Abstract
Purpose: To study and analyze the clinical effectiveness of miniprobe endoscopic ultrasonography (mEUS) in upper gastrointestinal eminence lesions caused by tumors. Methods: We recruited 210 patients admitted to our hospital from March 2018 to June 2020 as the study cohort, all of whom were diagnosed with upper gastrointestinal eminence lesions caused by tumors using routine endoscopies. To further clarify the type of disease, mEUS was used in all the patients along with high-quality nursing interventions. The results and the patients’ anxiety and depression levels before and after the examinations were analyzed. Results: After their admission, the patients’ SAS and SDS scores were high. Their scores decreased significantly after the nursing (P < 0.05). There were 75 patients with esophageal disease diagnosed among the 223 patients with upper gastrointestinal eminence lesions, accounting for 33.63% of the total. 114 patients with gastric disease were found, accounting for 51.12%; and there were 5 cases of cardia disease, accounting for 2.24% of the total. Another 29 cases of duodenal disease were detected, accounting for 13%. According to the determinations of the pathological types, the main disease types were stromal tumors and leiomyomas, with 79 cases (35.43%) and 53 cases (23.77%). The mEUS examinations in the 210 patients detected a total of 223 eminence lesions. In total, the 213 lesions were consistent with the pathological examinations, and the total diagnosis coincidence rate was 95.52% (213/223). Conclusion: mEUS examinations combined with nursing care applied to tumor-induced upper gastrointestinal swelling lesions can eliminate patient tension and anxiety and other unhealthy emotions and can improve patient compliance with the examinations. mEUS examinations can show the hierarchical structure of the upper gastrointestinal wall and accurately locate any upper gastrointestinal swelling lesions, effectively clarify the nature of the disease, and have a guiding significance for the differential diagnosis and treatment of the disease.
Keywords: Small probe ultrasound, raised lesions of the upper digestive tract, tumors, clinical value
Introduction
Upper gastrointestinal eminence lesions are a common morphologic lesion found in clinical practice, and they are chiefly caused by mucosal and submucosal tissue tumors, extramural viscera, and tumor compression [1]. Conventional gastroscopy can diagnose most diseases of the upper gastrointestinal tract by observing the mucosal surface of the upper gastrointestinal tract combined with a pathological examination by biopsy. Despite its clinical success, conventional gastroscopy has a number of limitations. An upper gastrointestinal eminence lesion can be easily detected using conventional gastroscopy, but only the superficial mucosal lesions can be identified using gastroscopy and biopsy. The diagnostic effect of the disease etiology, size, nature, and other conditions is not ideal, and the disease with a complete and continuous mucosal surface and a deep lesion location cannot be clearly diagnosed [2-4]. mEUS can be administered through the endoscopic biopsy orifice, and the nature of the lesion can be defined by demonstrating the shape, size, position, edge, and echo of the lesion. mEUS has the advantages of less pain and a higher detection rate, etc. [5,6]. With evolving medical care, the prevalence of mEUS has been increasing in the diagnosis of upper gastrointestinal eminence lesions such as in the duodenum and the esophagus [7]. The lesions may lead to patients’ negative emotions such as tension and anxiety, which may adversely affect the diagnostic examination to a certain extent. Consequently, combining the examination with nursing care in the diagnosis can effectively eliminate or alleviate the patients’ negative emotions and effectively improve the diagnostic accuracy [8,9]. In this study, 210 patients admitted to our hospital from March 2018 to June 2020, all of whom were diagnosed through conventional endoscopies with tumor-induced upper gastrointestinal eminence lesions, were investigated and analyzed to determine the clinical effectiveness of mEUS combined with nursing care. The report is as follows.
Experimental
General material
From March 2018 to June 2020, 210 patients admitted to our hospital who were diagnosed through routine endoscopies with tumor-induced eminence lesions in their upper gastrointestinal tracts were recruited as the study cohort. The cohort included 122 male patients and 88 female patients. The subjects ranged in age from 23 to 78 years old, with an average age of (46.35 ± 4.74) years old. The patients underwent routine gastroscopies, and a total of 223 upper gastrointestinal eminence lesions were observed. This study was approved by our hospital’s ethics committee, and all the patients were explained the content of the study, and they voluntarily participated in it and signed the informed consent forms.
