Abstract
Objective: This research investigated the combined analgesic effects of intercostal nerve block and intravenous patient-controlled analgesia (IV-PCA) on patients after lung cancer surgery. Methods: 95 patients with thoracoscopic radical resection of lung cancer from April 2017 to July 2020 were enrolled as the research objects, and randomly divided into observation-group (n=50) and control-group (n=45) by random number table. The control-group received intravenous patient-controlled analgesia (IV-PCA), and the observation group received combinative treatment of intercostal nerve block and IV-PCA. The changes of VAS scores and Ramsay sedation scores postoperatively, the satisfaction with analgesia of patients, the number of IV-PCA pump compressions and the incidence of postoperative anaesthetic-related adverse reactions were compared between the two groups. Results: The VAS score of the observation-group was markedly lower than that of the control-group 2 h, 4 h, 8 h, 12 h and 24 h after surgery (P<0.05). There was no statistically significant difference in Ramsay sedation scores between the two groups 2 h, 4 h, 8 h, 12 h and 24 h after surgery (P>0.05). The satisfaction score of analgesia and the times of IV-PCA pump compressions of the observation group were obviously less than those of the control group (P<0.05). The incidences of nausea and emesia, bradycardia and somnolence between the two groups of objects were statistically insignificant (P>0.05). Conclusion: The combinative treatment of intercostal nerve block and IV-PCA is safe and have obviously postoperative analgesic effect on patients undergoing thoracoscopic resection of lung cancer.
Keywords: Intercostal nerve block, intravenous controlled analgesia, lung cancer, analgesic effect
Introduction
Lung cancer is one of the most common malignant tumors over the world. In recent years, with the continuous development and progress of the varying examination technologies, an increasing number of patients have been diagnosed in early stages [1]. Compared with traditional thoracotomy, the thoracoscopic radical resection has been recognized as the preferred method for the treatment of lung cancer. This surgical method has merits of minimally invasiveness, less postoperative pain and impact on pulmonary and immunizing functions, rapid postoperative recovery and fewer complications [2,3]. Intercostal nerve block is one of the common methods adopted in postoperative analgesia. It has low risk and failure, and less discomfort in patients compared with epidural block [4,5]. In addition, the single use of intravenous patient-controlled analgesia (IV-PCA) is prone to cause serious adverse reactions in patients due to the high drug concentration. Such adverse reaction will not only affect the postoperative recovery of patients, but also increases the incidence of postoperative complications and mortality [6,7]. Therefore, to further improve the analgesic effect of thoracoscopic radical resection of lung cancer, this study investigated and evaluated the combinative treatment of intercostal nerve block and IV-PCA on postoperative analgesic treatment after of lung cancer surgery.
Materials and methods
Research objects
95 patients with thoracoscopic radical resection of lung cancer in our hospital from April 2017 to July 2020 were selected as the research objects, and randomly divided into observation-group (n=50) and control-group (n=45) by random number table. The study was implemented after acquiring approval of the hospital ethics committee.
Inclusion and exclusion criteria
Inclusion criteria: (1) Patients aged between 18 to 70 years; (2) Patients that were scheduled for thoracoscopic radical resection; (3) Patients diagnosed by clinical pathology as non-small cell lung cancer; (4) Patients classified as stage I~II by American Society of Anesthesiologists (ASA) grading; (5) Patients without experience of drug allergy or opioid abuse; and (6) Those who were willing to accept the trail and signed the informed consent form.
Exclusion criteria: (1) Patients that allergic to the drugs involved in this study; (2) Patients with abnormal coagulation function; (3) Patients with liver or kidney dysfunction; (4) Patients with experience of mental illness; or (5) Patients with a history of chronic pain.
