Abstract
The increased proliferation and migration of airway smooth muscle cells (ASMCs) are essential factors in the development of asthma. Long noncoding RNAs (lncRNAs) play key roles in the pathogenesis of various diseases, including asthma. A growing body of evidence indicates that lncRNA FTX regulates proliferation and migration in multiple cell types and the progression of various diseases. However, the role of FTX in asthma is still not yet fully understood. Therefore, we explored the role of FTX in the proliferation and migration of ASMCs stimulated by platelet-derived growth factor BB (PDGF-BB) in vitro, as well as the underlying molecular mechanisms. Here, it is demonstrated that the expression of FTX in ASMCs treated with PDGF-BB is significantly up-regulated, and FTX knockout effectively represses the proliferation and migration and promotes the apoptosis of ASMCs induced by PDGF-BB. Mechanistically, FTX can inhibit the proliferation and migration of ASMCs caused by PDGF-BB by targeting miR-590-5p, and FTX over-expression reverses the inhibitory effect. Furthermore, JAK2 is a direct target of the FTX/miR-590-5p signal axis, the over-expression of which reverses the inhibitory effect on the proliferation and migration and the apoptosis-inducing effect of miR-590-5p in ASMCs. Collectively, these results highlight the crucial regulatory role of the FTX/miR-590-5p/JAK2 axis in ASMC proliferation, migration, and apoptosis.
Keywords: Asthma, airway smooth muscle cells, FTX, miR-590-5p, JAK2
Introduction
Asthma is a chronic disease worldwide, involving a variety of inflammatory cells in chronic airway disease. It occurs in people of all ages and has become a serious public health threat [1,2]. Airflow obstruction, chronic airway inflammation, and remodeling are considered the major characteristics of asthma [3]. The proliferation and hypertrophy of airway smooth muscle cells (ASMC) are important factors contributing to airway remodeling [4]. In addition, fibrinolytic cytokines are highly up-regulated in various asthmatic ASMCs with inflammation, including transforming growth factor-β (TGF-β) and connective tissue growth factor (CTGF) [5,6].
It has been suggested that a subset of non-coding RNAs (ncRNAs) play vital roles in ASMC. FTX has been reported in a variety of tumors and diseases such as gastric cancer, lung cancer, myocardial infarction, and osteosarcoma [7-10]. For instance, zinc finger protein X-linked (ZFX) attenuates the effect of the FTX/miR-144 axis by contacting the miR-144 sponge. The inhibition of FTX promotes cell proliferation, migration and invasion, and tumor growth [7]. The serum FTX levels are decreased in patients with myocardial ischemia/reperfusion (I/R) injuries or in hypoxia/reoxygenation (H/R)-stimulated H9c2 cells. Moreover, FTX can mitigate hypoxia/reoxygenation-induced cardiomyocyte injury through the miR-410-3p/Fmr1 axis [9].
In this study, we demonstrated that the regulatory axis of FTX, miR-590-5p, and JAK2 is effective at controlling the proliferation and migration of ASMCs during the development of asthma. Our findings hypothesize a novel FTX/miR-590-5p/JAK2 pathway in the pathogenesis of asthma.
Material and methods
Isolation and culture of ASMCs
ASMCs were separated from healthy segments of the main trachea of three patients in hospital pneumonectomies. The study was approved by the Ethics Committee of Longgang Central Hospital (Shenzhen, China), and the patients signed the informed consent forms. ASM bundles were isolated from the surrounding tissue, cut into 0.5 mm3 pieces, and placed in a DMEM medium including 20% fetal bovine serum (FBS; Sigma, USA), 100 μg/mL penicillin (sigma, USA) and 100 μg/mL streptomycin (sigma, USA), cultured at 37°C with 5% CO2. The ASMCs were identified using morphological observation under an inverted optical microscope (Olympus, Inc., Japan) and by immunofluorescence staining. The cell sera from passages 4-6 were deprived for 24 h before the treatment with 25 ng/mL PDGF-BB [11].
Real-time PCR
Total RNA from the cells was extracted using TRIzol reagent following the manufacturer’s instructions (Sigma, USA). For our analysis of the FTX and the translation initiation factor 5p expressions, the cDNA were synthesized using PrimeScript™ RT reagent kits (Takara, Dalian, China), and then added to Master Mix for subsequent PCR. GAPDH was used as a reference. To analyze the miRNA expressions, cDNA was synthesized using taqMan MicroRNA Reverse Transcription Kit (Applied Biosystems). The transcription was performed using an ABI7300 system for real-time quantitative PCR (qRT-PCR) (Applied Biosystems). U6 snRNA was used as a reference. All the primers we used are listed in Table 1.