Methods
After their admission, the upper gastrointestinal eminence lesion patients were examined using mEUS, and they underwent high-quality nursing intervention.
Nursing intervention
The nursing intervention mainly included: 1) Psychological nursing. Due to their lack of understanding of the examination methods and the disease itself, the patients may be prone to anxiety, tension, and other negative emotions, which can lead to decreased cooperation, seriously affecting the examination progress and effect and delaying the timing of the treatment. The nursing staff explained the disease to the patients with a kind and gentle attitude, informed them of the necessity of the examinations, and the importance of eliminating their negative emotions and improving their treatment compliance. 2) Preparation before the examinations. The nursing staff learned each patient’s disease history and other general information, confirmed whether the patient has any serious organic diseases, and ensured that the work of recording the histories of their allergies and contraindications was done well. Before the examination, the patients were ordered to take oral expelling agents, their dentures were removed, and the elderly patients and the patients with cardiovascular and cerebrovascular diseases had their vital signs monitored, and they underwent nursing interventions such as oxygen inhalation. 3) Equipment preparation. The interface between endoscopic ultrasonography and the host machine were correctly connected. The light sources, endoscope buttons, and negative pressure suctions were checked to see whether they were in good condition. The temperature of the water injection was kept constant at 37°C. 4) Intervention measures during the examination. During the examination, the patient was in a kneeling position on the left side, the collar was kept relaxed, a square towel was put under the patient, the patient’s head was kept still, the patient’s mouth was cleaned promptly, and the patient was instructed not to swallow saliva to avoid coughing. During the process, the patients were assisted to adjust their body positions to ensure the best lesion image. Attention was paid to vital sign monitoring, and timely preparations were made for the treatment. 5) Nursing intervention after the examination. After the examination, the nurses cleaned each patient’s face and mouth and assisted him to get out of bed. Health and dietary guidance were given to the patients, and the physician was notified promptly if the patient had abdominal distension or other symptoms for the corresponding treatment. The patients could leave with their family if they had no symptoms.
Examining methods
The patient fasted for 6-8 hours before the surgery, and water was forbidden for 3 hours prior to the surgery. After the patient was given intravenous anesthesia and the lesion location was determined using gastroscopy, a small probe was inserted into the endoscopic biopsy orifice for the ultrasonic diagnosis. An Olympus EU ME 2 was used as the electronic endoscope host, and an Olympus UM 3R was used as the probe, with a diameter of 2.5 mm and a frequency of 12 MHZ and 20 MHZ. The morphology, level of origin, location, infiltration, echo, and the edge of the mucosal lesions of the upper digestive tract were strictly recorded through continuous flushing or the direct contact method, and a diagnosis was made according to the characteristics of the ultrasonic images. Among the 210 patients, 147 of them underwent endoscopic treatment such as high-frequency electrocoagulation resection and mucosal resection. 48 patients underwent a pathological diagnosis through an endoscopic biopsy. 15 patients underwent a surgical resection and received a pathological diagnosis.
Observation indexes
Before and after their admission and examinations, all the patients were assessed using the Self-Rating Anxiety Scale (SAS) and the Self-rating Depression Scale (SDS) [10]. Each of the 20 items was divided into 4 grades based on the frequency of the defined symptoms. The scores of the 20 items were added up to get a total rough score and then converted into a standard total score according to the formula.
The results of the mEUS examinations, the endoscopic manifestations, and the pathological examinations were statistically analyzed.
Statistical methods
The data were processed and analyzed using SPSS 21.0 software. The measurement data in the study were analyzed using t tests and expressed as (x̅ ± s). The enumeration data were expressed as n (%) using descriptive statistics. P < 0.05 was considered statistically significant. GraphPad prism 8 software was used for the graphing.
Results
Comparison of the SAS and SDS scores before and after the nursing
Before the nursing, the patients’ SAS and SDS scores were high. After the nursing, the scores were significantly reduced (P < 0.05). See Figure 1A, 1B for details.