Methods
The control-group underwent IV-PCA therapy: The objects received preoperative intramuscular injection of 0.1 g phenobarbital sodium and subcutaneous injection of 0.5 mg atropine. After the patient entered into the surgery room, we established the venous access for patient, and routinely monitored the vital indicators of blood pressure, heart rate, blood oxygen saturation, etc. The intravenous injection of midazolam (0.04 mg/kg), propofol (1.0~1.5 mg/kg), fentanyl (0.4 μg/kg), and vecuronium (0.1 mg/kg) was used as anesthesia induction. After anesthesia induction, target controlled infusion of 2~4 μg/ml propofol was performed to maintain anesthesia. The patients received intravenous infusion of fentanyl and vecuronium intermittently to maintain analgesia state and muscle relaxation. The anesthesia depth BIS of patient was held at 45-55, and blood pressure and heart rate were controlled within ±20% of the baseline value. After surgery, the patients received IV-PCA of Fentanyl (15 μg/kg diluted to 120 ml with 0.9% sodium chloride solution). The infusion speed was set as 2 ml/h, the additional dose was 1.0 ml, and the duration was 30 min. In addition to the treatment implemented in control-group, the observation-group received nerve block before the end of surgery. We used 5 ml of 0.375% ropivacaine under direct vision of thoracoscopy and placed it in intercostal cavity of the thoracic drainage tube and the surgical incision for nerve block.
Indexes observation
Postoperative pain of the two groups of objects was evaluated and scored 2 h, 4 h, 8 h, 12 h and 24 h after surgery by Visual Analogue Scale (VAS). The range of scale was from 0 to 10 points, with 0 point referred as painless and a score of 10 points indicated severe pain. The higher score represented the higher degree of pain.
The sedative effects of the two groups were evaluated by Ramsay Sedation Scale and compared 2 h, 4 h, 8 h, 12 h and 24 h after surgery. 1 point referred that the patient was restless and irritable; 2 points referred the patient can be cooperate quietly; a score of 3 indicated that the patient was sleepy and could follow instructions; 4 points indicated the patient was in sleep and could wake up; 5 points referred as sluggish breathing and reaction; and 6 points referred as the patient was in deep sleep and could not be able to awake.
The scores of the two groups of patients on analgesia satisfaction were recorded 48 hours after surgery. 1 point referred that the patient was completely painless and very satisfied; 2 points referred occasionally mild pain and satisfied; 3 points referred mild pain and occasionally moderate pain, with basically satisfied; 4 points referred continuous pain of beyond moderate degree, and was unsatisfied; a score of 5 point indicated that the patient had consistently severe pain and was extremely unsatisfied.
The times of postoperative IV-PCA pump pressing were compared between the two sets of objects.
The incidence of adverse reactions related to postoperative anesthesia, including nausea and emesia, bradycardia and somnolence, were compared between the two groups.
Statistical analysis
Data processing and analysis was conducted via SPSS 22.0 (IBM Corp). The comparison of measurement data and enumeration data were by t-test and χ 2 test respectively. The statistically significant of difference was fixed by P<0.05. The graphic software was Excel 2007.
Results
Clinical data
There was insignificant difference in gender, age, BMI and ASA grade between the two sets of objects (P>0.05) (Table 1).
Table 1.
Comparison of clinical data between the two groups
| Group | Number of cases | Gender | Age (yd, x̅±s) | BMI (kg/m2, x̅±s) | ASA classification | ||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| Male | Female | I | II | ||||
| Observation group | 50 | 31 | 19 | 51.28±6.39 | 23.74±4.20 | 28 | 22 |
| Control group | 45 | 30 | 15 | 50.97±7.20 | 24.10±3.79 | 25 | 20 |
| t/χ 2 | - | 0.2244 | 0.2223 | 0.4368 | 0.0019 | ||
| P | - | 0.6357 | 0.8245 | 0.6633 | 0.9652 | ||
Comparison of postoperative VAS scores
The VAS scores in the two groups of patients 4 h, 8 h, 12 h and 24 h after surgery had a remarkably rise than those 2 h after surgery (P<0.05); At 2 h, 4 h, 8 h, 12 h and 24 h after surgery, and the observation group had dramatically lower score than the control group (P<0.05) (Table 2 and Figure 1).
Table 2.
Comparison of postoperative VAS scores between the two groups (points, x̅±s)
| Group | Number of cases | 2 h after surgery | 4 h after surgery | 8 h after surgery | 12 h after surgery | 24 h after surgery |
|---|---|---|---|---|---|---|
| Observation group | 50 | 1.75±0.47 | 2.43±0.72* | 2.85±0.93* | 3.64±1.20* | 3.29±0.92* |
| Control group | 45 | 3.82±0.94 | 4.62±1.21* | 5.21±1.52* | 5.74±1.43* | 5.49±1.33* |
| t | - | 13.780 | 10.845 | 9.229 | 7.779 | 9.453 |
| P | - | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
Note: Compared with 2 h after surgery;
P<0.05.
