Abstract
Objective: Osteoarthritis (OA) is one of the most common chronic diseases, which is characterized by cartilage degeneration, subchondral osteosclerosis, and synovitis. Accumulating evidence has shown that galangin, a flavonoid derived from medicinal herbs, exhibits numerous pharmacological activities in various diseases. This study aimed to investigate the effects of galangin on interleukin (IL)-1β-induced inflammation in mouse chondrocytes and explore the underlying mechanisms. Methods: In this study, we investigated the protective effects of galangin on IL-1β-induced inflammatory response in vitro using the CCK-8 assay, RT-qPCR, western blotting, and immunofluorescence staining. In addition, the therapeutic effects of galangin on the anterior cruciate ligament transection (ACLT) mouse model were also explored in vivo. Results: Galangin treatment suppressed the expression of IL-1β-induced inflammatory cytokines, such as nitric oxide synthase, cyclooxygenase-2, TNF-α, and IL-6. Furthermore, galangin attenuated hypertrophic conversion and the extracellular matrix degradation via inhibiting the expression of catabolic enzymes. Mechanistically, galangin inhibited the activation of the JNK and ERK MAPK pathways and nuclear factor kappa-B (NF-κB) signaling pathway. In addition, galangin treatment ameliorated cartilage degeneration in an OA model in vivo. Conclusion: Galangin suppressed the IL-β-induced inflammatory response in vitro and ameliorated cartilage degeneration in vivo via inhibiting the NF-κB pathway and JNK and ERK pathways, suggesting its potential as an effective candidate for the treatment of OA.
Keywords: Galangin, osteoarthritis, IL-1β, NF-κB, chondrocyt
Introduction
Osteoarthritis (OA) is one of the most common chronic diseases, which causes pain and dysfunction among the aged [1,2]. Cartilage degeneration, subchondral osteosclerosis, and synovitis are considered as the main pathological features of OA [3,4]. It is widely accepted that chondrocytes, the only cell type in the articular cartilage, are closely related to OA development. Chondrocytes maintain the balance between anabolic metabolism and catabolic metabolism in the extracellular matrix (ECM) which includes type II collagen and proteoglycans [5-7]. Therefore, the apoptosis of chondrocytes and the degradation of ECM jointly promoted the development of OA [8]. Recent studies have reported that pro-inflammatory factors, such as IL-1β and TNF-α, have been found to be increased and activated in the development of OA [9,10]. Moreover, IL-1β exerts inflammatory effects by regulating the release of inflammatory cytokines such as IL-6, inducible nitric oxide synthase (iNOS), and cyclooxygenase-2 (COX-2). Type II collagen is degraded by collagenase or matrix metalloproteinases (MMPs) [11]. Proteoglycans are cleaved by several members of a disintegrin and metalloproteinase with thrombospondin motifs (ADAMTS) family, most probably by ADAMTS5 [12]. IL-1β is considered as a central factor in OA progression and IL-1β stimulation markedly increases the expression of MMPs and ADAMTS5 [13]. Therefore, inhibiting the IL-1β-induced inflammation might be an effective method for treating OA.
Previous studies have demonstrated that OA-related inflammation is mediated by several classical signaling pathways, such as MAPK pathways and NF-κB signaling pathway [14]. The NF-κB signaling pathway is considered to play a vital role in the development of OA and can be activated by IL-1β stimulation [15]. Thus, disruption of the inflammatory signaling pathways may be effective in the treatment of OA.
Galangin, a natural compound derived from medicinal herbs, has been demonstrated to possess multiple pharmacological effects, including anti-cancer, anti-inflammatory, and anti-oxidant activities through several signaling pathways [16-18]. Furthermore, a recent study reported that galangin alleviated collagen-induced arthritis via inhibiting the JNK, p38, and NF-κB pathways [19]. However, its potential effect on OA remains unknown. Therefore, we investigated the inhibitory effects of galangin on IL-1β-stimulated inflammation and the underlying mechanisms in vitro. We also determined the therapeutic effects of galangin in the anterior cruciate ligament transection (ACLT) mouse model of OA in vivo.
Material and methods
Reagents
Galangin (purity > 99%) was obtained from MCE (New Jersey, USA) and diluted in dimethyl sulfoxide for storage. Dulbecco’s modified Eagle’s medium (DMEM/F12) and fetal bovine serum (FBS) were obtained from Gibco (Rockville, MD, USA). Primary antibodies against MMP3, MMP13, p65, p-p65, iNOS, COX-2, aggrecan, and ADAMT5 were acquired from Abcam (Cambridge, UK). Antibodies against type X collagen, type II collagen, ERK, p-ERK, p38, p-p38, JNK, p-JNK, PI3K, AKT, p-AKT, IκBα, and β-actin were obtained from Cell Signaling Technology (Danvers, MA, USA). The secondary antibodies were purchased from Abcam. Type II collagenase was obtained from Sigma (St. Louis, MO, USA). Safranin O, Fast Green, and hematoxylin and eosin (H&E) solutions were obtained from Solarbio (Beijing, China).
Primary mouse chondrocyte culture
Mouse chondrocytes were extracted from the knee joints of 5-day-old C57BL/6 mice. Cartilage tissue was cut into pieces and then digested with 10 mL 0.25% collagenase II for 8 h under 5% CO2. Following this, the tissue was washed by PBS and seeded onto tissue culture flasks with DMEM/F12, 10% FBS, and antibiotics at 5% CO2 and 37°C. After the confluency reached 80-90%, the cells were passaged and only passage 1 and 2 were used for the subsequent experiments.