Figure 1.
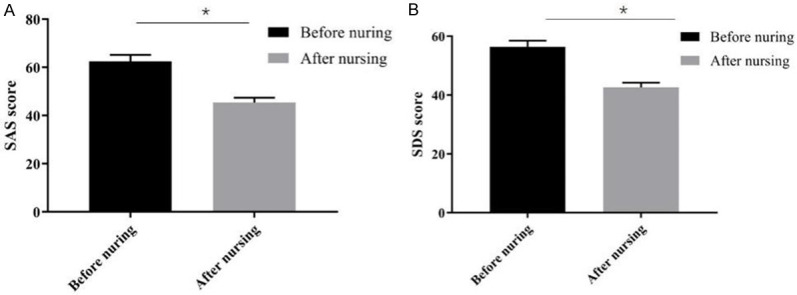
Comparison of the patients’ SAS and SDS scores before and after the nursing. Note: *P < 0.05.
Results of the mEUS examinations
Among the 223 upper gastrointestinal eminence lesions, 75 esophageal lesions were detected, accounting for 33.63% of the total. Also, there were 114 cases of gastric lesions, accounting for 51.12%, and five cases of cardiac lesions, accounting for 2.24%. Twenty-nine of the lesions (13%) were duodenal lesions. According to the pathological types, the main disease types were stromal tumors and leiomyoma, and of these there were 79 cases (35.43%) and 53 cases (23.77%), respectively. See Table 1 for details.
Table 1.
Results of the mEUS examinations
| Results | Gullet | Stomach | Cardia | Duodenum | Total |
|---|---|---|---|---|---|
| Stromal tumor | 35 | 37 | 2 | 5 | 79 |
| Leiomyoma | 24 | 25 | 1 | 3 | 53 |
| Tumor-like hyperplasia | 1 | 0 | 0 | 0 | 1 |
| Polyp | 4 | 11 | 2 | 4 | 21 |
| Heterotopic pancreas | 0 | 17 | 0 | 0 | 17 |
| Malignant tumor | 3 | 2 | 0 | 0 | 5 |
| Lipomyoma | 1 | 3 | 0 | 2 | 6 |
| Cyst | 1 | 3 | 0 | 10 | 14 |
| Papilloma | 0 | 0 | 0 | 2 | 2 |
| Lymphoma | 0 | 0 | 0 | 1 | 1 |
| Hemangioma | 2 | 1 | 0 | 0 | 3 |
| Outer cavity pressure | 4 | 15 | 0 | 3 | 22 |
| Total | 75 | 114 | 5 | 29 | 223 |
Results and features of the mEUS examinations
A total of 79 stromal tumor cases were diagnosed, and 76 of the cases were consistent with the pathology, so the coincidence rate was 96.20% (76/79). The main ultrasonic features were uniform or non-uniform hyperechoic. There were a total of 53 cases of leiomyoma, 50 of which were confirmed by pathology, so the coincidence rate was 94.34% (50/53). The main ultrasonic features were uniform low echoes, clear boundaries, and elliptic or irregular shapes. There was 1 case of neoplastic hyperplasia that met the pathological diagnosis for a coincidence rate of 100% (1/1). The main ultrasonic features were hyperechoic, uniform mucosal layers and localized thickening. A total of 21 polyps were diagnosed through pathology in 19 cases, so the coincidence rate was 90.48% (19/21). The main ultrasonic features were hyperechoes of the mucous layer or the mucous muscle layer, with clear boundaries and protrusions inward. A total of 17 cases of ectopic pancreas were diagnosed by pathology, 16 of which were consistent with the diagnosis, for a coincidence rate of 94.12% (16/17). The main ultrasonic features were a non-uniform medium-high echo, no echo, and an unclear boundary. A total of 5 cases of malignant tumors were diagnosed by pathology, and 5 cases were consistent, for a coincidence rate of 100% (5/5). The main ultrasonic features were an uneven echo, an irregular shape, an unclear boundary, and thickened, fuzzy, or interrupted tube walls. A total of 6 lipomas were diagnosed, and 5 of them were consistent with the pathology, so the coincidence rate was 83.33% (5/6). The main ultrasonic feature was that the submucosa presented as uniformly hyperechoic with clear boundaries. There were 14 cases of cysts and 13 of them were consistent with the pathological diagnosis, for a coincidence rate of 92.86% (5/6). The main ultrasonic feature was that the