Abstract
Objective: The dysfunction of vascular smooth muscle cells (VSMCs) has been revealed to be closely linked with the pathogenesis of cardiovascular diseases in diabetes. Recently, circular RNAs (circRNAs) were found to regulate the behaviors of VSMCs. Here, we attempted to study the role of circLRP6 in VSMCs under diabetes condition. Methods: Human VSMCs were cultured under the condition of normal glucose (NG) or high glucose (HG). VSMC viability and proliferation were estimated by CCK-8 and 5-ethynyl-2’-deoxyuridine (EdU) incorporation assays. VSMC migration and invasion were assessed via wound-healing and transwell experiments. Protein expression of HMGA1 was measured in VSMCs using western blot and immunofluorescence analysis. Relative expressions of circLRP6, miR-545-3p, and HMGA1 mRNA were estimated in VSMCs using qRT-PCR. The co-localization of circLRP6 and miR-545-3p was verified by fluorescence in situ hybridization (FISH) analysis. Binding sequence of miR-545-3p in circLRP6 or HMGA1 was predicted using StarBase tool, and verified by RNA immunoprecipitation and dual-luciferase reporter experiments. Results: HG exposure promoted VSMC proliferation, migration and invasion, upregulated the circLRP6 expression, and downregulated HMGA1 expression. Knockdown of circLRP6 or overexpression of miR-545-3p abrogated the HG-caused VSMC proliferation, migration and invasion. CircLRP6 severed as a miR-545-3p sponge, and HMGA1 was targeted by miR-545-3p. MiR-545-3p inhibitor blocked the suppressive effects of si-circLRP6 on VSMC in the presence of HG. Conclusion: These findings suggest that circRNA LRP6 promotes HG-induced VSMC proliferation and migration through regulating miR-545-3p/HMGA1 signaling axis.
Keywords: Vascular smooth muscle cells, circLRP6, miR-545-3p, HMGA1, high glucose
Introduction
Diabetes mellitus (DM), a common type of chronic metabolic disorders, is featured by hyperglycemia caused by the deficiency of insulin [1]. The prevalence of DM has been found to be sharply elevated in recent decades, and the number of DM patients between the ages of 20 and 79 is predicted to reach 642 million by 2040 [2]. As one of the most common complications caused by long-term hyperglycemia, cardiovascular disease (CVD) is a primary cause of mortality among DM patients, accounting for over 75% of mortality among type 2 DM patients [3]. Vascular smooth muscle cells (VSMCs) are well known to be plastic to alter their morphology and growth state, thus performing contractile and synthetic functions [4]. VSMCs display organized and differentiated phenotype under physiological condition, while they can switch to a proliferative phenotype upon extracellular stimuli [5]. Studies have suggested that diabetes or high glucose (HG) exposure could trigger excessive VSMC proliferation and migration, contributing to the increased incidence of diabetic vascular disorders [5,6]. Thus, preventing VSMCs from abnormal proliferation and migration could be a valuable intervention option for the treatment of diabetes-related CVD.
As an emerging subfamily of non-coding RNAs (ncRNAs), circular RNAs (circRNAs) exist widely and stably in eukaryotes with a unique circular structure [7]. CircRNAs have been well documented to be linked with diverse pathological and physiological activities by serving as pivotal molecular regulators [8,9]. Their expression patterns can reflect alterations in biological events that link with disease initiation [10]. A high-throughput RNA sequencing performed recently has found 112 circRNAs dysregulated in platelet-derived growth factor treated VSMCs compared to normal control VSMCs [11]. In another study, a total of 983 differentially expressed circRNAs were identified in HG treated VSMCs by a circRNA microarray [12]. These findings indicated that circRNAs act as an important participant in the proliferation of VSMCs. Moreover, multiple circRNAs have been shown to control VSMC proliferation and migration under pathological environments through diverse mechanisms [13,14]. CircRNA LRP6 (circLRP6) derived from LRP6 gene was previously revealed to be correlated with the development of various diseases [15,16]. Recently, circLRP6 was found to be enriched in both mouse and human VSMCs using RNA sequencing and bioinformatics, and silencing of circLRP6 repressed VSMC migration and proliferation [17]. However, whether circLRP6 exhibits biological functions in the diabetes-related CVDs remains undetermined.
Therefore, this study was performed to explore the biological functions of circLRP6 in HG-induced excessive VSMC proliferation and migration. Meanwhile, we attempted to explore the potential mechanisms by which circLRP6 functions in VSMCs under HG exposure.
Materials and methods
VSMC treatment
Human VSMCs were supplied by the ATCC (USA) and cultured in DMEM (Invitrogen, USA) containing 5.5 mM glucose (normal glucose, NG) or 30 mM glucose (high glucose, HG). Cells were kept in a humidified condition with 5% CO2 at 37°C.
Cell viability and proliferation assessment
For CCK-8 assay, VSMCs (2×105/well) were subjected to CCK-8 reagent incubation for 2 h after seeding in 96-well plates for 24 h. The optical density of each well was tested by a microplate reader (BMG Labtech, Germany) at 450 nm. For EdU incorporation assay, VSMCs were incubated with EdU (50 μM) for 12 h and fixed with 4% paraformaldehyde, followed by the detection of EdU incorporation using anti-EdU antibody (Promega, USA). DAPI (Sigma, USA) was used to label cell nuclei. Images were observed with a fluorescence microscope (Nikon, Japan).
Cell migration and invasion assessment
For wound-healing analysis, VSMCs were cultured until 100% confluence in 6-well plates. A scratch was then created on the cell surface, and cells were cultured for another 48 h. The width of scratch was measured after 0 h and 48 h of scratching. Transwell chambers (BD Bioscience, USA) coated with Matrigel (BD Bioscience) were employed to evaluate cell invasion of VSMCs. Complete DMEM (500 μL) was added into the lower chamber, and serum-free DMEM (200 μL) containing 2×105 VSMCs were added into the upper chamber. After 24 h of culture, cells invaded into the lower compartment were fixed by ethanol followed by the staining with crystal violet.
