Abstract
The aim of this study is to investigate the risk factors of diabetic foot ulcer (DFU) in patients with Type 2 diabetes. Baseline characteristics of DFU-free patients with Type 2 diabetes were retrospectively collected and DFU was identified during the follow-up. Incidence of DFU was calculated and cumulative incidence was estimated by Kaplan-Meier method. Cox regression model was used to explore factors associated with DFU. A total of 980 patients were included with a median follow-up time of 28.7 months. 259 (26.4%) patients developed DFU with an incidence rate of 11.3 per 100 person-years. The cumulative incidences of DFU at 1 year and 2 years during the follow-up were 5.4% (95% CI 3.9-6.9%) and 14.1% (95% CI 11.7-16.5%), respectively. Cox regression analysis indicated that factors associated with developing DFU included age (hazard ratio (HR)=1.06, 95% CI 1.05-1.07, per 1-year increase), body mass index (HR=1.05, 95% CI 1.02-1.07), higher level of education (HR=0.77, 95% CI 0.60-0.98), hypertension (HR=1.90, 95% CI 1.47-2.45), hyperlipidemia (HR=2.63, 95% CI 2.02-3.43), coronary heart disease (HR=2.88, 95% CI 2.22-3.75), heart failure (HR=2.47, 95% CI 1.91-3.20), stroke (HR=2.44, 95% CI 1.86-3.19), diabetic retinopathy (HR=1.86, 95% CI 1.40-2.48), diabetic kidney disease (HR=1.89, 95% CI 1.41-2.53), diabetic neuropathy (HR=1.73, 95% CI 1.31-2.30), poor glycemic control (HR=1.13, 95% CI 1.07-1.19, per 1% glycosylated hemoglobin increase), and course of diabetes (HR=1.01, 95% CI 1.00-1.01, per 1-month increase). The results showed a relatively high incidence of DFU, and revealed several baseline characteristics identified as risk factors of developing DFU.
Keywords: Diabetes mellitus, diabetic foot ulcer, incidence, risk factors, cohort studies
Introduction
Foot ulceration is a rather common complication of diabetes that affects the lower extremities [1]. It is estimated that about 34% patients with diabetes (either Type 1 or Type 2) develop foot ulcer in their lifetime [2]. As a major cause of morbidity, it is reported that about two-thirds of all nontraumatic amputations performed in the United States were due to diabetic foot ulcer (DFU), and about 25% hospitalizations among patients with diabetes were related to infected or ischemic DFU [3,4]. At the same time, diabetic patients with DFU also have poor prognosis, which is associated with a 2.5-fold risk of death compared with those without DFU [5]. Recent report indicates that the 1-, 2-, and 5-year survival of patients with DFU was 81%, 69%, and 29%, respectively [6].
Although considerable advances were made over the past two decades [7] and there are several relevant national and international guidances [8,9], DFU still remains a major health care problem [10,11], and one of the reasons is that DFU is widely unappreciated. Based on current grading schemes, the more severe the ulcers, the worse the prognosis [12-14]. The severity of ulcer is found to be associated with the time to first expert assessment, suggesting that the longer the elapsed time to expert assessment, the more severe the ulcers and the worse the clinical outcomes [15]. Investigations indicate that delayed diagnosis and treatment of DFU is not rare in real practice [16,17]. In addition, researches on DFU are also limited compared to other diabetes complications. Given these, to increase early diagnosis and treatment might be the currently most practical way to improve prognosis of DFU.
Several risk factors were identified for DFU, such as peripheral neuropathy, diabetic retinopathy, and diabetic nephropathy [18]. For some factors, such as hypertension, however, controversial findings were reported between studies [19,20], suggesting that more researches are necessary. To provide additional evidence about risk factors for developing DFU, the study aimed to investigate the risk factors of DFU in a cohort of patients with Type 2 diabetes.