Figure 1.
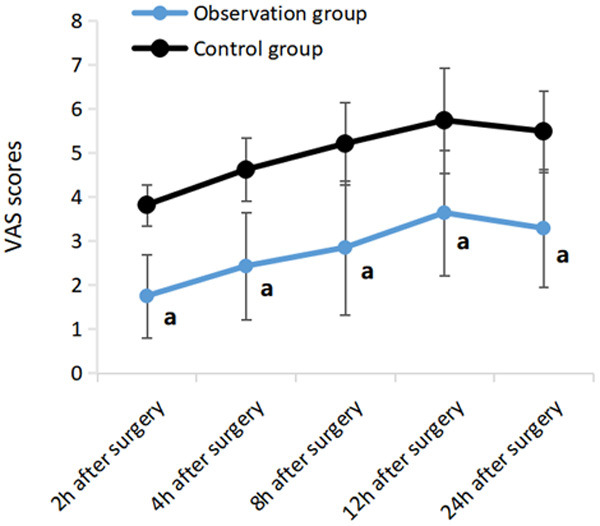
Comparison of postoperative VAS scores between the two groups. Note: Compared with control group, a P<0.05.
Comparison of Ramsay Sedation score after surgery
There was no significant change in the Ramsay sedation scores of the two groups of patients at each time point (P>0.05); and there was no statistically significant difference in Ramsay sedation scores between the two groups 2 h, 4 h, 8 h, 12 h and 24 h after surgery (P>0.05) (Table 3).
Table 3.
Comparison of Ramsay Sedation score between the two groups after surgery (points, x̅±s)
| Group | Number of cases | 2 h after surgery | 4 h after surgery | 8 h after surgery | 12 h after surgery | 24 h after surgery |
|---|---|---|---|---|---|---|
| Observation group | 50 | 2.39±0.52 | 2.45±0.33 | 2.33±0.39 | 2.26±0.47 | 2.24±0.60 |
| Control group | 45 | 2.31±0.49 | 2.38±0.45 | 2.37±0.48 | 2.30±0.56 | 2.21±0.54 |
| t | - | 0.769 | 0.870 | 0.448 | 0.378 | 0.255 |
| P | - | 0.444 | 0.386 | 0.656 | 0.706 | 0.799 |
Comparison of patients’ satisfaction with analgesia and times of IV-PCA pump compressions
The satisfaction score of analgesia and the times of IV-PCA pump compressions of the observation group were obviously less than those of the control group (P<0.05) (Table 4).
Table 4.
Comparison of patients’ satisfaction with analgesia and times of IV-PCA pump compressions (x̅±s)
| Group | Number of cases | Score of analgesic satisfaction | Times of IV-PCA pump compressions |
|---|---|---|---|
| Observation group | 50 | 1.85±0.64 | 14.37±4.20 |
| Control group | 45 | 3.02±0.93 | 27.96±8.49 |
| t | - | 7.202 | 10.040 |
| P | - | <0.001 | <0.001 |
Comparison of adverse reactions
The incidences of nausea and emesia, bradycardia and somnolence between the two groups of objects were statistically insignificant (P>0.05) (Table 5).
Table 5.
Comparison of adverse reactions between the two groups [n (%)]
| Group | Number of cases | Nausea and emesia | Bradycardia | Somnolence |
|---|---|---|---|---|
| Observation group | 50 | 6 (12.00) | 3 (6.00) | 2 (4.00) |
| Control group | 45 | 7 (15.56) | 1 (2.22) | 4 (8.89) |
| χ 2 | - | 0.254 | 0.163 | 0.309 |
| P | - | 0.615 | 0.686 | 0.578 |
Discussion
Lung cancer is among the most frequently occurred malignancies in the world, of which non-small cell lung cancer is the most common histological type and accounts for about 80% of all lung cancers [8]. In 2006, National Comprehensive Cancer Network (NCCN) included video-assisted thoracoscopy in its guidelines as the standard surgical procedure for lung cancer treatment for the first time. At present, the clinical application of video-assisted thoracoscopic radical resection of lung cancer has gradually been clinically recognized [9,10]. Video-assisted thoracoscopic radical resection of lung cancer has less surgical trauma and rapid postoperative recovery, which can meet the requirements of modern minimally invasive surgical treatment [11,12]. However, the postoperative pain of thoracic surgery is more severe, which is about 4 times compared to the surface surgery. Patients are often unable to take deep breath and cough after surgery due to pain. This kind of situation can easily lead to poor expectoration and adverse reactions such as atelectasis, pneumonia, respiratory failure and hypoxemia, poor postoperative healing and rehabilitation of patients [13-15]. Therefore, good analgesic measures are of great significance to patients after video-assisted thoracoscopy.