Table 1.
The primer sequences of U6 snRNA
| Primer | Sequence |
|---|---|
| FTX-forward | 5’-TGAAATATAGGTGACATAAGCTC-3’ |
| FTX-reverse | 5’-GACGTGAAAATACTTATTCGAG-3’ |
| miR-590-5p-forward | 5’-CTCATGAACTAAATTTAAGCTT-3’ |
| miR-590-5p-reverse | 5’-GACGTGAAAATACTTATTCGAG-3’ |
| GAPDH-forward | 5’-GGGCTCATCTGAAGGGTGGTGCTA-3’ |
| GAPDH-reverse | 5’-GTGGGGGAGACAGAAGGGAACAGA-3’ |
| U6-foward | 5’-CTCGCTTCGGCAGCACA-3’ |
| U6-reverse | 5’-AACGCTTCACGAATTTGCGT-3’ |
Cell transfection
To silence the lncRNA expression, the specific siRNA targeting FTX were synthesized by RiboBio (Guangzhou, China) as negative controls. A specific siRNA targeting FTX was obtained from RiboBio (Guangzhou, China) as a negative control. In addition, the plasmid for FTX was also synthesized by RiboBio to enhance the FTX expression. ASMC was transiently transfected with si-FTX, si-NC and lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s protocol [12].
CCK-8 proliferation
A Cell Counting Kit 8 (CCK-8, Dojindo, Kumamoto, Japan) was used to measure the cell proliferation and cell toxicity of the transfected cells according to manufacturer’s instructions. The cells were seeded into a 96-well plate (2×103 cells per well), and CCK-8 reagent (10 µL) was added to the cells and cultured at 37°C for 18 h. The absorbance was measured at 450 nm with a spectrophotometer (Bio-Rad) at different times.
Transwell assays
The 24-well Transwell (8 μm pore size, Corning, NY, USA) assay was used to measure the cell migration ability. Briefly, 200 μL of FBS-free DMEM medium was added to the upper chamber of the incubator to neutralize 3×104 ASMCs cells. The lower chamber was filled with 600 μL of DMEM medium containing FBS with or without 25 ng/mL PDGF-BB. The cells were incubated at 37°C and 5% CO2.
Western blot assay
The ASMCs were mixed with a protease inhibitor and lysed in a RIPA buffer (Thermo Scientific, Waltham, USA). A BCA protein detection kit (Beyotime, Shanghai, China) was used to measure the protein concentration. The lysed protein samples were transferred to a polyvinylidene fluoride membrane using SDS-PAGE. The membrane was incubated with a primary anti-FTX antibody (1:1000) (Abcam, CA, USA) and a secondary antibody (1:3000) (Abcam, CA, USA). Finally, the Western blots were measured using ECL kits (Pierce, CA, USA) and a ChemiDoc XRS + imager (Bio-Rad).
Luciferase reporter assays
The sequence of the potential binding sites within miR-590-5p and the 3’-UTR of FTX and JAK2 were inserted into a pGL3-basic vector. pGL3 with the pRL-TK Vector (Promoga, Madison, USA) was respectively transfected into the cells using Lipofectamine 2000 (Invitrogen, Carlsbad, USA). The Dual Luciferase Reporter Assay System was used to perform luciferase reporter assays at 48 h after the transfection.
RNA pull-down
The miR-590-5p and FTX probes labeled by Biotin were obtained by transcription in vitro separately (Roche, Switzerland). The sense hybridization probes were synthesized at the same time and were used as the control. The probes were added into cytoplasmic extract from the ASMCs and incubated to form the miRNA-lncRNA compound separately. Then it was separated using magnetic beads labeled with avidin. Finally, a qRT-PCR analysis was performed to determine the enrichment of miR-590-5p and FTX fragments in the immunoprecipitated RNA.
Statistical analysis
The statistical analysis was performed using GraphPad Prism 7.0. All the values and data are presented as the means ± SD. t-tests were used for the comparisons between two groups. The differences between multiple samples were analyzed using one-way ANOVA. P<0.05 was considered statistically significant.