Cytotoxicity assay
The cytotoxicity of galangin on the chondrocytes was determined using the CCK-8 assay (Thermo Scientific, Waltham, MA, USA). Chondrocytes were seeded onto 96-well plates at a density of 1×104 cells/well for 24 h. Then, the cells were incubated with different concentrations of galangin (0, 0.5, 1, 2, 4, 8, 16, or 32 μM) for 24 or 48 h. Finally, cells were incubated with 10 μL of the CCK-8 reagent for 2 h. The absorbance was detected at 450 nm by a microplate reader (BioTek Instruments, Winooski, VT, USA).
Western blot analysis
Total proteins were isolated with RIPA lysis buffer, and their concentration was determined using the BCA protein assay kit (Beyotime, Shanghai, China). The total proteins were separated using sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and subsequently transferred to polyvinylidene difluoride (PVDF) membranes. After blocking with 5% nonfat milk at 37°C for 1 h, the membranes were incubated with the indicated primary antibodies at 4°C for 24 h, and then incubated with the secondary antibodies for 2 h. Finally, the bands were detected via Enhanced Chemiluminescence Reagent (Beyotime, Jiangsu, China) and the intensity was quantified using the Image J (Bio-Rad).
Quantitative RT-PCR
The total RNA was extracted by RNAiso Plus (Takara Bio, Otsu, Japan). First-strand cDNA was synthesized using 1000 ng of extracted RNA template. Then qPCR was performed with a 10-µL reaction volume using the SYBR Green-based Real-time PCR Master Mix (Takara Bio) under the cycling parameters: 35 cycles at 95°C for 15 s, 60°C for 60 s. The level of each gene was normalized to β-actin. The following primers were used to amplify the target genes: β-actin (forward: 5’-AGCCATGTACGTAGCCATCC-3’, reverse: 5’-CTCTCAGCAGTGGTGGTGAA-3’); IL-6 (forward: 5’-TAGTCCTTCCTACCCCAATTTCC-3’, reverse: 5’-TTGGTCCTTAGCCACTCCTTC-3’); TNF-α (forward: 5’-CAGGCGGTGCCTATGTCTC-3’, reverse: 5’-CGATCACCCCGAAGTTCAGTAG-3’); MMP9 (forward: 5’-GCAGAGGCATACTTGTACCG-3, reverse: 5’-TGATGTTATGATGGTCCCACTTG-3’); MMP3 (forward: 5’-ACATGGAGACTTTGTCCCTTTTG-3’, reverse: 5’-TTGGCTGAGTGGTAGAGTCCC-3’); MMP13 (forward: 5’-TGTTTGCAGAGCACTACTTGAA-3’, reverse: 5’-CAGTCACCTCTAAGCCAAAGAAA-3’).
Immunofluorescence staining
The cells were fixed with 4% paraformaldehyde and permeabilized with 0.1% triton X-100. Next, the cells were blocked by 5% BSA and incubated with primary antibodies against MMP3 (1:200) and type II collagen (1:200) for 24 h. The chondrocytes were then incubated with secondary antibody for 1 h and with DAPI in the dark for 10 min. In the end, images were observed by a microscope (Olympus, Tokyo, Japan).
Animal experiments
All animal experiments were approved by Animal Care and Use Committee of the Zhejiang University (No. ZJU20200114). Eighteen 9-week-old C57BL/6 female mice were obtained from the Animal Center of the Chinese Academy of Sciences (Shanghai, China). Mice were randomly divided into three groups (sham group, ACLT group, and ACLT + galangin treatment group). An OA model was established by performing surgical anterior cruciate ligament transection of the mouse right knee joint [20]. Subsequently, mice in ACLT + galangin treatment group received intragastric administration of galangin (10 mg/kg/d) based on previously published articles [17,21]. The mice were sacrificed 8 weeks post-surgery and knee joints were collected for the following analysis.
Histological analysis
The samples were coronally sectioned and the cartilage destruction was evaluated using Hematoxylin and eosin staining and Safranin O staining. Images were obtained by a high-quality microscope. The Osteoarthritis Research Society International (OARSI) scoring system was used to measure the cartilage damage. The expression of MMP13 was detected using immunohistochemical analysis.
Statistical analysis
Experiments were performed independently at least three times and the results were presented as the mean ± SD. Statistical analyses were performed using SPSS 20.0 statistical software program (IBM Corporation, Armonk, NY, USA). Data were analyzed by oneway analysis of variance followed by Tukey’s post-hoc test to assess statistical differences between different experimental groups. P values < 0.05 were considered statistically significant.
Results
Effects of galangin on chondrocyte viability
The chemical structure of galangin is shown in Figure 1A. Mouse chondrocytes morphology is displayed in Figure 1B. The CCK-8 assay was performed to assess the potential toxicity of different concentrations of galangin on mouse chondrocytes. As shown in Figure 1C, 1D, galangin treatment at specific concentrations (0-16 μM) did not affect the viability of chondrocytes. Therefore, we used galangin concentrations of 4, 8, and 16 μM in the following experiments.
Figure 1.
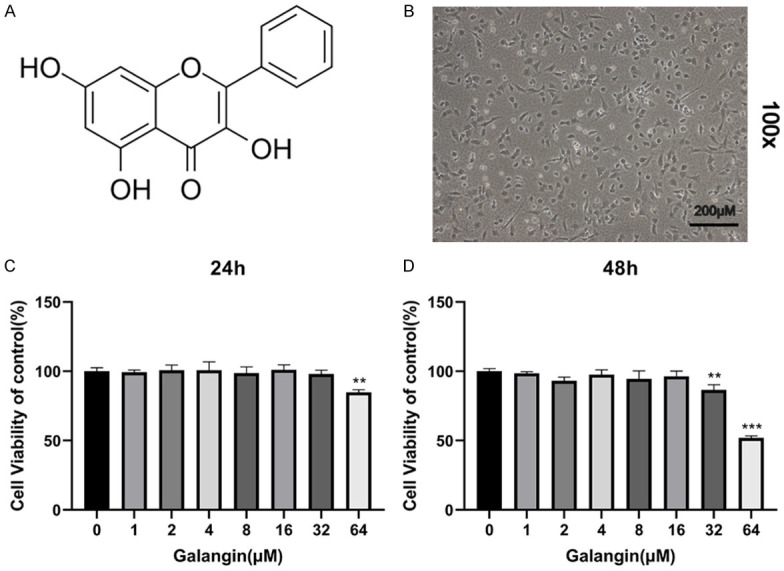
Effects of galangin on the viability of chondrocytes. A. Chemical structure of galangin. B. Representative images of mouse chondrocytes (magnification: 100×, scale bar: 200 µm). C, D. The cytotoxicity of various concentrations of galangin on chondrocytes was determined at 24 and 48 h using the CCK-8 assay. All data are presented as mean ± SD. **P < 0.01, ***P < 0.001 vs. control group, n = 3.
