Abstract
Objective: To explore the diagnostic value of miR-153 and miR-203 in patients with cervical cancer (CC). Methods: A total of 136 CC and suspected CC patients admitted to our hospital were enrolled in this prospective study. Among them, 80 cases had CC, 56 cases with cervical intraepithelial neoplasia, and 40 cases with cervicitis. The normal cervical tissues of the above 176 patients were taken as a control and the expression levels of miR-153, miR-203 and human papillomavirus (HPV) DNA in CC tissues were detected. Results: The relative expressions of miR-153 and miR-203 in the CC group were significantly lower than those in the cervicitis and cervical intraepithelial neoplasia groups (all P<0.01). The area under the ROC curve of miR-153 for the diagnosis of CC was 0.883 (95% CI: 0.828-0.938, P<0.001). When the cut-off value of miR-153 expression level in the diagnosis of CC was 0.27, its Youden index, specificity, and sensitivity were 0.748, 0.863, and 0.885, respectively. The area under the ROC curve of miR-203 for the diagnosis of CC was 0.752 (95% CI: 0.680-0.825, P<0.001). When the cut-off value of miR-203 expression level in the diagnosis of CC was 0.51, its Youden index, specificity, and sensitivity were 0.478, 0.615, and 0.863, respectively. Compared with the cervical intraepithelial neoplasia group, HPV infection rate in the CC group was significantly higher (P<0.001). The relative expression levels of miR-153 and miR-203 in HPV-positive patients were significantly lower than those in HPV-negative patients (P<0.001). Significantly lower levels of miR-153 and miR-203 were found in patients with myometrial infiltration, FIGO III-IV stage, and lymphatic metastasis (P<0.05). Conclusion: The expressions of miR-152 and miR-203 are down-regulated in patients with CC, which has diagnostic value for CC. The expressions of miR-153 and miR-203 are correlated with HPV infection and aggressiveness of tumor.
Keywords: miR-153, miR-203, cervical cancer, human papillomavirus infection, diagnosis, correlation
Introduction
Cervical cancer (CC) is a common gynecologic malignancy, and its incidence continues to increase in recent years. It ranks as fourth among female malignant tumors, causing great harm to women’s health [1,2]. Recent studies have shown that there is a certain correlation between the incidence of CC and the national economy. The incidence of CC is significantly reduced in high-income countries compared to that of low and middle-income countries. CC in low and middle-income countries was already at an advanced-stage when it was diagnosed [3]. In China, there is also late diagnosis and delayed treatment of CC, and the optimum time for surgical treatment is lost when the diagnosis is at advanced stage, with high recurrence rate of advanced CC after surgical treatment. Studies have shown that there are still a considerable number of CC patients who are diagnosed at advanced stage in China, and they often relapse after surgery, leading to poor prognosis. Therefore, early clinical diagnosis and radical surgery can effectively treat CC and improve the prognosis of patients [4,5]. Clinical studies have shown that over 94% of CC patients are infected with human papillomavirus (HPV); however, less than 1% of HPV patients develop CC [6].
Studies have found that miRNA plays a crucial role in the regulation of tumorigenesis and development [7,8]. miR-153 is a member of this family. It is reported that miR-153 has a low expression level in pancreatic cancer, breast cancer, ovarian cancer, and colorectal cancer, mainly by promoting apoptosis of tumor cells to inhibit tumor production [9,10]. Previous studies have shown that miR-203 can also inhibit tumor proliferation and it can inhibit tumor cell metastasis and invasion of colorectal cancer [11]. Another study has found that miR-203a-5p can inhibit the growth, proliferation, and invasion of CC tumor cells, but the role of miR-203 in CC is less reported [12]. Based on the above research, we studied the miR-153 and miR-203 expression in CC patients and their correlation with HPV infection to determine their diagnostic value for CC.
Materials and methods
General information
The Ethics Committee of our hospital approved this study and all patients signed an informed consent form. A total of 136 CC patients and suspected CC patients admitted to our hospital from March 2017 to August 2020 were enrolled in this prospective study. The patients included in the study were between 25-71 years old. The pathologic results confirmed that 80 patients had CC, with an average age of 43.1±10.0 years, and 56 patients had cervical intraepithelial neoplasia, with an average age of 42.8±9.8 years. In addition, 40 patients with cervicitis were enrolled into the study at the same period, and their average age was 43.6±9.7 years.
