Abstract
Objective: To investigate the potential miRNA targeting FGF18, and its role in regulating the proliferation, apoptosis and inflammation in human primary chondrocytes. Methods: The normal human chondrocytes were induced by IL-1β to mimic OA in vitro. qPCR and Western blotting were performed to evaluate the expression of FGF18. Target Scan analysis was performed to predict the miRNA targeting FGF18. Then, the expression of miR-590-5p was quantified by qPCR in IL-1β-induced chondrocytes. After transfection of miR-590-5p mimics or inhibitors, CCK-8 assay was conducted to determine the cell viability and apoptosis-related proteins, and cartilage degeneration related biomarkers were assayed by qPCR and Western blotting. The levels of tumor necrosis factor (TNF)-α, interleukin (IL)-6 and IL-8 were determined by ELISA. The targeting relationship between miR-590-5p and FGF18 was assayed by luciferase reporter assay in IL-1β-induced chondrocytes. Results: Target Scan analysis predicted that FGF18 is directly targeted by miR-590-5p. miR-590-5p was up-regulated, whereas FGF18 expression was inhibited in IL-1β-induced chondrocytes. miR-590-5p mimics reduced the expression of FGF18 protein, inhibited the cell viability of chondrocytes, and promoted secretion of inflammatory factors in chondrocytes, while miR-590-5p inhibitors increased FGF18 levels in IL-1β-treated chondrocytes. Furthermore, expression of inflammatory factors in chondrocytes was reduced by miR-590-5p inhibitors. The luciferase reporter assay showed that miR-590-5p could target FGF18. Conclusions: miR-590-5p promotes OA progression by targeting FGF18, which serves as a potential therapeutic target for OA.
Keywords: Chondrocytes, FGF18, miR-590-5p, osteoarthritis
Introduction
Osteoarthritis (OA) is a chronic inflammation-related joint disease with a complex pathogenesis. It often shows the symptoms of repeated attacks of joint pain, joint movement disorders and continuous aggravation [1,2]. At present, there is no effective drug to prevent osteoarthritis associated with articular cartilage degeneration. It is well documented that several risk factors including aging, obesity, trauma, and genetic predisposition are associated with osteoarthritis, but the pathogenesis is not fully elucidated [3-5]. Chondrocytes are the only cells found in cartilage tissue that participate in the production of cartilage extracellular matrix (ECM), which plays an important role in the maintenance of cartilage structure and function [6,7]. Therefore, it is of great significance to reveal the mechanism of chondrocyte injury and pathogenesis of OA.
It is well known that FGF18, a member of the fibroblast growth factor family, plays an important role in bone development, cartilage formation and osteogenesis during the growth period [8-10]. FGF18 has been shown to have significant anabolic effects on chondrocytes in cartilaginous tissues. More importantly, previous data suggested that lower expression of FGF18 may contribute to cartilage degradation [8]. Thus, FGF18 has been considered as a potential therapeutic target in the treatment of OA. According to the phase II clinical data published, recombinant human FGF-18 protein (rhFGF18) can effectively induce the proliferation of chondrocytes and promote the production of extracellular matrix, suggesting the good potential to promote the repair of joint diseases [11]. However, underlying mechanism for regulating FGF18 has not been fully elucidated.
Recently, FGF18 was demonstrated to be targeted by few miRNAs mainly in tumor models. miRNAs are highly conserved non-coding RNAs with a length of about 19-25 bp [12]. They can bind with the 3’UTR of target gene, thus regulating the expression of target gene at the post transcriptional level [13,14]. Of note, FGF-18 is overexpressed in many types of cancer and is demonstrated to promote tumor progression [15]. It is reported to be targeted by miR-590-5p and miR-139 in gastric cancer model and hepatocellular carcinoma cells [16,17]. Until now, only few miRNAs including miR-21-5p and miR595 were identified to target FGF-18 during OA development [18,19]. Whether other more miRNAs could regulate FGF-18 in chondrocytes and serve as potential target in OA still remains unclear.
At present, increasing evidence shows that various kinds of miRNAs have been identified as regulators of OA progression and play vital roles during cartilage formation and remodeling [20-24]. On one hand, the levels of several miRNA, e.g., miR-140, miR-335-5p, miR-93, miR-17-5p and miR-19b-3p, were found to be significantly reduced in OA group than those in control group, and these miRNA could protect against chondrocyte apoptosis, inflammation, and modulate extracellular matrix (ECM) homeostasis via different targets [22,25-28]. For example, Li et al. found that miR-140 targeting Smad1 can protect cartilage from injury in antigen-induced arthritis models [22]. Furthermore, miR-17-5p and miR-19b-3p were demonstrated to inhibit OA progression by targeting EZH2 in IL-1β stimulated chondrocytes [28]. On the other hand, several miRNAs, e.g., miR-634, miR-103, miR-27a, miR-181a-5p, and miR-204, were identified to be up-regulated in OA cartilage, and antagomir or antisense oligonucleotides of these miRNAs could attenuate osteoarthritis [23,24,29-31]. For example, Nakamura A et al. demonstrated that LNA-miR-181a-5p exhibited cartilage-protective effects in vitro and in cartilage explants [30]. Therefore, miRNAs may serve as novel therapeutic targets for OA. Of note, Prasadam et al. found that miR-590-5p was significantly increased in OA model [32], while the target genes have not been identified yet in chondrocytes.
In this present study, the miRNA target prediction was performed, then we investigated the expression levels of FGF18 and miR-590-5p in IL-1β-induced chondrocytes and investigated their influences on OA development, as well as the molecular mechanisms underlying miR-590-5p in OA in vitro. The results implied that miR-590-5p played a progressive role in OA, and it might be a potential therapeutic target for this disease.
Materials and methods
OA chondrocytes induced by IL-1β
The human chondrocyte cells (Procell, China) were induced by IL-1β to develop into IL-1β-induced chondrocytes. Cells were maintained in DMEM/F12 containing 10% fetal bovine serum (FBS, Gibco, USA) at 37°C in a humidified atmosphere of 5% CO2 and 95% air, and the medium was changed every 2 days. The chondrocytes were plated to 6-well plates and cultured for 24 h. Then, these cells were stimulated with IL-1β (10 ng/mL, Peprotech, US) to produce a cellular OA model.