Methods
Patients
The study included DFU-free patients with Type 2 diabetes. We used the following inclusive and exclusive criteria. Inclusive criteria: (1) patients who once visited the diabetic clinic of Wuhan University People’s Hospital (Hanchuan People’s Hospital) between January 1 2015 and December 31 2016; (2) patients with a diagnosis record of Type 2 diabetes (identified by screening hospitalization records in the diabetic clinic); (3) the first available hospitalization record for patients who had more than 1 hospitalization record between January 1 2015 and December 31 2016. Exclusive criteria: (1) patients who had any diagnosis records of DFU in the hospitalization records within 30 days after the baseline hospitalization; (2) patients who did not have any other hospital admission records after the baseline hospitalization until December 31 2019. The inclusion of the study population was shown in Figure 1. The study received approval from the Institutional review board of Wuhan University People’s Hospital (Hanchuan People’s Hospital) and informed consent was waived.
Figure 1.
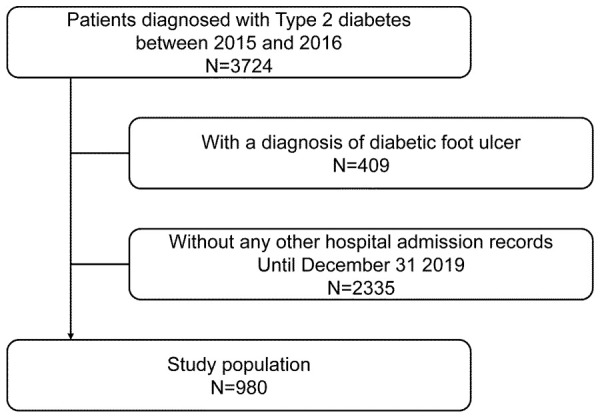
Inclusion of the study population.
Baseline characteristics
We collected the below baseline characteristics by screening the record of the baseline hospitalization: age, sex, body mass index (BMI), education (lower, or equal to or higher than high school), comorbid hypertension, hyperlipidemia, coronary heart disease, heart failure, stroke, diabetic retinopathy, diabetic kidney disease, diabetic neuropathy, admission hemoglobin A1C, course of diabetes, and type of treatment (oral hypoglycemic drugs, insulin, or both). All the baseline characteristics were retrieved directly from the recorded data via free text.
Clinical outcome
DFU was the clinical outcome of the study, which was identified by screening data retrieved from electronic health records of Wuhan University People’s Hospital (Hanchuan People’s Hospital) up to December 31 2019 via free text. If a patient had DFU diagnosis record(s) in hospitalization records, the admission date of the first hospitalization would be considered as the date of diagnosis of DFU. If no DFU diagnosis record was found until December 31 2019, the patient will be censored at the date of admission of the last hospitalization.
Statistical analysis
Continuous variables were presented as mean ± standard deviation (SD) or median and inter quartile range; categorical variables were presented as frequency and percentages. Comparisons between two groups were examined by student t test or Kruskal-Wallis H test for continuous variables, and Chi-squared test or fisher’s exact test for categorical variables. The incidence rate of DFU was calculated by dividing the total number of cases to the total observation time. Cumulative incidence of DFU for the entire cohort was estimated by Kaplan-Meier method. Univariable Cox regression analysis was used to explore factors associated with DFU. A P value <0.05 was declared to be statistical significance.
Results
Baseline characteristics
A total of 980 patients were included retrospectively with a median follow-up time of 28.7 (15.2-41.1) months. The average age was 61.49±12.38 years and 47.55% were female. The average BMI was 25.06±5.69 kg/m2 and 60.82% had an education level of high school or above. Hypertension (49.39%) and hyperlipidemia (48.06%) were the most two frequent comorbidities. The patients had an average Hemoglobin A1C of 7.97±2.35% and a median course of diabetes of 139 (60-238) months, and oral hypoglycemic drugs (37.76%) was the most frequent treatment (Table 1). Compared with patients with a course of diabetes less than 10 years, patients with a course of diabetes ≥10 years had a higher average age (64.81±10.50 versus 57.34±13.28 years, P<0.001), and more comorbidities (Table 2).
Table 1.