Results
FTX is upregulated in ASMCs stimulated by PDGF-BB
To verify the effect of the PDGF-BB stimulation on the FTX expression in the ASMCs, the ASMCs were cultured at 37°C for 24 h in a medium containing 25 ng/mL PDGF-BB, then collected to quantify the expression of FTX using RT-PCR. As shown in Figure 1, the FTX expression was significantly increased upon PDGF-BB stimulation in a time-dependent manner, indicating that FTX might be involved in the ASMC regulation activated by PDGF-BB.
Figure 1.

The expressions of FTX, miR-590-5p, and JAK2 changed by the PDGF-BB induction. The measurement of the FTX expressions after treatment with PDGF-BB for 6 h, 12 h, 18 h, and 24 h separately using qPCR. Untreated ASMCs were used as a control. **P<0.01; ***P<0.001.
FTX knockdown inhibits ASMC proliferation and migration, and increases cell apoptosis induced by PDGF-BB
To explore the role of FTX in the ASMCs, we transfected the ASMCs with si-FTX and si-NC. The FTX level in si-FTX group was significantly reduced compared to the FTX level in si-NC (Figure 2A), and this was confirmed using RT-PCR. CCK8 and Transwell assays were subsequently performed to assess the cell proliferation and migration. This showed that the PDGF-BB treatment significantly increased the proliferation and migration of ASMCs, but the effect was reversed by the FTX knockdown (Figure 2B, 2C). Moreover, the flow cytometry results showed that the PDGF-BB significantly decreased the percentage of the apoptotic cells, which was partially rescued by si-FTX (Figure 2D), suggesting that FTX relieved the effect of the PDGF-BB treatment on the proliferation, migration, and apoptosis of ASMCs.
Figure 2.
The FTX knockdown decreased the proliferation and metastasis and increased the apoptosis of the ASMCs induced by PDGF-BB. A. The measurement of the FTX expressions in the different groups using qPCR. B. The cell viability measurements of the different groups using CCK8. C. The valuation of the cell migration abilities of the different groups using CCK8. The scale is 100×. D. The measurement of the apoptotic levels using flow cytometry. Si-FTX was transfected into the ASMCs to silence the FTX expression, and si-NC was used as a control. **P<0.01; ***P<0.001.
FTX targets miR-590-5p
Many studies have found that lncRNAs reduce the expression of mRNAs by competing shared microRNAs (miRNAs) with mRNAs. Nucleocytoplasmic separation experiments have confirmed that FTX is mainly expressed in cytoplasms (Figure 3A), indicating that FTX may perform its functions in the above way. Therefore, we screened the potential miRNA through the online software Starbase and found the binding site of miR-590-5p in FTX (Figure 3B). As shown in Figure 3C, miR-590-5p expression increased compared with the control, and it was negatively correlated with the FTX expression, which indicated that FTX may perform its function in the above way, and that FTX may be the molecular sponge of miR-590-5p. Therefore, we studied the interaction between FTX and mir-590-5p. miR-590-5p is over-expressed in ASMCs, and miR-NC was used as a negative control (Figure 3D). The miR-590-5p overexpression inhibited the Luciferase activity carrying the wild-type FTX vector, but not the vector with the mutated FTX (Figure 3E). To further explore the mechanism of interaction between FTX and miR-590-5p, an RNA pull-down was performed. Compared with IgG RIP, Ago2 enriched both the FTX and miR-590-5p fragments (Figure 3F). In addition, the FTX fragments were enriched with a miR-590-5p probe (Figure 3G). In addition, the miR-590-5p fragments were enriched with an FTX probe (Figure 3H). Overall, these data indicate that FTX regulates the expression of miR-590-5p through direct binding.
Figure 3.