Galangin inhibits IL-1β-induced inflammatory response
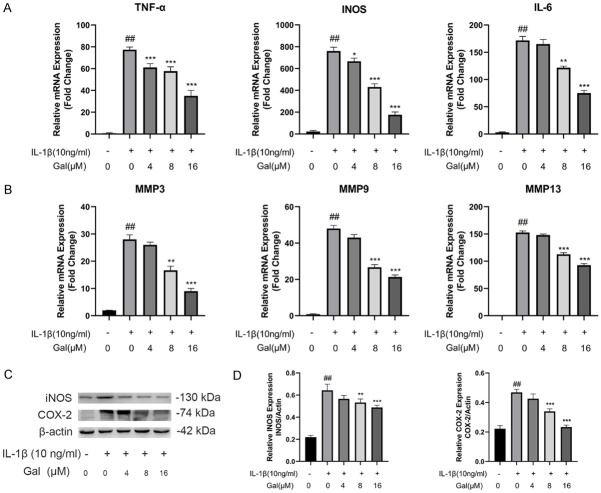
To assess the protective effect of galangin on IL-1β-induced inflammatory response, western blotting and qPCR experiments were performed to detect the expression of inflammatory factors. IL-1β stimulation significantly increased mRNA levels of inflammation-related genes such as TNF-α, IL-6, and iNOS, while galangin treatment reversed this effect in a dose-dependent manner (Figure 2A). Consistent with qPCR results, western blotting demonstrated that galangin inhibited the release of inflammatory cytokines, such as iNOS and COX-2 (Figure 2C, 2D).
Figure 2.
Galangin suppressed IL-1β-induced inflammation. A, B. Chondrocytes were incubated with or without IL-1β (10 ng/mL) and with indicated concentrations of galangin (0, 4, 8, 16 μM) for 24 h. The expressions of TNF-α, INOS, IL-6, MMP3, MMP9, and MMP13 were detected via qPCR. C, D. The expression of iNOS and COX-2 was measured via western blot analysis. All data are presented as mean ± SD. Significant differences among groups are indicated as ##P < 0.001 vs. control group; *P < 0.05, **P < 0.01, and ***P < 0.001 vs. IL-1β alone treatment group, n = 3.
Galangin alleviates IL-1β-induced hypertrophic conversion and ECM degradation
Type II collagen is an essential component of the extracellular matrix [22], whereas, type X collagen is considered as a marker protein of hypertrophic cartilage [23]. Thus, we explored the effects of galangin on ECM components using immunofluorescence staining and western blotting. We observed that IL-1β markedly suppressed the expression of type II collagen and increased the expression of type X collagen. However, galangin treatment reversed these effects, particularly at 8 and 16 μM (Figure 3A, 3B). Similarly, immunofluorescence staining indicated that galangin reduced IL-1β-induced type II collagen degradation (Figure 3C, 3D). In addition, IL-1β stimulation markedly increased ADAMTS5 and MMP3 protein expression, which was inhibited by galangin treatment. The results revealed the therapeutic effect of galangin on aggrecan, which is another major component of ECM (Figure 4A, 4B). Similarly, RT-PCR and immunofluorescence assay results were consistent with these findings (Figures 2B, 4C and 4D). Thus, above results demonstrated that galangin alleviated IL-1β-induced hypertrophic conversion and ECM degradation.
Figure 3.
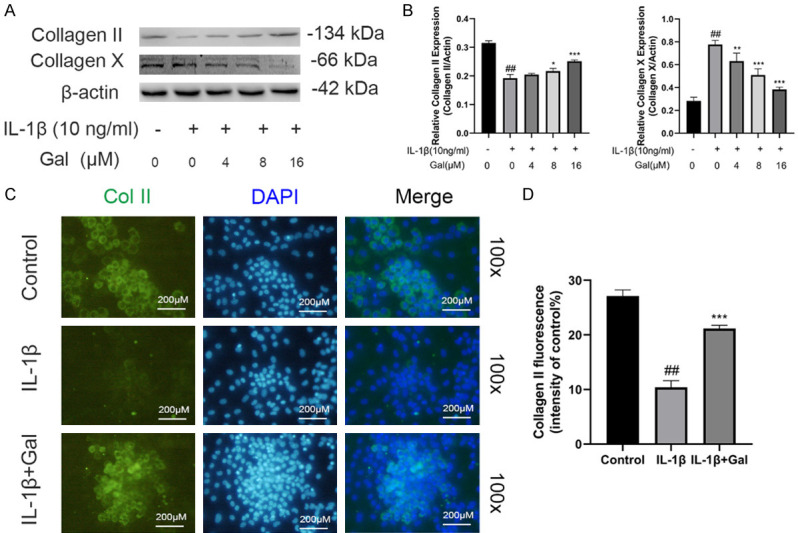
Galangin reduced IL-1β-induced hypertrophic conversion of chondrocytes. A, B. Collagen II and collagen X expression was detected by western blotting. C, D. Representative immunofluorescence image of collagen II with DAPI staining, and the fluorescence intensities were quantified by Image J (magnification: 100×, scale bar: 200 µm). All data are presented as mean ± SD. Significant differences among groups are indicated as ##P < 0.001 vs. control group; *P < 0.05, **P < 0.01, ***P < 0.001 vs. IL-1β alone treatment group, n = 3.
