Abstract
Objective: The aim of this investigation was to determine the influence of anti-tuberculosis (anti-TB) drugs plus cycloserine (CS) on the sputum negative conversion rate, adverse reactions and inflammatory factors in the treatment of multidrug-resistant tuberculosis (MDR-TB). Methods: Seventy patients with MDR-TB who were referred to Nanjing Hospital Affiliated with Nanjing University of Traditional Chinese Medicine from April 2017 to April 2020 were assigned into the research group (RG; 38 cases) for anti-TB drugs plus CS, and the control group (CG; 32 cases) for conventional anti-TB drugs. The two groups were compared in their sputum negative conversion rate, incidence of adverse reactions, and foci absorption rate after 6, 12 and 24 months of treatment. The levels of inflammatory factors; tumor necrosis factor (TNF-α), interleukin-6 (IL-6) and interferon-γ (IFN-γ), both pre- and post-treatment were detected. Also, pre- and post-treatment, pulmonary function (PF) indexes (forced expiratory volume in 1 s/forced vital capacity, FEV1/FVC; FEV1; peak expiratory flow, PEF), and the scores of anxiety and depression (self-rating anxiety/depression scale, SAS/SDS), as well as Pittsburgh Sleep Quality Index (PSQI) were compared. Results: After 6, 12 and 24 months of treatment, the sputum negative conversion rate and foci absorption rate were higher in the RG than in the CG (both P<0.05). The RG presented with fewer adverse reactions, lower TNF-α, IL-6 and IFN-γ levels, higher FEV1, FEV1/FVC and PEF, and lower SAS, SDS and PSQI scores than the CG, post treatment (all P<0.05). Conclusions: While helping to raise the sputum negative conversion rate, improve prognosis, and reduce adverse reactions, anti-TB drugs plus CS can also inhibit the release of inflammatory factors, improve PF and alleviate negative emotion and sleep disorders.
Keywords: Cycloserine, multidrug-resistant tuberculosis, sputum negative conversion rate, adverse reactions, inflammatory factors
Introduction
Tuberculosis (TB) is a chronic infectious disease caused by Mycobacterium TB infection, with pulmonary TB being the most pervasive type [1,2]. Multi-drug resistant tuberculosis (MDR-TB) refers to TB that is at least resistant to both isoniazid and rifampicin [3,4]. Due to irregular chemotherapy, HIV infection, poverty and the biological characteristics of Mycobacterium TB, MDR-PTB is rapidly increasing and spreading all over the world [5,6]. Studies have shown that inflammatory factors play an important role in the control of Mycobacterium tuberculosis. For example, tumor necrosis factor (TNF-α) and interleukin-6 (IL-6) have strong pro-inflammatory activity and can aggravate tissue damage through pro-inflammatory effects [7]. MDR-PTB has become a serious social and public health problem due to the prolonged course of disease, high treatment cost, poor curative effect and high mortality rate, which seriously affects the physical and mental health as well as the life and safety of patients [8]. As mentioned earlier, the mortality rate of MDR-TB is very high in clinical treatment, and it is difficult to use conventional treatments to achieve a high treatment efficacy. Meanwhile, the treatment cost is also overwhelmingly high, so most patients have to give up treatment as they cannot bear the large cost [9-11]. Thus, finding a suitable, safe and effective treatment has important implications [12].
Cycloserine (CS) is a broad-spectrum antibiotic, which is a D-alanine analogue. It can inhibit alanine racemase and synthetase, which in turn affects the formation of cell wall mucopeptides and reduces the cell wall resistance of Mycobacterium TB, thus playing a role of sterilization and bacteriostasis [13]. Given the drug resistance of first-line TB drugs, second-line TB drugs play an increasingly vital role in the treatment of drug-resistant TB [14]. CS, is a second-line oral anti-TB drug, and it has outstanding anti-TB effects and many other merits [15]. It has low drug resistance rates, without cross resistance with other anti-TB drugs. Apart from that, the drug has strong tissue penetration and wide distribution, and is distributed in various tissues and body fluids. Moreover, the toxic side effects of CS on the liver are relatively mild, so that it can be used even if the liver function of patients is damaged [16,17]. Nowadays, combined medication has become a crucial medication scheme for epidemic treatment [18]. Nevertheless, there are only a few studies on CS plus anti-TB drugs in the treatment of MDR-TB [19].
