00) | 20 (40.00) | 17 (34.00) |
| χ2 | 0.713 | 1.500 | 2.767 | 0.694 | 6.832 | |
| P | 0.398 | 0.221 | 0.096 | 0.405 | 0.009 |
Comparison of the follow-up results
The median time to disease progression in the OG was longer than it was in the CG (P < 0.05). The OG had lower 1- and 2-year recurrence rates and higher 1- and 2-year survival rates than the CG (P < 0.05), indicating that DEB-TACE loaded with epirubicin and raltitrexed can prolong the disease progression, reduce the recurrence rate, and improve the survival rate (Table 5).
Table 5.
Comparison of the follow-up results [x̅±s, n (%)]
| Group | n | Median time to disease progression (months) | Recurrent | Survival rate | ||
|---|---|---|---|---|---|---|
|
|
|
|||||
| 1 year | 2 year | 1 year | 2 year | |||
| Observation group | 50 | 13.71±1.92 | 2 (4.00) | 4 (8.00) | 43 (86.00) | 25 (50.00) |
| Control group | 50 | 8.36±1.76 | 8 (16.00) | 12 (24.00) | 35 (70.00) | 15 (30.00) |
| χ2 | 14.524 | 4.000 | 4.762 | 6.522 | 4.167 | |
| P | 0.000 | 0.046 | 0.029 | 0.011 | 0.041 | |
Discussion
Hepatic artery embolization chemotherapy is used to treat PHC by selectively blocking or reducing the blood supply to the lesion, causing ischemic and hypoxic necrosis, while killing the cancer cells through the injection of chemotherapeutic drugs [10-12]. However, this therapy often results in incomplete tumor vascular embolization and an inadequate collateral flow, which affects the therapeutic effect [13]. DEB-TACE loaded with microspheres were used in this study for the embolization, which can continuously embolize tumor vessels and slowly release chemotherapeutic drugs to maintain higher drug concentrations in the liver tumor tissues, prolong the drug’s duration of action, and improve the chemotherapeutic effects [14]. The present study showed that the efficacy of DEB-TACE for the treatment of intermediate and advanced PHC is superior to that of cTACE, a finding that is basically consistent with the results of a previous study [15]. It was also found that the DEB-TACE loaded chemotherapeutic drug was less detrimental to liver function [16].
AFP and CEA are highly expressed in the serum of patients with primary liver cancer and are important tumor markers of PHC [17,18]. MIF can inhibit the activity of macrophages and natural killer cells [19]. In this study, the serum AFP, CEA, and MIF levels in the OG were significantly lower than they were in the CG at 1 month after the intervention, while the lesion diameter in the OG was smaller than it was in the CG, suggesting that DEB-TACE loaded with chemotherapeutic agents is superior to cTACE in the inhibition of PHC. This is due to the fact that hepatic artery embolization chemotherapy exerts the dual effect of physical embolization and chemotherapeutic drugs, which enhances the killing effect on the tumor tissue cells, thereby reducing the tumor load, the tumor markers, and the related macrophage factor levels.
The evidence shows [20] that the DEB-TACE complication rate is significantly lower than the cTAC complication rate at the same dose of adriamycin. In this study, the incidence of abnormalities in the routine blood results in the OG was lower than it was in the CG in terms of bone marrow suppression and granulocytopenia. It indicated that the slow release of epirubicin with raltitrexed using DEB-TACE resulted in lower drug concentrations in the blood and reduced the risk of hematologic toxicity [21]. Also, the median time to disease progression in the OG was longer than it was in the CG, the 1- and 2-year recurrence rates in the OG were lower than they were in the CG, and the 1- and 2-year survival rates in the OG were higher than they were in the CG. This is due to the fact that the iodine oil deposited in the tumor is removed after the cTACE treatment, allowing the recanalization of the embolized vessels. This, combined with the increased secretions of the angiogenic factors due to local tissue ischemia and hypoxia after the embolization, leads to a regeneration of the tumor vessels and the formation of collateral circulation [22,23]. Tumor vascular regeneration and recanalization promote disease progression and recurrence [24]. In contrast, DEB-TACE loaded with microsphere embolization can avoid these issues, thus reducing recurrence and improving survival.
There are still some shortcomings to this study. The study design was too simple, as only patients undergoing embolization chemotherapy were recruited, and the efficacy of patients undergoing comprehensive chemotherapy was not compared. At the same time, the small study cohort may have a certain impact on the study results. Further multi-center research with a large sample will be carried out in the future.
In summary, DEB-TACE double-loaded with epirubicin with raltitrexed can improve the short-term outcomes, reduce the tumor load, decrease the incidence of adverse reactions, and improve survival.