Inclusion criteria: (1) patients met the diagnostic criteria for CC, cervical intraepithelial neoplasia, or cervicitis [13]; (2) patients were aged 18-75 years old; (3) patients received cervical biopsy or radical cervical resection in our hospital to obtain cervical tissue samples, and the diseased and normal tissues of their cervix were sampled separately.
Exclusion criteria: (1) patients with incomplete clinical data; (2) patients with severe heart, liver, and kidney related diseases; (3) patients with mental illness or cerebrovascular disease; (4) patients with other cancers or not primary CC; (5) patients participating in clinical research for other projects.
Methods
Determination of the relative expression levels of miR-153 and miR-203 in cervical tissues
RT-PCR technology was used to reverse transcribe miRNA into cDNA using a reverse transcription kit (Fernentas, Canada), which was used as a template for DNA amplification. The expression levels of miR-153 and miR-203 in cervical tissue samples were determined by probe-based fluorescence quantitative PCR. The 2-ΔΔCT method was applied to calculate relative expression levels and experiments were repeated in triplicate to take the average value. U6 was used as an internal control. The primer sequences are shown in Table 1.
Table 1.
Primers for RT-PCR
| Primer | Forward primers 5’-3’ | Reverse primers 5’-3’ |
|---|---|---|
| miR-153 | UUGCAUAGUCACAAAAGUGAUC | TCCACCACCCAGTTGCTGTA |
| miR-203 | GUGAAAUGUUUAGGACCACUAG | CCAGUGGUUCUUAACAGUUCAAC |
| U6 | CGGGTTTGTTTTGCATTTGT | AGTCCCAGCATGAACAGCTT |
Detection of the HPV DNA in cervical tissue samples
The PCR amplifications were performed to amplify HPV DNA and the second-generation hybrid capture methods were used to identify 14 high-risk types of HPV. In the hybridization reactions, the DNA load was used to determine HPV infection in the cervical tissue samples. When the DNA load ≥1 ng/L, the sample is HPV-positive; otherwise, it is HPV-negative [6].
Statistical analysis
Statistical analysis was conducted by statistical software SPSS 17.0. Normally distributed measured data were expressed as x̅ ± sd and the measured data conforming to the homogeneity of variance were tested by t-test. Independent sample t test was performed for comparison between groups; paired sample t test was used for intra-group comparison. The counted data were expressed as a percentage and tested by the Pearson chi-square test. The ROC curve was used to estimate the value of miR-153 and miR-203 in diagnosing CC, and the pictures were drawn by Medcalc software. Differences with P<0.05 were considered significant.
Results
Comparison of general information in the three groups
The expressions of tumor markers CEA, Ca199, and Ca125 in the CC group were significantly higher than those in the cervicitis group and cervical intraepithelial neoplasia group (P<0.001). The remaining clinical characteristics were not different (P>0.05, Table 2).
Table 2.
Comparison of general information in the three groups (x̅ ± sd)
| Characteristic | Cervicitis group (n=40) | Cervical intraepithelial neoplasia group (n=56) | CC group (n=80) | χ2/F | P |
|---|---|---|---|---|---|
| Age (years) | 43.6±9.7 | 42.8±9.8 | 43.1±10.0 | 0.077 | 0.926 |
| BMI | 23.82±1.62 | 24.22±1.46 | 23.99±1.58 | 0.811 | 0.446 |
| Comorbidity (n, %) | |||||
| Hypertension | 6 (15.00) | 9 (16.07) | 15 (18.75) | 0.320 | 0.852 |
| Coronary heart disease | 2 (5.00) | 4 (7.14) | 10 (12.50) | 2.192 | 0.334 |
| Type 2 diabetes | 4 (10.00) | 8 (14.29) | 12 (15.00) | 0.595 | 0.942 |
| Hyperlipidemia | 3 (7.50) | 5 (8.23) | 13 (16.25) | 2.648 | 0.266 |
| Cerebral infarction | 3 (7.50) | 6 (10.71) | 10 (12.50) | 0.693 | 0.707 |
| CEA (μg/L) | 0.92±0.43 | 1.22±0.74 | 1.72±0.93***,### | 17.231 | <0.001 |
| Ca199 (kU/L) | 3.44±1.34 | 4.22±1.93 | 5.63±2.64***,### | 15.765 | <0.001 |
| Ca125 (kU/L) | 5.41±1.73 | 7.92±2.74 | 13.6±2.62***,### | 32.458 | <0.001 |
| CRP (mg/L) | 7.60±2.62 | 7.93±2.83 | 7.13±2.44 | 0.876 | 0.445 |
| Smoking (n %) | 4 (10.00) | 8 (14.29) | 14 (17.50) | 1.224 | 0.521 |
Note: Compared with the cervicitis group;
P<0.001.