submucosa was uniform and echoless, with clear boundaries and a complete capsule. There were 2 cases of papilloma, and pathological diagnosis showed that the 2 cases were consistent. The coincidence rate was 100% (2/2). Lymphoma was diagnosed in 1 case, and the coincidence rate was 100% (1/1). The main ultrasonic features were an extremely low echo, round-like, and unclear boundaries. There were three cases of hemangioma. The pathological diagnosis showed that the 3 cases were consistent, so the coincidence rate was 100% (1/1). The main ultrasonic features were no echo and clear boundaries. A total of 22 cases were diagnosed, and 22 cases were consistent with the pathology for a coincidence rate of 100% (22/22). The main ultrasonic features were a non-uniform low echo, visible external organs or tissues, and a complete structure of the wall of the digestive tract. The overall diagnostic coincidence rate was 95.52% (213/223) in the mEUS examinations of the 210 patients. See the details in Table 2.
Table 2.
Results and features of the mEUS examinations
| Disease type | mEUS examination | Pathologic diagnosis | Coincidence rate |
|---|---|---|---|
| Stromal tumor | 79 | 76 | 96.20% |
| Leiomyoma | 53 | 50 | 94.34% |
| Tumor-like hyperplasia | 1 | 1 | 100% |
| Polyp | 21 | 19 | 90.48% |
| Heterotopic pancreas | 17 | 16 | 94.12% |
| Malignant tumor | 5 | 5 | 100% |
| Lipomyoma | 6 | 5 | 83.33% |
| Cyst | 14 | 13 | 92.86% |
| Papilloma | 2 | 2 | 100% |
| Lymphoma | 1 | 1 | 100% |
| Hemangioma | 3 | 3 | 100% |
| Outer cavity pressure | 22 | 22 | 100% |
| Total | 223 | 213 | 95.52% |
Comparison of mEUS examination and pathological examination results
A total of 223 lesions were detected in the mEUS examinations in the 210 patients. A total of 213 lesions were consistent with the pathological examination, and the total diagnostic coincidence rate was 95.52% (213/223). A comparison of the two examination results (excluding the patients with a 100% coincidence rate in the two examination results) is shown in Table 3.
Table 3.
Comparison of mEUS examinations and the pathological examination results
| mEUS examination | |||||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Stromal tumor | Leiomyoma | Polyp | Heterotopic pancreas | Lipomyoma | Cyst | ||
| Pathological examination | Stromal tumor | 76 | 0 | 0 | 0 | 0 | 0 |
| Leiomyoma | 0 | 50 | 0 | 0 | 0 | 0 | |
| Polyp | 0 | 0 | 19 | 0 | 0 | 0 | |
| Heterotopic pancreas | 1 | 3 | 0 | 16 | 0 | 0 | |
| Myxadenoma | 0 | 0 | 0 | 0 | 0 | 1 | |
| Lipomyoma | 0 | 0 | 0 | 0 | 5 | 0 | |
| Cyst | 0 | 0 | 0 | 0 | 0 | 13 | |
| Tubular adenoma | 0 | 0 | 1 | 0 | 0 | 0 | |
| Inflammatory hyperplasia | 0 | 0 | 1 | 1 | 0 | 0 | |
| Few cells | 2 | 0 | 0 | 0 | 1 | 0 | |
Extracavitary compression organs and lesions distribution features
A total of 22 cases of extracavitary compression lesions were confirmed through the mEUS examinations and the pathological examination, of which the spleen compression of gastric fundus accounted for 40.91% (9/22), ranking first. Vascular compression of the esophagus ranked second, accounting for 22.73% (5/22). The distribution characteristics of the extracellular compression organs and lesions are shown in Table 4.