Cell transfection
Vectors and oligonucleotides were synthesized by and purchased from Guangzhou RiboBio Co., Ltd. The following vectors and oligonucleotides were used for transfection: miR-590-5p mimic (miR-590-5p, 5’-GAGCUUAUUCAUAAAA GUGCAG-3’; 50 nM), mimic negative control (miR-NC, 5’-UUUGUACUACACAA AAGUACUG-3’; 50 nM), miR-590-5p inhibitor (in-miR-590-5p, 5’-CUGCACUUUU AUGAAUAAGCUC-3’; 100 nM), inhibitor control (in-miR-NC, 5’-GAGCUUAUUC AUAAAAGUGCAG-3’; 100 nM), FGF18 overexpression vector (FGF; 50 nM), and FGF18 mutation overexpression vector (FGF18-mut; 50 nM). At 70% confluency, cells were transfected using Lipofectamine® 3000 (Invitrogen; Thermo Fisher Scientific, Inc.), according to the manufacturer’s protocol.
qRT-PCR assay
Total RNA was extracted using RNA isolater Tatol RNA Extraction Reagent (Vazyme, China). 1 µg of total RNA was then reversely transcribed into cDNA by means of the HiScript® II 1st Strand cDNA Synthesis Kit (Vazyme, China) in line with the manufacturer’s instructions. PCR was performed on Light Cycler 480 system (Roche, Switzerland) and amplified detection was conducted using ChamQ SYBR qPCR Master Mix (Vazyme, China). The levels of miRNAs were measured by qRT-PCR using miDETECT A Track™ miRNA qRT-PCR Kit (Guangzhou RiboBio Co., Ltd.). The primers for miR-590-5p and U6 small nuclear RNA were obtained from RiboBio Company (Guangzhou, China). The sequences were covered by a patent. For the quantification of the relative expression of each mRNA and miRNA, the threshold cycle (Ct) values were normalized against the endogenous references β-actin and U6. The 2-ΔΔCT method was used. The primer sequences are presented in Table 1.
Table 1.
Primers sequence
| Species | Gene | Primer (5’ to 3’) |
|---|---|---|
| Human | β-actin | F: CTCCATCCTGGCCTCGCTGT |
| R: GCTGTCACCTTCACCGTTCC | ||
| Human | Bax | F: CGGGTTGTCGCCCTTTTCTA |
| R: GAGGAAGTCCAATGTCCAGCC | ||
| Human | Caspase-3 | F: ACAACAACGAAACCTCCGTGGAT |
| R: GTACCATTGCGAGCTGACATTCC | ||
| Human | Bcl-2 | F: ATCGCCCTGTGGATGACTGA |
| R: GAGACAGCCAGGAGAAATCAAAC | ||
| Human | ADAMTs5 | F: CGGGATCGCTTAGTAACTGTGGT |
| R: ACCGCCAGAGTAGAGTTGGTCT | ||
| Human | Collagen II | F: CTCATCGCCACGGTCCTACA |
| R: ACCACGGTCACCTCTGGGT | ||
| Human | FGF-18 | F: CAAGGAGTGCGTGTTCATTGAGA |
| R: CGGGATCGCTTAGTAACTGTGGT |
Cell viability assay
Cell viability was determined by Cell Counting Kit-8 assay. After being stimulated by IL-1β, the chondrocytes were transfected with NC or miR-590-5p mimics/inhibitors, the CCK8 solution (10 μl/well) was added to the culture medium. Then, after incubating for another 1 h at 37°C in humidified 95% air and 5% CO2, the optical density (450 nm) was recorded.
Enzyme-linked immunosorbent assay (ELISA)
Culture supernatant was collected and the concentrations of TNF-α, IL-6 and IL-8 were measured by ELISA (Boster Biological Technology, China) following the manufacturer’s instruction, and were normalized to cell protein concentrations.
Dual luciferase report assay
The binding sequences between miR-590-5p and FGF18 were predicted using Target Scan software. The FGF18 3’-untranslated region (3’-UTR) containing wild-type (WT) or mutant (MUT) binding sites for miR-590-5p (Guangzhou RiboBio Co., Ltd.) were inserted into a pmirGLO vector (Promega Corporation). Subsequently, the human FGF18 3’-UTR fragments and miR-590-5p were co-transfected into chondrocytes using Lipofectamine 3000 (Invitrogen). Transfection efficiency was normalized using the pRL-TK vector. A Dual Luciferase Assay Kit (Promega, USA) was used to measure luciferase activities at 48 h after transfection. Renilla luciferase activity was used for normalization.
Western blot assay
The chondrocytes in culture were lysed using the RIPA buffer (Beyotime Biotechnology, China). Total protein was separated by SDS-PAGE electrophoresis and transferred to 0.45 µm PVDF membranes. Membranes were blocked in TBS-T buffer containing 5% nonfat milk for 1 h and incubated overnight at 4°C with the following primary antibodies: FGF18 (#ab169615, Abcam, 1:1000), anti-Bcl2 (#ab117115, Abcam, 1:1000), anti-Bax (#ab182733, Abcam, 1:1000), anti-caspase 3 (#ab32150, Abcam, 1:1000), anti-ADAMTS5 (#ab41037, Abcam, 1:1000), anti-collagen II (#ab188570, Abcam, 1:1000), and β-actin (#ab8227, Abcam. 1:1000) was the internal control. Then the membranes were washed using TBST and hybridized with the horseradish peroxidase (HRP)-linked antibody goat anti-rabbit IgG (#BA1054, Boster Biological Technology, 1:5000) or goat anti-mouse IgG (#BA1051, Boster Biological Technology, 1:5000) for 2 h. Signal detection was carried out with an ECL system (#E411-04/05, Vazyme). The intensity of the bands was quantified using Image J software (National Institutes of Health, USA).
Statistical analysis
SPSS 21.0 software was applied to analyze the data. The measurement data was expressed as mean ± standard deviation (SD). The statistical comparisons were performed using unpaired Student’s t test or one-way ANOVA. The enumeration data is presented as percentage or rate, and chi square test was used for the comparison between two groups. P < 0.05 was determined significant difference.
Results
miR590-5p was upregulated in IL-1β-treated chondrocytes and was predicted to bind with FGF18 transcripts
To identify the miRNA candidates regulating FGF18 and explore the mechanism in OA, the chondrocytes were treated with IL-1β in vitro to mimic the inflammatory milieu of OA cartilage. As shown in Figure 1A, chondrocyte viability was decreased when cells were treated with 10, 30, 100 ng/ml IL-1β at the indicted time points. Of note, we found that the mRNA and protein levels of FGF18 were significantly downregulated in IL-1β-treated chondrocytes compared with those of the control group (Figure 1B, 1C).