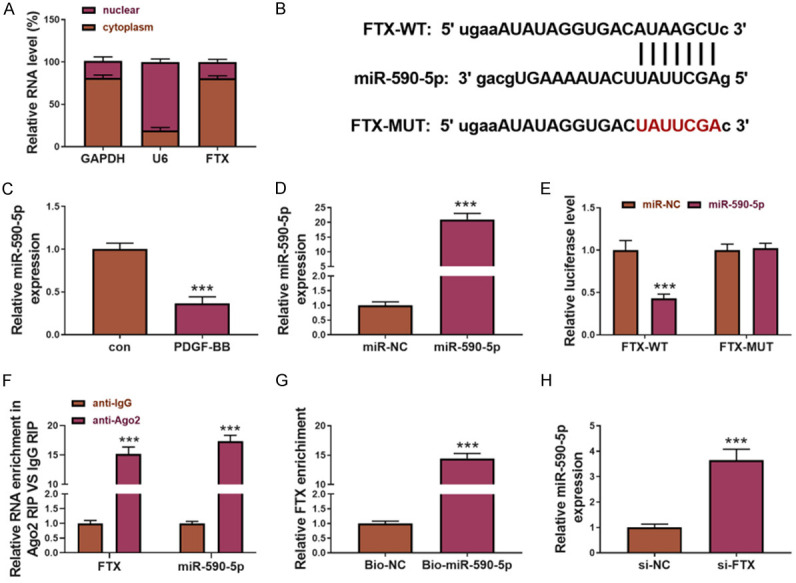
miR-590-5p was the target of FTX. (A) FTX subcellular localization using nucleocytoplasmic separation. U6 and GAPDH were used as the markers of the nuclei and the cytoplasms separately. (B) The FIX and miR-590-5p binding sites were predicted using Starbase. (C) The measurement of the miR-590-5p expression by qPCR. (D) The quantification of the miR-590-5p expression in the miR-590-50-overexpressed ASMCs using qPCR. miR-NC was used as control. (E) miR-590-5p inhibited the Luciferase activity carrying the wild JAK2 3’-UTR binding site but not those carrying the mutated JAK2 3’-UTR binding site. miR-NC was used as a control. Immunoprecipitation and RNA pull-down were performed to study the interaction between FTX and miR-590-5p. (F) Ago2 enriched more FTX and miR-590-5p fragments than IgG. The miR-590-5p probe enriched more FTX fragments (G) and the FTX probe enriched more miR-590-5p fragments (H).
The FTX/miR-590-5p axis regulates the proliferation, migration, and apoptosis of PDGF-BB-stimulated ASMCs
Considering the inhibitory effect of FTX on the miR-590-5p expression, we hypothesized that FTX can inhibit the function of miR-590-5p in ASMCs. We transfected ASMCs with a miR-590-5p inhibitor to suppress its expression (Figure 4A). CCK8 and Transwell analyses were performed to evaluate the cell proliferation and the migration of the ASMCs. The results showed that the FTX knockdown significantly inhibited the proliferation and migration of the PDGF-BB-induced ASMCs, while the miR-590-5p knockdown reversed these effects (Figure 4B and 4C). In addition, the FTX knockdown significantly promoted the apoptosis of the ASMCs induced by PDGF-BB, which were rescued by miR-590-5p downregulation (Figure 4D). These data indicate that FTX participates in ASMCs’ proliferation, migration, and apoptosis in PDGF-BB-treated ASMCs by inhibiting the miR-590-5p expression.
Figure 4.
The FTX/miR-590-5p axis increased cell proliferation and migration and decreased apoptosis. A. miR-590-5p expression by qRT-PCR in the different groups. B. An analysis of the cell proliferation abilities of the ASMCs in the different groups using CCK8. C. Evaluation of the cell migration abilities of the ASMCs in the different groups using TransWell. The scale is 100×. D. The apoptosis levels of the ASMCs in different groups using flow cytometry. Anti-miR-590-5p functions as an inhibitor of miR-590-5p expression, si-FTX as an inhibitor of the FTX expression. Anti-NC was used as a control. **P<0.01; ***P<0.001.