Baseline characteristic of the study population
| Variable | Statistics |
|---|---|
| Age (years) | 61.49±12.38 |
| Sex | |
| Male | 514 (52.45%) |
| Female | 466 (47.55%) |
| BMI (kg/m2) | 25.06±5.69 |
| Education | |
| Lower than High school | 384 (39.18%) |
| High school or above | 596 (60.82%) |
| Calendar year | |
| 2015 | 502 (51.22%) |
| 2016 | 478 (48.78%) |
| Comorbidities | |
| Hypertension | 484 (49.39%) |
| Hyperlipidemia | 471 (48.06%) |
| Coronary heart disease | 429 (43.78%) |
| Heart failure | 437 (44.59%) |
| Stroke | 142 (14.49%) |
| Diabetic retinopathy | 150 (15.31%) |
| Diabetic kidney disease | 133 (13.57%) |
| Diabetic neuropathy | 149 (15.20%) |
| Hemoglobin A1C (%) | 7.97±2.35 |
| Course of diabetes (months) | 139 (60-238) |
| Type of treatment | |
| Oral hypoglycemic drugs | 370 (37.76%) |
| Insulin | 272 (27.76%) |
| Combination | 338 (34.49%) |
Abbreviation: BMI, body mass index.
Table 2.
Baseline characteristic of the study population stratified by course of diabetes
| Variable | <10 years (n=436) | ≥10 years (n=544) | P value |
|---|---|---|---|
| Age (years) | 57.34±13.28 | 64.81±10.50 | <0.001 |
| Sex | 0.284 | ||
| Male | 237 (54.36%) | 277 (50.92%) | |
| Female | 199 (45.64%) | 267 (49.08%) | |
| BMI (kg/m2) | 25.06±5.76 | 25.05±5.65 | 0.990 |
| Education | 0.302 | ||
| Lower than High school | 163 (37.39%) | 221 (40.62%) | |
| High school or above | 273 (62.61%) | 323 (59.38%) | |
| Calendar year | 0.113 | ||
| 2015 | 211 (48.39%) | 291 (53.49%) | |
| 2016 | 225 (51.61%) | 253 (46.51%) | |
| Comorbidities | |||
| Hypertension | 206 (47.25%) | 278 (51.10%) | 0.230 |
| Hyperlipidemia | 185 (42.43%) | 286 (52.57%) | 0.002 |
| Coronary heart disease | 169 (38.76%) | 260 (47.79%) | 0.005 |
| Heart failure | 158 (36.24%) | 279 (51.29%) | <0.001 |
| Stroke | 41 (9.40%) | 101 (18.57%) | <0.001 |
| Diabetic retinopathy | 39 (8.94%) | 111 (20.40%) | <0.001 |
| Diabetic kidney disease | 48 (11.01%) | 85 (15.62%) | 0.036 |
| Diabetic neuropathy | 32 (7.34%) | 117 (21.51%) | <0.001 |
| Hemoglobin A1C (%) | 7.99±2.31 | 7.95±2.38 | 0.795 |
| Course of diabetes (months) | 52.50 (26.00-86.00) | 226.50 (170.00-280.25) | <0.001 |
| Type of treatment | <0.001 | ||
| Oral hypoglycemic drugs | 208 (47.71%) | 162 (29.78%) | |
| Insulin | 139 (31.88%) | 133 (24.45%) | |
| Combination | 89 (20.41%) | 249 (45.77%) |
Abbreviation: BMI, body mass index.
Occurrence of DFU during the follow-up
259 (26.4%) patients developed DFU with an incidence rate of 11.3 per 100 person-years. The cumulative incidences of DFU at 6 months, 1 year, and 2 years during the follow-up were 2.2% (95% CI 1.3-3.1%), 5.4% (95% CI 3.9-6.9%), and 14.1% (95% CI 11.7-16.5%), respectively, which were estimated by Kaplan-Meier curves (Figure 2).
Figure 2.