Figure 1.
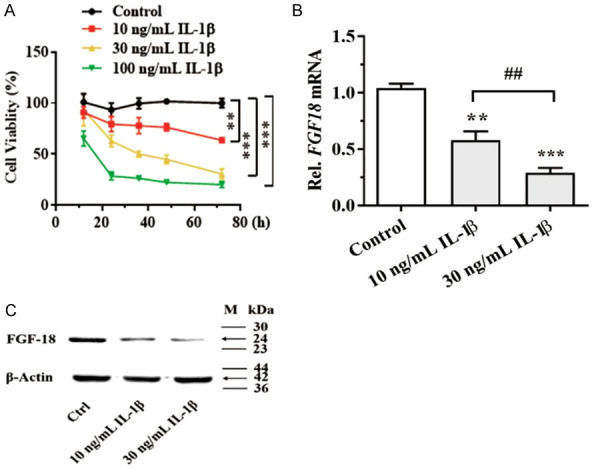
Human chondrocytes were treated with IL-1β. A: Cell viability was determined using the CCK-8 assay; B: FGF18 mRNA level was assayed by qPCR after IL-1β treatment for 24 h; C: FGF18 protein level was assayed by western blot after IL-1β treatment for 48 h. **P < 0.01 and ***P < 0.001 versus control; ##P < 0.01, 10 ng/mL versus 30 ng/mL.
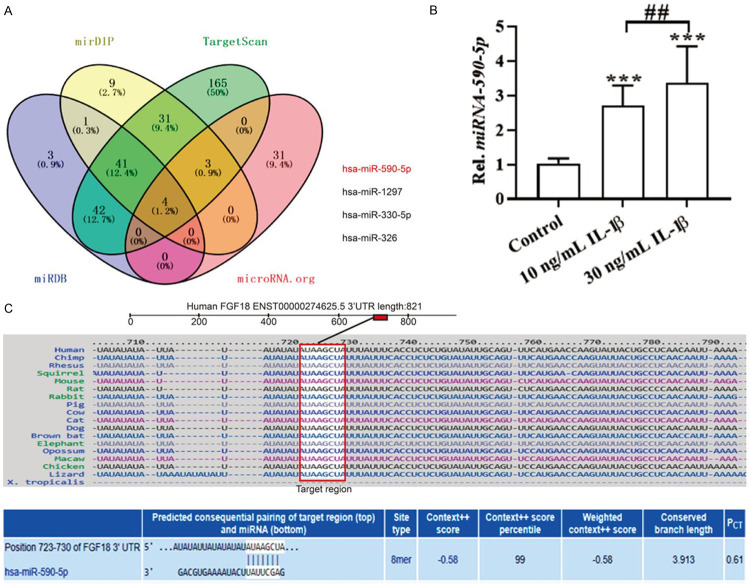
With the aim of identifying miRNAs regulating FGF18, the 3’UTR of FGF18 was analyzed using the algorithm Target Scan from Target Scan Human 7.2 (http://www.targetscan.org/vert_72/), mirDIP (http://ophid.utoronto.ca/mirDIP/index.jsp#r), microRNA.org (http://www.microrna.org/microrna/getGeneForm.do), and miRDB (http://mirdb.org/index.html). And the database cross-check showed that 4 miRNAs including hsa-miR-590-5p, hsa-miR-1297, hsa-miR-330-5p and hsa-miR-326 may target FGF18 mRNA 3’-UTR (Figure 2A). Considering that miR-590-5p has been reported to be up-regulated in OA [32], here we focused on the relationship between FGF18 and miR-590-5p. Importantly, bioinformatics analysis revealed that the 3’-UTR sequences of FGF18 mRNA (UGGUGA) had a complementary binding site for miR-590-5p (AUAAGCUA) (Figure 2C). Furthermore, we found that the expression of miR-590-5p was significantly up-regulated in IL-1β-treated chondrocytes (Figure 2B). These data indicated that miR-590-5p, predicted to bind to the FGF18 transcripts, was promoted in IL-1β-stimulated human chondrocyte cells.
Figure 2.
miR590-5p was predicted to bind with the FGF18 transcripts. A: Four different algorithms, including mitarget scan, mirDIP, microRNA.org, and miRDB were used to predict FGF18 mRNA-binding miRNA candidates; B: miR-590-5p was assayed by qPCR after IL-1β treatment for 24 h in human chondrocytes; C: The binding sites between miR-590-5p and the 3’UTR region of FGF18 predicted by PITA/miRanda. ***P < 0.001 versus control; ##P < 0.01, 10 ng/mL versus 30 ng/mL.
miR590-5p mimics inhibited the cell viability and promoted apoptosis of IL-1β-induced chondrocytes
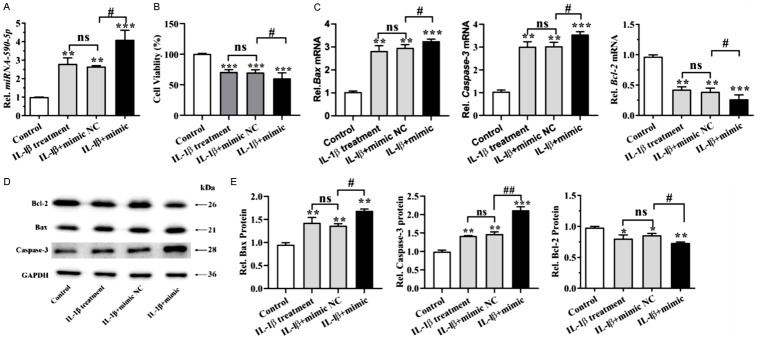
To investigate the role of miR-590-5p in IL-1β-treated OA model, chondrocytes were transfected with miR-590 mimics. After transfection with miR590-5p mimics for 24 h, qPCR revealed that the expression level of miR590-5p was significantly upregulated in IL-1β-induced human chondrocytes (Figure 3A). To determine the effect of miR-590-5p on viability of human chondrocytes, CCK-8 assay was applied. As shown in Figure 3B, the relative cell viability was decreased by 29.1% in the IL-1β-treated cells. Furthermore, cell viability in the miR-590-5p mimics group was significantly decreased compared with cells treated with IL-1β alone or cells transfected with the negative control (Figure 3B).