Western blot
VSMC proteins were extracted by RIPA buffer (Beyotime, China). Proteins (50 μg) were then isolated in 10% SDS-PAGE, and target proteins were transferred to nitrocellulose membrane. After 2 h of incubation with low-fat milk (5%), the membranes were incubated overnight with primary antibodies against HMGA1 (Rabbit, 1:10000, ab129153, Abcam) and GAPDH (1:5000, ab8245, Abcam). Afterwards, the membranes were incubated for 2 h with indicated HRP-conjugated secondary antibody. Enhanced chemiluminescence reagent (EMD Millipore, USA) was applied to detect signals, followed by the analysis using Image J software.
Immunofluorescence analysis
After fixation, VSMCs were washed with PBS and subsequently incubated with normal donkey serum containing 0.5% Triton X-100 for 2 h. Then, primary antibodies against HMGA1 (Rabbit, 1:500, ab129153, Abcam) was added into the PBS and incubated overnight. After washing with PBS, VSMCs were incubated with donkey-anti-rabbit-488 (1:200, Abcam) secondary antibody for 2 h.
Quantitative real-time PCR (RT-PCR) analysis
TRIzol reagent (Thermo Fisher Scientific, USA) was employed to extract total RNAs of VSMCs and 5 μg of RNA samples were subjected to reverse transcription into cDNA using Gibco BRL kit (Life Technologies, USA). PCR process was conducted in the presence of indicated primers and cDNA with the help of SYBR-GreenPCR kit (Takara, Japan) on the ABI7500 Fast Real-Time PCR System (PE Applied Biosystems). Sequences of primers used in this study were listed as follows:
circLRP6: F: 5’-CAAGATTGAGGCAGGCAGTG-3’, R: 5’-GCTCCAGTCAGTCCAGTACA-3’; HMGA1: F: 5’-AAGGGGCAGACCCAAAAA-3’, R: 5’-TCCAGTCCCAGAAGGAAGC-3’; MiR-545-3p: 5’-TGGCTCAGTTCAGCAGGAAC-3’, R: universal reverse prime; U6: F: 5’-CGCTTCGGCAGCACATATACTAAAATTGGAAC-3’, R: 5’-GCTTCACGAATTTGCGTGTCATCCTTGC-3’; GAPDH: F: 5’-GCATCCTGGGCTACACTG-3’, R: 5’-TGGTCGTTGAGGGCAAT-3’.
Fluorescence in situ hybridization (FISH)
The co-localization of circLRP6 and miR-545-3p in VSMCs was verified by FISH assay using Cy3-labeled circLRP6 and FITC-labeled miR-545-3p probes according to the protocols of the Fluorescent In Situ Hybridization Kit (Geneseed, China). To determine the localization of circLRP6, only Cy3-labeled circLRP6 was used. U3 and GAPDH were applied as control.
Cell transfection
CircLRP6 overexpression vector was established by sub-cloning the circLRP6 sequence into the pLCDH-ciR vector (Geenseed Biotech, China, sequence: TGATCAAATAGCAGAGCTCTCTGGCTAACTAGAGAACCCACTGCTTACTGGCTTATCGAAATTAATACGACTCACTATAGGGAGACCCAAGCTGGCTAGAAAAGTGCTGAGATTACAGGCGTGAGCCACCACCCCCGGCCCACTTTTTGTAAAGGTACGTACTAATGACTTTTTTTTTATACTTCAGGCAGGCAGTGGTTAAAGGTTCCCTTCCACATCCTTTTGCCTTGACGTTATTTGAGGACATATTGTACTGGACTGACTGGAGCACACACTCCATTTTGGCTTGCAACAAGTATACTGGTGAGGGTCTGCGTGAAATCCATTCTGACATCTTCTCTCCCATGGATATACATGCCTTCAGCCAACAGAGGCAGCCAAATGCCACAAATCCATGTGGAATTGACAATGGGGGTTGTTCCCATTTGTGTTTGATGTCTCCAGTCAAGCCTTTTTATCAGTGTGCTTGCCCCACTGGGGTCAAACTCCTGGAGAATGGAAAAACCTGCAAAGATGGTGCCACAGAATTATTGCTTTTAGCTCGAAGGACAGACTTGAGACGCATTTCTTTGGATACACCAGATTTTACAGACATTGTTCTGCAGTTAGAAGACATCCGTCATGCCATTGCCATAGATTACGATCCTGTGGAAGGCTACATCTACTGGACTGATGATGAAGTGAGGGCCATACGCCGTTCATTTATAGATGGATCTGGCAGTCAGTTTGTGGTCACTGCTCAAATTGCCCATCCTGATGGTATTGCTGTGGACTGGGTTGCACGAAATCTTTATTGGACAGACACTGGCACTGATCGAATAGAAGTGACAAGGCTCAATGGGACCATGAGGAAGATCTTGATTTCAGAGGACTTAGAGGAACCCCGGGCTATTGTGTTAGATCCCATGGTTGGGTACATGTATTGGACTGACTGGGGAGAAATTCCGAAAATTGAGCGAGCAGCTCTGGATGGTTCTGACCGTGTAGTATTGGTTAACACTTCTCTTGGTTGGCCAAATGGTTTAGCCTTGGATTATGATGAAGGCAAAATATACTGGGGAGATGCCAAAACAGACAAGATTGAGGTAAGAAGCAAGGAAAAGAATTAGGCTCGGCACGGTAGCTCACACCTGTAATCCCAGCATAGCTTAAGTTTAAACCCGCTGATCAGCCTCGACTGTGCCTTCTAGTTGCCAGCCATCTGTTGTTTGCCCCTCCCCCGTGCCTTCCTTGACCCTGGAAGGTGCCACTCCCACTGTCCTTTCCTAATAAAATGAGGAAATTGCATCGCATTGTCTGAGTAGGTGTCATTCTATTCTGGGGGGTGGGGTGGGGCAGGACAGCAAGGGGGAGGATTGGGAAGACAATAGCAGGCATGCTGGGGATGCGGTGGGCTCTATGGCTTCTGAGGCGGAAAGAACCAGCTGGGGCTCTAGGGGGTATCCCCACGCGCCCTGTAGCGGCGCATTAAGCGCGGCGGGTGTGGTGGTTACGCGCAGCGTGACCGCTACACTTGCCAGCGCCCTAGCGCCCGCTCCTTTCGCTTTCTTCCCTTCCTTTCTCGCCACGTTCGCCGGCTTTCCCCGTCAAGCTCTAAATCGGGGGCTCCCTTTAGGGTTCCGATTTAGTGCTTTACGGCACCTCGACCCCAAAAAACTTGATTAGGTGATGGTTCACGTAGTGGGCCATCGCCCTGATAGACGGTTTTCGCCCTTTGACGTTGGAGTCCACGTTCTTTAATAGTGACTCTTGTTCCAAACTGGAACAACA). The siRNA targeting back splice junction of circLRP6 (si-circLRP6, sequence: AGA TTG AGG CAG GCA GTG GTT) was generated by Geenseed Biotech, and a scramble siRNA was used as negative control (si-NC, sequence: CAG UAC UUU UGU GUA GUA CAA). MiR-545-3p mimic and inhibitor were provided by GenePharma (Shanghai, China). Cell transfection was executed using Lipofectamine 3000 (Invitrogen, USA).