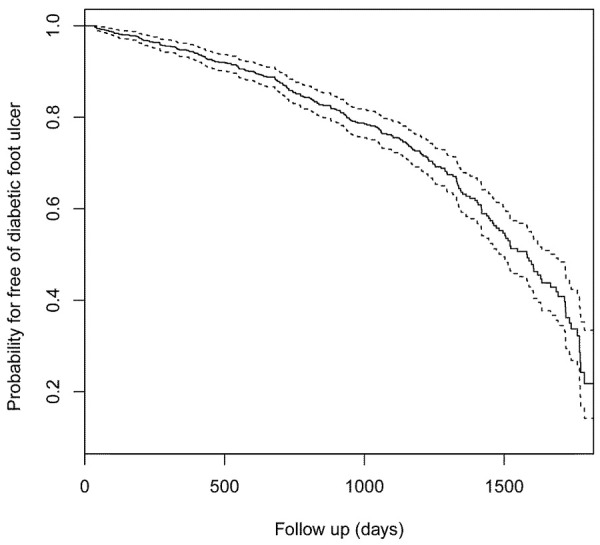
Kaplan-Meier curves displaying the estimated probability for free of diabetic foot ulcer. The dashed lines indicated the 95% confidence interval.
Risk factors associated with DFU
Cox regression analysis (Table 3) indicated that factors associated with developing DFU included elderly (hazard ratio (HR)=1.06, 95% CI 1.05-1.07, per 1-year increase), body mass index (HR=1.05, 95% CI 1.02-1.07, per 1-kg/m2 increase), higher level of education (HR 0.77, 95% CI 0.60-0.98), hypertension (HR 1.90, 95% CI 1.47-2.45), hyperlipidemia (HR 2.63, 95% CI 2.02-3.43), coronary heart disease (HR 2.88, 95% CI 2.22-3.75), heart failure (HR 2.47, 95% CI 1.91-3.20), stroke (HR 2.44, 95% CI 1.86-3.19), diabetic retinopathy (HR 1.86, 95% CI 1.40-2.48), diabetic kidney disease (HR 1.89, 95% CI 1.41-2.53), diabetic neuropathy (HR 1.73, 95% CI 1.31-2.30), poor glycemic control (HR 1.13, 95% CI 1.07-1.19, per 1% glycosylated hemoglobin increase), and course of diabetes (HR 1.01, 95% CI 1.00-1.01, per 1-month increase).
Table 3.
Factors associated with DFU
| Variable | Hazard ratio | 95% confidence interval | P value |
|---|---|---|---|
| Age (years) | 1.06 | 1.05-1.07 | <0.001 |
| Sex | |||
| Male | Reference | ||
| Female | 1.18 | 0.93-1.51 | 0.175 |
| BMI (kg/m2) | 1.05 | 1.02-1.07 | <0.001 |
| Education | |||
| Lower than High school | Reference | ||
| High school or above | 0.77 | 0.60-0.98 | 0.036 |
| Hypertension | |||
| No | Reference | ||
| Yes | 1.90 | 1.47-2.45 | <0.001 |
| Hyperlipidemia | |||
| No | Reference | ||
| Yes | 2.63 | 2.02-3.43 | <0.001 |
| Coronary heart disease | |||
| No | Reference | ||
| Yes | 2.88 | 2.22-3.75 | <0.001 |
| Heart failure | |||
| No | Reference | ||
| Yes | 2.47 | 1.91-3.20 | <0.001 |
| Stroke | |||
| No | Reference | ||
| Yes | 2.44 | 1.86-3.19 | <0.001 |
| Diabetic retinopathy | |||
| No | Reference | ||
| Yes | 1.86 | 1.40-2.48 | <0.001 |
| Diabetic kidney disease | |||
| No | Reference | ||
| Yes | 1.89 | 1.41-2.53 | <0.001 |
| Diabetic neuropathy | |||
| No | Reference | ||
| Yes | 1.73 | 1.31-2.30 | 0.001 |
| Hemoglobin A1C (%) | 1.13 | 1.07-1.19 | <0.001 |
| Course of diabetes (months) | 1.01 | 1.00-1.01 | <0.001 |
| Type of treatment | |||
| Oral hypoglycemic drugs | Reference | ||
| Insulin | 0.84 | 0.60-1.16 | 0.280 |
| Combination | 1.27 | 0.96-1.67 | 0.095 |
Abbreviation: BMI, body mass index.