Figure 4.
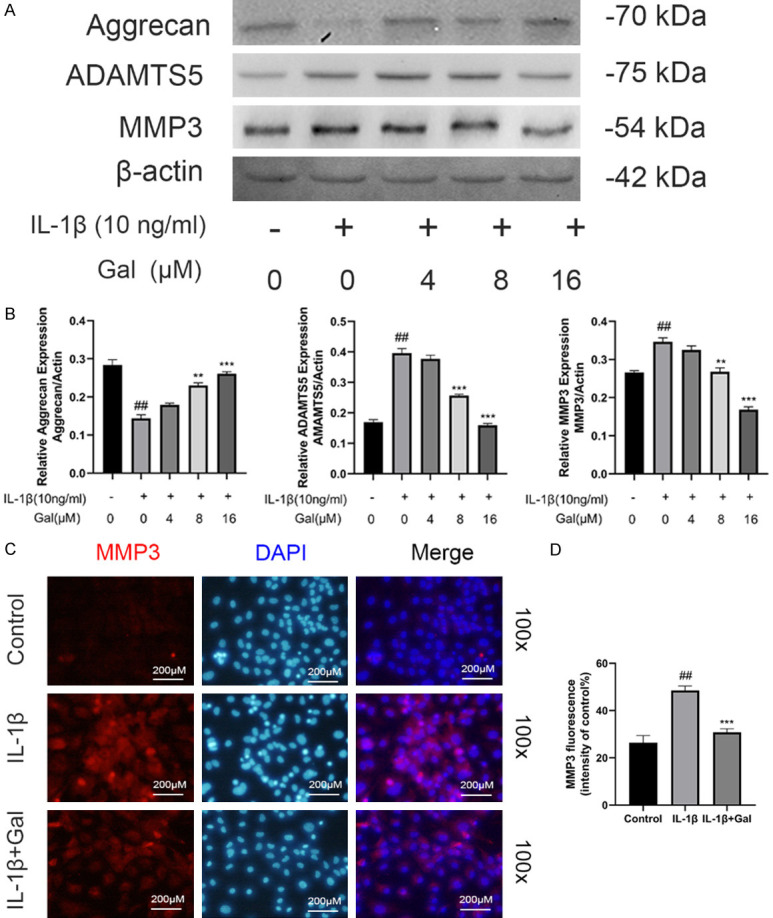
Galangin inhibited IL-1β-stimulated ECM degradation. A. The levels of ECM-related proteins, aggrecan, MMP3, and ADAMTS5, were assessed by western blotting. B. The expression of ECM proteins relative to β-actin was detected by Image J. C, D. Representative immunofluorescence image of MMP3 with DAPI staining, and the fluorescence intensities were quantified by Image J (magnification: 100×, scale bar: 200 µm). All data are presented as mean ± SD. Significant differences among groups are indicated as ##P < 0.001 vs. control group; **P < 0.01, and ***P < 0.001 vs. IL-1β alone treatment group, n = 3.
Galangin suppresses IL-1β-induced NF-κB activation
The NF-κB pathway has been demonstrated to play a vital role in modulating the ECM metabolism and inflammation in the development of OA [24]. We further explored the protective effect of galangin on IL-1β-induced activation of the NF-κB signaling pathway. Western blotting results revealed that IL-1β stimulation markedly promoted the IκBα degradation and p65 phosphorylation, while galangin treatment reversed these effects at 15 and 30 min (Figure 5A, 5B). These results indicated that IL-1β-induced NF-κB activation was suppressed by galangin.
Figure 5.
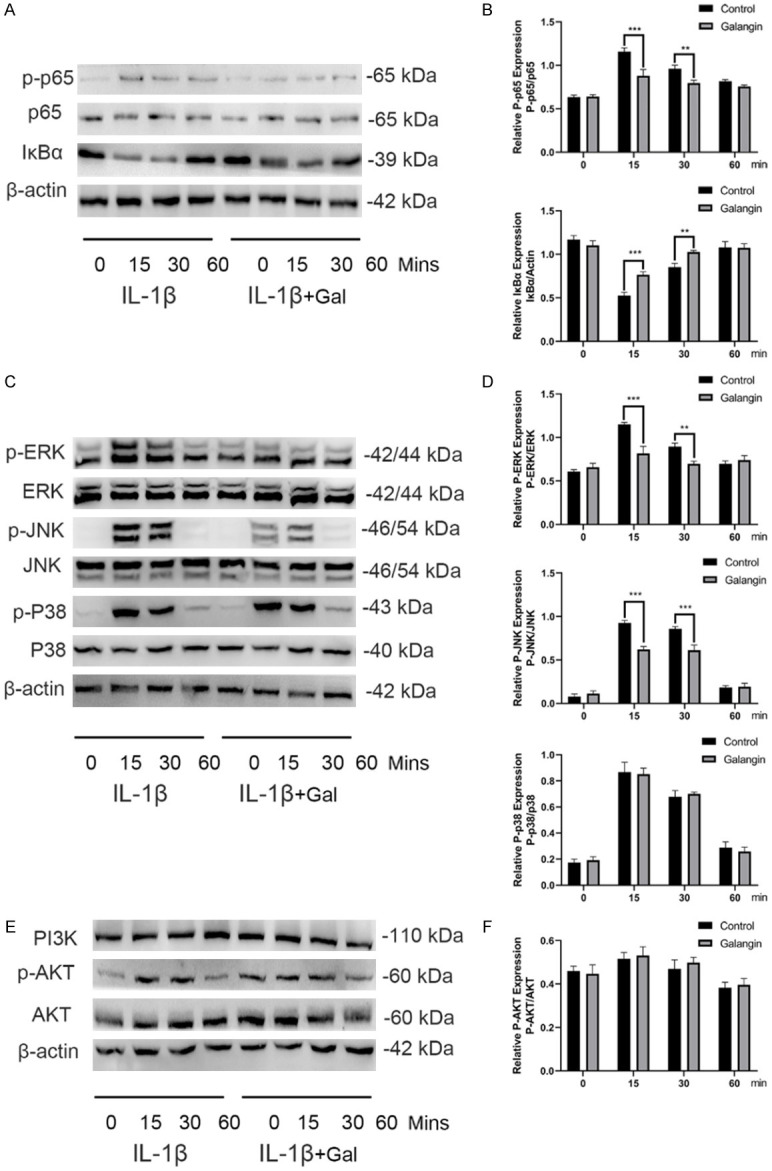
Galangin inhibited IL-1β-stimulated activation of the NF-κB pathway and the JNK and ERK pathways. A, B. The expression of p-p65, p65, IκBα, and β-actin was detected via western blotting and quantified by image J. C, D. The expression of p-ERK, ERK, p-JNK, JNK, p-p38, and p38 was detected by western blotting and quantified by Image J. E, F. The expression of PI3K, p-AKT, and AKT was detected via western blotting and quantified by Image J. All data are presented as mean ± SD. Significant differences among groups are indicated as *P < 0.05, **P < 0.01, and ***P < 0.001 vs. IL-1β treatment group, n = 3.