In this study, we administered CS plus anti-TB drugs to patients with MDR-TB, so as to explore the influence of this treatment regimen on the sputum negative conversion rate, adverse reactions and inflammatory factors in patients.
Materials and methods
General information
Seventy patients who were referred to the Nanjing Hospital Affiliated to Nanjing University of Traditional Chinese Medicine from April 2017 to April 2020, with MDR-TB were assigned into the research group (RG) for anti-TB drugs plus CS, and the control group (CG) for conventional anti-TB drugs. The RG consisted of 38 patients (21 males and 17 females, mean age 46.86±7.14 years ranging between 30-68 years), while the CG was composed of 32 patients (20 males and 12 females, mean age 47.69±8.06 years ranging between 35-70 years).
Inclusion and exclusion criteria
Inclusion criteria
(1) Patients who met the diagnostic criteria of MDR-TB [20]; (2) The Ethics Committee of Nanjing Hospital Affiliated with Nanjing University of Traditional Chinese Medicine, approved this study, and all patients and their families were informed and signed the fully informed consent.
Exclusion criteria
(1) Patients who were allergic to the study medication; (2) Patients with severe organ diseases such as in the heart, liver and kidney, or those with malignant tumors and end-stage diseases; (3) Patients with cognitive impairment, language or hearing impairment; (4) Patients with mental illness or family history of mental illness.
Treatment methods
The CG was treated with conventional anti-TB drugs: Patients in an intensive phase were given pyrazinamide (PZA) 1.6 g/d + amikacin (AMK) 0.75 g/d + levofloxacin (LEV) 0.75 g/d + protionamide (0.5 g/d for body mass <60 kg and 0.75 g/d for body mass ≥60 kg) + p-aminosalicylic acid (PAS) 8.0 g/d for 6 months. Patients in a consolidation phase were administered with the following drugs for 18 months: PZA 1.6 g/d + LEV 0.75 g/d + protionamide (0.5 g/d for body mass <60 kg and 0.75 g/d for body mass ≥60 kg) + PAS 8.0 g/d.
The RG was treated with CS plus anti-TB drugs: Patients in an intensive phase were treated with PZA 1.6 g/d + AMK 0.75 g/d + LEV 0.75 g/d + protionamide (0.5 g/d for body mass <60 kg and 0.75 g/d for body mass ≥60 kg) + CS (0.5 g/d for body mass <60 kg and 0.7 g/d for body mass ≥60 kg) for 6 months, while those in a consolidation phase were given PZA 1.6 g/d + LEV 0.75 g/d + protionamide (0.5 g/d for body mass <60 kg and 0.75 g/d for body mass ≥60 kg) + CS (0.5 g/d for body mass <60 kg and 0.7 g/d for body mass ≥60 kg) for 18 months.
All patients took vitamin B6 orally at 150 mg/d simultaneously during treatment.
Endpoints
(1) Sputum negative conversion rate: The sputum negative conversion rates after 6, 12 and 24 months of treatment were compared between the two groups.
(2) Foci absorption rate: The foci absorption rate was compared with the evaluation criteria for the efficacy as follows: Significant absorption: X-ray examination shows that the cavity in the focus area was closed, and the absorption was >2/3. Absorption: the cavity in the lesion area was obviously reduced, and the absorption was less than 2/3. Ineffective: there was no significant change in the size and cavity of the lesion area. Deteriorated: the focus area and cavity were enlarged.
(3) Incidence of adverse reactions: The incidence of adverse reactions during treatment was compared between the two series.
(4) Inflammatory factors: Serum tumor necrosis factor-α (TNF-α), interleukin (IL)-6 and interferon (IFN)-γ levels were measured by ELISA before and after treatment strictly following the kit instructions of human TNF-α ELISA, human IL-6 ELISA and human IFN-γ ELISA (Shanghai Jingkang Bioengineering Co., Ltd., Shanghai, China, JK-(a)-1446, JK-(a)-0023 and JK-ELISA-01321).
(5) Pulmonary function (PF) indexes: Forced expiratory volume in 1 s (FEV1), FEV1/forced vital capacity (FVC) and peak expiratory flow (PEF) were recorded and compared before and after treatment.