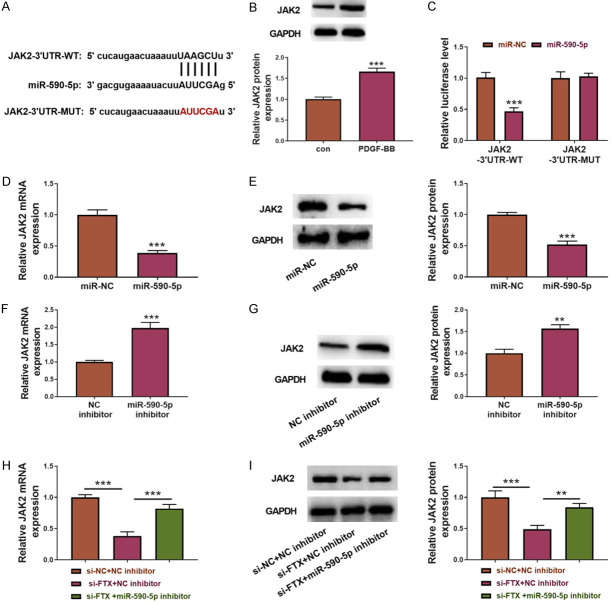
JAK2 is a direct target of the FTX/miR-590-5p signal axis
The binding site of miR-590-5p in the JAK2 3’non-coding region was found through Starbase (Figure 5A). The PDFG-BB stimulation increased the JAK2 protein levels, which were positively correlated with the FTX expression and negatively correlated with the miR-590-5p expression, indicating that JAK2 may be a direct target of the FTX/miR-590-5p axis (Figure 5B). Using a Luciferase reporter assay, we found that the over-expression of miR-590-5p inhibited the Luciferase activity in the ASMCs carrying a wild JAK2 3’-UTR binding site but not those carrying a mutated JAK2 3’-UTR binding site (Figure 5C). Next, we transfected the ASMCs with miR-NC, miR-590-5p mimics, and an NC inhibitor or a miR-590-5p inhibitor to analyze the effect of the miR-590-5p on JAK2 expression. The over-expression of miR-590-5p in the ASMCs decreased the mRNA and protein levels of JAK2, but the miR-590-5p inhibitor increased the JAK2 expression (Figure 5D-G). In addition, the FTX knockdown decreased the JAK2 expression and the protein levels, which were reversed by co-transfecting the anti-miR-590-5p (Figure 5H, 5I). Taken together, these data indicate that FTX functions as a competing endogenous RNA (ceRNA) of miR-590-5p to regulate the JAK2 expression.
Figure 5.
JAK2 is a direct target of the FTX/miR-590-5p axis. (A) The binding site of miR-590-5p in JAK2 3’UTR. (B) The protein levels of JAK2 in the ASMCs before and after the PDGF-BB treatment using Western blot. (C) miR-590-5p inhibited the Luciferase activity which was fused with wild JAK2-3’UTR (JAK2-3’UTR-WT), but the effect disappeared when the binding site was mutated. miR-590-5p decreased the JAK2 mRNA level (D) and the JAK2 protein level (E), but the anti-miR-590-5p had the opposite effect (F and G). (H) The examination of the JAK2 mRNA level (I) and the protein level (H) of the ASMCs in the different groups. Anti-miR-590-5p functions as an inhibitor of miR-590-5p expression, si-FTX as an inhibitor of FTX expression. Anti-NC and si-NC were used as controls. **P<0.01; ***P<0.001.
miR-590-5p/JAK2 regulates the proliferation, migration and apoptosis of the ASMCs
According to the above results, FTX/miR590-5p/JAK2 may together form a ceRNA network. The role of this ceRNA network in regulating asthma progression was studied. JAK2 was over-expressed in the ASMCs, and the empty vector was used as a control, and the increased levels of the JAK2 mRNA and protein were confirmed (Figure 6A). Furthermore, the colony forming ability, cell viability, and apoptosis of the ASMCs in the different groups were examined to evaluate the function of JAK2. As shown in Figure 6B-D, the miR-590-5p over-expression inhibited the cell viability and clone forming abilities of the ASMCs, but it increased the apoptosis induced by the PDGF-BB. When we transfected the ASMCs with both miR-590-5p and JAK2, the decreased cell viability and colony forming ability, as well as the increased apoptosis induced by the miR-590-5p over-expression were reversed (Figure 6B-D). In summary, the miR-590-5p/JAK2 axis plays a negative role in the proliferation and migration of ASMCs.
Figure 6.
The role of the miR-590-5p/JAK2 axis in inhibiting the cell proliferation and migration and promoting apoptosis. A. The protein level of JAK2 using western blot in the JAK2-overexpression ASMCs. B. Analysis of the cell proliferation abilities of the ASMCs in the different groups using CCK8. C. Evaluation of the cell migration abilities of ASMCs in the different groups using TransWell. The scale is 100×. D. The apoptosis levels of the ASMCs in the different groups using flow cytometry. miR-590-5p indicated the overexpression of miR-590-5p, as well as JAK2. miR-NC and Vector were used as the controls for miR-590-5p and the JAK2-overexpressions separately. **P<0.01; ***P<0.001.