Figure 3.
miR590-5p overexpression inhibited the cell viability and promoted apoptosis of IL-1β-induced chondrocytes. Human chondrocytes were treated with 10 ng/mL IL-1β, and then transfected with NC or miR590-5p mimics (100 nM) for 24 h. A: miRNA-590-5p was detected by RT-PCR assay. B: Cell viability was determined using the CCK-8 assay. C: The mRNA levels of Bcl-2, Bax and Caspase-3 were detected by RT-PCR assay. D: Western blot analysis was carried out to detect Bcl-2, Bax and Caspase-3. E: Bar graph shows quantification results for Bcl-2, Bax and Caspase-3. *P < 0.05, **P < 0.01 and ***P < 0.001; #P < 0.05, ##P < 0.01, IL-1β+ mimics versus IL-1β mimics NC.
To further determine the effects of the miR-590-5p on cell apoptosis of chondrocytes, the apoptosis of chondrocytes was induced by IL-1β (10 ng/ml) to mimic the pathological conditions of OA, and pro-apoptotic and anti-apoptotic genes were assayed. Importantly, the mRNA level of pro-apoptotic genes (Bax, caspase-3) in groups co-treated with IL-1β and miR-590-5p mimics was higher than that in groups incubated with IL-1β alone, while the expression of the anti-apoptotic protein Bcl-2 was decreased after miR590-5p mimics treatment (Figure 3C). Alternatively, we obtained a similar result when detecting the protein levels of Bax, caspase-3 and Bcl-2 (Figure 3D, 3E). These data indicated that miR590-5p mimics inhibited the cell viability and promoted apoptosis of IL-1β-treated chondrocytes.
miR590-5p inhibitors increased the cell viability and inhibited apoptosis of IL-1β-induced chondrocytes
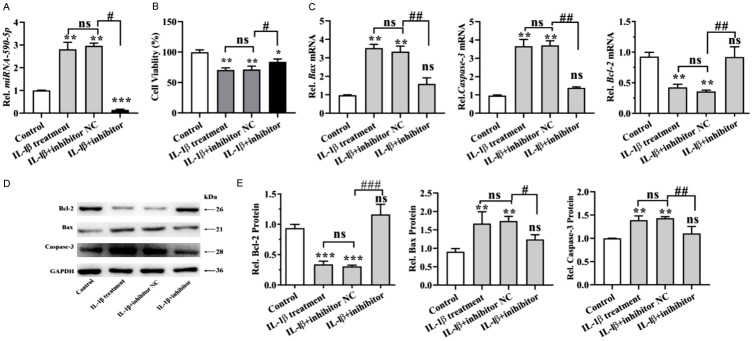
To further investigate the effect of miR-590-5p on IL-1β-stimulated human chondrocytes cells, miR-590-5p inhibitors were transfected. As shown in Figure 4A, the expression of miR-590-5p was dramatically decreased as compared to NC group. By using CCK-8 assay, we found that miR-590-5p inhibitors rescued the inhibition of proliferation induced by IL-1β (Figure 4B). Moreover, IL-1β notably upregulated the mRNA levels of Bax and caspase 3, and downregulated the mRNA level of Bcl-2 in human chondrocytes cells; however, these IL-1β-induced changes in apoptosis-related protein expressions were reversed following transfection with miR-590-5p inhibitors (Figure 4C). Furthermore, we obtained the similar results when detected the protein levels of Bax, caspase-3 and Bcl-2 (Figure 4D, 4E). These results indicated that miR590-5p inhibitors could increase cell viability and inhibit apoptosis of IL-1β-induced human chondrocytes cells.
Figure 4.
miR590-5p inhibition promoted the cell viability and inhibited apoptosis of IL-1β-induced chondrocytes. Human chondrocytes were treated with 10 ng/mL IL-1β, and then transfected with NC or miR590-5p inhibitors (100 nM) for 24 h. A: The expression of miRNA-590-5p was detected by qPCR assay. B: CCK-8 assay was performed to evaluate the viability of chondrocytes cells. C: The mRNA levels of Bcl-2, Bax and Caspase-3 were detected by RT-PCR assay. D: Western blot analysis was carried out to detect Bcl-2, Bax and Caspase-3. E: Bar graph shows quantification results for Bcl-2, Bax and Caspase-3. *P < 0.05, **P < 0.01 and ***P < 0.001; #P < 0.05, ##P < 0.01, ##P < 0.01, IL-1β+ inhibitors versus IL-1β+ inhibitors NC.
miR590-5p regulated inflammatory response and cartilage degeneration related biomarkers in IL-1β-induced chondrocytes
To investigate whether miR-590-5p could regulate IL-1β-induced inflammatory injury in human chondrocytes, ELISA was used to detect the relevant indicators. As shown in Figure 5A-C, IL-1β significantly increased the levels of IL-6, IL-8 and TNF-α in the supernatants of human chondrocytes. Moreover, these IL-1β-induced changes were further promoted by miR-590-5p overexpression (Figure 5A-C). However, IL-1β-induced inflammatory responses were reversed by miR-590-5p inhibition, as the enhancement of TNF-α, IL-6, and IL-8 secretion was inhibited by miR-590-5p inhibitors (Figure 5D-F). These results illustrated that miR-590-5p could promote IL-1β-induced inflammatory response in human chondrocytes cells.
Figure 5.
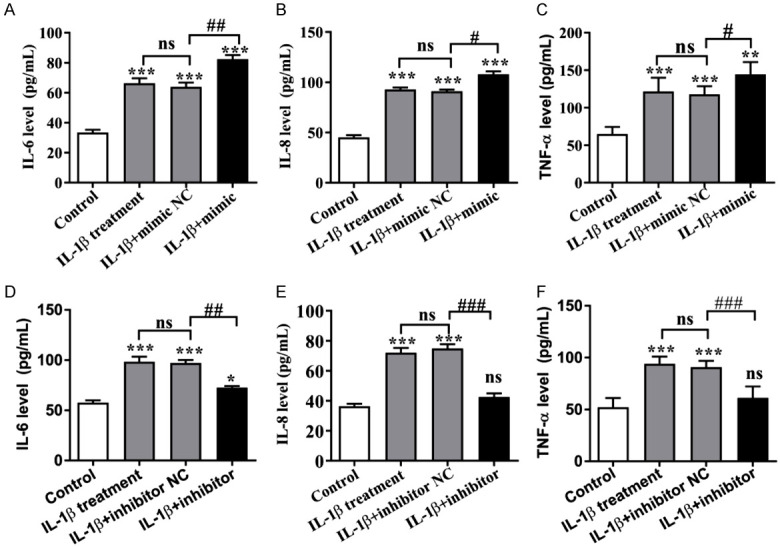
Human chondrocytes were treated with 10 ng/mL IL-1β, and then transfected with miR590-5p mimics or inhibitors (100 nM) for 48 h. miR590-5p promoted the IL-1β-induced inflammatory response in chondrocytes. A: The level of IL-6 in chondrocytes treated by miR590-5p mimics; B: The level of IL-8 in chondrocytes treated by miR590-5p mimics; C: The level of TNF-α in chondrocytes treated by miR590-5p mimics; D: The level of IL-6 in chondrocytes treated by miR590-5p inhibitor; E: The level of IL-8 in chondrocytes treated by miR590-5p inhibitor; F: The level of TNF-α in chondrocytes treated by miR590-5p inhibitor; *P < 0.05, **P < 0.01 and ***P < 0.001; #P < 0.05, ##P < 0.01 and ###P < 0.001, IL-1β+ mimics or inhibitors versus IL-1β+ NC.