RNA immunoprecipitation (RIP)
EZ-Magna RIP™ Kit (Millipore, USA) was used in RIP assay. In brief, VMSCs were lysed in RIP buffer and the cell lysates were collected and incubated with magnetic beads conjugated with IgG or Ago2 (Millipore). After purification, immunoprecipitated RNA was examined by qRT-PCR.
Dual-luciferase reporter assay
The wild type (WT) miR-545-3p binding sequences of circLRP6 and HMGA1 were respectively sub-cloned into pmiRGLO-basic vectors (Genecreate, China), referred to as: circLRP6-WT and HMGA1-WT. Mutant miR-545-3p sequence was also inserted into pmiRGLO-basic vectors as negative control, referred to as: circLRP6-MUT and HMGA1-MUT. To verify the interaction between miR-545-3p and circLRP6, miR-545-3p mimic or miR-NC was co-transfected into VSMCs with circLRP6-WT, circLRP6-MUT or empty pmiRGLO vector using lipofectamine 3000. After 24 h of transfection, the activities of firefly and Renilla luciferase were assessed. Interplay between miR-545-3p and HMGA1 was verified as mentioned above.
Statistical analysis
Data were expressed as mean ± standard deviation. Student’s t test or one way ANOVA was conducted to evaluate significant difference using Graphpad Prism (Version 7.0, USA). P value less than 0.05 was considered statistically significant.
Results
HG exposure increased circLRP6 level in VSMCs
We first estimated the influences of HG exposure on cell viability and proliferation of VSMCs. CCK-8 result indicated that cell viability was significantly increased in HG (30 mM)-exposed VSMCs compared with the NG (5.5 mM)-treated VSMCs (Figure 1A). Consistently, EdU incorporation assay suggested that HG exposure increased VSMC proliferation compared with the NG group (Figure 1B). Next, the influences of HG exposure on VSMC migration and invasion were estimated. Compared to NG group, both the migratory and invasive abilities of VSMCs were dramatically increased after 48 h of exposure to HG (Figure 1C and 1D). To determine the potential roles of circLRP6 in VSMCs under diabetic condition, the expression of circLRP6 was examined in NG- and HG-treated VSMCs. Relative expression of circLRP6 was found to be upregulated in HG-treated VSMCs compared with that in NG-treated VSMCs (Figure 1E). Additionally, we demonstrated that circLRP6 was primarily localized in the cytoplasm of VSMCs using FISH (Figure 1F). These findings indicated that circLRP6 may play a role in the abnormal behavior of VSMCs under diabetic condition.
Figure 1.
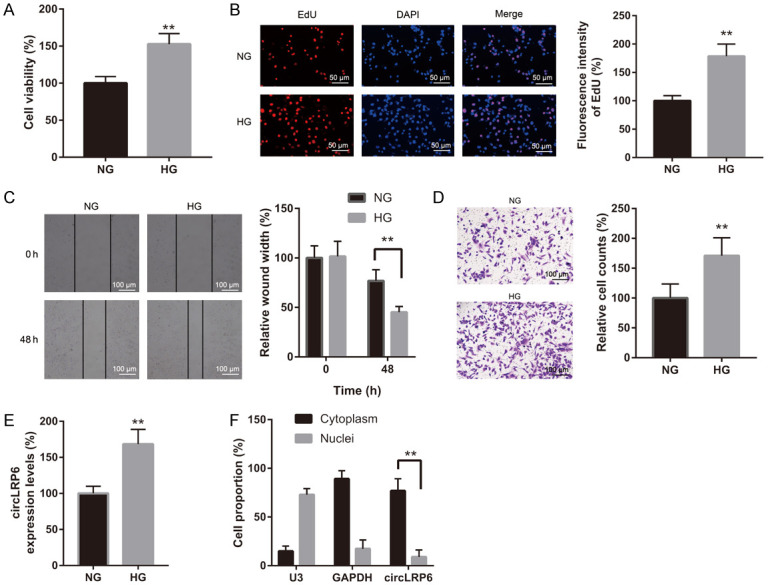
HG exposure increased circLRP6 expression while decreased HMGA1 expression in VSMCs. (A) CCK-8 assay and (B) EdU staining were employed to estimate proliferation of VSMCs under NG and HG condition. (C and D) Effects of HG exposure on the migratory and invasive abilities of VSMCs were assessed by wound-healing and transwell assays. (E) CircLRP6 level in NG- and HG-treated VSMCs was measured via qRT-PCR. (F) The subcellular localization of circLRP6 in VSMCs was determined by FISH assay.