Discussion
This study investigated the incidence of DFU and risk factors associated with DFU among a cohort of patients with prevalent Type 2 diabetes. In this study, among 980 individual Type 2 diabetic patients, 259 (26.4%) developed DFU during the follow-up with an incidence rate of 11.3 per 100 person-years. Several baseline characteristics including an older age, a higher BMI, lower level of education, poor glycemic control evaluated by Hemoglobin A1C, and various comorbidities were found to be associated with an increased risk of developing DFU. These findings might further raise clinicians’ awareness of DFU and help to promote its early diagnosis.
The incidences of DFU have been reported in various studies. Adem et al [21] reported that the incidence of DFU was 4 cases per 100 person-years of observation in a cohort of patients with newly diagnosed diabetes from a hospital in Ethiopia. Iwase et al [22] investigate patients with type 2 diabetes attending an outpatient diabetes clinic in Japan and report a DFU incidence rate of 0.29/100 person-years. Abbott et al [23] reported an average annual incidence of 2.2% in a community-based patient cohort from United Kingdom. Compared with these reported incidences, the incidence in our study (11.3 per 100 person-years) was much higher. This could be related to the different study population in our study, where prevalent Type 2 diabetic patients from a hospital with a median course of diabetes of about 10 years were studied. In our study, when the courses of diabetes were counted into observation time, the incidence of DFU was about 3.5 cases per 100 person-years (data not shown above), which was quite close to the incidences reported by Adem et al [21]. In addition, differences in diabetic care between countries might also explain the differences in the reported incidences [24].
Several baseline characteristics were found to be associated with an increased risk of developing DFU. We found the risk of DFU was increased with the increase of age, which was consistent with the findings from other studies [25,26], but since our study only investigated prevalent diabetic patients, we were unable to study the association between the age of the onset of diabetes and DFU. We found there was no significant association between sex and DFU, and this was consistent with the study from Dinh et al [27], which suggests that women has the same risk of developing DFU as men when they have neuropathy or other risk factors. Increased BMI was associated with increased risk of DFU, which was also reported by other studies [21,28,29], with the hypothesis that obesity might increase atherosclerosis and decrease blood supply to lower extremities [21]. We found patients with higher level of education might have lower risk of DFU compared with patients with lower level of education. This variable was rarely investigated in similar studies, but the mechanism behind it could be that patients with higher level of education might receive diabetic education better which is proved to be associated with lower risk of DFU [30]. The several comorbidities we studied were all associated with increased risk of developing DFU, including hypertension, hyperlipidemia, coronary heart disease, heart failure, stroke, diabetic retinopathy, diabetic kidney disease, and diabetic neuropathy. In the study from Yazdanpanah et al [31], dyslipidemia was also reported as a risk factor for developing DFU. The mechanisms could be that these comorbidities shared some of pathogenesis of DFU [32]. Poor glycemic control evaluated by Hemoglobin A1C and longer course of diabetes are also reported as risk factors of DFU in other studies [33,34]. Insulin consumption is found as a risk factor for DFU [35], while the association was not statistically significant in our study. This might be due to the categorization of types of treatment in our study, since it could be observed that the combination of oral hypoglycemic drugs and insulin showed a hazard ratio of 1.27 which though not significant, was toward the direction of a risk factor.
The study had some limitations. First, the study used retrospectively collected data mainly based on free text, which might raise concerns about the validity of the diagnosis of DFU and several comorbidities studied. Second, the follow-up for the study outcome was based on hospitalization records, which meant we might include a study population with worse health status. Third, we only studied the baseline characteristics as potential risk factors of DFU, but some of the characteristics might change with time such as Hemoglobin A1C. Last, some other variables were not included in the study, which could also be risk factors of DFU, such as foot deformity.
In conclusion, the study observed relatively high incidences of DFU in a cohort of hospitalized patients with type 2 diabetes, and identified several baseline characteristics as risk factors of developing DFU. It provided information for health care providers, but further studies were still needed to reveal the mechanisms about the associations.
Disclosure of conflict of interest
None.