Discussion
Asthma is a chronic inflammatory lung disease that occurs in the bronchial airways. The worldwide prevalence of asthma in adults is estimated to be 1.2% to 25.5%, and in children 3.3% to 29.0% [13]. The proliferation and migration of ASMCs have important effects on the pathogenesis of asthma [14]. Growth factors such as PDGF and transforming growth factor are the main ASM remodeling factors, and their expressions are directly related to the severity of asthma [15,16]. PDGF-BB is a dimer subtype and is secreted by the inflammatory cells and the airway epithelial cells [17-19]. Halayko showed that PDGF-BB can drive the conversion of ASMCs from contractile phenotype to proliferation, migration, and synthetic phenotype, causing ASMCs’ proliferation and migration [20]. Therefore, we used PDGF-BB-induced ASMC proliferation and migration as a model to study asthma ASMC proliferation and migration in vitro.
In recent years, lncRNA in asthma has been widely studied. Several studies have found that lncRNAs are associated with a variety of human diseases, including cancer. Jin et al. revealed a novel FTX/miR-200a-3p/FOXA2 that competes with the endogenous RNA regulatory axis in lung cancer cells and found that the ectopic expression of FTX can inhibit cell proliferation and migration in the lungs, in vitro and in vivo [21]. Zuo et al. reported that FTX is involved in Nogo-66-induced neurite growth inhibition through the PDK1/PKB/GSK-3β pathway [22]. Huang et al. demonstrated that FTX in osteosarcoma can inhibit the development of osteosarcoma by regulating TXNRD1/miR-320a to affect cell proliferation and migration [10].
This study systematically reported the role of lncRNA FTX in ASMCs and further explored its proliferation and migration mechanisms. In the ASMCs of asthma patients, the FTX expression levels are elevated. In addition, the gain and loss of cell function indicates that lncRNA FTX promotes the proliferation and migration of ASMCs. Therefore, this study also supports the important role of FTX in the mechanism of asthma. In vascular smooth muscle cells, TUG1 promotes the proliferation and migration of hypertension cells through the miR-590-5p/FGF1 axis [12]. In mechanism studies, the interactions among miR-590-5p, FTX, and JAK2 were predicted by the Starbase database, and they were confirmed by our subsequent experiments (Figures 3 and 5). FTX can promote the cell proliferation and migration of ASMCs in asthma (Figure 2). It also revealed the crucial regulatory effect of the FTX/miR-590-5p/JAK2 axis on ASMC proliferation and migration (Figure 6), by which FTX regulates asthma progression. In airway smooth muscle, the miR-590-5p expression is significantly down-regulated, and the over-expression of miR-590-5p inhibits cell proliferation through the PDGF combination [23]. JAK2 is a non-receptor tyrosine protein kinase, and it is involved in cell proliferation, differentiation, apoptosis, and immune regulation [24]. Several studies have proved its promoting function in asthma. PM2.5 (diameter ≤2.5 μm) can cause respiratory inflammation and is the pathological basis of asthma or other respiratory diseases, in which JAK2/STAT3 contributes to the disease occurrence. Furthermore, pre-treatment with the JAK pathway inhibitor AG490 (JAK inhibitor) can reduce the respiratory inflammation stimulated by PM2.5, thereby reversing the asthma symptoms [25]. Kim et al. showed that Menthas pecies essential oil (MEO) can significantly reduce the phosphorylation of JAK2 and STAT3 induced by PM10, thus significantly inhibiting asthma under PM10 exposure [26]. The above research reports, along with our research, demonstrate that FTX/mir-590-5p/JAK2 is involved in asthma progression.
Conclusion
In this study, we demonstrated that the regulatory axis of FTX, miR-590-5p, and JAK2 controls the proliferation and migration of ASMCs in the development of asthma. This finding describes a novel FTX/miR-590-5p/JAK2 pathway in the pathogenesis of asthma. But this study only involved in vitro experiments and lacked studies in vivo, which should be optimized in further studies.
In summary, our study found that PDGF-BB induces the up-regulation of FTX in ASMCs, which regulates the function of ASMCs through the miR-590-5p/JAK2 axis. It suggests that FTX knockdown might be a new target for alleviating airway remodeling in asthma.
Acknowledgements
The study was approved by the Qingdao Municipal Hospital ethics committee (No. 2021ECPJ007), and the patients signed the informed consent.