Knockdown of circLRP6 repressed HG-induced VSMC proliferation, migration, and invasion
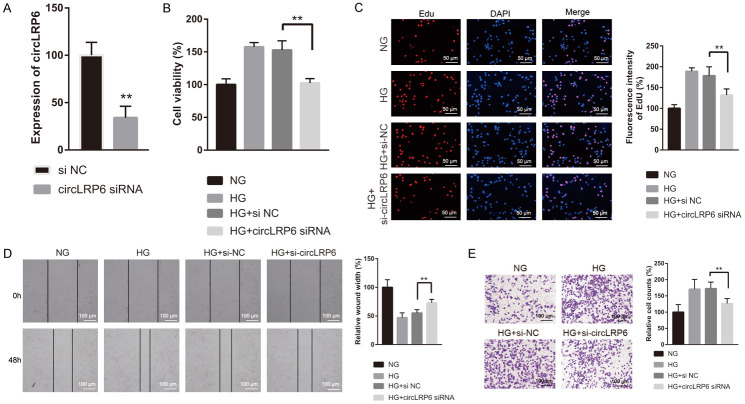
To assess the role of circLRP6 in HG induced VSMC proliferation, migration, and invasion, we silenced the expression of circLRP6 by introducing si-circLRP6, and qRT-PCR results verified the successful knockdown of circLRP6 in VSMCs (Figure 2A). By employing CCK-8, EdU incorporation, wound-healing, and transwell assays, we observed that the HG exposure-induced VSMC proliferation, migration, and invasion were all reversed by the silencing of circLRP6 (Figure 2B-E). These findings indicated that circLRP6 silencing abolished the pro-proliferative effects of HG on VSMCs.
Figure 2.
Knockdown of circLRP6 repressed HG induced VSMCs proliferation, migration, and invasion. (A) Knockdown efficiency of si-circLRP6 in VSMCs was examined via qRT-PCR after 24 h of transfection. After transfection with si-NC (negative control) or si-circLRP6 for 48 h in culture medium containing NG or HG, VSMCs were subjected to the analysis of cell proliferation (B and C), migration (D), and invasion (E).
CircLRP6 served as a sponge of miR-545-3p
To explore the regulatory mechanism of circLRP6 in VSMCs under diabetic condition, we focused on “miRNA sponges” since circLRP6 was found to be mainly located in the cytoplasm of VSMCs. StarBase (v2.0) tool was employed to predict miRNAs associated with circLRP6, and miR-545-3p was found to be a target miRNA of circLRP6 (Figure 3A). We then performed RIP assay using AGO2 antibody. qRT-PCR results indicated that endogenous circLRP6 was enriched in AGO2 group, implying that circLRP6 possesses the ability to sponge miRNAs (Figure 3B). Results from luciferase reporter experiment indicated that the luciferase activity driven by circLRP6-WT, but not pmiRGLO-control or circLRP6-MUT, was dramatically attenuated by miR-545-3p overexpression (Figure 3C). Luciferase activity was not affected by the transfection of NC mimic. By FISH, we found that the co-localization of circLRP6 and miR-545-3p was significantly elevated in the VSMCs transfected with circLRP6 and miR-545-3p mimic (Figure 3D). Moreover, a remarkable miR-545-3p upregulation was found in the circLRP6-silenced VSMCs (Figure 3E). These findings suggested that circLRP6 could negatively modulate the level of miR-545-3p by serving as a sponge of miR-545-3p.
Figure 3.
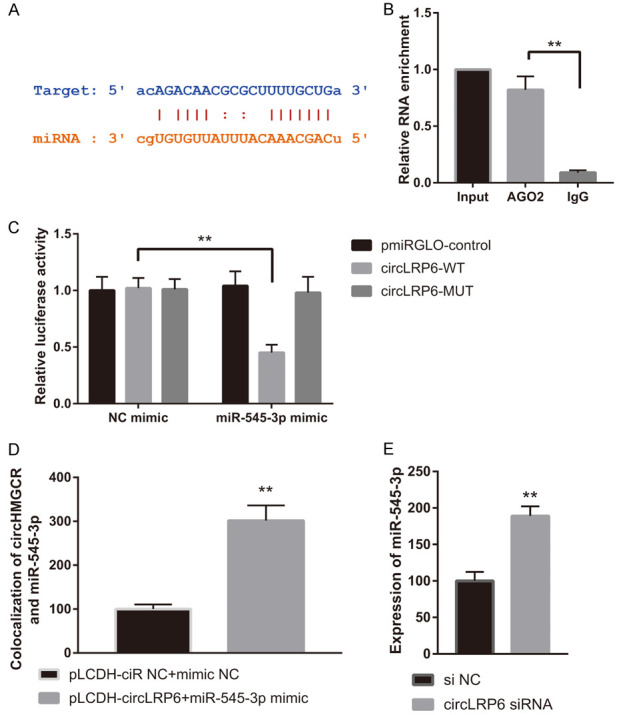
CircLRP6 served as a sponge of miR-545-3p. (A) StarBase showing the potential miR-545-3p sequences in circLRP6. (B) RIP and (C) dual-luciferase reporter assays were employed to test the interplay between circLRP6 and miR-545-3p in VSMCs. (D) The co-localization of circLRP6 and miR-545-3p in VSMCs. (E) miR-545-3p level in si-NC or si-circLRP6 treated VSMCs.
miR-545-3p targeted and negatively regulated HMGA1 expression
Next, we sought to screen the miR-545-3p target gene. Through StarBase (v2.0) prediction tool, HMGA1 was predicted to interact with miR-545-3p (Figure 4A). In luciferase reporter experiment, we observed a remarkable inhibition of luciferase intensity in the VSMCs co-transfected with miR-545-3p and HMGA1-WT, while luciferase activity was not affected by the transfection of NC mimic or the co-transfection of miR-545-3p and HMGA1-MUT (Figure 4B). Moreover, we found that HMAG1 were dramatically decreased in the VSMCs transfected with miR-545-3p mimic (Figure 4C and 4D). Conversely, the HMAG1 mRNA and protein were dramatically increased in the VSMCs transfected with miR-545-3p inhibitor compared to VSMCs transfected with NC inhibitor (Figure 4C and 4D). We also examined the expression of HMGA1 in si-NC or si-circLRP6-treated VSMCs by western blot and immunofluorescence analysis under NG and HG condition. Results indicated a significant upregulation of HMGA1 in HG-treated VSMCs compared to that in NG-treated VSMCs, while the HG-induced upregulation of HMGA1 was abolished by the transfection of si-circLRP6 (Figure 4E and 4F). These findings argued that miR-545-3p negatively modulated HMGA1 by binding to it, and circLRP6 could regulate the expression of HMGA1 through miR-545-3p.
Figure 4.