Disclosure of conflict of interest
None.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Wang H, Lu Z, Zhao X. Tumorigenesis, diagnosis, and therapeutic potential of exosomes in liver cancer. J Hematol Oncol. 2019;12:133. doi: 10.1186/s13045-019-0806-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sun Y, Ji S, Ji H, Liu L, Li C. Clinical efficacy analysis of transcatheter arterial chemoembolization (TACE) combined with radiofrequency ablation (RFA) in primary liver cancer and recurrent liver cancer. J BUON. 2019;24:1402–1407. [PubMed] [Google Scholar]
- 4.Liao HF, Chen LZ, Liu M. Serum expression of macrophage migration inhibition factor in patients with primary hepatocellular carcinoma and its clinical significance. J Chin Phys. 2009;11:1400–1401. [Google Scholar]
- 5.Farid K, Elalfy H, Abo El-Khair SM, Elgamal H, Besheer T, Elmokadem A, Shabana W, Abed S, Elegezy M, El-Khalek AA, El-Morsy A, Negm A, Elsamanoudy AZ, El Deek B, Amer T, El-Bendary M. Prognostic value of vascular endothelial growth factor in both conventional and drug eluting beads transarterial chemoembolization for treatment of unresectable hepatocellular carcinoma in HCV patients. Expert Rev Gastroenterol Hepatol. 2020;14:1203–1214. doi: 10.1080/17474124.2020.1823215. [DOI] [PubMed] [Google Scholar]
- 6.Ballı HT, Aikimbaev K. Super-selective transarterial chemoembolization of hepatocellular carcinoma with doxorubicin-eluting beads sized 40-75 microns: assessment of efficacy and safety. Diagn Interv Radiol. 2020;26:482–487. doi: 10.5152/dir.2020.19410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang H, Cao C, Wei X, Shen K, Shu Y, Wan X, Sun J, Ren X, Dong Y, Liu Y, Zhai B. A comparison between drug-eluting bead-transarterial chemoembolization and conventional transarterial chemoembolization in patients with hepatocellular carcinoma: a meta-analysis of six randomized controlled trials. J Cancer Res Ther. 2020;16:243–249. doi: 10.4103/jcrt.JCRT_504_19. [DOI] [PubMed] [Google Scholar]
- 8.Roth GS, Teyssier Y, Abousalihac M, Seigneurin A, Ghelfi J, Sengel C, Decaens T. Idarubicin vs doxorubicin in transarterial chemoembolization of intermediate stage hepatocellular carcinoma. World J Gastroenterol. 2020;26:324–334. doi: 10.3748/wjg.v26.i3.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu YH. Diagnostic criteria and treatment principles of primary hepatic carcinoma. J Chin Phys. 2000;28:22. [Google Scholar]
- 10.Choi JW, Lee JM, Lee DH, Yoon JH, Kim YJ, Lee JH, Yu SJ, Cho EJ. Radiofrequency ablation using internally cooled wet electrodes in bipolar mode for the treatment of recurrent hepatocellular carcinoma after locoregional treatment: a randomized prospective comparative study. PLoS One. 2020;15:e0239733. doi: 10.1371/journal.pone.0239733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bian LF, Zhao XH, Gao BL, Zhang S, Ge GM, Zhan DD, Ye TT, Zheng Y. Predictive model for acute abdominal pain after transarterial chemoembolization for liver cancer. World J Gastroenterol. 2020;26:4442–4452. doi: 10.3748/wjg.v26.i30.4442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Peng Z, Cao G, Hou Q, Li L, Ying S, Sun J, Zhou G, Zhou J, Zhang X, Ji W, Yu Z, Li T, Zhu D, Hu W, Ji J, Du H, Shi C, Guo X, Fang J, Han J, Gu W, Xie X, Sun Z, Xu H, Wu X, Hu T, Huang J, Hu H, Zheng J, Luo J, Chen Y, Yu W, Shao G. The comprehensive analysis of efficacy and safety of CalliSpheres(®) drug-eluting beads transarterial chemoembolization in 367 liver cancer patients: a multiple-center, cohort study. Oncol Res. 2020;28:249–271. doi: 10.3727/096504019X15766663541105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chang Y, Jeong SW, Young Jang J, Jae Kim Y. Recent updates of transarterial chemoembolilzation in hepatocellular carcinoma. Int J Mol Sci. 2020;21:8165. doi: 10.3390/ijms21218165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Peisen F, Maurer M, Grosse U, Nikolaou K, Syha R, Ketelsen D, Artzner C, Bitzer M, Horger M, Grözinger G. Predictive performance of the mHAP-II score in a real-life western cohort with hepatocellular carcinoma following trans-arterial chemoembolisation with drug-eluting beads (DEB-TACE) Eur Radiol. 