(6) SAS and SDS scores: Self-rating Anxiety Scale (SAS) and Self-rating Depression Scale (SDS) [21] evaluated the anxiety and depression of patients in the two series before and after treatment, with 50-70 points corresponding to mild anxiety, 71-90 corresponding to moderate anxiety, and >90 corresponding to severe anxiety on a 100-point scale. With a total score of 100 points, the score and corresponding depression evaluation was 50-70 points for mild depression, 71-90 for moderate depression, and >90 for severe depression. The severity of anxiety and depression was in positive association with the corresponding score.
(7) Pittsburgh Sleep Quality Index (PSQI) score: PSQI [22] was applied for sleep quality assessment of patients before and after treatment. There are 7 components (each scoring 0-3 points) and 18 items in the scale, and the cumulative score is the total score of PSQI, which ranges from 0 to 21. Very good sleep quality: 0-5 points; Good sleep quality: 6-10 points; Fair sleep quality: 11-15 points; Poor sleep quality: 16-21 points.
Statistical methods
Statistical analysis and figure illustration were performed by SPSS 24.0 (IBM Corp, Armonk, NY, USA) and GraphPad Prism 7 respectively. Recorded as [n (%)], the counting data were compared by Chi-square test between groups and analyzed by continuity correction Chi-square in case that the theoretical frequency in Chi-square test was less than 5. Expressed as means ± standard deviation (x̅ ± sd), the measurement data between groups were compared by independent sample t-test, and the intra-comparison pre- and post-treatment was made by paired T-test. The data at different time points within the group were analyzed by one-way ANOVA, and the subsequent pairwise comparisons were carried out by SNK-q method. Differences with p-values <0.05 were considered significant.
Results
General information
Significant statistical differences were absent with respect to patient general clinical baseline data such as gender, age, body mass index (BMI), course of disease, marriage, residence, educational background, smoking history, drinking history, hypertension history, and diabetes history (P>0.05) (Table 1).
Table 1.
Comparison of general information between the two groups ([n (%)], x ± sd)
| Classification | Research group (n=38) | Control group (n=32) | t/χ2 value | P value |
|---|---|---|---|---|
| Gender | 0.374 | 0.540 | ||
| Male | 21 (55.26) | 20 (62.50) | ||
| Female | 17 (44.74) | 12 (37.50) | ||
| Age (years old) | 46.86±7.14 | 47.69±8.06 | 0.456 | 0.649 |
| BMI (kg/m2) | 23.56±3.24 | 23.04±3.02 | 0.689 | 0.492 |
| Course of disease (month) | 11.04±1.08 | 10.85±1.12 | 0.720 | 0.473 |
| Marital status | 0.537 | 0.463 | ||
| Married | 28 (73.68) | 21 (65.62) | ||
| Single or widowed | 10 (26.32) | 11 (34.38) | ||
| Residence | 0.130 | 0.717 | ||
| Urban | 15 (39.47) | 14 (43.75) | ||
| Rural | 23 (60.53) | 18 (56.25) | ||
| Educational background | 0.320 | 0.571 | ||
| ≥ High school | 18 (47.37) | 13 (40.62) | ||
| < High school | 20 (52.63) | 19 (59.38) | ||
| History of smoking | 0.160 | 0.689 | ||
| Yes | 22 (57.89) | 17 (53.12) | ||
| No | 16 (42.11) | 15 (46.88) | ||
| History of drinking | 0.576 | 0.447 | ||
| Yes | 11 (28.95) | 12 (37.50) | ||
| No | 27 (71.05) | 20 (62.50) | ||
| History of hypertension | 0.472 | 0.491 | ||
| Yes | 8 (21.05) | 9 (28.12) | ||
| No | 30 (78.95) | 23 (71.88) | ||
| History of diabetes | 0.015 | 0.900 | ||
| Yes | 10 (26.32) | 8 (25.00) | ||
| No | 28 (73.68) | 24 (75.00) |
Comparison of sputum negative conversion rate
The RG present with remarkably higher sputum negative conversion rates than the CG after 6, 12 and 24 months post treatment (P<0.05) (Table 2).
Table 2.
Comparison of sputum negative conversion rate between the two groups [n (%)]
| Groups | At 6th month after treatment | At 12th month after treatment | At 24th month after treatment |
|---|---|---|---|
| Research group (n=38) | 25 (65.79) | 35 (92.11) | 38 (100.00) |
| Control group (n=32) | 13 (40.63) | 19 (59.38) | 23 (71.88) |
| χ2 | 4.433 | 10.550 | 12.260 |
| P | 0.035 | 0.001 | <0.001 |
Comparison of foci absorption rate
The post-treatment foci absorption rate was 97.37% in the RG, which was notably higher than 68.75% in the CG (P<0.01) (Table 3).