Galangin inhibits IL-1β-induced phosphorylation of the ERK and JNK signaling pathway
Previous studies reported that the MAPK and PI3K/AKT pathways were also associated with IL-1β-stimulated inflammatory response [25-27]. To further investigate whether galangin exerted protective effects via the MAPK and PI3K/AKT pathways, we detected the early activation and phosphorylation of these two pathways using western blot analysis. The data showed that IL-1β stimulation activated the ERK, JNK, and p38 signaling rapidly within 15 min, lasting for 30 min before the levels returned to baseline (Figure 5C). However, galangin treatment significantly suppressed the phosphorylation of JNK and ERK signaling, but not of p38 signaling, compared with the IL-1β alone treatment group. In addition, the activation of AKT was unaffected by galangin (Figure 5E). Statistical analysis for western blotting is presented in Figure 5D and 5F. In short, galangin suppressed IL-1β-induced inflammatory response via inhibiting the NF-κB pathway and the ERK and JNK pathway.
Galangin attenuates cartilage degeneration in an OA model in vivo
To determine the therapeutic effects of galangin on cartilage degeneration during OA progression in vivo, an ACLT-induced OA model was established. The cartilage destruction was evaluated using H&E and Safranin O staining. Results of the ACLT group exhibited severe proteoglycan loss and cartilage damage. However, galangin treatment reversed the effects (Figure 6A). Further, lower OARSI scores were measured in the ACLT + galangin treatment group (Figure 6B). Immunohistochemical analysis revealed that galangin treatment markedly reduced MMP13 expression in the cartilage (Figure 6C). In conclusion, galangin treatment attenuated cartilage degeneration of OA in vivo.
Figure 6.
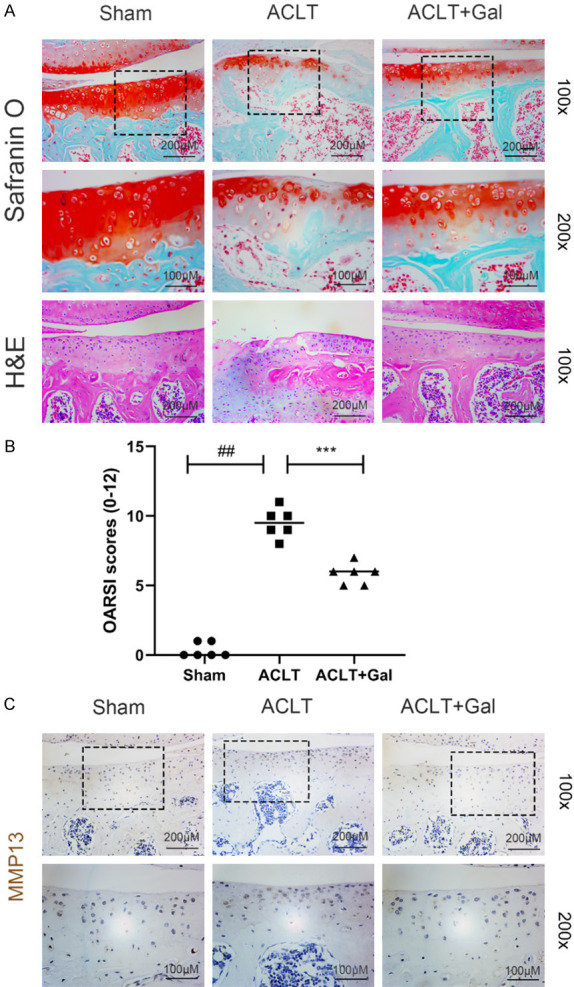
Galangin ameliorated OA progression in mouse ACLT model. A. Representative H&E and Safranin O staining of the cartilage tissues (magnification: 100× or 200×, scale bar: 100 µm or 200 µm). B. Cartilage OARSI scores. C. Immunohistochemical staining of MMP13 of the cartilage tissues (magnification: 100× or 200×, scale bar: 100 µm or 200 µm). All data are presented as mean ± SD. Significant differences among groups are indicated as ##P < 0.001 vs. sham group; ***P < 0.001 vs. ACLT group, n = 6.