Disclosure of conflict of interest
None.
References
- 1.Li H, Wang K, Huang H, Cheng W, Liu X. A meta-analysis of anti-interleukin-13 monoclonal antibodies for uncontrolled asthma. PLoS One. 2019;14:e0211790. doi: 10.1371/journal.pone.0211790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kaczmarek KA, Clifford RL, Knox AJ. Epigenetic changes in airway smooth muscle as a driver of airway inflammation and remodeling in asthma. Chest. 2019;155:816–824. doi: 10.1016/j.chest.2018.10.038. [DOI] [PubMed] [Google Scholar]
- 3.Wang Y, Xu J, Meng Y, Adcock IM, Yao X. Role of inflammatory cells in airway remodeling in COPD. Int J Chronic Obstruct Pulmon Dis. 2018;13:3341–3348. doi: 10.2147/COPD.S176122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bhalla A, Mukherjee M, Nair P. Airway eosinophilopoietic and autoimmune mechanisms of eosinophilia in severe asthma. Immunol Allergy Clin North Am. 2018;38:639–654. doi: 10.1016/j.iac.2018.06.003. [DOI] [PubMed] [Google Scholar]
- 5.Liang X, Wang J, Chen W, Ma X, Wang Y, Nagao N, Weng W, Huang J, Liu J. Inhibition of airway remodeling and inflammation by isoforskolin in PDGF-induced rat ASMCs and OVA-induced rat asthma model. Biomed Pharmacother. 2017;95:275–286. doi: 10.1016/j.biopha.2017.08.063. [DOI] [PubMed] [Google Scholar]
- 6.Mouraux S, Bernasconi E, Pattaroni C, Koutsokera A, Aubert JD, Claustre J, Pison C, Royer PJ, Magnan A, Kessler R, Benden C, Soccal PM, Marsland BJ, Nicod LP SysCLAD Consortium. Airway microbiota signals anabolic and catabolic remodeling in the transplanted lung. J Allergy Clin Immun. 2018;141:718–729. e717. doi: 10.1016/j.jaci.2017.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li H, Yao G, Zhai J, Hu D, Fan Y. LncRNA FTX promotes proliferation and invasion of gastric cancer via miR-144/ZFX axis. Onco Targets Therapy. 2019;12:11701–11713. doi: 10.2147/OTT.S220998. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 8.Wei J, Baode Z, Jilan S, Yan L, Yanling B, Hong W. LncRNA FTX promotes the tumorigenesis of lung adenocarcinoma by targeting miR-300. Panminerva Med. 2020 doi: 10.23736/S0031-0808.19.03823-0. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 9.Li L, Li L, Zhang YZ, Yang HY, Wang YY. Long non-coding RNA FTX alleviates hypoxia/reoxygenation-induced cardiomyocyte injury via miR-410-3p/Fmr1 axis. Eur Rev Med Pharmaco. 2020;24:396–408. doi: 10.26355/eurrev_202001_19938. [DOI] [PubMed] [Google Scholar]
- 10.Huang S, Zhu X, Ke Y, Xiao D, Liang C, Chen J, Chang Y. LncRNA FTX inhibition restrains osteosarcoma proliferation and migration via modulating miR-320a/TXNRD1. Cancer Biol Ther 2020: 21:379–387. doi: 10.1080/15384047.2019.1702405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Naveed SU, Clements D, Jackson DJ, Philp C, Billington CK, Soomro I, Reynolds C, Harrison TW, Johnston SL, Shaw DE, Johnson SR. Matrix metalloproteinase-1 activation contributes to airway smooth muscle growth and asthma severity. Am J Resp Crit Care. 2017;195:1000–1009. doi: 10.1164/rccm.201604-0822OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lin J, Feng X, Zhang J, Tong Z. Long noncoding RNA TUG1 promotes airway smooth muscle cells proliferation and migration via sponging miR-590-5p/FGF1 in asthma. Am J Transl Res. 2019;11:3159–3166. [PMC free article] [PubMed] [Google Scholar]
- 13.Kagen S, Garland A. Asthma and allergy mobile apps in 2018. Curr Allergy Asthm Rep. 2019;19:6. doi: 10.1007/s11882-019-0840-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhou H, Wu Q, Wei L, Peng S. Paeoniflorin inhibits PDGF-BB-induced human airway smooth muscle cell growth and migration. Mol Med Rep. 2018;17:2660–2664. doi: 10.3892/mmr.2017.8180. [DOI] [PubMed] [Google Scholar]