Compared with the cervical intraepithelial neoplasia group;
P<0.001.
CEA: Carcinoembryonic antigen; Ca199: Carbohydrate antigen Ca199; Ca125: Carbohydrate antigen Ca125; CRP: C-reactive protein; CC: cervical cancer.
Comparison of expression levels of miR-153 and miR-203 among groups
The present study demonstrated that relative expression levels of miR-153 and miR-203 in the CC group were significantly lower than those in the other three groups (P<0.01); furthermore, those in the cervical intraepithelial neoplasia group were significantly lower than those in the control and cervicitis groups (P<0.001, Table 3).
Table 3.
Comparison of expression levels of miR-153 and miR-203 in the three groups of patients and the normal group (x̅ ± sd)
| Group | n | Relative expression levels of miR-153 | Relative expression levels of miR-203 |
|---|---|---|---|
| Control group | 176 | 0.43±0.12 | 0.81±0.33 |
| Cervicitis group | 40 | 0.40±0.11 | 0.75±0.31 |
| Cervical intraepithelial neoplasia group | 56 | 0.33±0.09***,### | 0.47±0.21***,### |
| CC group | 80 | 0.21±0.08***,###,@@@ | 0.36±0.20***,###,@@ |
| F | 65.076 | 36.190 | |
| P | <0.001 | <0.001 |
Note: Compared with the control group;
P<0.001.
Compared with the cervicitis group;
P<0.001.
Compared with the cervical intraepithelial neoplasia group;
P<0.01;
P<0.001.
CC: cervical cancer.
Diagnostic values of miR-153 and miR-203 for CC
The area under the ROC curve of miR-153 for the diagnosis of CC was 0.883 (95% CI: 0.828-0.938, P<0.001). When the cut-off value of miR-153 expression level in the diagnosis of CC was 0.27 (2-ΔΔCT), its Youden index, specificity, and sensitivity were 0.748, 0.863 and 0.885, respectively. The area under the ROC curve of miR-203 for the diagnosis of CC was 0.752 (95% CI: 0.680-0.825, P<0.001). When the cut-off value of miR-203 expression level in the diagnosis of CC was 0.51 (2-ΔΔCT), its Youden index, specificity, and sensitivity were 0.478, 0.615, and 0.863, respectively (Figure 1).
Figure 1.
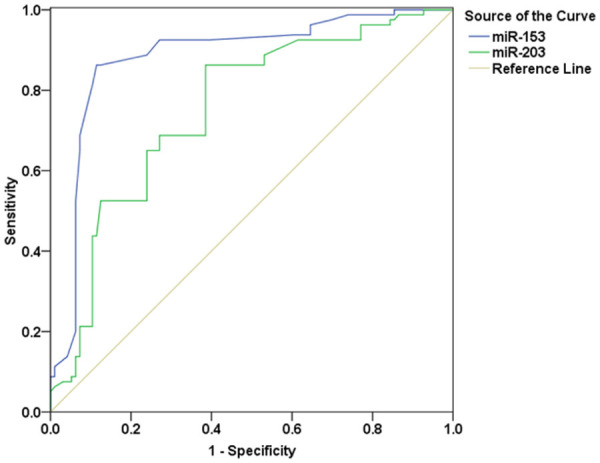
ROC curves of miR-153 and miR-203 for CC diagnosis. CC: cervical cancer.
Comparison of HPV infection in the three groups
Compared with the cervicitis group, HPV infection rates in the CC and cervical intraepithelial neoplasia groups were significantly higher (P<0.001), and the CC group had significantly higher HPV infection rate (P<0.001, Table 4).