Table 3.
Comparison of foci absorption rate between the two groups [n (%)]
| Groups | Significant absorption | Absorption | Ineffective | Deteriorated | Total absorptivity |
|---|---|---|---|---|---|
| Research group (n=38) | 27 (71.05) | 10 (26.32) | 1 (2.63) | 0 (0.00) | 37 (97.37) |
| Control group (n=32) | 9 (28.13) | 13 (40.63) | 10 (31.25) | 0 (0.00) | 22 (68.75) |
| χ2 | - | - | - | - | 10.740 |
| P | - | - | - | - | 0.001 |
Comparison of incidence of adverse reactions
The RG (18.42%) showed a noticeably lower incidence of adverse reactions than in the CG (53.13%) post treatment (P<0.01) (Table 4).
Table 4.
Comparison of incidence of adverse reactions between the two groups [n (%)]
| Groups | Gastrointestinal reaction | Hypothyroidism | Drug-induced liver injury | Central nervous system symptoms | Mental illness | Total incidence |
|---|---|---|---|---|---|---|
| Research group (n=38) | 1 (2.63) | 0 (0.00) | 1 (2.63) | 3 (7.90) | 2 (5.26) | 7 (18.42) |
| Control group (n=32) | 6 (18.75) | 4 (12.50) | 5 (15.62) | 1 (3.13) | 1 (3.13) | 17 (53.13) |
| χ2 | - | - | - | - | - | 9.286 |
| P | - | - | - | - | - | 0.002 |
Comparison of inflammatory factors
TNF-α, IL-6 and IFN-γ levels differed insignificantly between the two series before treatment, while after treatment, these parameters reduced notably and were noticeably lower in the RG than in the CG (P<0.001) (Figure 1).
Figure 1.
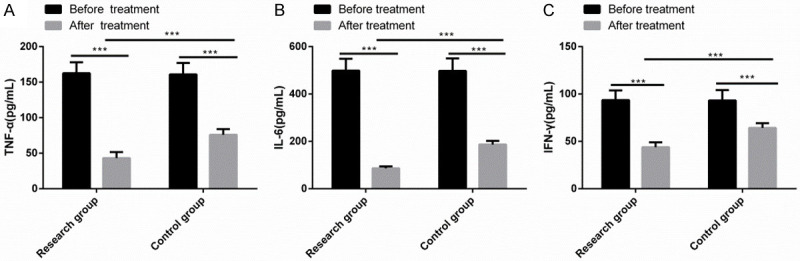
Comparison of inflammatory factors. TNF-α (A), IL-6 (B) and IFN-γ (C) levels differed insignificantly between the two series before treatment, while after treatment these parameters reduced notably and were noticeably lower in the RG than in the CG (P<0.001). Note: ***P<0.001.
Comparison of PF indexes
Significant differences were absent in FEV1, FEV1/FVC and PEF between the two series before treatment, while after treatment, these parameters increased significantly and were dramatically higher in the RG than in the CG (P<0.001) (Figure 2).
Figure 2.
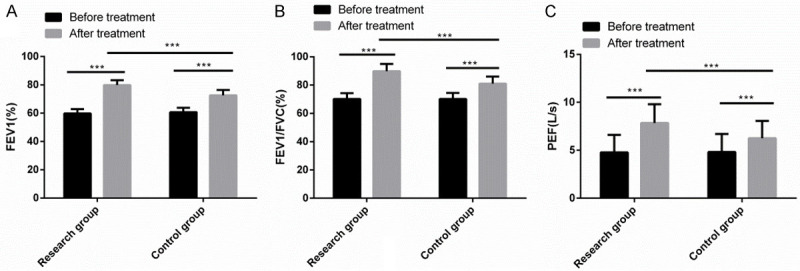
Comparison of pulmonary function indexes. There was no significant difference in FEV1, FEV1/FVC and PEF between the two groups before treatment. After treatment, FEV1, FEV1/FVC and PEF increased significantly, and levels in the research group were significantly higher than those in the control group. Note: ***P<0.001.