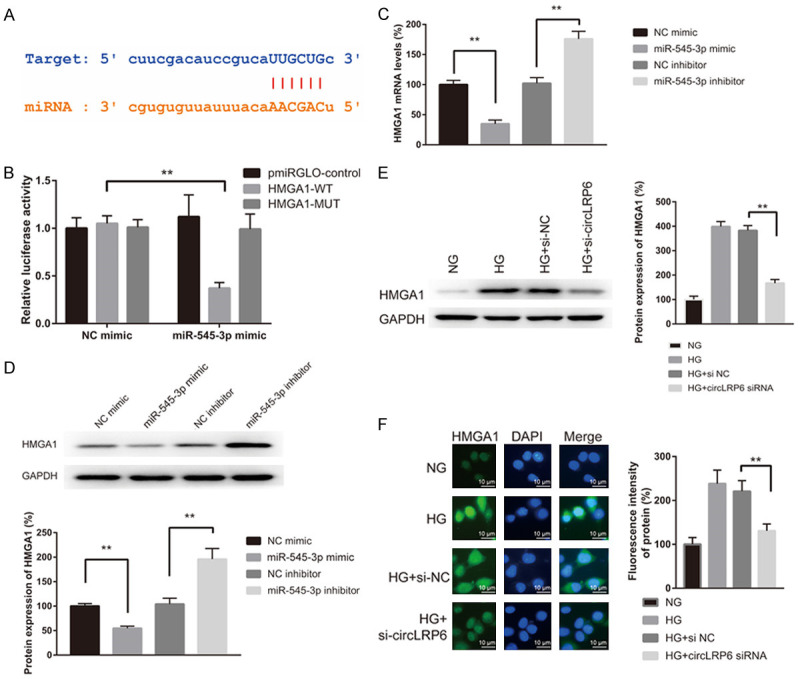
miR-545-3p negatively regulated HMGA1 expression by binding to it. (A) StarBase prediction showing the potential miR-545-3p binding sites in HMGA1. (B) Interplay between miR-545-3p and HMGA1 was tested by dual-luciferase reporter assay in VSMCs. (C and D) Relative mRNA and protein expression levels of HMGA1 in VSMCs treated with miR-545-3p mimic and inhibitor. NC mimic and NC inhibitor were used as negative control. (E) Western blot and (F) immunofluorescence analyses for HMGA1 in VSMCs treated with si-NC and si-circLRP6 under NG and HG condition for 24 h.
miR-545-3p overexpression repressed HG-induced VSMC proliferation, migration, and invasion
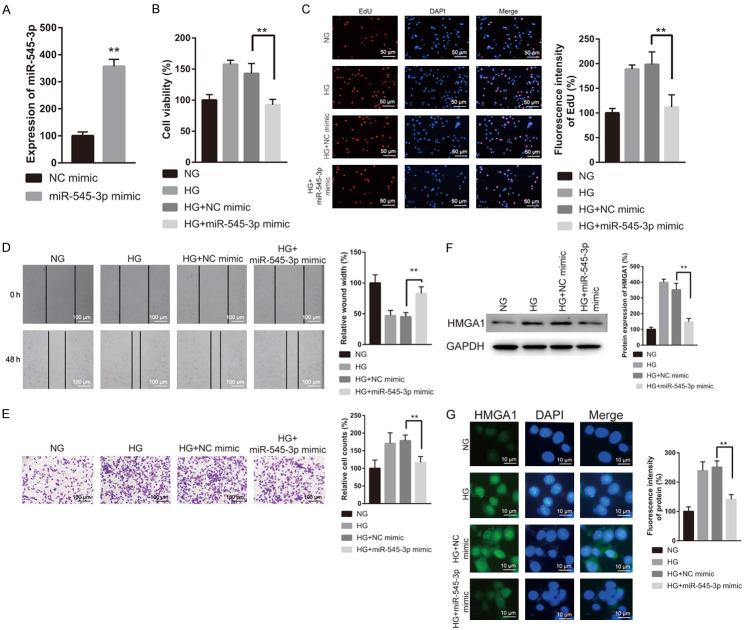
Subsequently, we evaluated the impacts of miR-545-3p on HG-induced cell proliferation, migration, and invasion of VSMCs. Compared to NC mimic group, miR-545-3p was upregulated in miR-545-3p overexpressed VSMCs (Figure 5A). Functionally, miR-545-3p mimic transfection abolished the increased cell proliferation, migration, and invasion of VSMCs induced by HG (Figure 5B-E). Additionally, the upregulation of HMGA1 protein induced by HG was abrogated by the transfection with miR-545-3p mimic (Figure 5F and 5G). These results indicated that miR-545-3p overexpression could reverse HG-induced VSMC proliferation, migration, and invasion by inhibiting HMGA1 expression.
Figure 5.
miR-545-3p overexpression repressed HG induced VSMCs proliferation, migration, and invasion. (A) Overexpression efficiency of miR-545-3p mimic in VSMCs. After 48 h of transfection with NC or miR-545-3p mimic under NG or HG condition, VSMCs were subjected to the analysis of cell proliferation (B and C), migration (D), and invasion (E). (F) Western blot and (G) immunofluorescence assays were carried out to measure the expression of HMGA1 in VSMCs transfected with NC mimic or miR-545-3p mimic under NG or HG condition.
CircLRP6/miR-545-3p/HMGA1 axis participated in the HG-induced VSMCs proliferation, migration, and invasion
Finally, rescue experiments were carried out to determine whether the effect of circLRP6 on HG-treated VSMCs was mediated by miR-545-3p/HMGA1 axis. To this end, HG-treated VSMCs were co-transfected with si-circLRP6 and miR-545-3p inhibitor followed by the examination of cell proliferation, migration, and invasion. Transfection with si-circLRP6 reversed the upregulation of HMGA1 mRNA and protein induced by HG exposure, while this phenomenon was abolished by the co-transfection of si-circLRP6 and miR-545-3p inhibitor (Figure 6A and 6B). In the functional analysis, miR-545-3p inhibitor blocked the suppressive effect of si-circLRP6 on VSMC proliferation, migration, and invasion in the presence of HG (Figure 6C-F). Taken together, circLRP6 repressed HG-induced VSMC proliferation, migration, and invasion by regulating miR-545-3p/HMGA1 axis.
Figure 6.