Table 4.
Comparison of HPV infection in the three groups (n, %)
| Group | HPV-positive |
|---|---|
| Cervicitis group (n=40) | 8 (20.00) |
| Cervical intraepithelial neoplasia group (n=56) | 27 (48.21)*** |
| CC group (n=80) | 78 (97.50)***,### |
| χ2 | 76.586 |
| P | <0.001 |
Note: compared with the cervicitis group;
P<0.001.
Compared with the cervical intraepithelial neoplasia group;
P<0.001.
HPV: Human papillomavirus; CC: cervical cancer.
Comparisons of expression levels of miR-153 and miR-203 under different HPV infection conditions
The relative expression levels of miR-153 and miR-203 in HPV-positive patients were significantly lower than those in HPV-negative patients (P<0.001, Table 5).
Table 5.
Expression of miR-153 and miR-203 under different HPV infection conditions (x̅ ± sd)
| Group | Relative expression level of miR-153 | Relative expression level of miR-203 |
|---|---|---|
| HPV-positive group (n=113) | 0.25±0.11 | 0.43±0.24 |
| HPV-negative group (n=63) | 0.36±0.11 | 0.59±0.30 |
| t | 6.238 | 4.055 |
| P | <0.001 | <0.001 |
Note: HPV: Human papillomavirus.
Correlation between the relative expression levels of miR-153 and miR-203 and the clinical and pathologic characteristics of 80 CC patients
Significantly lower expressions of miR-153 and miR-203 were found in patients with myometrial infiltration, FIGO III-IV stage, and lymphatic metastasis (P<0.05, Table 6).
Table 6.
Correlation between the relative expression levels of miR-153 and miR-203 and the clinical and pathologic characteristics of 80 patients with CC (x̅ ± sd)
| Characteristic | n | miR-153 | t | P | miR-203 | t | P |
|---|---|---|---|---|---|---|---|
| Age | 1.973 | 0.052 | 1.071 | 0.281 | |||
| ≥50 | 46 | 0.20±0.09 | 0.35±0.18 | ||||
| <50 | 34 | 0.23±0.06 | 0.39±0.21 | ||||
| Pathologic type | 0.584 | 0.561 | 0.102 | 0.919 | |||
| Squamous cancer | 71 | 0.21±0.08 | 0.37±0.19 | ||||
| Adenocarcinoma | 9 | 0.23±0.03 | 0.36±0.26 | ||||
| Histologic grade | 0.854 | 0.341 | 0.324 | 0.746 | |||
| Highly differentiated | 59 | 0.21±0.10 | 0.36±0.21 | ||||
| Poorly differentiated | 21 | 0.23±0.08 | 0.37±0.23 | ||||
| Muscle infiltration | 2.765 | 0.017 | 2.627 | 0.019 | |||
| Yes | 32 | 0.19±0.06 | 0.31±0.17 | ||||
| No | 48 | 0.25±0.10 | 0.39±0.23 | ||||
| FIGO stage | 3.114 | 0.008 | 3.411 | <0.001 | |||
| I-II | 65 | 0.24±0.11 | 0.38±0.24 | ||||
| III-IV | 15 | 0.18±0.07 | 0.32±0.19 | ||||
| Lymphatic metastasis | 3.476 | <0.001 | 4.014 | <0.001 | |||
| No | 61 | 0.25±0.11 | 0.38±0.25 | ||||
| Yes | 19 | 0.18±0.09 | 0.30±0.18 |
Note: CC: cervical cancer.
Discussion
The early diagnosis of malignant tumors determines the prognosis and quality of life of patients, which is getting more attention in clinical practice. How to find biomarkers quickly and effectively has become a focus of clinical research. More and more clinical studies have been conducted on the diagnostic value of miRNAs in tumor patients, and studies have shown that miRNAs are closely related to the occurrence and development of tumors [14,15]. Existing studies have found that miRNAs are abnormally expressed in CC patients, and different miRNAs play a role in inhibiting or promoting tumor cells in CC patients [16,17].