Comparison of SAS, SDS and PSQI scores
No significant differences were observed in SAS, SDS and PSQI scores between the two groups before treatment, but after treatment, the three scale scores declined observably, with lower parameters in the RG than in the CG (P<0.001) (Figures 3, 4).
Figure 3.
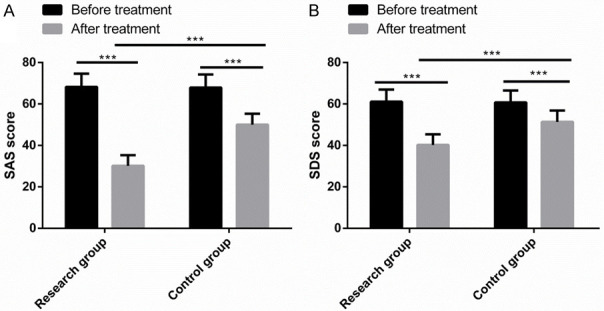
Comparison of SAS, SDS and PSQI scores. Before treatment, there was no significant difference in SAS score (A) and SDS score (B). After treatment, SAS, SDS decreased significantly, and the three scale scores in the research group were significantly lower than those in the control group. Note: ***P<0.001.
Figure 4.
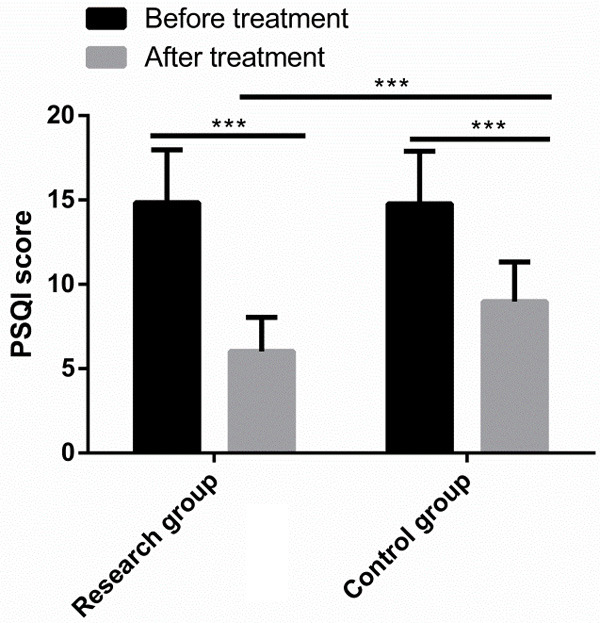
Comparison of PSQI scores. Before treatment, there was no significant difference in PSQI scores between the two groups. After treatment, PSQI scores decreased significantly in both groups, and were lower in the research group than in the control group. Note: ***P<0.001.
Discussion
The incidence of MDR-TB is increasing annually, and the clinical treatment effect is poor, with low sputum negative conversion rates and severe lung tissue destruction, which have become the Gordian knot in the clinical treatment of pulmonary TB [23,24]. Accordingly, finding new treatment schemes and drugs to improve clinical efficacy and avoid drug resistance is a pressing issue [25]. In the current research, we applied the regimen of CS plus anti-TB drugs to patients with MDR-TB and discussed its application effect, hoping to provide useful references for clinical practice.