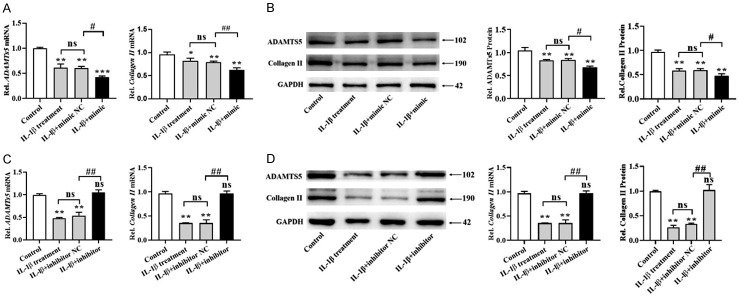
In addition, qPCR and Western blot were used to analyze the mRNA and protein levels of cartilage degeneration related biomarkers, including ADAMTs5 and collagen II. We found that IL-1β treatment reduced the expression of ADAMTs5 and collagen II, and miR-590-5p mimics further decreased the expression of these two genes at mRNA and protein levels (Figure 6A, 6B). Moreover, ADAMTs5 and collagen II levels downregulated by IL-1β were further reversed by miR-590-5p inhibitors (Figure 6C, 6D). These data suggested that miR-590-5p modulated cartilage degeneration related biomarkers in IL-1β-treated chondrocytes.
Figure 6.
miR-590-5p regulated cartilage degeneration related biomarkers in IL-1β-induced chondrocytes. Chondrocytes were treated with 10 ng/mL IL-1β, and then transfected with NC or miR590-5p mimics (100 nM) for 24 h. The mRNA (A) and protein (B) levels of ADAMTs5 and collagen II were detected by qPCR and Western blot. Then, human chondrocytes were treated with 10 ng/mL IL-1β, and then transfected with NC or miR590-5p inhibitors (100 nM) for 24 h. qPCR and Western blot were conducted to determine the mRNA (C) and protein (D) levels of ADAMTs5 and collagen II. *P < 0.05, **P < 0.01 and ***P < 0.001; #P < 0.05, ##P < 0.01 and ###P < 0.001, IL-1β+ mimics or inhibitors versus IL-1β+ NC.
FGF18 was proved to be a direct target of miR-590-5p in chondrocytes
The luciferase reporter assay was performed to validate whether FGF18 expression was directly modulated by miR-590-5p via interactions with potential binding sites in IL-1β-induced chondrocytes. As shown in Figure 7A, perfect base paring was found between the seed sequence of miR-590-5p and the 3’UTR of FGF 18. The results showed that human chondrocyte cells co-transfected with miR-590-5p mimic and WT FGF18 presented a significant decrease in luciferase activity in comparison with miR-NC group (Figure 7B). There was no obvious change in the luciferase activity in MUT FGF18 group either transfected with miR-590-5p mimic or miR-NC. Moreover, miR-590-5p inhibitors significantly promoted the luciferase activity of WT-FGF18, rather than MUT-FGF18 (P < 0.05) (Figure 7C). Furthermore, miR-590-5p mimics conspicuously down-regulated the mRNA and protein expressions of FGF18 in IL-1β-treated human chondrocytes (P < 0.05) (Figure 7D-F), while miR590-5p inhibitors up-regulated the mRNA and protein levels of FGF18 in IL-1β-treated human chondrocytes (P < 0.05) (Figure 7E, 7F). These data suggested that miR-590-5p directly regulated human chondrocytes expression through binding to FGF18 3’-UTR sequence.
Figure 7.
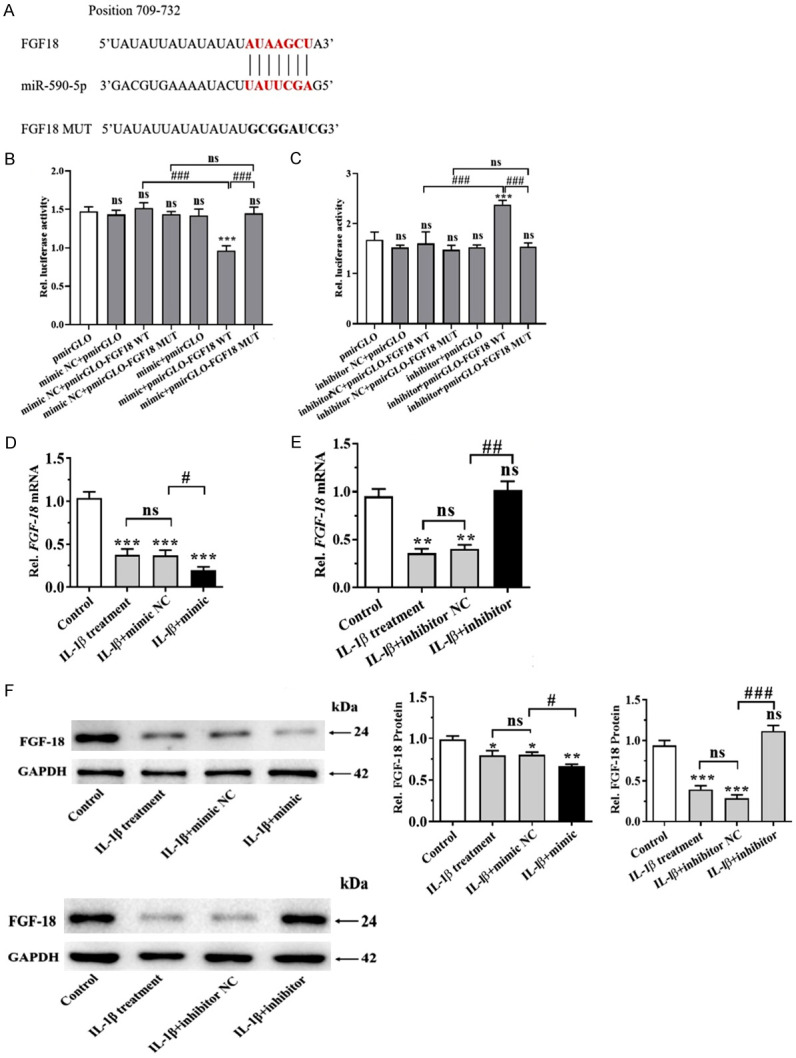
miR590-5p targeted FGF-18 in chondrocytes. A: Sequence alignment between miR-590-5p, WT and MUT of 3’-UTR of FGF18. B, C: Chondrocytes were transiently co-transfected with either a WT or mutant FGF18 3’UTR reporter plasmid and either a miR-590-5p mimics or inhibitors. The cells were harvested at 48 h after transfection, and then luciferase activity was measured by dual-luciferase reporter assay. D-F: Relative mRNA and protein expressions of FGF18 after transfection of miR-590-5p mimics or inhibitors were examined by qPCR or WB. *P < 0.05, **P < 0.01 and ***P < 0.001; #P < 0.05, ##P < 0.01 and ###P < 0.001, IL-1β+ mimics or inhibitors versus IL-1β+ NC.