In this study, it was shown that the expressions of miR-153 and miR-203 were both low in CC tissues. This suggests that the low expressions of miR-153 and miR-203 in patients with CC may be related to an antineoplastic effect. Previous studies have shown that the low expression of miR-153 in breast cancer tissues has an antitumor function. This may be achieved by regulating the TGF-β signaling pathway to inhibit the migration, invasion, and epithelial mesenchymal transition (EMT) of breast cancer cells [18]. Low expression of miR-153 also exists in glioma patients, and research has shown that the promotion of miR-153 expression can enhance the radiosensitivity of glioma cells [19]. Also, low expression of miR-153 in renal cancer patients can promote the proliferation and invasion of renal cancer cells [20]. A study on the effect of miR-153 in CC has indicated that miR-153 can inhibit the occurrence and development of CC cells through the targeted regulation of GALNT7 [21]. miR-203 also plays an inhibitory role in the occurrence and development of tumors, and a study has shown that miR-203 can inhibit the proliferation and migration of oral cancer cells [22]. miR-203 can inhibit the proliferation of glioma cancer cells by regulating alpha-interferon [23]. It has found that miR-203 showed a low expression in CC tissues, and in the case of folic acid deficiency, the level of miR-203 can be down-regulated to induce CC [24]. The above results show that the low expressions of miR-153 and miR-203 have anti-cancer effects, which is consistent with the results of this study. This study further showed that miR-153 and miR-203 had value for diagnosis of CC and could be used as early markers for the diagnosis of CC. In this study, the areas under the ROC curve of miR-153 and miR-203 for the diagnosis of CC were 0.883 and 0.752, respectively, and the specificity/sensitivity of miR-153 and miR-203 were 0.927/0.688 and 0.525/0.875, respectively. It suggests that both miR-153 and miR-203 have high diagnostic value. Previous studies showed that miR-153 level in patients with CC was significantly lower than that of the control group. The above results suggested that miR-153 may be a tumor suppressor gene, and its decreased expression may be involved in the occurrence and development of CC. The optimal cut-off value of serum miR-153 expression level in the diagnosis of CC was 1.31, and the area under the ROC curve was 0.817, respectively. The sensitivity and specificity were both good. These results suggested that miR-153 could be used as a biological indicator for the diagnosis of CC [25]. The sensitivity/specificity of miR-203 molecule in endometrial carcinoma and healthy people were 85.9%/74.6%, respectively, which had a high diagnostic value [26].
In this study, HPV infection of CC reached 97.50%, and more than 94% of CC patients were found to be infected with HPV in clinical studies [6]. Research has shown that HPV virus enhances its activity through the mediation of viral proteins E6 and E7, and can inhibit miR-203 to increase its life cycle [27]. As for miR-153, there is no relevant report on HPV infection at present, and its mechanism still has yet to be further clarified. Previous studies on the relationship between clinicopathologic features of CC and miR-153 and miR-203 have found that the low expressions of miR-153 and miR-203 are related to age, clinical stage, lymphatic metastasis, and myometrial invasion [28,29]. In this study, myometrial invasion, FIGO III-IV stage, and lymphatic metastasis were associated with the expressions of miR-153 and miR-203.
This study has some limitations. This was a single-center study with a small sample size, so it may be possible to further expand the sample size and conduct a multi-center randomized controlled study.
In conclusion, low expressions of miR-153 and miR-203 in patients with CC have certain diagnostic value for CC, and the expressions of miR-153 and miR-203 are correlated with HPV infection and tumor aggressiveness.
Acknowledgements
This work was supported by the General Science and Technology Projects of Huzhou City, Zhejiang Province for Clinical study of HPVE7 protein, a marker of cervical cancer (2018GY01).
Disclosure of conflict of interest
None.