The ideal analgesia method should be simple, easy to complete, with high success rate and less complications. Epidural analgesia is one of the commonly adopted methods and is very effective in most patients [16-18]. However, it can cause nausea, emesia, pruritus, uroschesis, headache, and back pain. Epidural block can cause hypotension and critical block in muscle motor function, which leads to delayed postoperative activity [19-21]. In addition, epidural analgesia is accompanied by certain risks, such as epidural perforation, hard hematoma, and infection. Intercostal nerve block is less dangerous, and easy to operate and practice. The previous complications associated with intercostal nerve block, such as anesthesia into the blood, pneumothorax, toxic reactions of local anesthetics, etc., have been well avoided by performing the surgery under direct vision to the chest cavity. Compared with epidural anesthesia, intercostal nerve block does not produce hematoma and rarely produces motion block [22-24]. This study evaluated the postoperative analgesic effect of intercostal nerve block combined with IV-PCA on lung cancer patients.
The results showed that the VAS score of observation-group was remarkably lower than that of control-group 2 h, 4 h, 8 h, 12 h, and 24 h after surgery; and the satisfaction of analgesia and the times of IV-PCA pump compressions in observation group were substantially lower than those in the control group. These results are consistent with those reported in previous studies [25,26], indicating that the intercostal nerve block combined with IV-PCA can evidently reduce the postoperative pain in patients undergoing video-assisted thoracoscopic radical resection of lung cancer, which can effectively improve their satisfaction with postoperative analgesia, and reduce the times of IV-PCA pump compressions. In addition, there was statistically insignificant difference in Ramsay Sedation Scale 2 h, 4 h, 8 h, 12 h and 24 h postoperatively between the two groups; and the incidences of nausea and emesis, bradycardia and somnolence between the two groups were statistically insignificant. This suggested the combinative application of intercostal nerve block and IV-PCA will not have any impact on the depth of sedation in patients, and would not increase the incidence of postoperative adverse reactions. The results of this study are similar to those reported in related studies [27,28], according to the analysis of its possible mechanism, ropivacaine, the local anesthetic drug for nerve block, can delay the conduction of nerve impulse by increasing the threshold of nerve action potential in patients, and thus reducing the increased rate of cellular action potential in body. Ultimately, the generation and transmission of nerve impulses can be blocked.
Due to the small quantity of subjects included in this study and the lack of in-depth discussion and analysis on the specific mechanism, it is suggested to further expand the sample size in subsequent studies in order to obtain more reliable clinical research results.
In conclusion, the combined treatment of intercostal nerve block and IV-PCA is safe and has obviously postoperative analgesic effect on patients undergoing thoracoscopic resection of lung cancer.
Disclosure of conflict of interest
None.