References
- 1.Armstrong DG, Boulton AJM, Bus SA. Diabetic foot ulcers and their recurrence. N Engl J Med. 2017;376:2367–2375. doi: 10.1056/NEJMra1615439. [DOI] [PubMed] [Google Scholar]
- 2.Boulton AJ, Armstrong DG, Albert SF, Frykberg RG, Hellman R, Kirkman MS, Lavery LA, Lemaster JW, Mills JL Sr, Mueller MJ, Sheehan P, Wukich DK American Diabetes Association; American Association of Clinical Endocrinologists. Comprehensive foot examination and risk assessment: a report of the task force of the foot care interest group of the American Diabetes Association, with endorsement by the American Association of Clinical Endocrinologists. Diabetes Care. 2008;31:1679–1685. doi: 10.2337/dc08-9021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ramsey SD, Newton K, Blough D, McCulloch DK, Sandhu N, Reiber GE, Wagner EH. Incidence, outcomes, and cost of foot ulcers in patients with diabetes. Diabetes Care. 1999;22:382–387. doi: 10.2337/diacare.22.3.382. [DOI] [PubMed] [Google Scholar]
- 4.Gregg EW, Sorlie P, Paulose-Ram R, Gu Q, Eberhardt MS, Wolz M, Burt V, Curtin L, Engelgau M, Geiss L 1999-2000 national health and nutrition examination survey. Prevalence of lower-extremity disease in the US adult population ≥40 years of age with and without diabetes: 1999-2000 national health and nutrition examination survey. Diabetes Care. 2004;27:1591–1597. doi: 10.2337/diacare.27.7.1591. [DOI] [PubMed] [Google Scholar]
- 5.Walsh JW, Hoffstad OJ, Sullivan MO, Margolis DJ. Association of diabetic foot ulcer and death in a population-based cohort from the United Kingdom. Diabet Med. 2016;33:1493–1498. doi: 10.1111/dme.13054. [DOI] [PubMed] [Google Scholar]
- 6.Brennan MB, Hess TM, Bartle B, Cooper JM, Kang J, Huang ES, Smith M, Sohn MW, Crnich C. Diabetic foot ulcer severity predicts mortality among veterans with type 2 diabetes. J Diabetes Complications. 2017;31:556–561. doi: 10.1016/j.jdiacomp.2016.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sattar N. Advances in the clinical management of type 2 diabetes: a brief history of the past 15 years and challenges for the future. BMC Med. 2019;17:46. doi: 10.1186/s12916-019-1281-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schaper NC, van Netten JJ, Apelqvist J, Bus SA, Hinchliffe RJ, Lipsky BA IWGDF Editorial Board. Practical guidelines on the prevention and management of diabetic foot disease (IWGDF 2019 update) Diabetes Metab Res Rev. 2020;36(Suppl 1):e3266. doi: 10.1002/dmrr.3266. [DOI] [PubMed] [Google Scholar]
- 9.Hingorani A, LaMuraglia GM, Henke P, Meissner MH, Loretz L, Zinszer KM, Driver VR, Frykberg R, Carman TL, Marston W, Mills JL, Murad MH. The management of diabetic foot: a clinical practice guideline by the Society for vascular surgery in collaboration with the American Podiatric Medical Association and the Society for Vascular Medicine. J Vasc Surg. 2016;63(Suppl):3S–21S. doi: 10.1016/j.jvs.2015.10.003. [DOI] [PubMed] [Google Scholar]
- 10.Chun DI, Kim S, Kim J, Yang HJ, Kim JH, Cho JH, Yi Y, Kim WJ, Won SH. Epidemiology and burden of diabetic foot ulcer and peripheral arterial disease in Korea. J Clin Med. 2019;8:748. doi: 10.3390/jcm8050748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Raghav A, Khan ZA, Labala RK, Ahmad J, Noor S, Mishra BK. Financial burden of diabetic foot ulcers to world: a progressive topic to discuss always. Ther Adv Endocrinol Metab. 2018;9:29–31. doi: 10.1177/2042018817744513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Armstrong DG, Lavery LA, Harkless LB. Validation of a diabetic wound classification system. The contribution of depth, infection, and ischemia to risk of amputation. Diabetes Care. 1998;21:855–859. doi: 10.2337/diacare.21.5.855. [DOI] [PubMed] [Google Scholar]