References
- 1.Wang S, Zhu H, Ding B, Feng X, Zhao W, Cui M, Xu Y, Shi M, Chen J, Jin H. Genetic variants in microRNAs are associated with cervical cancer risk. Mutagenesis. 2019;34:127–133. doi: 10.1093/mutage/gez005. [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69:7–34. doi: 10.3322/caac.21551. [DOI] [PubMed] [Google Scholar]
- 3.Velentzis LS, Smith MA, Simms KT, Lew JB, Hall M, Hughes S, Yuill S, Killen J, Keane A, Butler K, Darlington-Brown J, Hui H, Brotherton JML, Skinner R, Brand A, Roeske L, Heley S, Carter J, Bateson D, Frazer I, Garland SM, Guy R, Hammond I, Grogan P, Arbyn M, Castle PE, Saville M, Armstrong BK, Canfell K. Pathways to a cancer-free future: a protocol for modelled evaluations to maximize the future impact of interventions on cervical cancer in Australia. Gynecol Oncol. 2019;152:465–471. doi: 10.1016/j.ygyno.2018.12.019. [DOI] [PubMed] [Google Scholar]
- 4.Zhou J, Liu X, Wang C, Li C. The correlation analysis of miRNAs and target genes in metastasis of cervical squamous cell carcinoma. Epigenomics. 2018;10:259–275. doi: 10.2217/epi-2017-0104. [DOI] [PubMed] [Google Scholar]
- 5.Burki TK. Outcomes after minimally invasive surgery in cervical cancer. Lancet Oncol. 2018;19:e674. doi: 10.1016/S1470-2045(18)30840-4. [DOI] [PubMed] [Google Scholar]
- 6.Chan CK, Aimagambetova G, Ukybassova T, Kongrtay K, Azizan A. Human papillomavirus infection and cervical cancer: epidemiology, screening, and vaccination-review of current perspectives. J Oncol. 2019;2019:3257939. doi: 10.1155/2019/3257939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li L, Kou J, Zhong B. Up-regulation of long non-coding RNA AWPPH inhibits proliferation and invasion of gastric cancer cells via miR-203a/DKK2 axis. Hum Cell. 2019;32:495–503. doi: 10.1007/s13577-019-00277-x. [DOI] [PubMed] [Google Scholar]
- 8.Xia C, Liang S, He Z, Zhu X, Chen R, Chen J. Metformin, a first-line drug for type 2 diabetes mellitus, disrupts the MALAT1/miR-142-3p sponge to decrease invasion and migration in cervical cancer cells. Eur J Pharmacol. 2018;830:59–67. doi: 10.1016/j.ejphar.2018.04.027. [DOI] [PubMed] [Google Scholar]
- 9.Wu X, Li L, Li Y, Liu Z. MiR-153 promotes breast cancer cell apoptosis by targeting HECTD3. Am J Cancer Res. 2016;6:1563–1571. [PMC free article] [PubMed] [Google Scholar]
- 10.Liu F, Liu B, Qian J, Wu G, Li J, Ma Z. miR-153 enhances the therapeutic effect of gemcitabine by targeting Snail in pancreatic cancer. Acta Biochim Biophys Sin (Shanghai) 2017;49:520–529. doi: 10.1093/abbs/gmx039. [DOI] [PubMed] [Google Scholar]
- 11.Chen L, Gao H, Liang J, Qiao J, Duan J, Shi H, Zhen T, Li H, Zhang F, Zhu Z, Han A. miR-203a-3p promotes colorectal cancer proliferation and migration by targeting PDE4D. Am J Cancer Res. 2018;8:2387–2401. [PMC free article] [PubMed] [Google Scholar]
- 12.Dai SG, Guo LL, Xia X, Pan Y. Long non-coding RNA WT1-AS inhibits cell aggressiveness via miR-203a-5p/FOXN2 axis and is associated with prognosis in cervical cancer. Eur Rev Med Pharmacol Sci. 2019;23:486–495. doi: 10.26355/eurrev_201901_16860. [DOI] [PubMed] [Google Scholar]
- 13.Perkins RB, Guido RS, Castle PE, Chelmow D, Einstein MH, Garcia F, Huh WK, Kim JJ, Moscicki AB, Nayar R, Saraiya M, Sawaya GF, Wentzensen N, Schiffman M. 2019 ASCCP risk-based management consensus guidelines for abnormal cervical cancer screening tests and cancer precursors. J Low Genit Tract Dis. 2020;24:102–131. doi: 10.1097/LGT.0000000000000628. [DOI] [PubMed] [Google Scholar]