In this study, a significantly higher sputum negative conversion rate was determined in the RG after 6, 12 and 24 months of treatment as compared to in the CG. This indicates that CS plus anti-TB drugs can effectively improve the sputum negative conversion rate of patients, which is related to the antibacterial pharmacological action of CS, that is, high sensitivity and difficulty in developing drug resistance. Li Y et al. [26] found that CS in the treatment of MDR-TB can enormously improve the sputum negative conversion rate in the consolidation phase and enhance the treatment effect of patients, which is in line with our research results. Van der Walt ML et al. [27] reported that compared with those receiving ethambutol or terizidone, patients receiving CS had evidently higher sputum negative conversion rates, lower adverse reactions, higher treatment compliance and better clinical efficacy. Besides, our research determined distinctly higher foci absorption rates and a lower incidence of adverse reactions in the RG than in the CG, suggesting that CS plus anti-TB drugs can promote foci absorption and cavity healing, and reduce the adverse reaction rate of patients. This is related to the strong tissue penetration and relatively small toxic side effects of CS, which is similar to the research results of Van der Walt ML et al. As PF of MDR-TB patients is an essential index for efficacy and prognosis evaluation of patients [28], we also evaluated the PF of patients. Significantly elevated FEV1, FEV1/FVC and PEF were found in thje RG after treatment, which were also higher than those in the CG, indicating that CS plus anti-TB drugs can effectively enhance the PF of patients. In the research of Wang J et al. [29], it was observed that CS significantly enhanced the efficacy and the PF of patients with MDR-TB, but the adverse reactions in the central nervous system in patients deserves attention, which is in line with our research results. What’s more, inflammatory factors TNF-α, IL-6 and IFN-γ were determined to be evidently decreased in the RG after treatment and were also lower than those in the CG, indicating that CS plus anti-TB drugs can validly inhibit inflammatory reactions. Basingnaa et al. [30] revealed that in patients with MDR-TB, TNF-α, IL-6 and IFN-γ levels declined dramatically after anti-TB treatment, which agrees with our research results. Most MDR-TB patients will suffer from anxiety and depression, which will adversely affect their sleep quality [31]. In our investigation, the scores of SAS SDS, and PSQI decreased statistically in the RG after treatment and were distinctly lower than those in the CG, demonstrating that CS plus anti-TB drugs can greatly relieve the patients’ anxiety and depression and improve their sleep quality. Similarly, Khanal S et al. [32] found that patients with MDR-TB had a high risk of anxiety, depression and sleep disorders, which could be effectively alleviated by social psychological support.
To sum up, while effectively improving the sputum negative conversion rate, ameliorating the prognosis, and reducing adverse reactions, CS plus anti-TB drugs for MDR-TB patients can inhibit the release of inflammatory factors, improve PF, and palliate anxiety, depression and sleep disorders. However, there are still some shortcomings to be addressed. For example, we can further analyze the influencing factors of patients’ treatment compliance, and expand the sample size to explore the credibility of clinical efficacy and adverse reactions. In the future, we will conduct research from the above perspectives.
Acknowledgements
The General project of Nanjing Municipal Health Science and Technology Development Special Fund, project name: Preliminary study on the clinical characteristics and pathogenesis of pleural tuberculoma (2019YKK18150).
Disclosure of conflict of interest
None.
References
- 1.Cardona PJ. Pathogenesis of tuberculosis and other mycobacteriosis. Enferm Infecc Microbiol Clin. 2018;36:38–46. doi: 10.1016/j.eimc.2017.10.015. [DOI] [PubMed] [Google Scholar]