Table 4.
Organs under outer cavity pressure and the lesion distribution features
| Gullet | Fundus of stomach | Gastric body | Antrum | Duodeum | |
|---|---|---|---|---|---|
| Blood vessel | 5 | 0 | 0 | 0 | 0 |
| Trachea | 1 | 0 | 0 | 0 | 0 |
| Spine | 1 | 0 | 0 | 0 | 0 |
| Liver | 0 | 0 | 1 | 1 | 0 |
| Spleen | 0 | 9 | 0 | 0 | 0 |
| Gall bladder | 0 | 0 | 0 | 1 | 1 |
| Abdominal neoplasms | 0 | 0 | 1 | 1 | 0 |
| Total | 7 | 9 | 2 | 3 | 1 |
Discussion
Upper gastrointestinal eminence lesions have existed as a common clinical disease for decades, most of which are caused by mucosal and submucosal tissue lesions as well as a compression of the external organs or tissues, and most of the patients have a hidden onset without obvious clinical symptoms [10,11]. Most digestive tract diseases can be diagnosed and identified using a conventional gastroscopy combined with a biopsy. However, deep mucosal lesions of the upper digestive tract and the lesions outside the digestive tract or the swelling caused by organ compression remain a challenge. The blind acquisition of deep pathological tissue will greatly increase the chance of bleeding or perforation [12]. mEUS can insert a small probe through the endoscopy biopsy hole, directly inspect the lesion site through the endoscopy, obtain the lesion wall layer structure and the characteristics of the adjacent tissues through ultrasonic scanning, and indicate the source, level, echo, and other information about the lesion. It is of positive guiding significance in defining the nature of the lesion, diagnosing, differentiating the disease and treating the lesion [13].
MEUS examinations can demonstrate the tissue hierarchy of the tube wall, which is divided into 5 layers from the inside to the outside. The first layer is hyperechoic, which is generated in the mucosal layer and the mucosal surface. The second layer is the mucous muscle layer, whose nature is also hyperechoic. The third layer is the submucosa, and the fourth layer is the muscularis propria, both of which are hyperechoic. The fifth layer is hyperechoic and is produced in the serosal layer and the external tissues [14,15]. By analyzing the characteristics of echogenicity, the edge conditions, and the evenness of the ultrasonic images, the examination can play a vital role in scanning the disease lesions and the clear diagnosis and differentiation of the lesions. As most patients with upper gastrointestinal eminence lesions have an incorrect cognition of the disease and a low understanding of the examination, they are prone to produce negative emotions such as tension and anxiety, which reduces the examination compliance of the patients and affects the accuracy of the examination. Previous studies have established that the ultrasound examination of upper gastrointestinal eminence lesions can improve the patients’ awareness of the disease and its diagnosis and treatment measures by combining it with high-quality nursing intervention, relieving or even eliminating the patients’ tension, anxiety, and other negative emotions, and effectively improving the patients’ examination cooperation and examination accuracy [16].