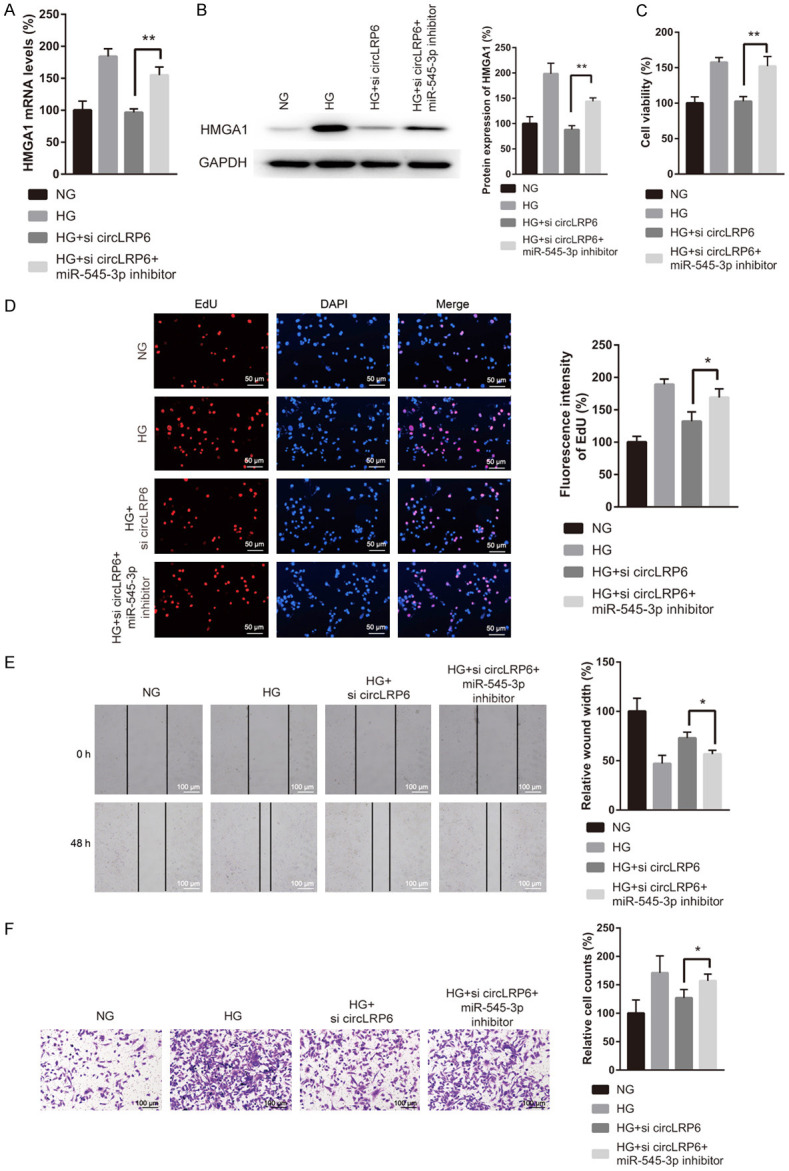
CircLRP6/miR-545-3p/HMGA1 axis participated in the HG induced VSMCs proliferation, migration, and invasion. (A and B) After co-transfection with si-circLRP6 and miR-545-3p inhibitor, VSMCs were subjected to the detection of HMGA1 mRNA and protein. The treated VSMCs were also subjected to the assessment of proliferation (C and D), migration (E), and invasion (F).
Discussion
Long-term hyperglycemia induced CVD-related complications, including hypertension, atherosclerosis and restenosis, are the primary causes of DM patients’ mortality [18,19]. As a key component of artery wall, the excessive VSMC growth and migration are fundamental processes in the pathological development of diabetic-related CVDs [20]. Herein, we revealed that HG exposure increased circLRP6 level in VSMCs, and knockdown of circLRP6 abolished the HG-induced VSMC proliferation and migration. Mechanistically, we revealed that circLRP6 could indirectly regulate HMGA1 expression by serving as miR-545-3p sponge. Moreover, rescue assays indicated that the promotive impact of circLRP6 on HG induced VSMC proliferation and migration was mediated by miR-545-3p/HMGA1 axis.
Accumulating evidences have suggested that the dysregulation of circRNAs may be detrimental to cardiovascular system by controlling VSMC proliferation and migration [21]. For instance, circCHFR was reported to be decreased in the ox-LDL treated VSMCs by microarray analysis and circCHFR silencing suppressed VSMC proliferation and migration in vitro by miR-37-/Cyclin D1 pathway [14]. Recently, exsomal circRNA-0070930 derived from human vascular endothelium was found to induce the senescence of VSMCs under HG condition by regulating miR-622/Krase ceRNA network [22]. Moreover, circWDR77 was reported to facilitate the proliferation and migration of VSMCs under HG condition through modulating miR-124/FGF-2 axis [12]. CircLRP6 was previously revealed to play a key role in epithelial-mesenchymal transition, autophagy, and proliferation of various tumor cells [23]. Recently, circLRP6 was reported to regulate high-glucose induced proliferation, oxidative stress, inflammatory response, and extracellular matrix of mesangial cells [24], indicating a key role of circLRP6 in the pathogenesis of diabetic nephropathy. Of note, circLRP6 was found to impact the proliferation and migration of VSMCs [17]. Given the fact that excessive proliferation of VSMCs has been demonstrated to be closely correlated with the pathogenesis of diabetic-related CVDs, we speculated that circLRP6 might be involved in the regulation of HG-induced proliferation and migration of VSMCs. Herein, we found that HG exposure increased the proliferation and migration of VSMCs. Meanwhile, circLRP6 was found to be upregulated under HG condition, which implied a close correlation between the expression level of circLRP6 and the proliferation and migration of VSMCs. Moreover, loss-of-function assays indicated that circLRP6 silencing repressed the proliferation and migration of VSMCs in vitro.