- 13.Ince P, Abbas ZG, Lutale JK, Basit A, Ali SM, Chohan F, Morbach S, Mollenberg J, Game FL, Jeffcoate WJ. Use of the SINBAD classification system and score in comparing outcome of foot ulcer management on three continents. Diabetes Care. 2008;31:964–967. doi: 10.2337/dc07-2367. [DOI] [PubMed] [Google Scholar]
- 14.Mills JL Sr, Conte MS, Armstrong DG, Pomposelli FB, Schanzer A, Sidawy AN, Andros G Society for Vascular Surgery Lower Extremity Guidelines Committee. The society for vascular surgery lower extremity threatened limb classification system: risk stratification based on wound, ischemia, and foot infection (WIfI) J Vasc Surg. 2014;59:220–234. e1–2. doi: 10.1016/j.jvs.2013.08.003. [DOI] [PubMed] [Google Scholar]
- 15.Smith-Strom H, Iversen MM, Igland J, Ostbye T, Graue M, Skeie S, Wu B, Rokne B. Severity and duration of diabetic foot ulcer (DFU) before seeking care as predictors of healing time: a retrospective cohort study. PLoS One. 2017;12:e0177176. doi: 10.1371/journal.pone.0177176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Manu C, Lacopi E, Bouillet B, Vouillarmet J, Ahluwalia R, Ludemann C, Garcia-Klepzig JL, Meloni M, De Buruaga VR, Sanchez-Rios JP, Edmonds M, Apelqvist J, Lazaro-Martinez JL, Van Acker K. Delayed referral of patients with diabetic foot ulcers across Europe: patterns between primary care and specialised units. J Wound Care. 2018;27:186–192. doi: 10.12968/jowc.2018.27.3.186. [DOI] [PubMed] [Google Scholar]
- 17.Mills JL, Beckett WC, Taylor SM. The diabetic foot: consequences of delayed treatment and referral. South Med J. 1991;84:970–974. [PubMed] [Google Scholar]
- 18.Banik PC, Barua L, Moniruzzaman M, Mondal R, Zaman F, Ali L. Risk of diabetic foot ulcer and its associated factors among Bangladeshi subjects: a multicentric cross-sectional study. BMJ Open. 2020;10:e034058. doi: 10.1136/bmjopen-2019-034058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cardoso HC, Zara ALSA, Rosa SSRF, Rocha GA, Rocha JVC, de Araújo MCE, Quinzani PF, Barbosa YP, Mrué F. Risk factors and diagnosis of diabetic foot ulceration in users of the Brazilian public health system. J Diabetes Res. 2019;2019:5319892. doi: 10.1155/2019/5319892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alex R, Ratnaraj B, Winston B, Samson Devakiruba DN, Samuel C, John J, Mohan VR, Prasad JH, Jacob K. Risk factors for foot ulcers in patients with diabetes mellitus - a short report from Vellore, South India. Indian J Community Med. 2010;35:183–185. doi: 10.4103/0970-0218.62582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Adem AM, Andargie AA, Teshale AB, Wolde HF. Incidence of diabetic foot ulcer and its predictors among diabetes mellitus patients at Felege Hiwot Referral Hospital, Bahir Dar, Northwest Ethiopia: a retrospective follow-up study. Diabetes Metab Syndr Obes. 2020;13:3703–3711. doi: 10.2147/DMSO.S280152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Iwase M, Fujii H, Nakamura U, Ohkuma T, Ide H, Jodai-Kitamura T, Sumi A, Komorita Y, Yoshinari M, Kitazono T. Incidence of diabetic foot ulcer in Japanese patients with type 2 diabetes mellitus: the Fukuoka diabetes registry. Diabetes Res Clin Pract. 2018;137:183–189. doi: 10.1016/j.diabres.2018.01.020. [DOI] [PubMed] [Google Scholar]
- 23.Abbott CA, Carrington AL, Ashe H, Bath S, Every LC, Griffiths J, Hann AW, Hussein A, Jackson N, Johnson KE, Ryder CH, Torkington R, Van Ross ER, Whalley AM, Widdows P, Williamson S, Boulton AJ North-West Diabetes Foot Care Study. The North-West Diabetes Foot Care Study: incidence of, and risk factors for, new diabetic foot ulceration in a community-based patient cohort. Diabet Med. 2002;19:377–384. doi: 10.1046/j.1464-5491.2002.00698.x. [DOI] [PubMed] [Google Scholar]