- 15.Vignola AM, Chanez P, Chiappara G, Merendino A, Pace E, Rizzo A, la Rocca AM, Bellia V, Bonsignore G, Bousquet J. Transforming growth factor-beta expression in mucosal biopsies in asthma and chronic bronchitis. Am J Resp Crit Care. 1997;156:591–599. doi: 10.1164/ajrccm.156.2.9609066. [DOI] [PubMed] [Google Scholar]
- 16.Puddicombe SM, Polosa R, Richter A, Krishna MT, Howarth PH, Holgate ST, Davies DE. Involvement of the epidermal growth factor receptor in epithelial repair in asthma. FASEB J. 2000;14:1362–1374. doi: 10.1096/fj.14.10.1362. [DOI] [PubMed] [Google Scholar]
- 17.Ohno I, Nitta Y, Yamauchi K, Hoshi H, Honma M, Woolley K, O’Byrne P, Dolovich J, Jordana M, Tamura G, et al. Eosinophils as a potential source of platelet-derived growth factor B-chain (PDGF-B) in nasal polyposis and bronchial asthma. Am J Resp Cell Mol. 1995;13:639–647. doi: 10.1165/ajrcmb.13.6.7576701. [DOI] [PubMed] [Google Scholar]
- 18.Shimizu S, Gabazza EC, Hayashi T, Ido M, Adachi Y, Suzuki K. Thrombin stimulates the expression of PDGF in lung epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2000;279:L503–510. doi: 10.1152/ajplung.2000.279.3.L503. [DOI] [PubMed] [Google Scholar]
- 19.Fredriksson L, Li H, Eriksson U. The PDGF family: four gene products form five dimeric isoforms. Cytokine Growth Factor Rev. 2004;15:197–204. doi: 10.1016/j.cytogfr.2004.03.007. [DOI] [PubMed] [Google Scholar]
- 20.Halayko AJ, Tran T, Gosens R. Phenotype and functional plasticity of airway smooth muscle: role of caveolae and caveolins. Proceed Am Thorac Soc. 2008;5:80–88. doi: 10.1513/pats.200705-057VS. [DOI] [PubMed] [Google Scholar]
- 21.Jin S, He J, Zhou Y, Wu D, Li J, Gao W. LncRNA FTX activates FOXA2 expression to inhibit non-small-cell lung cancer proliferation and metastasis. J Cell Mol Med. 2020;24:4839–4849. doi: 10.1111/jcmm.15163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zuo Y, Sun H, Song L, Hu Y, Guo F. LncRNA FTX involves in the Nogo-66-induced inhibition of neurite outgrowth through regulating PDK1/PKB/GSK-3β pathway. Cell Mol Neurobiol. 2020;40:1143–1153. doi: 10.1007/s10571-020-00803-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shi S, Jin L, Zhang S, Li H, Zhang B, Sun M. MicroRNA-590-5p represses proliferation of human fetal airway smooth muscle cells by targeting signal transducer and activator of transcription 3. Arch Med Sci. 2018;14:1093–1101. doi: 10.5114/aoms.2018.74538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yaru Y, Yang L, Ming Z, Li S. MiR-216a inhibits proliferation and promotes apoptosis of human airway smooth muscle cells by targeting JAK2. J Asthma. 2019;56:938–946. doi: 10.1080/02770903.2018.1509991. [DOI] [PubMed] [Google Scholar]
- 25.Xu Z, Wu H, Zhang H, Bai J, Zhang Z. Interleukins 6/8 and cyclooxygenase-2 release and expressions are regulated by oxidative stress-JAK2/STAT3 signaling pathway in human bronchial epithelial cells exposed to particulate matter ≤2.5 μm. J Appl Toxicol. 2020;40:1210–1218. doi: 10.1002/jat.3977. [DOI] [PubMed] [Google Scholar]
- 26.Kim MH, Park SJ, Yang WM. Inhalation of essential oil from mentha piperita ameliorates PM10-exposed asthma by targeting IL-6/JAK2/STAT3 pathway based on a network pharmacological analysis. Pharmaceuticals (Basel) 2021;14:2. doi: 10.3390/ph14010002. [DOI] [PMC free article] [PubMed] [Google Scholar]