- 2.de Vries G, Tsolova S, Anderson LF, Gebhard AC, Heldal E, Hollo V, Cejudo LS, Schmid D, Schreuder B, Varleva T, van der Werf MJ. Health system factors influencing management of multidrug-resistant tuberculosis in four European union countries-learning from country experiences. BMC Public Health. 2017;17:334. doi: 10.1186/s12889-017-4216-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Seaworth BJ, Griffith DE. Therapy of multidrug-resistant and extensively drug-resistant tuberculosis. Microbiol Spectr. 2017;5 doi: 10.1128/microbiolspec.tnmi7-0042-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Welekidan LN, Skjerve E, Dejene TA, Gebremichael MW, Brynildsrud O, Agdestein A, Tessema GT, Tonjum T, Yimer SA. Characteristics of pulmonary multidrug-resistant tuberculosis patients in Tigray Region, Ethiopia: a cross-sectional study. PLoS One. 2020;15:e0236362. doi: 10.1371/journal.pone.0236362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lange C, Dheda K, Chesov D, Mandalakas AM, Udwadia Z, Horsburgh CR Jr. Management of drug-resistant tuberculosis. Lancet. 2019;394:953–966. doi: 10.1016/S0140-6736(19)31882-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arockiaraj J, Karthik R, Michael JS, Amritanand R, David KS, Krishnan V, Sundararaj GD. ‘Need of the hour’: early diagnosis and management of multidrug resistant tuberculosis of the spine: an analysis of 30 patients from a “high multidrug resistant tuberculosis burden” country. Asian Spine J. 2019;13:265–271. doi: 10.31616/asj.2018.0073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gomez-Ayerbe C, Vivancos MJ, Moreno S. Multidrug-resistant tuberculosis: current epidemiology, therapeutic regimens, new drugs. Rev Esp Quimioter. 2016;29(Suppl 1):35–38. [PubMed] [Google Scholar]
- 8.Pontali E, Raviglione MC, Migliori GB and the writing group members of the Global TB Network Clinical Trials Committee. Regimens to treat multidrug-resistant tuberculosis: past, present and future perspectives. Eur Respir Rev. 2019;28:190035. doi: 10.1183/16000617.0035-2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Seung KJ, Keshavjee S, Rich ML. Multidrug-resistant tuberculosis and extensively drug-resistant tuberculosis. Cold Spring Harb Perspect Med. 2015;5:a017863. doi: 10.1101/cshperspect.a017863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Faridgohar M. Finding new ways to combat multidrug-resistant tuberculosis. Microb Drug Resist. 2020;26:71–80. doi: 10.1089/mdr.2018.0353. [DOI] [PubMed] [Google Scholar]
- 11.Sinshaw W, Kebede A, Bitew A, Tesfaye E, Tadesse M, Mehamed Z, Yenew B, Amare M, Dagne B, Diriba G, Alemu A, Getahun M, Fikadu D, Desta K, Tola HH. Prevalence of tuberculosis, multidrug resistant tuberculosis and associated risk factors among smear negative presumptive pulmonary tuberculosis patients in Addis Ababa, Ethiopia. BMC Infect Dis. 2019;19:641. doi: 10.1186/s12879-019-4241-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li Y, Sun F, Zhang W. Bedaquiline and delamanid in the treatment of multidrug-resistant tuberculosis: promising but challenging. Drug Dev Res. 2019;80:98–105. doi: 10.1002/ddr.21498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li C, Li GL, Luo Q, Li SJ, Wang RB, Lou YL, Lyu JX, Wan KL. A preliminary study on the molecular characteristics of D-cycloserine resistance of Mycobacterium tuberculosis. Zhonghua Liu Xing Bing Xue Za Zhi. 2017;38:240–243. doi: 10.3760/cma.j.issn.0254-6450.2017.02.021. [DOI] [PubMed] [Google Scholar]
- 14.Jangra MS, Chhabra M. Cycloserine induced suicidal tendencies and kanamycin induced ototoxicity in Indian MDR-TB patient: a case report. Curr Drug Saf. 2018;13:211–213. doi: 10.2174/1574886313666180605095842. [DOI] [PubMed] [Google Scholar]
- 15.Intini E, Kishore G, Richeldi L, Udwadia ZF. Neuropsychiatric reactions induced by cycloserine in the treatment of multidrug-resistant tuberculosis: what an Indian female patient tells us. BMJ Case Rep. 2019;12:e230993. doi: 10.1136/bcr-2019-230993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hwang TJ, Wares DF, Jafarov A, Jakubowiak W, Nunn P, Keshavjee S. Safety of cycloserine and terizidone for the treatment of drug-resistant tuberculosis: a meta-analysis. Int J Tuberc Lung Dis. 2013;17:1257–1266. doi: 10.5588/ijtld.12.0863. [DOI] [PubMed] [Google Scholar]
- 17.Chang MJ, Jin B, Chae JW, Yun HY, Kim ES, Lee YJ, Cho YJ, Yoon HI, Lee CT, Park KU, Song J, Lee JH, Park JS. Population pharmacokinetics of moxifloxacin, cycloserine, p-aminosalicylic acid and kanamycin for the treatment of multi-drug-resistant tuberculosis. Int J Antimicrob Agents. 2017;49:677–687. doi: 10.1016/j.ijantimicag.2017.01.024. [DOI] [PubMed] [Google Scholar]