In this study, 210 patients admitted to our hospital from March 2018 to June 2020, all of whom were diagnosed through conventional endoscopy with tumor-induced upper gastrointestinal eminence lesions, were investigated and analyzed to determine the clinical effectiveness of mEUS combined with nursing intervention for treating the disease. After the nursing intervention, it was found that the patients’ SAS and SDS scores were high after their admission. After the nursing, the scores decreased significantly (P < 0.05), indicating that high-quality nursing interventions for patients with upper gastrointestinal eminence lesions undergoing mEUS examination can effectively eliminate the patients’ negative emotions, so it is of positive significance for improving the quality of diagnosis. According to the mEUS examinations, 75 esophageal lesions were detected in 223 upper gastrointestinal eminence lesions, accounting for 33.63%. There were 114 cases of gastric lesions, accounting for 51.12%; Five cases of cardiac lesions were detected, accounting for 2.24%. Twenty-nine cases (13%) involved duodenal lesions. According to the pathological type, the main disease types were stromal tumors and leiomyoma, of which there were 79 cases (35.43%) and 53 cases (23.77%), respectively. The type and characteristics of the lesions are similar and consistent with those reported in many studies [17,18]. It was recognized that the eminence of upper gastrointestinal tract lesions gives priority to benign lesions, most of which were gastric lesions, accounting for 50%. Gullet lesions followed, accounting for about 25% to 35%. In terms of disease types, stromal tumors and leiomyoma were the main diseases, accounting for more than 50%. Compared with the pathological examination results, it was found that there were a total of 213 lesions consistent with the pathological examination, so the total diagnostic coincidence rate was 95.52% (213/223). In this study, the coincidence rate of the mEUS examination was similar and consistent with the previous reports [19]. mEUS examinations are confirmed to be of high diagnostic value. For lymphoma, hemangioma, papilloma, extracellular compression, and other lesions with obvious characteristics, the diagnostic compliance rate of the mEUS examination can reach 100%. For stromal tumors, leiomyoma, and other diseases, the coincidence rate is over 90%, which confirms that mEUS is of high value in the diagnosis of stromal tumors and leiomyoma. Studies have found that mEUS is highly accurate at finding pathological changes in the submucosal tissue, through endoscopic pathological changes can be clearly detected by their shapes, boundaries, and sizes. For lesions with small diameters and non-muscularis propria, endoscopic treatment can be performed, with positive effects on reducing the occurrence of complications. For lesions with a large diameter or muscularis propria, surgical resection can be performed. It was confirmed that mEUS examinations have an important influence on the differential diagnosis and treatment of diseases [20].
To sum up, the application of mEUS examinations combined with nursing intervention in tumor-induced upper gastrointestinal eminence lesions can eliminate patients’ anxiety and other adverse emotions and improve patients’ examination compliance. mEUS examinations can clearly demonstrate the hierarchical structure of the upper gastrointestinal wall, accurately locate the upper gastrointestinal eminence lesions, and effectively define the nature of the lesions. The study provides new insights on the differential diagnosis and treatment of such diseases.
Disclosure of conflict of interest
None.
References
- 1.Yokoyama A, Omori T, Yokoyama T. Changing trends in cancer incidence of upper aerodigestive tract and stomach in Japanese alcohol-dependent men (1993-2018) Cancer Med. 2020;9:837–846. doi: 10.1002/cam4.2737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Samor V, Sarowa D, Nayak P, Saini V, Kaur G, Rathi U. Rare foreign bodies of upper aerodigestive tract: a study of 30 cases. Int J Otorhinolaryngol Head Neck Surg. 2020 [Google Scholar]
- 3.Hua R, Liang G, Yang F. Meta-analysis of the association between dietary inflammatory index (DII) and upper aerodigestive tract cancer risk. Medicine (Baltimore) 2020;99:e19879. doi: 10.1097/MD.0000000000019879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Deng Y, Govers C, Tomassen M, Hettinga K, Wichers HJ. Heat treatment of β-lactoglobulin affects its digestion and translocation in the upper digestive tract. Food Chem. 2020;330:127184. doi: 10.1016/j.foodchem.2020.127184. [DOI] [PubMed] [Google Scholar]