Discussion
OA is a common chronic bone disease which is characterized by cartilage degeneration, subchondral osteosclerosis, and synovitis [28]. The apoptosis of chondrocytes and the degradation of ECM jointly initiate cartilage degradation and promote OA development [29]. At present, traditional drugs only alleviate clinical symptoms temporarily but fail to efficiently treat OA [30]. More effective agents are urgently required to attenuate the development of OA. Galangin has been demonstrated to exert anti-inflammatory and anticancer effects in several diseases [16-18]. Therefore, in this study, we investigated the effects of galangin on the IL-1β-induced inflammation and elucidated the underlying mechanisms.
The inflammatory response runs through the initiation and the development of cartilage destruction [31]. The pro-inflammatory factors, especially IL-1β, release a series of inflammatory cytokines such as iNOS and COX-2. iNOS and COX-2 subsequently promote the production of NO and prostaglandin E2 which cause non-bacterial inflammatory response in the cartilage [32,33]. Previous studies have indicated that inhibitors of iNOS and COX-2 could attenuate the development of OA. Our data determined that galangin treatment markedly reduced IL-1β-induced iNOS and COX-2 expression.
The ECM comprises two main components, aggrecan and type II collagen, which provide a metabolic microenvironment for cells and the integrity of cartilage tissue [34]. Type X collagen is considered as a marker protein of hypertrophic cartilage, and its excessive production causes cartilage ossification [35]. Accumulated evidence confirmed that IL-1β stimulation increased the expression of MMPs and ADAMTS, which are the key enzymes in ECM degradation [9,36,37]. MMP13 is responsible for collagen degradation [38], while ADAMTS5 plays an important role in cleaving aggrecan [39]. Our data indicated that galangin attenuated IL-1β-induced hypertrophic conversion by upregulating type II collagen and downregulating type X collagen expression. Similarly, galangin reduced the expression of catabolic enzymes, thereby ameliorating the loss of ECM components.
Mechanistically, the NF-κB pathway is a classical pathway in the modulating inflammation in OA development, which promotes chondrocyte apoptosis and ECM degradation. Once chondrocytes become simulated by IL-1β, IκBα is activated and p65 is released from the cytoplasm and then translocated to the nucleus, resulting in the production of inflammatory mediators and catabolic enzymes [40]. Herein, we confirmed that galangin dramatically suppressed the IL-1β-induced NF-κB activation. Previous studies confirmed that the MAPK and PI3K/AKT pathways are associated with IL-1β-stimulated inflammatory response [14,25]. Galangin has also been reported to alleviate collagen-induced arthritis via inhibiting MAPK pathway [19]. Thus, we assessed the effect of galangin on the MAPK and PI3K/AKT pathways. Our findings determined that galangin treatment significantly attenuated the phosphorylation of ERK and JNK, but not of p38. Additionally, the activation of PI3K/AKT pathway was not influenced by galangin.
Having confirmed the therapeutic effects of galangin in vitro, we established a mouse ACLT model to mimic cartilage damage of OA [20,41]. The ACLT group exhibited severe proteoglycan loss and cartilage erosion. However, galangin treatment significantly ameliorated cartilage destruction and decreased the OARSI scores.
In summary, our study revealed that galangin suppressed the IL-β-induced inflammatory response in vitro and ameliorated ACLT-induced cartilage degeneration in vivo via inhibiting the NF-κB pathway and JNK and ERK pathways (Figure 7). These findings indicate that galangin has potential as a novel agent for the treatment of OA.
Figure 7.
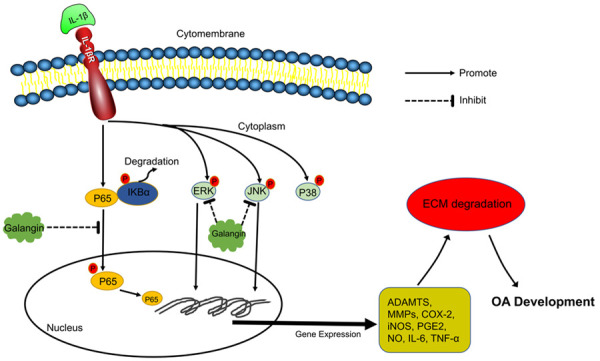
Schematic representation of the therapeutic effect of galangin on IL-1β-stimulated inflammation via inhibiting the NF-κB pathway and the JNK and ERK pathway.
Acknowledgements
This research was supported by the National Natural Science Foundation of China (grant number 81871801) and the Natural Science Foundation of Zhejiang Province (grant number LQ21H060001), and the Zhejiang Medical and Health Science and Technology Project (grant number 2019KY712).
Disclosure of conflict of interest
None.