References
- 1.Zheng Y, Wang H, Ma X, Cheng Z, Cao W, Shao D. Comparison of the effect of ultrasound-guided thoracic paravertebral nerve block and intercostal nerve block for video-assisted thoracic surgery under spontaneous-ventilating anesthesia. Rev Assoc Med Bras (1992) 2020;66:452–457. doi: 10.1590/1806-9282.66.4.452. [DOI] [PubMed] [Google Scholar]
- 2.Altıparmak B, Korkmaz Toker M, Uysal AI, Dere Ö, Uğur B. Evaluation of ultrasound-guided rhomboid intercostal nerve block for postoperative analgesia in breast cancer surgery: a prospective, randomized controlled trial. Reg Anesth Pain Med. 2020;45:277–282. doi: 10.1136/rapm-2019-101114. [DOI] [PubMed] [Google Scholar]
- 3.Sheets NW, Davis JW, Dirks RC, Pang AW, Kwok AM, Wolfe MM, Sue LP. Intercostal nerve block with liposomal bupivacaine vs epidural analgesia for the treatment of traumatic rib fracture. J Am Coll Surg. 2020;231:150–154. doi: 10.1016/j.jamcollsurg.2019.12.044. [DOI] [PubMed] [Google Scholar]
- 4.Hacıbeyoğlu G, Arıcan Ş, Ulukaya SO, Yılmaz R, Reisli R, Tuncer Uzun S. Evaluation of the efficacy of erector spinae plane block and intercostal nerve block in the postherpetic neuralgia. Agri. 2020;32:208–218. doi: 10.14744/agri.2020.87523. [DOI] [PubMed] [Google Scholar]
- 5.Boccara D, Picard F, Chaouat M, Mimoun M, Serror K. Postoperative pain control by intercostal nerve block after augmentation mammoplasty. Aesthetic Plast Surg. 2018;42:338–339. doi: 10.1007/s00266-017-0990-0. [DOI] [PubMed] [Google Scholar]
- 6.Kang CM, Kim WJ, Yoon SH, Cho CB, Shim JS. Postoperative pain control by intercostal nerve block after augmentation mammoplasty. Aesthetic Plast Surg. 2017;41:1031–1036. doi: 10.1007/s00266-017-0802-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huan S, Deng Y, Wang J, Ji Y, Yin G. Efficacy and safety of paravertebral block versus intercostal nerve block in thoracic surgery and breast surgery: a systematic review and meta-analysis. PLoS One. 2020;15:e0237363. doi: 10.1371/journal.pone.0237363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Park JW, Kim JH, Woo KJ. Intraoperative intercostal nerve block for postoperative pain control in pre-pectoral versus subpectoral direct-to-implant breast reconstruction: a retrospective study. Medicina (Kaunas) 2020;56:325. doi: 10.3390/medicina56070325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mogahed MM, Elkahwagy MS. Paravertebral block versus intercostal nerve block in non-intubated uniportal video-assisted thoracoscopic surgery: a randomised controlled trial. Heart Lung Circ. 2020;29:800–807. doi: 10.1016/j.hlc.2019.04.013. [DOI] [PubMed] [Google Scholar]
- 10.Zhu M, Gu Y, Sun X, Liu X, Chen W, Miao C. Ultrasound-guided intercostal nerve block following esophagectomy for acute postoperative pain relief in the postanesthesia care unit. Pain Pract. 2018;18:879–883. doi: 10.1111/papr.12689. [DOI] [PubMed] [Google Scholar]
- 11.Liu X, Zheng B, Huang C, Wu Q, Zhan L. Comment on “Comparison of the effect of ultrasound-guided thoracic paravertebral nerve block and intercostal nerve block for video-assisted thoracic surgery under spontaneous-ventilating anesthesia”. Rev Assoc Med Bras (1992) 2020;66:1009. doi: 10.1590/1806-9282.66.7.1009. [DOI] [PubMed] [Google Scholar]
- 12.Lee HJ, Park HS, Moon HI, Yoon SY. Effect of ultrasound-guided intercostal nerve block versus fluoroscopy-guided epidural nerve block in patients with thoracic herpes zoster: a comparative study. J Ultrasound Med. 2019;38:725–731. doi: 10.1002/jum.14758. [DOI] [PubMed] [Google Scholar]
- 13.Singh I, Yadav OK, Gupta S. Efficacy of intercostal nerve block with 0.25% bupivacaine in percutaneous nephrolithotomy: a prospective randomized clinical trial. Urol Ann. 2019;11:363–368. doi: 10.4103/UA.UA_141_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ohgoshi Y, Usui Y, Terada S, Takeda Y, Ohtsuka A, Matsuno K, Okuda Y. Visualization of injectate spread of intercostal nerve block: a cadaveric study. JA Clin Rep. 2018;4:65. doi: 10.1186/s40981-018-0204-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ahmed Z, Samad K, Ullah H. Role of intercostal nerve block in reducing postoperative pain following video-assisted thoracoscopy: a randomized controlled trial. Saudi J Anaesth. 2017;11:54–57. doi: 10.4103/1658-354X.197342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang W, Song W, Yang C, Sun Q, Chen H, Zhang L, Bu X, Zhan L, Xia Z. Ultrasound-guided pectoral nerve block I and serratus-intercostal plane block alleviate postoperative pain in patients undergoing modified radical mastectomy. Pain Physician. 2019;22:E315–E323. [PubMed] [Google Scholar]