- 5.Bohm M, Shin A, Teagarden S, Xu H, Gupta A, Siwiec R, Nelson D, Wo J. Risk factors associated with upper aerodigestive tract or coliform bacterial overgrowth of the small intestine in symptomatic patients. J Clin Gastroenterol. 2018;54:1. doi: 10.1097/MCG.0000000000001150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gianella P, Roncone S, Ala U, Bottero E, Cagnasso F, Cagnotti G, Bellino C. Upper digestive tract abnormalities in dogs with chronic idiopathic lymphoplasmacytic rhinitis. J Vet Intern Med. 2020;34:1845–1852. doi: 10.1111/jvim.15827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arens C, Betz C, Kraft M, Voigt-Zimmermann S. Narrow band imaging for early diagnosis of epithelial dysplasia and microinvasive tumors in the upper aerodigestive tract. HNO. 2017;65:5–12. doi: 10.1007/s00106-016-0284-x. [DOI] [PubMed] [Google Scholar]
- 8.Norris CD, Koontz NA. Secondary otalgia: referred pain pathways and pathologies. AJNR Am J Neuroradiol. 2020;41:2188–2198. doi: 10.3174/ajnr.A6808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Priyamvada S, Motwani G. A study on deep neck space infections. Indian J Otolaryngol Head Neck Surg. 2019;71:912–917. doi: 10.1007/s12070-019-01583-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dunstan DA, Scott N, Todd AK. Screening for anxiety and depression: reassessing the utility of the Zung scales. BMC Psychiatry. 2017;17:329. doi: 10.1186/s12888-017-1489-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chan SA, Alfonso KP, Comer BT. Upper aerodigestive tract frostbite from inhalation of automotive nitrous oxide. Ear Nose Throat J. 2018;97:E13–E14. doi: 10.1177/014556131809700903. [DOI] [PubMed] [Google Scholar]
- 12.Scheufele F, Schirren R, Friess H, Reim D. Selective decontamination of the digestive tract in upper gastrointestinal surgery: systematic review with meta-analysis of randomized clinical trials. BJS Open. 2020;4:1015–1021. doi: 10.1002/bjs5.50332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Godzhello E, Bulganina N, Khrustaleva M. Methodological and technical aspects of EUS-guided fine needle aspiration of the upper digestive tract lesions. Eksp Klin Gastroenterol. 2020;174:107–114. [Google Scholar]
- 14.Borch AM, Novovic S, Karran D, Hansen EF, Schmidt PN, Karstensen JG. Endoscopic ultrasound-guided establishment of transmural anastomoses in the upper gastrointestinal tract. Ugeskr Laeger. 2020;182:V01200013. [PubMed] [Google Scholar]
- 15.Yamamoto Y, Yahagi N, Yamamoto H, Ono H, Inoue H. Innovative therapeutic endoscopy in the upper gastrointestinal tract: review of Japan Gastroenterological Endoscopic Society Core Sessions. Dig Endosc. 2020;32:882–887. doi: 10.1111/den.13722. [DOI] [PubMed] [Google Scholar]
- 16.Sato T, Ueha R, Goto T, Yamauchi A, Kondo K, Yamasoba T. Expression of ACE2 and TMPRSS2 proteins in the upper and lower aerodigestive tracts of rats: implications on COVID 19 infections. Laryngoscope. 2021;131:E932–E939. doi: 10.1002/lary.29132. [DOI] [PubMed] [Google Scholar]
- 17.Di Mitri R, Mocciaro F, Antonini F, Scimeca D, Conte E, Bonaccorso A, Scibetta N, Unti E, Fornelli A, Giorgini S, Binda C, Macarri G, Larghi A, Fabbri C. Stylet slow-pull vs. standard suction technique for endoscopic ultrasound-guided fine needle biopsy in pancreatic solid lesions using 20 Gauge Procore™ needle: a multicenter randomized trial. Dig Liver Dis. 2020;52:178–184. doi: 10.1016/j.dld.2019.08.023. [DOI] [PubMed] [Google Scholar]
- 18.Nakai Y, Kogure H, Koike K. Double-guidewire technique for endoscopic ultrasound-guided pancreatic duct drainage. Dig Endosc. 2019;31:65–66. doi: 10.1111/den.13333. [DOI] [PubMed] [Google Scholar]
- 19.Varas Lorenzo MJ, Abad Belando R, Sánchez-Vizcaíno Mengual E. Miniprobe endoscopic sonography for gastrointestinal tract assessment: a case series of 1451 procedures. J Ultrasound Med. 2018;37:293–303. doi: 10.1002/jum.14330. [DOI] [PubMed] [Google Scholar]
- 20.Martin Cardona A, García A, Lopez E, Zabana Y, Aceituno M, Cassadó C, Horta D, Ruiz-Campos L, Alastruey C, Murcia X, Leiva O, Fernández-Bañares F, Comas M. P074 Histopathologic evaluation and lymphocyte subpopulations of the upper digestive tract of Crohn’s disease. J Crohns Colitis. 2020;14:S172–S173. [Google Scholar]