References
- 1.Chen D, Shen J, Zhao W, Wang T, Han L, Hamilton JL, Im HJ. Osteoarthritis: toward a comprehensive understanding of pathological mechanism. Bone Res. 2017;5:16044. doi: 10.1038/boneres.2016.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Glyn-Jones S, Palmer AJ, Agricola R, Price AJ, Vincent TL, Weinans H, Carr AJ. Osteoarthritis. Lancet. 2015;386:376–387. doi: 10.1016/S0140-6736(14)60802-3. [DOI] [PubMed] [Google Scholar]
- 3.Van Spil WE, Kubassova O, Boesen M, Bay-Jensen AC, Mobasheri A. Osteoarthritis phenotypes and novel therapeutic targets. Biochem Pharmacol. 2019;165:41–48. doi: 10.1016/j.bcp.2019.02.037. [DOI] [PubMed] [Google Scholar]
- 4.Rahmati M, Nalesso G, Mobasheri A, Mozafari M. Aging and osteoarthritis: central role of the extracellular matrix. Ageing Res Rev. 2017;40:20–30. doi: 10.1016/j.arr.2017.07.004. [DOI] [PubMed] [Google Scholar]
- 5.Lieberthal J, Sambamurthy N, Scanzello CR. Inflammation in joint injury and post-traumatic osteoarthritis. Osteoarthritis Cartilage. 2015;23:1825–1834. doi: 10.1016/j.joca.2015.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sherwood JC, Bertrand J, Eldridge SE, Dell’Accio F. Cellular and molecular mechanisms of cartilage damage and repair. Drug Discov Today. 2014;19:1172–1177. doi: 10.1016/j.drudis.2014.05.014. [DOI] [PubMed] [Google Scholar]
- 7.Heinegård D, Saxne T. The role of the cartilage matrix in osteoarthritis. Nat Rev Rheumatol. 2011;7:50–56. doi: 10.1038/nrrheum.2010.198. [DOI] [PubMed] [Google Scholar]
- 8.Abramson SB, Yazici Y. Biologics in development for rheumatoid arthritis: relevance to osteoarthritis. Adv Drug Deliv Rev. 2006;58:212–225. doi: 10.1016/j.addr.2006.01.008. [DOI] [PubMed] [Google Scholar]
- 9.Blasioli DJ, Kaplan DL. The roles of catabolic factors in the development of osteoarthritis. Tissue Eng Part B Rev. 2014;20:355–363. doi: 10.1089/ten.teb.2013.0377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kapoor M, Martel-Pelletier J, Lajeunesse D, Pelletier JP, Fahmi H. Role of proinflammatory cytokines in the pathophysiology of osteoarthritis. Nat Rev Rheumatol. 2011;7:33–42. doi: 10.1038/nrrheum.2010.196. [DOI] [PubMed] [Google Scholar]
- 11.Chao PZ, Hsieh MS, Cheng CW, Lin YF, Chen CH. Regulation of MMP-3 expression and secretion by the chemokine eotaxin-1 in human chondrocytes. J Biomed Sci. 2011;18:86. doi: 10.1186/1423-0127-18-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chabane N, Zayed N, Afif H, Mfuna-Endam L, Benderdour M, Boileau C, Martel-Pelletier J, Pelletier JP, Duval N, Fahmi H. Histone deacetylase inhibitors suppress interleukin-1beta-induced nitric oxide and prostaglandin E2 production in human chondrocytes. Osteoarthritis Cartilage. 2008;16:1267–1274. doi: 10.1016/j.joca.2008.03.009. [DOI] [PubMed] [Google Scholar]
- 13.Santangelo KS, Nuovo GJ, Bertone AL. In vivo reduction or blockade of interleukin-1β in primary osteoarthritis influences expression of mediators implicated in pathogenesis. Osteoarthritis Cartilage. 2012;20:1610–1618. doi: 10.1016/j.joca.2012.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Saklatvala J. Inflammatory signaling in cartilage: MAPK and NF-kappaB pathways in chondrocytes and the use of inhibitors for research into pathogenesis and therapy of osteoarthritis. Curr Drug Targets. 2007;8:305–313. doi: 10.2174/138945007779940115. [DOI] [PubMed] [Google Scholar]
- 15.Csaki C, Mobasheri A, Shakibaei M. Synergistic chondroprotective effects of curcumin and resveratrol in human articular chondrocytes: inhibition of IL-1beta-induced NF-kappaB-mediated inflammation and apoptosis. Arthritis Res Ther. 2009;11:R165. doi: 10.1186/ar2850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lu H, Yao H, Zou R, Chen XW, Xu HL. Galangin suppresses renal inflammation via the inhibition of NF-B, PI3K/AKT and NLRP3 in uric acid treated NRK-52E tubular epithelial cells. Biomed Res Int. 2019;2019:3018357. doi: 10.1155/2019/3018357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li Y, Tong L, Zhang J, Zhang Y, Zhang F. Galangin alleviates liver ischemia-reperfusion injury in a rat model by mediating the PI3K/AKT pathway. Cell Physiol Biochem. 2018;51:1354–1363. doi: 10.1159/000495553. [DOI] [PubMed] [Google Scholar]
- 18.Ciolino HP, Yeh GC. The flavonoid galangin is an inhibitor of CYP1A1 activity and an agonist/antagonist of the aryl hydrocarbon receptor. Br J Cancer. 1999;79:1340–1346. doi: 10.1038/sj.bjc.6690216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huh JE, Jung IT, Choi J, Baek YH, Lee JD, Park DS, Choi DY. The natural flavonoid galangin inhibits osteoclastic bone destruction and osteoclastogenesis by suppressing NF-κB in collagen-induced arthritis and bone marrow-derived macrophages. Eur J Pharmacol. 2013;698:57–66. doi: 10.1016/j.ejphar.2012.08.013. [DOI] [PubMed] [Google Scholar]