- 17.Wang Y, Cheng J, Yang L, Wang J, Liu H, Lv Z. Ropivacaine for intercostal nerve block improves early postoperative cognitive dysfunction in patients following thoracotomy for esophageal cancer. Med Sci Monit. 2019;25:460–465. doi: 10.12659/MSM.912328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bijkerk E, Cornelissen AJM, Sommer M, Van Der Hulst RRWJ, Lataster A, Tuinder SMH. Intercostal nerve block of the anterior cutaneous branches and the sensibility of the female breast. Clin Anat. 2020;33:1025–1032. doi: 10.1002/ca.23532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abu Elyazed MM, Abdelghany MS, Mostafa SF. The analgesic efficacy of pecto-intercostal fascial block combined with pectoral nerve block in modified radical mastectomy: a prospective randomized trial. Pain Physician. 2020;23:485–493. [PubMed] [Google Scholar]
- 20.Fiorelli S, Leopizzi G, Menna C, Teodonio L, Ibrahim M, Rendina EA, Ricci A, De Blasi RA, Rocco M, Massullo D. Ultrasound-guided erector spinae plane block versus intercostal nerve block for post-minithoracotomy acute pain management: a randomized controlled trial. J Cardiothorac Vasc Anesth. 2020;34:2421–2429. doi: 10.1053/j.jvca.2020.01.026. [DOI] [PubMed] [Google Scholar]
- 21.Kaushal B, Chauhan S, Saini K, Bhoi D, Bisoi AK, Sangdup T, Khan MA. Comparison of the efficacy of ultrasound-guided serratus anterior plane block, pectoral nerves II block, and intercostal nerve block for the management of postoperative thoracotomy pain after pediatric cardiac surgery. J Cardiothorac Vasc Anesth. 2019;33:418–425. doi: 10.1053/j.jvca.2018.08.209. [DOI] [PubMed] [Google Scholar]
- 22.Lei D, Sha Y, He L. Is ultrasound-guided thoracic paravertebral nerve block better than intercostal nerve block for video-assisted thoracic surgery under spontaneous-ventilating anesthesia? Rev Assoc Med Bras (1992) 2020;66:568. doi: 10.1590/1806-9282.66.5.568. [DOI] [PubMed] [Google Scholar]
- 23.Matchett G. Intercostal nerve block and neurolysis for intractable cancer pain. J Pain Palliat Care Pharmacother. 2016;32:114–117. doi: 10.3109/15360288.2016.1167804. [DOI] [PubMed] [Google Scholar]
- 24.Pedoto A, Amar D. Liposomal bupivacaine for intercostal nerve block: pricey or priceless? Semin Thorac Cardiovasc Surg. 2017;29:538–539. doi: 10.1053/j.semtcvs.2017.08.016. [DOI] [PubMed] [Google Scholar]
- 25.Chen N, Qiao Q, Chen R, Xu Q, Zhang Y, Tian Y. The effect of ultrasound-guided intercostal nerve block, single-injection erector spinae plane block and multiple-injection paravertebral block on postoperative analgesia in thoracoscopic surgery: a randomized, double-blinded, clinical trial. J Clin Anesth. 2020;59:106–111. doi: 10.1016/j.jclinane.2019.07.002. [DOI] [PubMed] [Google Scholar]
- 26.Zhan Y, Chen G, Huang J, Hou B, Liu W, Chen S. Effect of intercostal nerve block combined with general anesthesia on the stress response in patients undergoing minimally invasive mitral valve surgery. Exp Ther Med. 2017;14:3259–3264. doi: 10.3892/etm.2017.4868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yao F, Xu S, Zhang W, Xiong H, Han J, Zhu A. Impacts of different administration modes of dexmedetomidine with 0.5% ropivacaine on intercostal nerve block. Ann Palliat Med. 2020;9:447–450. doi: 10.21037/apm.2020.03.25. [DOI] [PubMed] [Google Scholar]
- 28.Song WQ, Wang W, Yang YC, Sun Q, Chen H, Zhang L, Bu XS, Zhan LY, Xia ZY. Parasternal intercostal block complementation contributes to postoperative pain relief in modified radical mastectomy employing pectoral nerve block I and serratus-intercostal block: a randomized trial. J Pain Res. 2020;13:865–871. doi: 10.2147/JPR.S237435. [DOI] [PMC free article] [PubMed] [Google Scholar]