Discussion
In the current study, we observed a significant downregulation of FGF18 and upregulation of miR-590-5p in IL-1β-treated human chondrocytes compared with the corresponding controls. Using an in vitro IL-1β-treated human chondrocyte model, we found that cell viability was strongly decreased, cell apoptosis and pro-inflammatory cytokines (TNF-α, IL-6 and IL-8) production were significantly increased in miR-590-5p-overexpressing cells compared to miR-NC cells, while miR-590-5p inhibitors produced the opposite results. Furthermore, luciferase activity validated that FGF18 was a target of miR-590-5p in chondrocytes.
miR-590-5p has been previously reported to be aberrantly expressed in several cancers and functioned as an onco-miR or an anti-onco-miR in various types of cancers including colorectal cancer and hepatocellular carcinoma [33,34]. Notably, by acting on different target genes, miR-590-5p plays a different role in many pathophysiological processes, such as cellular proliferation and apoptosis. Accumulating evidence has suggested the importance of miR-590 in progression of human cervical cancer, colorectal cancer, lung adenocarcinoma, and gastric cancer [35,36]. On the other hand, miR-590-5p has also been demonstrated to exert an anti-tumor role in colorectal cancer and breast cancer [33,37]. In the present study, we observed that the expression of miR590-5p was significantly increased in IL-1β-treated chondrocytes, which indicates involvement of miR-590-5p in the pathogenesis of OA. To determine the function of miR-590-5p in OA, we used mimics and inhibitors to manipulate the expression of miR590-5p. We observed that miR-590-5p over-expression remarkably reduced viability and significantly induced the secretion of inflammatory factors (IL-6, IL-8, and TNF-α) in IL-1β-treated chondrocytes, while miR-590-5p inhibitors could reverse these effects. In summary, we conclude that miRNA-590-5p may promote OA progression.
FGF18 is reported to be involved in chondrogenesis and cartilage repair with certain therapeutic effect and its mechanisms of action is supportive of evaluation for efficacy in osteoarthritis [9,38,39]. FGF18 can stimulate the proliferation and accumulation of type II collagen and proteoglycans in cultured porcine and adult human articular chondrocytes [8]. Previous studies demonstrated that FGF18 could stimulate the proliferation of chondrocytes through PI3K-AKT signaling in both monolayer and 3D culture and promote the accumulation of extracellular matrix (ECM) [9,40]. Recently, FGF18 was demonstrated to be regulated by several miRNAs and was identified as a target of miRNAs, e.g., miR-19, miR-21-5p in human chondrocytes [18,19], and miR-139 in hepatocellular carcinoma [17]. In the present study, we found that the levels of FGF18, ADAMTs5 and collagen II were significantly downregulated in IL-1β chondrocytes, miR-590-5p mimics further promoted the downregulation of these genes, while miR-590-5p inhibitors could reverse these effects. Luciferase reporter assay confirmed that FGF18 is a target of miR-590-5p in IL-1β-treated chondrocytes. Therefore, it was hypothesized that miR-590-5p inhibited the cell viability and promoted apoptosis of IL-1β-induced chondrocytes through targeting FGF18.
There are still some limitations existing in this study, such as insufficient sample, and single-center study. Subsequent studies will concentrate on the collection of an increased number of samples and animal experiments, and the multi-center study with long-term results. In addition, the effect of miR590-5p and FGF18 in the OA progression will be investigated by observing changes in indices at multiple time points, and to further confirm by a long-term follow-up study.
In summary, OA is a progressive degenerative disease characterized by cartilage degradation and chondrocyte apoptosis, the underlying mechanism may involve aberrant gene expressions and the associated responding biological events. In the present study, we connected miR-590-5p with the apoptosis, cell viability and FGF18 together, highlighting miRNA-590-5p silencing as an attractive therapeutic regimen in future clinical trials for OA patients.
Disclosure of conflict of interest
None.