CircRNAs are well known as pivotal gene regulators by acting as miRNA sponges, thereby impacting the development of CVDs [25,26]. Here, for the first time, we identified that circLRP6 could act as a sponge of miR-545-3p, a miRNA with important function in various human diseases, especially in cancers [27-29]. However, the role of miR-545-3p in CVDs has not been reported yet. Our findings showed that miR-545-3p overexpression could repress the proliferation and migration of VSMCs by targeting HMGA1. As a key nuclear factor, HMGA1 involves in the regulation of chromatin structure and transcription by targeting AT-rich sequences in the promoter area of DNA [30]. Evidences have shown that HMGA1 could influence the function of insulin by directly binding to the promoter of insulin receptor, thus participating in the initiation and progression of DM [31]. Studies also revealed that HMGA1 activation could promote HG-induced VSMC proliferation by upregulating PI3K/Akt signaling pathway [32]. Here, we revealed that HMGA1 was increased in HG treated VSMCs. Moreover, miR-545-3p inhibition was found to not only abolish the repressive effects of si-circLRP6 on HMGA1 expression under HG condition, but also block the suppressive effects of si-circLRP6 on VSMC proliferation, migration, and invasion in the presence of HG. These findings implied that circLRP6 maintained HMGA1 expression by sponging miR-545-3p to impact the proliferation and migration of VSMCs induced by HG.
In conclusion, our findings suggested that circLRP6/miR-545-3p/HMGA1 axis takes part in modulating VSMCs proliferation and migration under HG condition, thus indicating that circLRP6 might be a promising target for the treatment of diabetic-related CVDs.
Acknowledgements
Our study was approved by the Ethics Review Board of Guangzhou First People’s Hospital (K-2021-114-01).
Disclosure of conflict of interest
None.
References
- 1.Guthrie RA, Guthrie DW. Pathophysiology of diabetes mellitus. Crit Care Nurs Q. 2004;27:113–125. doi: 10.1097/00002727-200404000-00003. [DOI] [PubMed] [Google Scholar]
- 2.Zinman B. The international diabetes federation world diabetes congress 2015. Eur Endocrinol. 2015;11:66. doi: 10.17925/EE.2015.11.02.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mancini GBJ, Gupta M, Tsigoulis M, Cannon CP, Genest J, Ray KK, Santos RD, Watts GF, Raggi P. Detection of atherosclerotic cardiovascular disease influences the perceived need for aggressive lipid management. Atherosclerosis. 2017;263:112–118. doi: 10.1016/j.atherosclerosis.2017.06.006. [DOI] [PubMed] [Google Scholar]
- 4.Wang G, Jacquet L, Karamariti E, Xu Q. Origin and differentiation of vascular smooth muscle cells. J Physiol. 2015;593:3013–3030. doi: 10.1113/JP270033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shi J, Yang Y, Cheng A, Xu G, He F. Metabolism of vascular smooth muscle cells in vascular diseases. Am J Physiol Heart Circ Physiol. 2020;319:H613–H631. doi: 10.1152/ajpheart.00220.2020. [DOI] [PubMed] [Google Scholar]
- 6.Majesky MW, Dong XR, Regan JN, Hoglund VJ. Vascular smooth muscle progenitor cells: building and repairing blood vessels. Circ Res. 2011;108:365–377. doi: 10.1161/CIRCRESAHA.110.223800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kristensen LS, Andersen MS, Stagsted LVW, Ebbesen KK, Hansen TB, Kjems J. The biogenesis, biology and characterization of circular RNAs. Nat Rev Genet. 2019;20:675–691. doi: 10.1038/s41576-019-0158-7. [DOI] [PubMed] [Google Scholar]
- 8.Beermann J, Piccoli MT, Viereck J, Thum T. Non-coding RNAs in development and disease: background, mechanisms, and therapeutic approaches. Physiol Rev. 2016;96:1297–1325. doi: 10.1152/physrev.00041.2015. [DOI] [PubMed] [Google Scholar]
- 9.Altesha MA, Ni T, Khan A, Liu K, Zheng X. Circular RNA in cardiovascular disease. J Cell Physiol. 2019;234:5588–5600. doi: 10.1002/jcp.27384. [DOI] [PubMed] [Google Scholar]
- 10.Dube U, Del-Aguila JL, Li Z, Budde JP, Jiang S, Hsu S, Ibanez L, Fernandez MV, Farias F, Norton J, Gentsch J, Wang F Dominantly Inherited Alzheimer Network (DIAN) Salloway S, Masters CL, Lee JH, Graff-Radford NR, Chhatwal JP, Bateman RJ, Morris JC, Karch CM, Harari O, Cruchaga C. An atlas of cortical circular RNA expression in Alzheimer disease brains demonstrates clinical and pathological associations. Nat Neurosci. 2019;22:1903–1912. doi: 10.1038/s41593-019-0501-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tian J, Fu Y, Li Q, Xu Y, Xi X, Zheng Y, Yu L, Wang Z, Yu B, Tian J. Differential expression and bioinformatics analysis of CircRNA in PDGF-BB-induced vascular smooth muscle cells. Front Genet. 2020;11:530. doi: 10.3389/fgene.2020.00530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen J, Cui L, Yuan J, Zhang Y, Sang H. Circular RNA WDR77 target FGF-2 to regulate vascular smooth muscle cells proliferation and migration by sponging miR-124. Biochem Biophys Res Commun. 2017;494:126–132. doi: 10.1016/j.bbrc.2017.10.068. [DOI] [PubMed] [Google Scholar]
- 13.Huang Z, Li P, Wu L, Zhang D, Du B, Liang C, Gao L, Zhang Y, Yao R. Hsa_circ_0029589 knockdown inhibits the proliferation, migration and invasion of vascular smooth muscle cells via regulating miR-214-3p and STIM1. Life Sci. 2020;259:118251. doi: 10.1016/j.lfs.2020.118251. [DOI] [PubMed] [Google Scholar]