- 14.Dutta RK, Chinnapaiyan S, Unwalla H. Aberrant microRNAomics in pulmonary complications: implications in lung health and diseases. Mol Ther Nucleic Acids. 2019;18:413–431. doi: 10.1016/j.omtn.2019.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu KL, Tsai YM, Lien CT, Kuo PL, Hung AJ. The roles of MicroRNA in lung cancer. Int J Mol Sci. 2019;20:1611. doi: 10.3390/ijms20071611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ma L, Li LL. miR-145 contributes to the progression of cervical carcinoma by directly regulating FSCN1. Cell Transplant. 2019;28:1299–1305. doi: 10.1177/0963689719861063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.He B, Bai Y, Kang W, Zhang X, Jiang X. LncRNA SNHG5 regulates imatinib resistance in chronic myeloid leukemia via acting as a CeRNA against MiR-205-5p. Am J Cancer Res. 2017;7:1704–1713. [PMC free article] [PubMed] [Google Scholar]
- 18.Wang J, Liang S, Duan X. Molecular mechanism of miR-153 inhibiting migration, invasion and epithelial-mesenchymal transition of breast cancer by regulating transforming growth factor beta (TGF-β) signaling pathway. J Cell Biochem. 2019;120:9539–9546. doi: 10.1002/jcb.28230. [DOI] [PubMed] [Google Scholar]
- 19.Sun D, Mu Y, Piao H. MicroRNA-153-3p enhances cell radiosensitivity by targeting BCL2 in human glioma. Biol Res. 2018;51:56. doi: 10.1186/s40659-018-0203-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhou B, Zheng P, Li Z, Li H, Wang X, Shi Z, Han Q. CircPCNXL2 sponges miR-153 to promote the proliferation and invasion of renal cancer cells through upregulating ZEB2. Cell Cycle. 2018;17:2644–2654. doi: 10.1080/15384101.2018.1553354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xu QY, Zuo Y, Guo GL, Ren YH, Li XJ. Effects of miR-153 targeting GALNT7 on proliferation, migration and invasion of cervical cancer cells. J Qinghai Med Coll. 2018;39:247–251. [Google Scholar]
- 22.Kim JS, Choi DW, Kim CS, Yu SK, Kim HJ, Go DS, Lee SA, Moon SM, Kim SG, Chun HS, Kim J, Kim JK, Kim DK. MicroRNA-203 induces apoptosis by targeting Bmi-1 in YD-38 oral cancer cells. Anticancer Res. 2018;38:3477–3485. doi: 10.21873/anticanres.12618. [DOI] [PubMed] [Google Scholar]
- 23.Yang CH, Wang Y, Sims M, Cai C, He P, Häcker H, Yue J, Cheng J, Boop FA, Pfeffer LM. MicroRNA203a suppresses glioma tumorigenesis through an ATM-dependent interferon response pathway. Oncotarget. 2017;8:112980–112991. doi: 10.18632/oncotarget.22945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hao M, Zhao W, Zhang L, Wang H, Yang X. Low folate levels are associated with methylation-mediated transcriptional repression of miR-203 and miR-375 during cervical carcinogenesis. Oncol Lett. 2016;11:3863–3869. doi: 10.3892/ol.2016.4449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Su HE, Zhong QJ, Fu CL, Xue WX, Lin Y, Deng SL. The value of serum miR-153 and miR-142-3p expression in the diagnosis and prognosis of cervical cancer. J Chin Oncol. 2020;218:48–52. [Google Scholar]
- 26.Wang Y, He J, Wang HY, Lei X. Value of serum CA125 combined with miR-31 and miR-203 in diagnosis and prognosis of endometrial carcinoma. Mod Med J. 2019;317:81–85. [Google Scholar]
- 27.Melar-New M, Laimins LA. Human papillomaviruses modulate expression of microRNA 203 upon epithelial differentiation to control levels of p63 proteins. J Virol. 2010;84:5212–5221. doi: 10.1128/JVI.00078-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen XH, Qu JY, Lin Y, Chen XL. Expression and clinicopathological significance of lncRNA AWPPH and miR-203a in cervical carcinoma. Chin J Clin Exp Pathol. 2020;36:1052–1057. [Google Scholar]
- 29.Zhou CY, Du Y, Zhao J, Li L. Expression and clinical significance of miR-153 and survivin in cervical carcinoma. Chin J Clin Exp Pathol. 2020;36:311–315. [Google Scholar]