- 18.Mulubwa M, Mugabo P. Steady-state population pharmacokinetics of terizidone and its metabolite cycloserine in patients with drug-resistant tuberculosis. Br J Clin Pharmacol. 2019;85:1946–1956. doi: 10.1111/bcp.13975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mulubwa M, Mugabo P. Amount of cycloserine emanating from terizidone metabolism and relationship with hepatic function in patients with drug-resistant tuberculosis. Drugs R D. 2019;19:289–296. doi: 10.1007/s40268-019-00281-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dheda K, Gumbo T, Maartens G, Dooley KE, Murray M, Furin J, Nardell EA, Warren RM Lancet Respiratory Medicine drug-resistant tuberculosis Commission group. The Lancet Respiratory Medicine Commission: 2019 update: epidemiology, pathogenesis, transmission, diagnosis, and management of multidrug-resistant and incurable tuberculosis. Lancet Respir Med. 2019;7:820–826. doi: 10.1016/S2213-2600(19)30263-2. [DOI] [PubMed] [Google Scholar]
- 21.Dunstan DA, Scott N, Todd AK. Screening for anxiety and depression: reassessing the utility of the Zung scales. BMC Psychiatry. 2017;17:329. doi: 10.1186/s12888-017-1489-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mollayeva T, Thurairajah P, Burton K, Mollayeva S, Shapiro CM, Colantonio A. The Pittsburgh sleep quality index as a screening tool for sleep dysfunction in clinical and non-clinical samples: a systematic review and meta-analysis. Sleep Med Rev. 2016;25:52–73. doi: 10.1016/j.smrv.2015.01.009. [DOI] [PubMed] [Google Scholar]
- 23.Marks SM, Mase SR, Morris SB. Systematic review, meta-analysis, and cost-effectiveness of treatment of latent tuberculosis to reduce progression to multidrug-resistant tuberculosis. Clin Infect Dis. 2017;64:1670–1677. doi: 10.1093/cid/cix208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.de Vos M, Derendinger B, Dolby T, Simpson J, van Helden PD, Rice JE, Wangh LJ, Theron G, Warren RM. Diagnostic accuracy and utility of fluorotype MTBDR, a new molecular assay for multidrug-resistant tuberculosis. J Clin Microbiol. 2018;56:e00531-18. doi: 10.1128/JCM.00531-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Prasad R, Gupta N, Banka A. Multidrug-resistant tuberculosis/rifampicin-resistant tuberculosis: principles of management. Lung India. 2018;35:78–81. doi: 10.4103/lungindia.lungindia_98_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li Y, Wang F, Wu L, Zhu M, He G, Chen X, Sun F, Liu Q, Wang X, Zhang W. Cycloserine for treatment of multidrug-resistant tuberculosis: a retrospective cohort study in China. Infect Drug Resist. 2019;12:721–731. doi: 10.2147/IDR.S195555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van der Walt ML, Shean K, Becker P, Keddy KH, Lancaster J. Treatment outcomes and adverse drug effects of ethambutol, cycloserine, and terizidone for the treatment of multidrug-resistant tuberculosis in South Africa. Antimicrob Agents Chemother. 2020;65:e00744-20. doi: 10.1128/AAC.00744-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Munoz-Torrico M, Rendon A, Centis R, D’Ambrosio L, Fuentes Z, Torres-Duque C, Mello F, Dalcolmo M, Perez-Padilla R, Spanevello A, Migliori GB. Is there a rationale for pulmonary rehabilitation following successful chemotherapy for tuberculosis? J Bras Pneumol. 2016;42:374–385. doi: 10.1590/S1806-37562016000000226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang J, Pang Y, Jing W, Chen W, Guo R, Han X, Wu L, Yang G, Yang K, Chen C, Jiang L, Cai C, Dou Z, Diao L, Pan H, Wang J, Du F, Xu T, Wang L, Li R, Chu N. Efficacy and safety of cycloserine-containing regimens in the treatment of multidrug-resistant tuberculosis: a nationwide retrospective cohort study in China. Infect Drug Resist. 2019;12:763–770. doi: 10.2147/IDR.S194484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Basingnaa A, Antwi-Baffour S, Nkansah DO, Afutu E, Owusu E. Plasma levels of cytokines (IL-10, IFN-gamma and TNF-alpha) in multidrug resistant tuberculosis and drug responsive tuberculosis patients in Ghana. Diseases. 2018;7:2. doi: 10.3390/diseases7010002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huque R, Elsey H, Fieroze F, Hicks JP, Huque S, Bhawmik P, Walker I, Newell J. “Death is a better option than being treated like this”: a prevalence survey and qualitative study of depression among multi-drug resistant tuberculosis in-patients. BMC Public Health. 2020;20:848. doi: 10.1186/s12889-020-08986-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Khanal S, Elsey H, King R, Baral SC, Bhatta BR, Newell JN. Development of a patient-centred, psychosocial support intervention for multi-drug-resistant tuberculosis (MDR-TB) care in Nepal. PLoS One. 2017;12:e0167559. doi: 10.1371/journal.pone.0167559. [DOI] [PMC free article] [PubMed] [Google Scholar]