- 14.Yang L, Yang F, Zhao H, Wang M, Zhang Y. Circular RNA circCHFR facilitates the proliferation and migration of vascular smooth muscle via miR-370/FOXO1/Cyclin D1 pathway. Mol Ther Nucleic Acids. 2019;16:434–441. doi: 10.1016/j.omtn.2019.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zheng S, Qian Z, Jiang F, Ge D, Tang J, Chen H, Yang J, Yao Y, Yan J, Zhao L, Li H, Yang L. CircRNA LRP6 promotes the development of osteosarcoma via negatively regulating KLF2 and APC levels. Am J Transl Res. 2019;11:4126–4138. [PMC free article] [PubMed] [Google Scholar]
- 16.Wang J, Zhu W, Tao G, Wang W. Circular RNA circ-LRP6 facilitates Myc-driven tumorigenesis in esophageal squamous cell cancer. Bioengineered. 2020;11:932–938. doi: 10.1080/21655979.2020.1809922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hall IF, Climent M, Quintavalle M, Farina FM, Schorn T, Zani S, Carullo P, Kunderfranco P, Civilini E, Condorelli G, Elia L. Circ_Lrp6, a circular RNA enriched in vascular smooth muscle cells, acts as a sponge regulating miRNA-145 function. Circ Res. 2019;124:498–510. doi: 10.1161/CIRCRESAHA.118.314240. [DOI] [PubMed] [Google Scholar]
- 18.Henning RJ. Type-2 diabetes mellitus and cardiovascular disease. Future Cardiol. 2018;14:491–509. doi: 10.2217/fca-2018-0045. [DOI] [PubMed] [Google Scholar]
- 19.Glovaci D, Fan W, Wong ND. Epidemiology of diabetes mellitus and cardiovascular disease. Curr Cardiol Rep. 2019;21:21. doi: 10.1007/s11886-019-1107-y. [DOI] [PubMed] [Google Scholar]
- 20.Fiorentino TV, Prioletta A, Zuo P, Folli F. Hyperglycemia-induced oxidative stress and its role in diabetes mellitus related cardiovascular diseases. Curr Pharm Des. 2013;19:5695–5703. doi: 10.2174/1381612811319320005. [DOI] [PubMed] [Google Scholar]
- 21.Sun J, Zhang Z, Yang S. Circ_RUSC2 upregulates the expression of miR-661 target gene SYK and regulates the function of vascular smooth muscle cells. Biochem Cell Biol. 2019;97:709–714. doi: 10.1139/bcb-2019-0031. [DOI] [PubMed] [Google Scholar]
- 22.Wang S, Zhan J, Lin X, Wang Y, Wang Y, Liu Y. CircRNA-0077930 from hyperglycaemia-stimulated vascular endothelial cell exosomes regulates senescence in vascular smooth muscle cells. Cell Biochem Funct. 2020;38:1056–1068. doi: 10.1002/cbf.3543. [DOI] [PubMed] [Google Scholar]
- 23.Xue J, Chen C, Luo F, Pan X, Xu H, Yang P, Sun Q, Liu X, Lu L, Yang Q, Xiao T, Dai X, Luo P, Lu J, Zhang A, Liu Q. CircLRP6 regulation of ZEB1 via miR-455 is involved in the epithelial-mesenchymal transition during arsenite-induced malignant transformation of human keratinocytes. Toxicol Sci. 2018;162:450–461. doi: 10.1093/toxsci/kfx269. [DOI] [PubMed] [Google Scholar]
- 24.Chen B, Li Y, Liu Y, Xu Z. circLRP6 regulates high glucose-induced proliferation, oxidative stress, ECM accumulation, and inflammation in mesangial cells. J Cell Physiol. 2019;234:21249–21259. doi: 10.1002/jcp.28730. [DOI] [PubMed] [Google Scholar]
- 25.Miao L, Yin RX, Zhang QH, Liao PJ, Wang Y, Nie RJ, Li H. A novel circRNA-miRNA-mRNA network identifies circ-YOD1 as a biomarker for coronary artery disease. Sci Rep. 2019;9:18314. doi: 10.1038/s41598-019-54603-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhu K, Hu X, Chen H, Li F, Yin N, Liu AL, Shan K, Qin YW, Huang X, Chang Q, Xu GZ, Wang Z. Downregulation of circRNA DMNT3B contributes to diabetic retinal vascular dysfunction through targeting miR-20b-5p and BAMBI. EBioMedicine. 2019;49:341–353. doi: 10.1016/j.ebiom.2019.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li L, Qiu X, Sun Y, Zhang N, Wang L. SP1-stimulated miR-545-3p inhibits osteogenesis via targeting LRP5-activated Wnt/beta-catenin signaling. Biochem Biophys Res Commun. 2019;517:103–110. doi: 10.1016/j.bbrc.2019.07.025. [DOI] [PubMed] [Google Scholar]
- 28.Changjun L, Feizhou H, Dezhen P, Zhao L, Xianhai M. MiR-545-3p/MT1M axis regulates cell proliferation, invasion and migration in hepatocellular carcinoma. Biomed Pharmacother. 2018;108:347–354. doi: 10.1016/j.biopha.2018.09.009. [DOI] [PubMed] [Google Scholar]
- 29.Cosin-Tomas M, Antonell A, Llado A, Alcolea D, Fortea J, Ezquerra M, Lleo A, Marti MJ, Pallas M, Sanchez-Valle R, Molinuevo JL, Sanfeliu C, Kaliman P. Plasma miR-34a-5p and miR-545-3p as early biomarkers of Alzheimer’s disease: potential and limitations. Mol Neurobiol. 2017;54:5550–5562. doi: 10.1007/s12035-016-0088-8. [DOI] [PubMed] [Google Scholar]
- 30.Wang Y, Hu L, Zheng Y, Guo L. HMGA1 in cancer: cancer classification by location. J Cell Mol Med. 2019;23:2293–2302. doi: 10.1111/jcmm.14082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chiefari E, Nevolo MT, Arcidiacono B, Maurizio E, Nocera A, Iiritano S, Sgarra R, Possidente K, Palmieri C, Paonessa F, Brunetti G, Manfioletti G, Foti D, Brunetti A. HMGA1 is a novel downstream nuclear target of the insulin receptor signaling pathway. Sci Rep. 2012;2:251. doi: 10.1038/srep00251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang Q, Chen L, Zhao Z, Wu Y, Zhong J, Wen G, Cao R, Zu X, Liu J. HMGA1 mediated high-glucose-induced vascular smooth muscle cell proliferation in diabetes mellitus: association between PI3K/Akt signaling and HMGA1 expression. DNA Cell Biol. 2018;37:389–397. doi: 10.1089/dna.2017.3957. [DOI] [PubMed] [Google Scholar]