References
- 1.Grassel S, Muschter D. Recent advances in the treatment of osteoarthritis. F1000Res. 2020;9:F1000 Faculty Rev-325. [Google Scholar]
- 2.Goldring SR, Goldring MB. Changes in the osteochondral unit during osteoarthritis: structure, function and cartilage-bone crosstalk. Nat Rev Rheumatol. 2016;12:632–644. doi: 10.1038/nrrheum.2016.148. [DOI] [PubMed] [Google Scholar]
- 3.Barnett R. Osteoarthritis. Lancet. 2018;391:1985. doi: 10.1016/S0140-6736(18)31064-X. [DOI] [PubMed] [Google Scholar]
- 4.Lieben L. Osteoarthritis: osteophyte formation involves PAR2. Nat Rev Rheumatol. 2016;12:70–71. doi: 10.1038/nrrheum.2016.6. [DOI] [PubMed] [Google Scholar]
- 5.Deyle GD, Allen CS, Allison SC, Gill NW, Hando BR, Petersen EJ, Dusenberry DI, Rhon DI. Physical therapy versus glucocorticoid injection for osteoarthritis of the knee. N Engl J Med. 2020;382:1420–1429. doi: 10.1056/NEJMoa1905877. [DOI] [PubMed] [Google Scholar]
- 6.Charlier E, Deroyer C, Ciregia F, Malaise O, Neuville S, Plener Z, Malaise M, de Seny D. Chondrocyte dedifferentiation and osteoarthritis (OA) Biochem Pharmacol. 2019;165:49–65. doi: 10.1016/j.bcp.2019.02.036. [DOI] [PubMed] [Google Scholar]
- 7.Latourte A, Kloppenburg M, Richette P. Emerging pharmaceutical therapies for osteoarthritis. Nat Rev Rheumatol. 2020;16:673–688. doi: 10.1038/s41584-020-00518-6. [DOI] [PubMed] [Google Scholar]
- 8.Ellsworth JL, Berry J, Bukowski T, Claus J, Feldhaus A, Holderman S, Holdren MS, Lum KD, Moore EE, Raymond F, Ren H, Shea P, Sprecher C, Storey H, Thompson DL, Waggie K, Yao L, Fernandes RJ, Eyre DR, Hughes SD. Fibroblast growth factor-18 is a trophic factor for mature chondrocytes and their progenitors. Osteoarthritis Cartilage. 2002;10:308–320. doi: 10.1053/joca.2002.0514. [DOI] [PubMed] [Google Scholar]
- 9.Yao X, Zhang J, Jing X, Ye Y, Guo J, Sun K, Guo F. Fibroblast growth factor 18 exerts anti-osteoarthritic effects through PI3K-AKT signaling and mitochondrial fusion and fission. Pharmacol Res. 2019;139:314–324. doi: 10.1016/j.phrs.2018.09.026. [DOI] [PubMed] [Google Scholar]
- 10.Antunes BP, Vainieri ML, Alini M, Monsonego-Ornan E, Grad S, Yayon A. Enhanced chondrogenic phenotype of primary bovine articular chondrocytes in fibrin-hyaluronan hydrogel by multi-axial mechanical loading and FGF18. Acta Biomater. 2020;105:170–179. doi: 10.1016/j.actbio.2020.01.032. [DOI] [PubMed] [Google Scholar]
- 11.Dahlberg LE, Aydemir A, Muurahainen N, Guhring H, Fredberg Edebo H, Krarup-Jensen N, Ladel CH, Jurvelin JS. A first-in-human, double-blind, randomised, placebo-controlled, dose ascending study of intra-articular rhFGF18 (sprifermin) in patients with advanced knee osteoarthritis. Clin Exp Rheumatol. 2016;34:445–450. [PubMed] [Google Scholar]
- 12.Turchinovich A, Weiz L, Langheinz A, Burwinkel B. Characterization of extracellular circulating microRNA. Nucleic Acids Res. 2011;39:7223–7233. doi: 10.1093/nar/gkr254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rupaimoole R, Slack FJ. MicroRNA therapeutics: towards a new era for the management of cancer and other diseases. Nat Rev Drug Discov. 2017;16:203–222. doi: 10.1038/nrd.2016.246. [DOI] [PubMed] [Google Scholar]
- 14.Li L, Song Y, Shi X, Liu J, Xiong S, Chen W, Fu Q, Huang Z, Gu N, Zhang R. The landscape of miRNA editing in animals and its impact on miRNA biogenesis and targeting. Genome Res. 2018;28:132–143. doi: 10.1101/gr.224386.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wei W, Mok SC, Oliva E, Kim SH, Mohapatra G, Birrer MJ. FGF18 as a prognostic and therapeutic biomarker in ovarian cancer. J Clin Invest. 2013;123:4435–4448. doi: 10.1172/JCI70625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang J, Zhou Y, Huang T, Wu F, Pan Y, Dong Y, Wang Y, Chan AKY, Liu L, Kwan JSH, Cheung AHK, Wong CC, Lo AKF, Cheng ASL, Yu J, Lo KW, Kang W, To KF. FGF18, a prominent player in FGF signaling, promotes gastric tumorigenesis through autocrine manner and is negatively regulated by miR-590-5p. Oncogene. 2019;38:33–46. doi: 10.1038/s41388-018-0430-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang L, Yin D, Wang Y, Cao L. Inhibition of the growth of hepatocellular carcinoma cells through fibroblast growth factor 18 suppressed by miR-139. Oncol Rep. 2017;38:2565–2571. doi: 10.3892/or.2017.5869. [DOI] [PubMed] [Google Scholar]
- 18.Wang XB, Zhao FC, Yi LH, Tang JL, Zhu ZY, Pang Y, Chen YS, Li DY, Guo KJ, Zheng X. MicroRNA-21-5p as a novel therapeutic target for osteoarthritis. Rheumatology (Oxford) 2019:kez102. doi: 10.1093/rheumatology/kez102. [DOI] [PubMed] [Google Scholar]
- 19.Wang Y, Yang T, Liu Y, Zhao W, Zhang Z, Lu M, Zhang W. Decrease of miR-195 promotes chondrocytes proliferation and maintenance of chondrogenic phenotype via targeting FGF-18 pathway. Int J Mol Sci. 2017;18:975. doi: 10.3390/ijms18050975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang B, Ni J, Long H, Huang J, Yang C, Huang X. IL-1beta-induced miR-34a up-regulation inhibits Cyr61 to modulate osteoarthritis chondrocyte proliferation through ADAMTS-4. J Cell Biochem. 2018;119:7959–7970. doi: 10.1002/jcb.26600. [DOI] [PubMed] [Google Scholar]
- 21.Hu W, Zhang W, Li F, Guo F, Chen A. miR-139 is up-regulated in osteoarthritis and inhibits chondrocyte proliferation and migration possibly via suppressing EIF4G2 and IGF1R. Biochem Biophys Res Commun. 2016;474:296–302. doi: 10.1016/j.bbrc.2016.03.164. [DOI] [PubMed] [Google Scholar]