- 24.Guell C, Unwin N. Barriers to diabetic foot care in a developing country with a high incidence of diabetes related amputations: an exploratory qualitative interview study. BMC Health Serv Res. 2015;15:377. doi: 10.1186/s12913-015-1043-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fawzy MS, Alshammari MA, Alruwaili AA, Alanazi RTR, Alharbi JAM, Almasoud AMR, Alshammari RA, Toraih EA. Factors associated with diabetic foot among type 2 diabetes in Northern area of Saudi Arabia: a descriptive study. BMC Res Notes. 2019;12:51. doi: 10.1186/s13104-019-4088-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sarinnapakorn V, Sunthorntepwarakul T, Deerochanawong C, Niramitmahapanya S, Napartivaumnuay N. Prevalence of diabetic foot ulcers and risk classifications in type 2 diabetes mellitus patients at Rajavithi Hospital. J Med Assoc Thai. 2016;99(Suppl 2):S99–105. [PubMed] [Google Scholar]
- 27.Dinh T, Veves A. The influence of gender as a risk factor in diabetic foot ulceration. Wounds. 2008;20:127–131. [PubMed] [Google Scholar]
- 28.Mariam TG, Alemayehu A, Tesfaye E, Mequannt W, Temesgen K, Yetwale F, Limenih MA. Prevalence of diabetic foot ulcer and associated factors among adult diabetic patients who attend the diabetic follow-up clinic at the University of Gondar Referral Hospital, North West Ethiopia, 2016: institutional-based cross-sectional study. J Diabetes Res. 2017;2017:2879249. doi: 10.1155/2017/2879249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sarfo-Kantanka O, Kyei I, Mbanya JC, Owusu-Ansah M. Diabetes-related foot disorders among adult Ghanaians. Diabet Foot Ankle. 2018;9:1511678. doi: 10.1080/2000625X.2018.1511678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mohammad NA, Khresheh RM. Evaluate the effect of education interventions in the prevention of diabetic foot ulcers through knowledge of the disease and self-care practices in Saudi Arabia. Open Access Maced J Med Sci. 2018;6:2206–2213. doi: 10.3889/oamjms.2018.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yazdanpanah L, Shahbazian H, Nazari I, Hesam S, Ahmadi F, Cheraghian B, Arti HR, Mohammadianinejad SE. Risk factors associated with diabetic foot ulcer-free survival in patients with diabetes. Diabetes Metab Syndr. 2018;12:1039–1043. doi: 10.1016/j.dsx.2018.06.020. [DOI] [PubMed] [Google Scholar]
- 32.Tuttolomondo A, Maida C, Pinto A. Diabetic foot syndrome as a possible cardiovascular marker in diabetic patients. J Diabetes Res. 2015;2015:268390. doi: 10.1155/2015/268390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xiang J, Wang S, He Y, Xu L, Zhang S, Tang Z. Reasonable glycemic control would help wound healing during the treatment of diabetic foot ulcers. Diabetes Ther. 2019;10:95–105. doi: 10.1007/s13300-018-0536-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Al-Rubeaan K, Al Derwish M, Ouizi S, Youssef AM, Subhani SN, Ibrahim HM, Alamri BN. Diabetic foot complications and their risk factors from a large retrospective cohort study. PLoS One. 2015;10:e0124446. doi: 10.1371/journal.pone.0124446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yazdanpanah L, Shahbazian H, Nazari I, Arti HR, Ahmadi F, Mohammadianinejad SE, Cheraghian B, Hesam S. Incidence and risk factors of diabetic foot ulcer: a population-based diabetic foot cohort (ADFC Study)-two-year follow-up study. Int J Endocrinol. 2018;2018:7631659. doi: 10.1155/2018/7631659. [DOI] [PMC free article] [PubMed] [Google Scholar]