2020;30:3782–3792. doi: 10.1007/s00330-020-06734-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhou TY, Zhou GH, Zhang YL, Nie CH, Zhu TY, Wang HL, Chen SQ, Wang BQ, Yu ZN, Wu LM, Zheng SS, Sun JH. Drug-eluting beads transarterial chemoembolization with CalliSpheres microspheres for treatment of unresectable intrahepatic cholangiocarcinoma. J Cancer. 2020;11:4534–4541. doi: 10.7150/jca.39410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sun J, Zhou G, Xie X, Gu W, Huang J, Zhu D, Hu W, Hou Q, Shi C, Li T, Zhang X, Ji W, Ying S, Peng Z, Zhou J, Yu Z, Ji J, Du H, Guo X, Fang J, Han J, Xu H, Sun Z, Yu W, Shao G, Wu X, Hu H, Li L, Zheng J, Luo J, Chen Y, Cao G, Hu T. Efficacy and safety of drug-eluting beads transarterial chemoembolization by CalliSpheres(®) in 275 hepatocellular carcinoma patients: results from the Chinese CalliSpheres(®) transarterial chemoembolization in liver cancer (CTILC) study. Oncol Res. 2020;28:75–94. doi: 10.3727/096504019X15662966719585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang Y, Zhang MW, Fan XX, Mao DF, Ding QH, Zhuang LH, Lv SY. Drug-eluting beads transarterial chemoembolization sequentially combined with radiofrequency ablation in the treatment of untreated and recurrent hepatocellular carcinoma. World J Gastrointest Surg. 2020;12:355–368. doi: 10.4240/wjgs.v12.i8.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shi Q, Chen D, Zhou C, Liu J, Huang S, Yang C, Xiong B. Drug-eluting beads versus lipiodol transarterial chemoembolization for the treatment of hypovascular hepatocellular carcinoma: a single-center retrospective study. Cancer Manag Res. 2020;12:5461–5468. doi: 10.2147/CMAR.S255960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Luo J, Zheng J, Shi C, Fang J, Peng Z, Huang J, Sun J, Zhou G, Li T, Zhu D, Xu H, Hou Q, Ying S, Sun Z, Du H, Xie X, Cao G, Ji W, Han J, Gu W, Guo X, Shao G, Yu Z, Zhou J, Yu W, Zhang X, Li L, Hu H, Hu T, Wu X, Chen Y, Ji J, Hu W. Drug-eluting beads transarterial chemoembolization by CalliSpheres is effective and well tolerated in treating intrahepatic cholangiocarcinoma patients: a preliminary result from CTILC study. Medicine (Baltimore) 2020;99:e19276. doi: 10.1097/MD.0000000000019276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Matsui Y, Fujiwara H, Hiraki T, Iguchi T, Komaki T, Tanaka T, Yagi T, Gobara H, Kanazawa S. Histological findings in non-tumoral liver and tumor after chemoembolization with drug-eluting beads. Minim Invasive Ther Allied Technol. 2020;29:217–223. doi: 10.1080/13645706.2019.1626250. [DOI] [PubMed] [Google Scholar]
- 21.Wang TC, Zhang ZS, Xiao YD. Determination of risk factors for pain after transarterial chemoembolization with drug-eluting beads for hepatocellular carcinoma. J Pain Res. 2020;13:649–656. doi: 10.2147/JPR.S246197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chiu SH, Chang PY, Shih YL, Huang WY, Ko KH, Chang WC, Huang GS. Efficacy and safety of supplemental transarterial chemoembolization through extrahepatic collateral arteries with drug-eluting beads: treatment for unresectable hepatocellular carcinoma. Drug Des Devel Ther. 2020;14:5029–5041. doi: 10.2147/DDDT.S266470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grumme J, Werncke T, Meine TC, Becker LS, Kloeckner R, Maschke SK, Kirstein MM, Vogel A, Wacker FK, Meyer BC, Hinrichs JB, Rodt T. Transarterial chemoembolization for hepatocellular carcinoma: quality of life, tumour response, safety and survival comparing two types of drug-eluting beads. Abdom Radiol (NY) 2020;45:3326–3336. doi: 10.1007/s00261-019-02349-w. [DOI] [PubMed] [Google Scholar]
- 24.Pipa-Muñiz M, Sanmartino S, Mesa A, Álvarez-Navascués C, González-Diéguez ML, Cadahía V, Rodríguez JE, Vega F, Rodríguez M, Costilla-García SM, Varela M. The development of early ascites is associated with shorter overall survival in patients with hepatocellular carcinoma treated with drug-eluting embolic chemoembolization. BMC Gastroenterol. 2020;20:166. doi: 10.1186/s12876-020-01307-x. [DOI] [PMC free article] [PubMed] [Google Scholar]