- 22.Li C, Hu Q, Chen Z, Shen B, Yang J, Kang P, Zhou Z, Pei F. MicroRNA-140 suppresses human chondrocytes hypertrophy by targeting SMAD1 and controlling the bone morphogenetic protein pathway in osteoarthritis. Am J Med Sci. 2018;355:477–487. doi: 10.1016/j.amjms.2018.01.004. [DOI] [PubMed] [Google Scholar]
- 23.Cui X, Wang S, Cai H, Lin Y, Zheng X, Zhang B, Xia C. Overexpression of microRNA-634 suppresses survival and matrix synthesis of human osteoarthritis chondrocytes by targeting PIK3R1. Sci Rep. 2016;6:23117. doi: 10.1038/srep23117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cai C, Min S, Yan B, Liu W, Yang X, Li L, Wang T, Jin A. MiR-27a promotes the autophagy and apoptosis of IL-1beta treated-articular chondrocytes in osteoarthritis through PI3K/AKT/mTOR signaling. Aging (Albany NY) 2019;11:6371–6384. doi: 10.18632/aging.102194. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 25.Si HB, Zeng Y, Liu SY, Zhou ZK, Chen YN, Cheng JQ, Lu YR, Shen B. Intra-articular injection of microRNA-140 (miRNA-140) alleviates osteoarthritis (OA) progression by modulating extracellular matrix (ECM) homeostasis in rats. Osteoarthritis Cartilage. 2017;25:1698–1707. doi: 10.1016/j.joca.2017.06.002. [DOI] [PubMed] [Google Scholar]
- 26.Zhong G, Long H, Ma S, Shunhan Y, Li J, Yao J. miRNA-335-5p relieves chondrocyte inflammation by activating autophagy in osteoarthritis. Life Sci. 2019;226:164–172. doi: 10.1016/j.lfs.2019.03.071. [DOI] [PubMed] [Google Scholar]
- 27.Ding Y, Wang L, Zhao Q, Wu Z, Kong L. MicroRNA93 inhibits chondrocyte apoptosis and inflammation in osteoarthritis by targeting the TLR4/NFkappaB signaling pathway. Int J Mol Med. 2019;43:779–790. doi: 10.3892/ijmm.2018.4033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li Y, Yuan F, Song Y, Guan X. miR-17-5p and miR-19b-3p prevent osteoarthritis progression by targeting EZH2. Exp Ther Med. 2020;20:1653–1663. doi: 10.3892/etm.2020.8887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li F, Yao J, Hao Q, Duan Z. miRNA-103 promotes chondrocyte apoptosis by down-regulation of Sphingosine kinase-1 and ameliorates PI3K/AKT pathway in osteoarthritis. Biosci Rep. 2019;39:BSR20191255. doi: 10.1042/BSR20191255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nakamura A, Rampersaud YR, Nakamura S, Sharma A, Zeng F, Rossomacha E, Ali SA, Krawetz R, Haroon N, Perruccio AV, Mahomed NN, Gandhi R, Rockel JS, Kapoor M. microRNA-181a-5p antisense oligonucleotides attenuate osteoarthritis in facet and knee joints. Ann Rheum Dis. 2019;78:111–121. doi: 10.1136/annrheumdis-2018-213629. [DOI] [PubMed] [Google Scholar]
- 31.Kang D, Shin J, Cho Y, Kim HS, Gu YR, Kim H, You KT, Chang MJ, Chang CB, Kang SB, Kim JS, Kim VN, Kim JH. Stress-activated miR-204 governs senescent phenotypes of chondrocytes to promote osteoarthritis development. Sci Transl Med. 2019;11:eaar6659. doi: 10.1126/scitranslmed.aar6659. [DOI] [PubMed] [Google Scholar]
- 32.Prasadam I, Batra J, Perry S, Gu W, Crawford R, Xiao Y. Systematic identification, characterization and target gene analysis of microRNAs involved in osteoarthritis subchondral bone pathogenesis. Calcif Tissue Int. 2016;99:43–55. doi: 10.1007/s00223-016-0125-7. [DOI] [PubMed] [Google Scholar]
- 33.Zhou Q, Zhu Y, Wei X, Zhou J, Chang L, Sui H, Han Y, Piao D, Sha R, Bai Y. MiR-590-5p inhibits colorectal cancer angiogenesis and metastasis by regulating nuclear factor 90/vascular endothelial growth factor A axis. Cell Death Dis. 2016;7:e2413. doi: 10.1038/cddis.2016.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen M, Wu L, Tu J, Zhao Z, Fan X, Mao J, Weng Q, Wu X, Huang L, Xu M, Ji J. miR-590-5p suppresses hepatocellular carcinoma chemoresistance by targeting YAP1 expression. EBioMedicine. 2018;35:142–154. doi: 10.1016/j.ebiom.2018.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim CW, Oh ET, Kim JM, Park JS, Lee DH, Lee JS, Kim KK, Park HJ. Hypoxia-induced microRNA-590-5p promotes colorectal cancer progression by modulating matrix metalloproteinase activity. Cancer Lett. 2018;416:31–41. doi: 10.1016/j.canlet.2017.12.018. [DOI] [PubMed] [Google Scholar]
- 36.Chu Y, Ouyang Y, Wang F, Zheng A, Bai L, Han L, Chen Y, Wang H. MicroRNA-590 promotes cervical cancer cell growth and invasion by targeting CHL1. J Cell Biochem. 2014;115:847–853. doi: 10.1002/jcb.24726. [DOI] [PubMed] [Google Scholar]
- 37.Zhou L, Zhao LC, Jiang N, Wang XL, Zhou XN, Luo XL, Ren J. MicroRNA miR-590-5p inhibits breast cancer cell stemness and metastasis by targeting SOX2. Eur Rev Med Pharmacol Sci. 2017;21:87–94. [PubMed] [Google Scholar]
- 38.Tachmazidou I, Hatzikotoulas K, Southam L, Esparza-Gordillo J, Haberland V, Zheng J, Johnson T, Koprulu M, Zengini E, Steinberg J, Wilkinson JM, Bhatnagar S, Hoffman JD, Buchan N, Suveges D arcOGEN Consortium. Yerges-Armstrong L, Smith GD, Gaunt TR, Scott RA, McCarthy LC, Zeggini E. Identification of new therapeutic targets for osteoarthritis through genome-wide analyses of UK Biobank data. Nat Genet. 2019;51:230–236. doi: 10.1038/s41588-018-0327-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xie Y, Zinkle A, Chen L, Mohammadi M. Fibroblast growth factor signalling in osteoarthritis and cartilage repair. Nat Rev Rheumatol. 2020;16:547–564. doi: 10.1038/s41584-020-0469-2. [DOI] [PubMed] [Google Scholar]
- 40.Haanes KA, Edvinsson L. Pathophysiological mechanisms in migraine and the identification of new therapeutic targets. CNS Drugs. 2019;33:525–537. doi: 10.1007/s40263-019-00630-6. [DOI] [PubMed] [Google Scholar]