- 20.Glasson SS, Blanchet TJ, Morris EA. The surgical destabilization of the medial meniscus (DMM) model of osteoarthritis in the 129/SvEv mouse. Osteoarthritis Cartilage. 2007;15:1061–1069. doi: 10.1016/j.joca.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 21.Aladaileh SH, Abukhalil MH, Saghir SAM, Hanieh H, Alfwuaires MA, Almaiman AA, BinJumah M, Mahmoud AM. Galangin activates Nrf2 signaling and attenuates oxidative damage, inflammation, and apoptosis in a rat model of cyclophosphamide-induced hepatotoxicity. Biomolecules. 2019;9:346. doi: 10.3390/biom9080346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhong L, Huang X, Karperien M, Post JN. Correlation between gene expression and osteoarthritis progression in human. Int J Mol Sci. 2016;17:1126. doi: 10.3390/ijms17071126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van der Kraan PM, van den Berg WB. Chondrocyte hypertrophy and osteoarthritis: role in initiation and progression of cartilage degeneration? Osteoarthritis Cartilage. 2012;20:223–232. doi: 10.1016/j.joca.2011.12.003. [DOI] [PubMed] [Google Scholar]
- 24.Roman-Blas JA, Jimenez SA. NF-kappaB as a potential therapeutic target in osteoarthritis and rheumatoid arthritis. Osteoarthritis Cartilage. 2006;14:839–848. doi: 10.1016/j.joca.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 25.Qian YQ, Feng ZH, Li XB, Hu ZC, Xuan JW, Wang XY, Xu HC, Chen JX. Downregulating PI3K/Akt/NF-κB signaling with allicin for ameliorating the progression of osteoarthritis: in vitro and vivo studies. Food Funct. 2018;9:4865–4875. doi: 10.1039/c8fo01095a. [DOI] [PubMed] [Google Scholar]
- 26.Xue JF, Shi ZM, Zou J, Li XL. Inhibition of PI3K/AKT/mTOR signaling pathway promotes autophagy of articular chondrocytes and attenuates inflammatory response in rats with osteoarthritis. Biomed Pharmacother. 2017;89:1252–1261. doi: 10.1016/j.biopha.2017.01.130. [DOI] [PubMed] [Google Scholar]
- 27.Prasadam I, Friis T, Shi W, van Gennip S, Crawford R, Xiao Y. Osteoarthritic cartilage chondrocytes alter subchondral bone osteoblast differentiation via MAPK signalling pathway involving ERK1/2. Bone. 2010;46:226–235. doi: 10.1016/j.bone.2009.10.014. [DOI] [PubMed] [Google Scholar]
- 28.Gregori D, Giacovelli G, Minto C, Barbetta B, Gualtieri F, Azzolina D, Vaghi P, Rovati LC. Association of pharmacological treatments with long-term pain control in patients with knee osteoarthritis: a systematic review and meta-analysis. JAMA. 2018;320:2564–2579. doi: 10.1001/jama.2018.19319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mobasheri A, Rayman MP, Gualillo O, Sellam J, van der Kraan P, Fearon U. The role of metabolism in the pathogenesis of osteoarthritis. Nat Rev Rheumatol. 2017;13:302–311. doi: 10.1038/nrrheum.2017.50. [DOI] [PubMed] [Google Scholar]
- 30.Giercksky KE, Huseby G, Rugstad HE. Epidemiology of NSAID-related gastrointestinal side effects. Scand J Gastroenterol Suppl. 1989;163:3–8. doi: 10.3109/00365528909091168. [DOI] [PubMed] [Google Scholar]
- 31.Martel-Pelletier J. Pathophysiology of osteoarthritis. Osteoarthritis Cartilage. 1999;7:371–373. doi: 10.1053/joca.1998.0214. [DOI] [PubMed] [Google Scholar]
- 32.Chowdhury TT, Bader DL, Lee DA. Dynamic compression counteracts IL-1beta induced iNOS and COX-2 activity by human chondrocytes cultured in agarose constructs. Biorheology. 2006;43:413–429. [PubMed] [Google Scholar]
- 33.Amin AR, Dave M, Attur M, Abramson SB. COX-2, NO, and cartilage damage and repair. Curr Rheumatol Rep. 2000;2:447–453. doi: 10.1007/s11926-000-0019-5. [DOI] [PubMed] [Google Scholar]
- 34.Poole AR, Kobayashi M, Yasuda T, Laverty S, Mwale F, Kojima T, Sakai T, Wahl C, El-Maadawy S, Webb G, Tchetina E, Wu W. Type II collagen degradation and its regulation in articular cartilage in osteoarthritis. Ann Rheum Dis. 2002;61(Suppl 2):ii78–ii81. doi: 10.1136/ard.61.suppl_2.ii78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shen G. The role of type X collagen in facilitating and regulating endochondral ossification of articular cartilage. Orthod Craniofac Res. 2005;8:11–17. doi: 10.1111/j.1601-6343.2004.00308.x. [DOI] [PubMed] [Google Scholar]
- 36.Maldonado M, Nam J. The role of changes in extracellular matrix of cartilage in the presence of inflammation on the pathology of osteoarthritis. Biomed Res Int. 2013;2013:284873. doi: 10.1155/2013/284873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wong M, Carter DR. Articular cartilage functional histomorphology and mechanobiology: a research perspective. Bone. 2003;33:1–13. doi: 10.1016/s8756-3282(03)00083-8. [DOI] [PubMed] [Google Scholar]
- 38.Baker J, Falconer AMD, Wilkinson DJ, Europe-Finner GN, Litherland GJ, Rowan AD. Protein kinase D3 modulates MMP1 and MMP13 expression in human chondrocytes. PLoS One. 2018;13:e0195864. doi: 10.1371/journal.pone.0195864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Song RH, Tortorella MD, Malfait AM, Alston JT, Yang Z, Arner EC, Griggs DW. Aggrecan degradation in human articular cartilage explants is mediated by both ADAMTS-4 and ADAMTS-5. Arthritis Rheum. 2007;56:575–585. doi: 10.1002/art.22334. [DOI] [PubMed] [Google Scholar]
- 40.Surh YJ, Chun KS, Cha HH, Han SS, Keum YS, Park KK, Lee SS. Molecular mechanisms underlying chemopreventive activities of anti-inflammatory phytochemicals: down-regulation of COX-2 and iNOS through suppression of NF-kappa B activation. Mutat Res. 2001;480-481:243–268. doi: 10.1016/s0027-5107(01)00183-x. [DOI] [PubMed] [Google Scholar]
- 41.Hayami T, Pickarski M, Zhuo Y, Wesolowski GA, Rodan GA, Duong LT. Characterization of articular cartilage and subchondral bone changes in the rat anterior cruciate ligament transection and meniscectomized models of osteoarthritis. Bone. 2006;38:234–243. doi: 10.1016/j.bone.2005.08.007. [DOI] [PubMed] [Google Scholar]